Abstract
Cardiac tissue engineering offers the promise of creating functional tissue replacements for use in the failing heart or for in vitro drug screening. The last decade has seen a great deal of progress in this field with new advances in interdisciplinary areas such as developmental biology, genetic engineering, biomaterials, polymer science, bioreactor engineering, and stem cell biology. We review here a selection of the most recent advances in cardiac tissue engineering, including the classical cell-scaffold approaches, advanced bioreactor designs, cell sheet engineering, whole organ decellularization, stem-cell based approaches, and topographical control of tissue organization and function. We also discuss current challenges in the field, such as maturation of stem cell-derived cardiac patches and vascularization.
Motivation for cardiac tissue engineering
Cardiovascular diseases (CVD) accounted for 34% of all deaths in the United States with an associated cost of an alarming $503.2 billion in 2010 alone [1]. These staggering figures greatly motivate research into new therapeutic interventions. In terms of myocardial infarction (MI) and heart failure, conventional treatment options are limited by the inability of myocardium to regenerate after injury and a shortage of donor organs available for transplantation. Recently, tissue- and cell-based strategies have come to the forefront as viable alternatives for treatment of heart disease. One of these novel approaches is cardiac tissue engineering.
Main cardiac tissue engineering approaches
In vitro approaches are aimed at organizing cardiomyocytes into a functional tissue; specifically, one capable of generating force during contraction (2-4 mN/mm2) and propagating electrical signals (~25cm/s). To be clinically relevant, an engineered cardiac tissue must have functional and morphological properties similar to that of native myocardium and remain viable after implantation. One of the main challenges identified during in vitro cultivation was that oxygen diffusion, coupled with a high oxygen demand by cardiomyocytes, limited the viable tissue thickness to ~200μm [2]. To provide sufficient oxygen supply, perfusion bioreactors delivering culture medium supplemented with an oxygen carrier were utilized in conjunction with channeled poly(glycerol sebacate) [3] scaffolds to engineer thick and compact cardiac constructs. Second generation perfusion bioreactors incorporate electrical stimulation [4] or mechanical stimulation [5] into a single system.
In a pioneering approach, Eschenhagen and Zimmermann generated an engineered heart tissue (EHT) by seeding a mix of collagen I, extracellular matrix proteins (Matrigel), and neonatal rat cardiomyocytes into lattices or circular molds. Upon spontaneous remodeling of the liquid reconstitution mixture and cyclic mechanical stimulation, spontaneously and synchronously contracting solid EHTs were generated after one to two weeks of cultivation [6]. These studies clearly demonstrated the importance of physical stimuli in improving morphological, functional, and mechanical properties of the EHT. By stacking three of these ring-shaped constructs into a flower-like structure followed by auxotonic mechanical stimulation and implantation in a rat MI model (Figure 1(A-E)), electrophysiological studies indicated electrical coupling with the native tissue and improved diastolic and systolic function compared to the sham-operated rats [7]. This landmark study clearly demonstrated for the first time that implantation of EHTs can result in functional improvement upon MI. Alternatively, suprathreshold electrical field stimulation (Figure 1(F-I)) can be utilized to induce synchronous contractions of cardiomyocytes in porous collagen scaffolds resulting in the formation of mature myocardium with elongated, viable cells aligned in parallel [8,9,10].
Figure 1. Main cardiac tissue engineering approahces.
