Abstract
Objective
Stresses to skeletal muscle often result in injury. A subsequent bout of the same activity performed days or even weeks after an initial bout results in significantly less damage. The underlying causes of this phenomenon, termed the “repeated bout effect” (RBE), are unclear. This study compared the protective effect of two different injury protocols on the ankle dorsiflexors in the rat. We hypothesized that the RBE would occur soon after the initial injury and persist for several weeks, and that the RBE would occur even if the second injury was performed under different biomechanical conditions than the first.
Design
In this controlled laboratory study, the dorsiflexor muscles in the left hindlimbs of adult, male Sprague-Dawley rats (N = 75) were subjected to 10 repetitions of large-strain lengthening contractions or 150 repetitions of small-strain lengthening contractions.
Results
Both protocols induced a significant (P < 0.001) and similar loss of isometric torque (~50%) following the first bout of contractions. The RBE occurred as early as 2 days after injury and remained high for 14 days (P < 0.001), but diminished by 28 days and was lost by 42 days. The small-strain contractions offered a protective effect against a subsequent large-strain contraction, but not vice-versa. Although the RBE did not occur sooner than day 2, the early recovery following a second large strain injury performed 8 h after the first was two-fold greater than following a single injury.
Conclusions
The RBE is both rapid in onset and prolonged, and some but not all injuries can protect against different types of subsequent injury.
Keywords: Skeletal Muscle, Eccentric, Injury, Adaptation, Lengthening, Repeated Bout Effect
Mechanical stresses to skeletal muscle, particularly during maximal lengthening contractions, often result in injury. However, a subsequent bout of the same activity performed days or even weeks after an initial bout results in significantly less damage. The “damage” can be measured in a number of ways (discussed below), but the end result is that the second injury results in less loss of muscle function. The underlying mechanisms of this phenomenon, termed the “repeated bout effect” (RBE), are unclear, but are likely a combination of contributions from neural, mechanical, and cellular adaptations. This could include such changes as a shift in recruitment of motor unit firing or in the length-tension curve due to the addition of sarcomeres, the disruption of passive elements, or adaptations in the inflammatory response.
Lengthening contractions occur when the force exerted on a contracting muscle exceeds the force generated by that muscle. They can produce twice as much force as that generated by shortening (concentric) or isometric contractions. Consequently, lengthening contractions are more likely to produce damage1 than either isometric or concentric contractions2. This damage is often accompanied by reduced contractile force, which recovers over the next several days to weeks3, 4. Such injuries can be accompanied by structural changes9, the presence of muscle-specific proteins in the blood3–5, increased edema and fiber swelling5, 6, inflammation4, 7, 8, soreness3, decreased range of motion4,5, myofiber necrosis and regeneration9, 10.
Paradoxically, although high-force lengthening contractions are associated with injury, they can also provide significant protection against future injury7 in what is known as the RBE. Several aspects of muscle damage are ameliorated due to the RBE. For example, compared to the first bout, a second bout of lengthening contractions is associated with a decreased loss of contractile force, less soreness, and a reduction in the amount of muscle proteins in the blood5. However, little is known about the conditions that result in the protective adaptation.
Some of the difficulties associated with defining the RBE are likely to be due to the differences in the methods used to induce injury and the markers used to assess muscle damage. We have therefore established standard conditions to induce and measure both injury and the RBE. For the purposes of this study, we define muscle injury as a loss of contractile function and we define a RBE as occurring when muscle shows a smaller loss of force following a second injury, compared to the first. Here we address three questions regarding the RBE: 1) How soon after an initial injury will a RBE be conferred? 2) How long does the RBE last? 3) How specific is the RBE to the nature of the initial injury? We compared two injuries induced by different levels of strain and the number of lengthening contractions. In one injury model, 10 repetitions of contractions were performed while the muscle underwent a large strain, and in the other, 150 repetitions were performed while the muscle was subjected to a small strain. Our laboratory has previously shown that the recovery of muscle after a small-strain injury (SSI) requires myogenesis, whereas recovery from a large-strain injury (LSI) does not11. Based on observations on humans12–14, we hypothesized that the RBE would occur soon after either injury and persist for several weeks. We further predicted that the RBE would occur even if the second injury was performed under different biomechanical conditions than the first injury. Our results are consistent with both of these predictions.
METHODS
Animals
Adult male Sprague-Dawley rats (N = 75, 416 ± 32 g, Charles River, Wilmington, MA) were housed in a pathogen-free environment at constant temperature of 22.0 °C and a 12:12 h light-dark cycle. Experiments were performed on 12–14 week-old rats weighing 416 ± 32 g. All measurements of contractile function, induction of injury, and tissue collection were performed under general anesthesia induced by 2% isoflurane inhalation (VetEquip, Pleasanton, CA). Animals were assigned to one of two groups: a large strain injury induced by several repetitions (LSI) or a small strain injury induced by many repetitions (SSI). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland, School of Medicine.