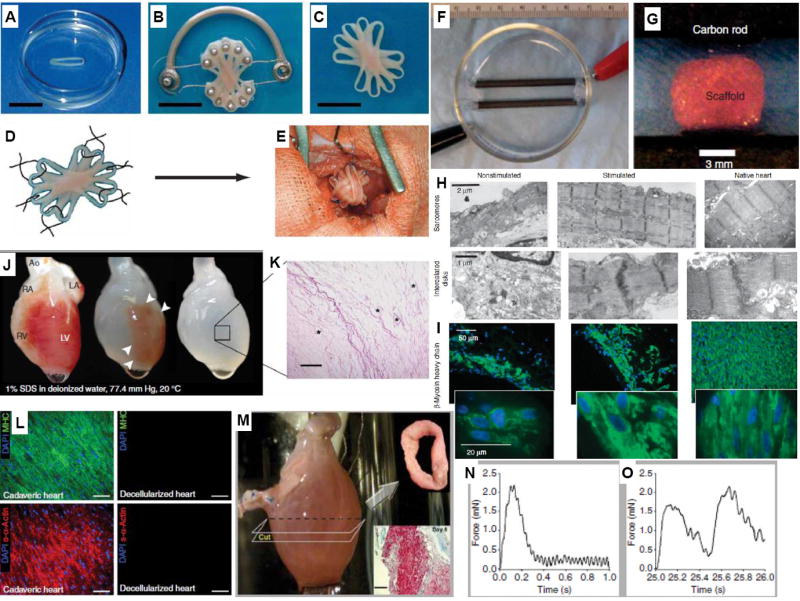
(A) Stacking five single EHTs (B) on a custom-made device facilitated EHT fusion and allowed contractions under auxotonic load resulting in synchronously contracting (C) multiloop EHTs ready for in vivo engraftment. (D) Six single-knot sutures served to fix multiloop EHTs on (E) the recipient hearts ((A-E), with permission from [7] ). (F) Photograph of an assembled electrical stimulation chamber developed by Radisic and colleagues for applying pulsatile electrical field stimuli to (G) cardiac cells seeded on scaffolds. (H) Representative transmission electron micrographs of stimulated and non-stimulated constructs after 8 days of cultivation, compared with neonatal rat ventricles. (I) Stimulated constructs and neonatal ventricles contained higher levels of β-Myosin heavy chain staining compared to non-stimulated constructs ((F-I), with permission from [8]). (J) Decellularized cadaveric rat heart. (K) H&E staining indicates no intact cells or nuclei with large vascular conduits maintained (black asterisks) Scale bar, 200 μm. (L) Immunofluorescence staining of cadaveric and SDS-decellularized rat heart thin sections showing the presence or absence, respectively, of DAPI-positive nuclei (purple), cardiac α-myosin heavy chain (green) and sarcomeric α-actin (red). Scale bars, 50 μm. (M) Recellularized whole rat heart at day 4 of perfusion culture, showing cross-sectional ring harvested for functional analysis (day 8, Upper Insert), and Masson’s trichrome staining of a ring thin section showing cells throughout the thickness of the wall (Lower Insert). Scale bar, 100 μm. Force generation in recellularized left ventricular rings after (N) 1-Hz and (O) 2-Hz electrical stimulation ((J-O), with permission from [20**]).
A notable method for developing functional EHT uses no scaffold at all. Instead, cardiomyocyte monolayers are stacked to form a functional tissue. Release of undamaged monolayers was made possible by seeding cardiac cells on poly(N-isopropylacrylamide)-grafted polystyrene dishes and then lowering the temperature to 20°C, thus inducing hydrophobic/hydrophilic switch of the surface. After transplantation of the cell sheets onto infarcted rat hearts, cardiac performance was significantly improved and successful engraftment was observed [11]. Using this method, 1cm-thick myocardium was created by serial re-operations and implantations of multiple sheets [12]. The cell sheet method has also been used to assess the capacity of Sca-1-positive resident cardiac progenitor cells (CPCs) in attenuating MI in mice and embryonic stem cell (ESC) derived SSEA-1-positive cardiac progenitors in rhesus monkeys, both showing functional improvements and cardiac differentiation of transplanted cells [13,14]. Importantly, a clinical trial using autologous skeletal myoblasts in cell sheets has been initiated in Japan with successful treatment of one patient with dilated cardiomyopathy [15].
Revascularization alone is often not sufficient to restore systolic function post-MI. Restoration of the normal ellipsoidal shape of the left ventricle from its decompensated, globular form with the use of a Dacron (polyethylene terephthalate) patch demonstrated immediate improvement in ejection fraction with sustained benefit and minimal morbidity [16]. More recently Gaudette and colleagues [17] demonstrated that the use of an FDA-approved extracellular matrix (ECM) from porcine urinary bladder outperformed the benefits seen with Dacron in a canine full thickness right ventricular defect model [17,18] . Porcine heart decellularized matrix hydrogels can also enhance maturation of human embryonic stem cell (hESC)-derived cardiomyocytes [19]. Importantly, decellularization by detergent perfusion of the whole cadaveric rat [20**] (Figure 1(J-O)), pig and human hearts [21] opens the way to engineering of the entire organ.
Vascularization
In native cardiac tissue, capillaries (~7μm diameter) are spaced at an average distances of ~ 20μm such that each myofiber is located between two capillaries [22]. The need to supply sufficient oxygen and nutrients to engineered tissues has motivated strategies to promote blood vessel formation, including 1) cell tri-culture, 2) use of growth factors and peptides and 3) engineering of novel proangiogenic scaffolds.
In the context of synthetic scaffolds, pre-treatment with cardiac fibroblasts was effective in improving survival of subsequently seeded cardiomyocytes and the functional properties of the EHT [3,23]. Sequential cultivation of fibroblasts and endothelial cells followed by cardiomyocytes also improved function of microscale cardiac organoids compared to simultaneous tri-culture [24,25]. Zimmermann and colleagues achieved completely defined cultivation conditions (serum and Matrigel free) by utilizing a whole rat heart cell population and culture medium containing insulin, triiodothyronine and other growth factors [26]. Initiation of pre-vascular networks by sandwiching endothelial cells (ECs) between cardiac cell sheets has also been achieved. These vascular structures connected to the host vessels and enabled improved sheet vascularization upon implantation in an MI model [27,28].
As an alternative to cell tri-culture, angiogenic growth factors, such as vascular endothelial growth factor (VEGF), can be incorporated into biomaterials for tissue engineering. Current delivery strategies include soluble factors, microparticles, as well as physical and covalent immobilization. Release of multiple growth factors from microparticles is required to achieve stable vasculature and homing of intravenously-administered progenitors [29,30]. Small peptides and molecules such as thymosin β4 [31] or ascorbic acid [32] can also be utilized to enhance angiogenesis.
Covalent immobilization of growth factors can localize growth factor activity and prolong receptor/ligand signaling to promote rapid vascularization [33]. A fusion protein, consisting of VEGF and a collagen-binding domain (CBD-VEGF), was able to bind to a collagen-rich matrix in infarcted hearts, thus increasing capillary density and reducing the scar size as compared to phosphate buffered saline (PBS) and VEGF controls. We covalently immobilized VEGF and angiopoietin-1 onto collagen scaffolds [34] (Figure 2(A)) to improve proliferation of H5V endothelial cells [34] and primary rat aortic endothelial cells [35], leading to tube formation. Upon implantation of scaffolds with uniformly immobilized VEGF165 into right ventricular free wall defects in rat hearts, enhanced angiogenesis and patch stability was observed compared to VEGF-free controls (Figure 2(B-G))[36]. An alginate scaffold capable of sustained release of several growth factors in conjunction with heterotopic transplantation onto the omentum was used to pre-vascularize an EHT prior to MI repair in rats [37*].
Figure 2. Vascularization and topographical cues for engineered cardiac tissues.
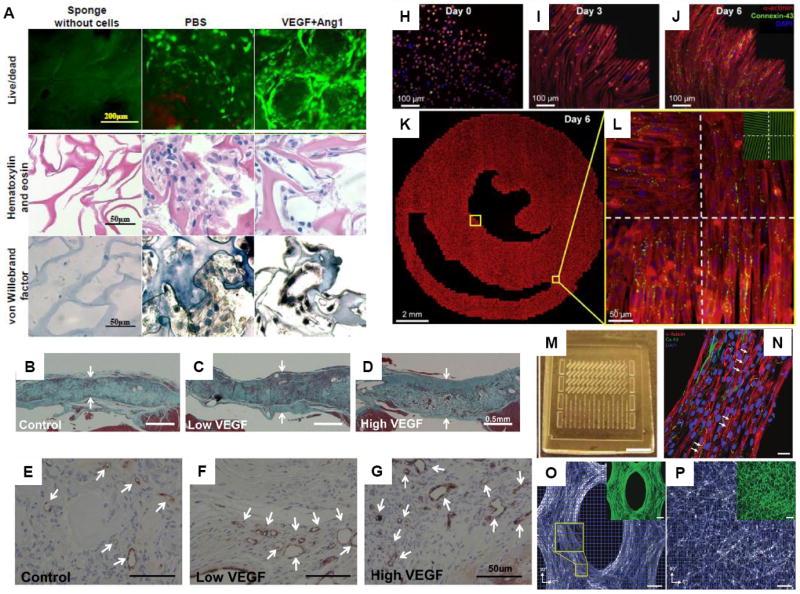
(A) Tube formation after 7-day cultivation of endothelial cells on collagen scaffolds with covalently immobilized vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang1). Images of PBS control group (scaffold with no growth factor) and VEGF+Ang1 group (scaffold with immobilized VEGF and Ang1) with live/dead staining (CFDA stains live cells green, PI stains dead cells red), hematoxylin and eosin staining, and von Willebrand factor staining. Sponges without cells are also shown. Random clusters of cells were found in PBS control group, compared to circular structures formed by elongated thin cells with nuclei in the peripheral position in the VEGF+Ang1 group ((A) with permission from [34]). (B-G) Implantation of collagen scaffolds with covalently immobilized VEGF to replace a full right ventricular free wall defect in rat hearts (B-D) Masson’s trichrome staining at 28 days after repair of right ventricular free wall defect shows higher patch thickness for patches with low dose VEGF (14.5 ± 1.4 ng VEGF) and high dose VEGF (97.2 ± 8.0 ng VEGF), compared to control patch with no VEGF. Arrows indicate thickness of the patch. (E-G) CD31 staining shows more CD31-positive vascular structures (indicated by arrows) for patch with high dose VEGF, compared to control low dose VEGF patch ((B-G) with permission from [36]). (H-L) Formation of realistic cardiac microstructure using micropatterned anisotropic slice cultures that replicate the natural fiber directions in ventricular cross sections. (H-J) The cells attached, spread and aligned to the micropatterned fibronectin lines and formed confluent cardiac fibers by Day 6. (K) A composite image of the entire micropatterned slice culture as well as (L) close-up images of four sections with the underlying fibronectin pattern (green, inset) is shown ((H-L) with permission from [44*]). (M-P) PDMS molds with arrays of (M) mesoscopic posts were used to fabricate three-dimensional muscle tissue architectures. (N) After 2 weeks of cultivation, tissue constructs were formed with densely aligned and striated cardiomyocytes, showing a high level of connexin-43 expression.. Directions of cell alignment in subregions (blue squares) were obtained to show more aligned cells in (O) PDMS molds with posts compared to (P) those without posts. (P,O) Inset shows the same image with F-actin stain (green) ((M-P) with permission from [47] ).