Injury
Injury was induced in vivo in the ankle dorsiflexor muscles by a series of lengthening contractions, as previously described11, 15–17. Briefly, with the animal supine, the hindlimb was stabilized by placing a 21 gauge needle through the head of the tibia. The needle was secured by a vice, and the foot was secured onto a plate. The plate turned around an axis that was attached to a stepper motor, a potentiometer to measure range of motion of the ankle, and a torque sensor. A custom program was used to initiate contractile activation prior to ankle rotation. The left hindlimb was designated as the experimental side, and the right hindlimb was designated as the control side.
Contractions of the dorsiflexor muscles were elicited by depolarizing the peroneal nerve with subcutaneous needle electrodes (Chalgren 30 ga electrodes, part 223-012-24TP, Jari Electrode, Gilroy, CA). Impulses of 1 ms duration were generated by an S48 square pulse stimulator (Grass Instruments, West Warwick, RI); a PSIU6 stimulation isolation unit (Grass Instruments), placed between the electrode and stimulator. Current amplitude was restricted to a maximum of 15 mA. Pulse amplitude was adjusted to obtain maximal tetanic torque, and 300 ms trains of varying pulse frequencies were used to generate a torque-frequency curve. Functional data were recorded from each group of animals. Maximum tetanic torque recorded at each time point was expressed as a percentage of the maximum tetanic torque measured for each animal before injury.
We used two different injury models, which we have previously described, that result in comparable force loss12, 18. For the LSI (N = 63), the foot was placed with the ankle at 80° (with the foot orthogonal to the leg considered 90°) and moved into plantar flexion through a 90° arc of motion at an angular velocity of 900°/sec, beginning 200 ms after tetanic stimulation of the tibialis anterior (TA). There was a 1 min rest between repetitions. For SSI (N = 12), 150 lengthening contractions were equally spaced apart over 30 min, through a smaller arc of motion, and with the muscle in a shorter start position. The ankle was set at 70° and moved into plantar flexion through a 40° arc at 900°/s. Ankle movement was initiated 200 ms after the onset of tetanic contraction.
Contractile Function
The maximal isometric torque of the dorsiflexor muscle group, predominantly generated by the tibialis anterior (TA) muscles8, was measured prior to muscle injury and again 3 min (“0 days”) and 1, 2, 3, 5, 7, 14, 28 and 42 days after the injury. The TA muscle was used because of its easy accessibility, the ability to compare results to previous literature, and the relatively small angle of pennation of its muscle fibers, which reduces the contribution of angular rotation during eccentric contraction. Following an initial LSI, a second LSI was performed at 8 hours, or 1, 2, 3, 5, 7, 14, 28, or 42 days later. Contractile function was measured before and after each bout to determine how long after the initial injury the RBE started. To determine the specificity of the RBE, three days after a LSI, when the muscle was functionally recovered, or 10 days after the initial LSI, the dorsiflexors were injured a second time following either the LSI or the SSI protocol. In addition, 10 days after SSI, a second SSI was performed. Contractile function (maximal isometric torque) was recorded before and after each injury.
Histology
After functional data were collected, TA muscles were harvested from the anesthetized rat, snap frozen in liquid nitrogen, and stored at −80°C. Cross sections (10 µm thick) were cut from the middle third of the TA and either stained with hematoxylin and eosin (H&E) for morphological analysis or processed for immunostaining for the identification of inflammatory cells. Immunohistochemistry was performed as described39. Briefly, sections were air dried and then fixed in cold acetone. After air drying again, sections were washed in phosphate buffered saline (PBS) and quenched with 0.3% hydrogen peroxide. Sections were incubated with buffer containing 3% bovine serum albumin and then with primary antibodies overnight at 4°C. Macrophages were labeled with an ED1 (CD68) and ED2 (CD163) antibody (1:200; Serotec, Raleigh, NC). After washing with PBS, sections were incubated with biotinylated horse anti-mouse secondary antibody that had been pre-adsorbed with rat IgG (1:200; Vector Laboratories, Burlingame, CA). Sections were subsequently washed in PBS, incubated with avidin D horseradish peroxidase (1:1,000), and developed using the 3-amino-9-ethylcarbazole (AEC) kit (Vector Laboratories). Sections were viewed with a 20× objective and pictures were taken with a digital camera (AxioCam HR using AxioVision 3.0, Carl Zeiss, Germany).
Statistical Analysis
Values are reported as mean ± standard deviation. Data were compared across groups or time points using one-way ANOVA with Holm-Sidak post-hoc analysis or Student’s t-test. For groups that did not pass tests of normality and sphericity, the nonparametric Kruskal-Wallis one-way ANOVA on ranks with Dunn’s post-hoc method or the Mann-Whitney rank sum test was used. Differences between groups were considered significant if P<0.05.