Vascularization can be further promoted with appropriate geometries [32,38*]. Madden et al. [38*] used microtemplating to make tissue engineered scaffolds consisting of interconnected pores of 30-40μm in diameter that promoted angiogenesis in a rat MI model. Seeding cells in subcutaneously implanted chambers around an arteriovenous loop is an effective method to engineer vascularized myocardium in vivo [39,40]. Implanting myoblasts into the chamber at day 7, at which point capillary growth was well-established, rather than day 0 led to improved myoblast survival. This clearly indicates the importance of seeding cells on well-established vascular beds for tissue regeneration.
Topographical control of engineered tissue structure and function
The heart tissue has complex macro- to nano-scale structural organization important for cardiac function. The myocytes and myofibers that make up the myocardium are aligned in parallel such that the force of contraction and impulse propagation velocity are higher along the long axis of the fiber [41*]. To enable appropriate pump function, myofiber orientation varies along the depth of the ventricular wall. Our recent work [42,43] and that of other groups has focused on reproducing this aligned structure [41*,44*]. Nanopatterning of PEG hydrogels [41*], rotary spinning of polymer nanofibers [45](Figure 2(H-L)) or stamping of ECM protein lanes on thin poly(dimethylsiloxane) (PDMS) films [41*,46] can be used to provide anisotropic cues for elongation of cardiomyocytes. High-fidelity two-dimensional (2D) models of ventricular cross-sections were created by projecting three-dimensional (3D) maps of local cardiac fiber directions onto soft-litography masks to produce angled parallel lines of fibronectin and guide cardiomyocyte alignment [44*]. In another approach, Bian et al.[47] fabricated PDMS molds that contained arrays of mesoscopic posts to guide compaction of cardiomyocyte-seeded hydrogels. By defining the size, elongation and spacing of the posts, two goals were achieved: 1) the enhancement in the diffusion of nutrients to the cells due to the introduction of pores, and 2) the local guidance of three-dimensional cell alignment due to spatial patterning of mechanical tension (Figure 2(M-P)). Alternatively, micro-organoids can be cultivated using gel compaction around two posts [48]. It is becoming apparent now that small topographical cues can affect a large number of different properties in cardiomyocytes including cell attachment, biomechanical stresses, structural remodeling, cell hypertrophy, ion channel remodeling, atrial natriuretic peptide release and binucleation [49,50].
Tissue engineering of stem-cell derived myocardium
Cardiac tissue engineering requires large cell numbers, especially in the case of whole organ engineering, in order to ensure appropriate physiological cell densities (~108 cells/cm3) and, in turn, contractile function. Since adult cardiomyocytes are terminally differentiated and are generally considered to have minimal proliferative capacity [51] alternative cell sources are required to provide millions of cells required for true regeneration. To this end, stem-cell derived cardiomyocytes can be employed. Promising studies report generation of cardiac-like cells from resident stem cells [52,53]. However, only pluripotent stem cells such as ESC or induced pluripotent stem cells (iPSC) can be expanded to sufficient numbers using existing technologies.
Early attempts at engineering ESC-derived myocardium used mouse ESC and embryoid body/serum differentiation. ESC-derived cardiomyocytes were seeded into circular molds with Matrigel and collagen I along with mechanical stimulation to produce the EHT [54]. To engineer cardiac tissues from progenitor cells, Domian et al employed a double fluorescent reporter system to generate cardiomyocytes from the Islet-1 (Isl1)-positive cardiac progenitor population followed by generation of beating thin films (Figure 3(A-E))[55].
Figure 3. Stem cells in cardiac tissue engineering.
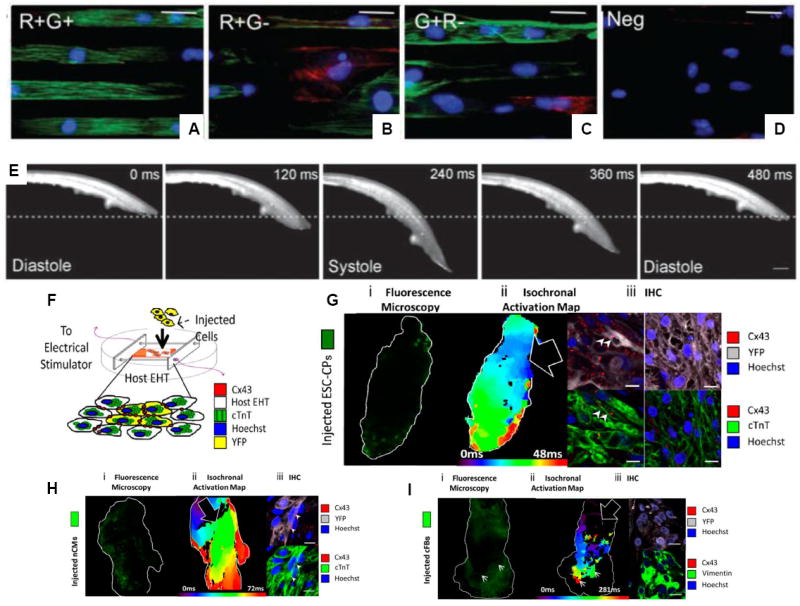
(A-E) Double transgenic mice (Isl1-dsRed and Nkx-2.5-eGFP) were used to create embryonic stem cells with a defined myogenic identity. (A) ESC-derived progenitor cells from the R+G+ population grown on micropatterned fibronectin lanes showed striated sarcomeric α-actinin staining (green) and little smooth muscle myosin heavy chain staining (red). (B) R+G- cells and (C) G+R- cells (D) Negative control exhibited less cardiac-like cells. (E) R+G+ progenitors were used to generate contractile muscular thin films (MTF) ((A-E) with permission from [55]). (F-I) Engineered heart tissues (EHTs) were used as surrogate tissues to evaluate integration of the injected ESC-derived cell populations. (F) Schematic depicting the EHT and injected cells. (G) Left Isochronal activation maps depict the speed of electrical impulses through the EHT, showing that ESC-derived cardiac progenitors improve conduction velocity and impulse propagation in EHTs. Right , Immunohistochemical staining of the injected EHT shows YFP+ injected cells (purple) and expression of key cardiac markers including Connexin-43 (Cx43, red) and cardiac troponin T (cTnT, green) as well as nuclear counterstaining (DAPI, blue). Conversely, impulse propagation is uniform but slower in EHTs injected with (H) neonatal cardiomyocytes (nCMs) and is even blocked by the addition of (I) cardiac fibroblasts (cFBs) ((F-I) with permission from [65*]).
The derivation of cardiomyocytes from human ESC and iPSC lines has decidedly shifted the focus of cardiac tissue engineering toward human cell work [56,57,58,59,60**]. Recent studies with hESC-derived cardiomyocytes from Murry and colleagues[59,60**] and Levenberg and colleagues [56,58] clearly point to the consensus that tri-culture of cardiomyocytes, endothelial cells and mesenchymal cells such as fibroblasts is required for survival and integration of EHT with the host myocardium[24,58]. In those cases, pre-formed donor-derived vessels functionally integrated with the host coronary vasculature and enabled graft survival.
Engineered tissue as a model system for in vitro studies
One of the main strengths of the tissue engineering approach, combined with the use of human cardiomyocytes, lies in the ability to create models of healthy and diseased myocardium. Towards this goal, Eschenhagen and colleagues developed a simple technique to construct a series of fibrin-based EHTs and automatically evaluate contractile activity [61**] . Dose-dependent responses were measured in the presence of four drugs with known proarrhythmic or cardiotoxic effects, thereby validating the utility of such a system as a high-through put method for drug screening and disease modeling [61**].
In vivo myocardial cell transplantation studies have utilized various cell types including embryonic, fetal and neonatal cardiomyocytes (CMs), skeletal myoblasts, bone marrow stem cells, and resident cardiac progenitors (reviewed in [62]). One of the main limitations in clinical studies has been low cell retention rate, although functional improvements have been noted [63]. The complexity of the in vivo environment and the heterogeneity of native cardiac tissue have made it difficult to isolate cell effects on heart function. Thus we hypothesized that EHT could be used as an in vitro model to study integration and differentiation potential of stem and progenitor cells in a cardiac environment [64,65*]. We injected R1 Flk1+/PDGFRα+ cardiac progenitors and assessed their potential to functionally integrate with the host EHT. These cardiac progenitors were able to appropriately differentiate into cardiomyocytes and integrate into EHT while mouse-ESC derived cardiomyocytes exhibited limited integration potential (Figure (3(F-O))[65*]. Additionally EHT was instrumental in enabling identification of residual undifferentiated cell activity in mouse ESC derived populations [64].
Challenges and future studies
The field of cardiac tissue engineering is rapidly advancing. Although the results to date are exceedingly encouraging, much remains to be done in order to develop clinically relevant approaches. Since the in vivo studies conducted thus far used different cell sources, biomaterials, animal models, delivery times post-infarction and experimental time frames, a direct comparison between the methods cannot be achieved. While all reported studies have shown some form of improvement, complete myocardial regeneration has not been achieved. Perhaps, a valid question to be answered in the future is: What is the required level of myocardial regeneration in terms of survival and attenuation of symptoms?
The discovery of human iPSCs[66] and ability to generate cardiomyocytes from them [67**] offers the possibility to engineer an autologous cardiac patch of clinically relevant size. Bioreactors will also need to be developed for large scale production of such cells (>108 cells/patient/patch). Careful consideration is required so that all non-human components (i.e. serum, Matrigel) are replaced. Sophisticated techniques such as mechanical and electrical stimulation have been developed to control the level of differentiation and maturation of primary rat cardiomyocytes in the EHT. It remains to be seen if the same techniques can be utilized to mature cardiomyocytes derived from pluripotent stem cells. Another critical question is: How mature and developed should the cardiac patch be to enable appropriate integration with the host myocardium? Additionally, it will be useful to generate different subtypes of human cardiomyocytes, with pacemaking-, atrial-, ventricular-, or Purkinje-like phenotypes, so that replacement therapies can be extended over a wide range of heart diseases.
In contrast to cultivating grafts for cardiac repair, the basic pre-requisite for developing effective, high-throughput methods for pharmacological and developmental studies is the miniaturization of the EHT. We anticipate that microfluidic devices and BioMEMS (Micro-Electro-Mechanical Systems) will enable this goal.
Conclusions
Heart disease remains the number one cause of mortality and morbidity in North America and myocardial tissue engineering may offer a new treatment option. In vitro approaches focus on recreating the physiological conditions found in the body. Bioreactor technologies have enabled this through application of biochemical, electrical, and mechanical stimuli necessary for cell survival and function. Oxygen delivery and vascularization have been addressed through the use of perfusion bioreactors, cell tri-culture, as well as angiogenic growth factor immobilization and delivery techniques. Cell sheet engineering and topographical guidance represent novel approaches to manipulate structure and function of the EHT. Meanwhile, the discovery of human iPSCs offers the promise of generating millions of autologous cardiomyocytes required for engineering of a clinically relevant heart patch.
Highlights
The review highlights recent advances in cardiac tissue engineering.
We cover classical cell-scaffold approaches and advanced bioreactor designs.
Cell sheet engineering and whole organ decellularization.
Stem-cell based approaches and topographical control of tissue organization and function.
Challenges in the field include stem cell control, maturation and tissue vascularization.
Acknowledgments
Financial support for our work is provided by an NSERC Discovery Grant (RGPIN 326982-10), NSERC Strategic Grant (STPGP 381002-09), NSERC-CIHR Collaborative Health Research Grant (CHRPJ 385981-10) and HSFO Grant-in-Aid (T6946).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rohin K. Iyer, Email: rohin.iyer@utoronto.ca.
Loraine L. Y. Chiu, Email: loraine.chiu@utoronto.ca.
Lewis A. Reis, Email: lewis.reis@utoronto.ca.
Milica Radisic, Email: m.radisic@utoronto.ca.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 3.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 4.Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods. 2010;16:1417–1426. doi: 10.1089/ten.TEC.2010.0068. [DOI] [PubMed] [Google Scholar]
- 5.Brown MA, Iyer RK, Radisic M. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol Prog. 2008;24:907–920. doi: 10.1002/btpr.11. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 8.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu LL, Iyer RK, King JP, Radisic M. Biphasic Electrical Field Stimulation Aids in Tissue Engineering of Multicell-Type Cardiac Organoids. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, Imanishi Y, Shimizu T, Okano T, Matsuda H. Tissue Cardiomyoplasty Using Bioengineered Contractile Cardiomyocyte Sheets to Repair Damaged Myocardium: Their Integration with Recipient Myocardium. Transplantation. 2005;80:1586–1595. doi: 10.1097/01.tp.0000181163.69108.dd. [DOI] [PubMed] [Google Scholar]
- 12.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–712. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Bellabas L, Brinon B, Vanneaux V, Pradeau P, et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122:S118–123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 15.Sawa Y. [Myocardial regeneration for heart failure] Nippon Rinsho. 68:719–725. [PubMed] [Google Scholar]
- 16.Di Donato M, Sabatier M, Dor V, Gensini GF, Toso A, Maioli M, Stanley AW, Athanasuleas C, Buckberg G. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiovasc Surg. 2001;121:91–96. doi: 10.1067/mtc.2001.111379. [DOI] [PubMed] [Google Scholar]
- 17.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144–149. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 18.Kelly DJ, Rosen AB, Schuldt AJ, Kochupura PV, Doronin SV, Potapova IA, Azeloglu EU, Badylak SF, Brink PR, Cohen IS, et al. Increased myocyte content and mechanical function within a tissue-engineered myocardial patch following implantation. Tissue Eng Part A. 2009;15:2189–2201. doi: 10.1089/ten.tea.2008.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. Entire hearts were decellularized by detergent perfusion, keeping the vasculature and extracellular matrix components intact. The hearts were then reseeded with cardiac or endothelial cells and were shown to generate pump function, demonstrating the potential of the technique for use in whole organ engineering strategies.
- 21.Taylor DA. From stem cells and cadaveric matrix to engineered organs. Curr Opin Biotechnol. 2009;20:598–605. doi: 10.1016/j.copbio.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Korecky B, Hai CM, Rakusan K. Functional capillary density in normal and transplanted rat hearts. Can J Physiol Pharmacol. 1982;60:23–32. doi: 10.1139/y82-003. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer RK, Chui J, Radisic M. Spatiotemporal tracking of cells in tissue-engineered cardiac organoids. J Tissue Eng Regen Med. 2009;3:196–207. doi: 10.1002/term.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer RK, Chiu LL, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- 26.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72–78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 27.Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 29.Saif J, Schwarz TM, Chau DY, Henstock J, Sami P, Leicht SF, Hermann PC, Alcala S, Mulero F, Shakesheff KM, et al. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vasc Biol. 2010;30:1897–1904. doi: 10.1161/ATVBAHA.110.207928. [DOI] [PubMed] [Google Scholar]
- 30.Jay SM, Shepherd BR, Andrejecsk JW, Kyriakides TR, Pober JS, Saltzman WM. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials. 2010;31:3054–3062. doi: 10.1016/j.biomaterials.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart N, Risebro CA, Clark JE, Ehler E, Miquerol L, Rossdeutsch A, Marber MS, Riley PR. Thymosin beta4 facilitates epicardial neovascularization of the injured adult heart. Ann N Y Acad Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- 32.Martinez EC, Wang J, Gan SU, Singh R, Lee CN, Kofidis T. Ascorbic acid improves embryonic cardiomyoblast cell survival and promotes vascularization in potential myocardial grafts in vivo. Tissue Eng Part A. 2010;16:1349–1361. doi: 10.1089/ten.TEA.2009.0399. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Ding L, Zhao Y, Sun W, Chen B, Lin H, Wang X, Zhang L, Xu B, Dai J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009;119:1776–1784. doi: 10.1161/CIRCULATIONAHA.108.800565. [DOI] [PubMed] [Google Scholar]
- 34.Chiu LL, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Chiu LL, Weisel RD, Li RK, Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med. 2011;5:69–84. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- 36.Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- *37.Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci U S A. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. Cardiac patches were made by seeding neonatal heart cells into alginate scaffolds containing proangiogenic factors. The patches were first prevascularized in the omental cavity prior to suturing onto infarcted hearts, demonstrating functional and mechanical integration as well as prevention of ventricular dysfunction.
- *38.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. Methacrylate-based hydrogel scaffolds created by microtemplating were used to guide the organization of cardiomyocytes into bundles and stromal cells into interconnected pores. Scaffolds were shown to support the growth of hESC-derived cardiomyocytes for up to 2 weeks in vitro. Acellular scaffolds implanted in vivo were shown to be proangiogenic and promoted vessel infiltration as well as reduced scarring and a shift toward M2 macrophage phenotype, indicating mitigation of the inflammatory response.
- 39.Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 40.Tilkorn DJ, Bedogni A, Keramidaris E, Han X, Palmer JA, Dingle AM, Cowling BS, Williams MD, McKay SM, Pepe L, et al. Implanted myoblast survival is dependent on the degree of vascularization in a novel delayed implantation/prevascularization tissue engineering model. Tissue Eng Part A. 2010;16:165–178. doi: 10.1089/ten.TEA.2009.0075. [DOI] [PubMed] [Google Scholar]
- *41.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107:565–570. doi: 10.1073/pnas.0906504107. PEG hydrogels were patterned with nanoscale topographical features mimicking the archictecture of matrix fibers found in the ECM of the native heart. Cells grown on patterned gels exhibited significantly improved organization, contraction strength, and conduction velocity, suggesting nanoscale features may exercise important influences on cardiac cells.
- 42.Au HT, Cheng I, Chowdhury MF, Radisic M. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 2007;28:4277–4293. doi: 10.1016/j.biomaterials.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Au H, Cui B, Chu Z, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009 doi: 10.1039/b810034a. [DOI] [PubMed] [Google Scholar]
- *44.Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96:3873–3885. doi: 10.1016/j.bpj.2009.02.019. Microcontact printing was employed to pattern nanometer-sized fibronectin lanes onto glass coverslips simulating a cardiac fiber-like topography These nanoscale patterns guided the anistropic orientation of cardiomyocyte monolayers and had a significant influence over conduction velocity compared to unpatterned cells, but did not affect action potential duration.
- 45.Badrossamay MR, McIlwee HA, Goss JA, Parker KK. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010;10:2257–2261. doi: 10.1021/nl101355x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alford PW, Feinberg AW, Sheehy SP, Parker KK. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials. 2010;31:3613–3621. doi: 10.1016/j.biomaterials.2010.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian W, Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401–1412. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci U S A. 2009;106:10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung CY, Bien H, Sobie EA, Dasari V, McKinnon D, Rosati B, Entcheva E. Hypertrophic phenotype in cardiac cell assemblies solely by structural cues and ensuing self-organization. FASEB J. 2010 doi: 10.1096/fj.10-168625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 52.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 53.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 54.Guo XM, Zhao YS, Chang HX, Wang CY, E LL, Zhang XA, Duan CM, Dong LZ, Jiang H, Li J, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113:2229–2237. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 55.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 57.Chen QZ, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, Chrzanowski W, Boccaccini AR, Ali NN, Knowles JC, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885–3893. doi: 10.1016/j.biomaterials.2010.01.108. [DOI] [PubMed] [Google Scholar]
- 58.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 59.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. Scaffold-free cardiac patches were created by self-association of orbitally mixed suspension cultures of human embryonic stem cell-derived cardiomyocytes. Patches based on enriched cardiomyocytes alone did not survive well in vivo, but patches precultured with human endothelial cells and fibroblasts formed larger, beating constructs that anastomosed with the host vasculature and could be electrically paced.
- **61.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schworer A, Uebeler J, Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. EHTs based on neonatal rat heart cells were seeded within a fibrin/Matrigel/thrombin mixture and cast into rectangular molds around deformable silicone posts, whose deflection could be monitored to estimate contractile force generation. The EHTs could be grown in a high throughput manner and assayed for their response to proarrhythmic and cardiotoxic drugs, suggesting the utility of the technique as a drug screening platform for EHT validation.
- 62.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 63.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 64.Dengler J, Song H, Thavandiran N, Masse S, Wood GA, Nanthakumar K, Zandstra PW, Radisic M. Engineered heart tissue enables study of residual undifferentiated embryonic stem cell activity in a cardiac environment. Biotechnol Bioeng. 2010 doi: 10.1002/bit.22987. [DOI] [PubMed] [Google Scholar]
- *65.Song H, Yoon C, Kattman SJ, Dengler J, Masse S, Thavaratnam T, Gewarges M, Nanthakumar K, Rubart M, Keller GM, et al. Interrogating functional integration between injected pluripotent stem cell-derived cells and surrogate cardiac tissue. Proc Natl Acad Sci U S A. 2010;107:3329–3334. doi: 10.1073/pnas.0905729106. EHTs were used as a surrogate tissue for injection of cardiac cells to mimic cell injection into host myocardium. Injection of cardiac fibroblasts into EHTs resulted in blocked action potential signal propagation, while injection of ES-derived cardiomyocytes or neonatal cardiomyocytes did not markedly improve or diminish EHT function. On the other hand, injection of ES cell-derived cardiac progenitors (Flk1+/PDGFα+) into EHTs improved tissue organization, conduction velocity, excitation threshold, and maximum capture rate. This study demonstrates the utility of EHTs as in vitro tools for the study of cell injection of various cell types.
- 66.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- **67.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. iPSCs were characterized for their differentiation potential into cardiomyocytes. Atrial, ventricular, and nodal phenotypes could be produced with comparable fidelity to hESC-derived cardiomyocytes, but there was residual transgene expression of OCT4 and NANOG in iPSC - derived cardiomyocytes. This landmark study describes one of the first attempts to completely characterize the cardiac differentiation potential of iPSCs, highlighting their potential for autologous cell therapy as well their some of their inherent limitations.