RESULTS
Characterization of Injured Fibers
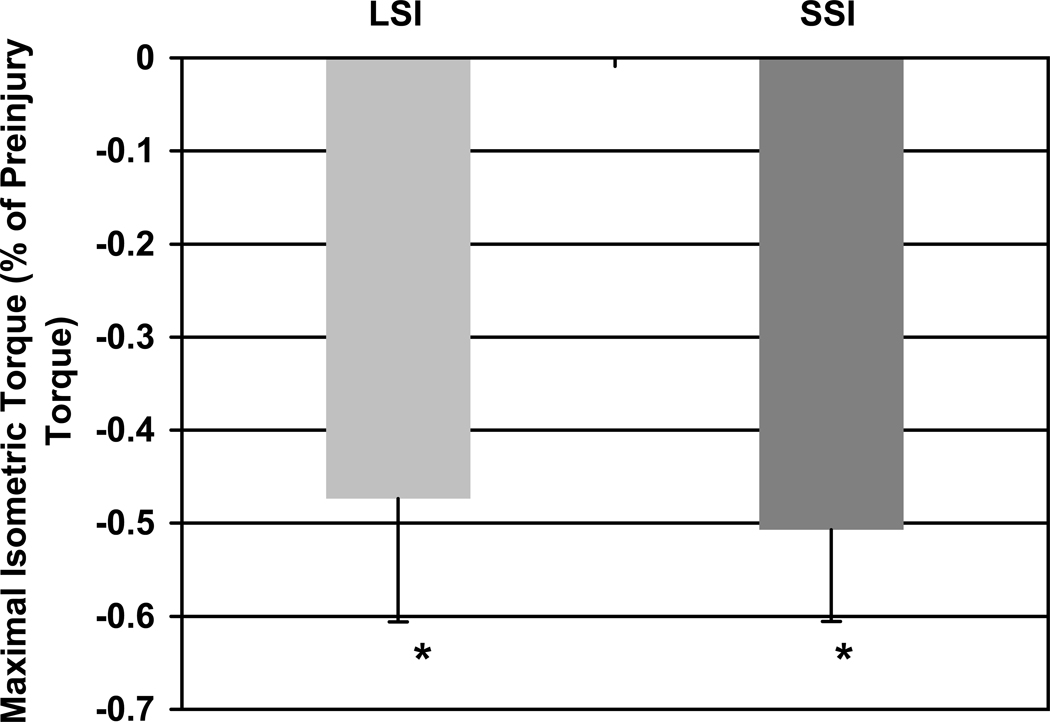
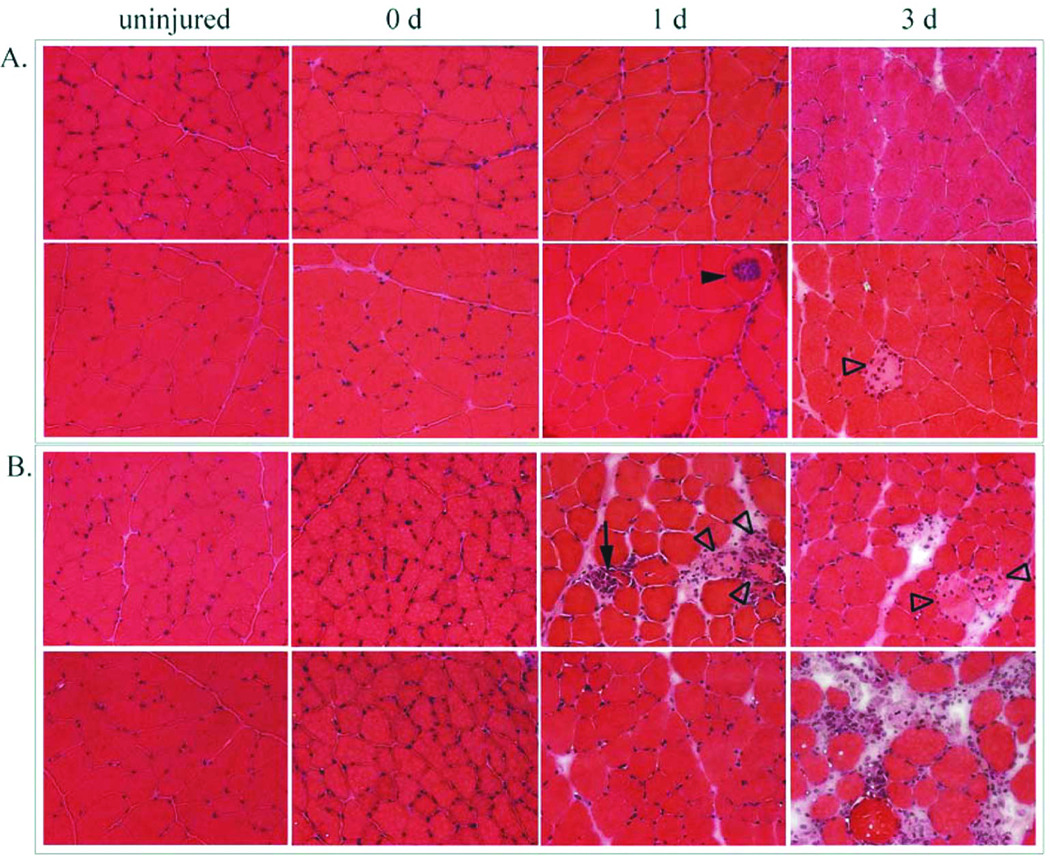
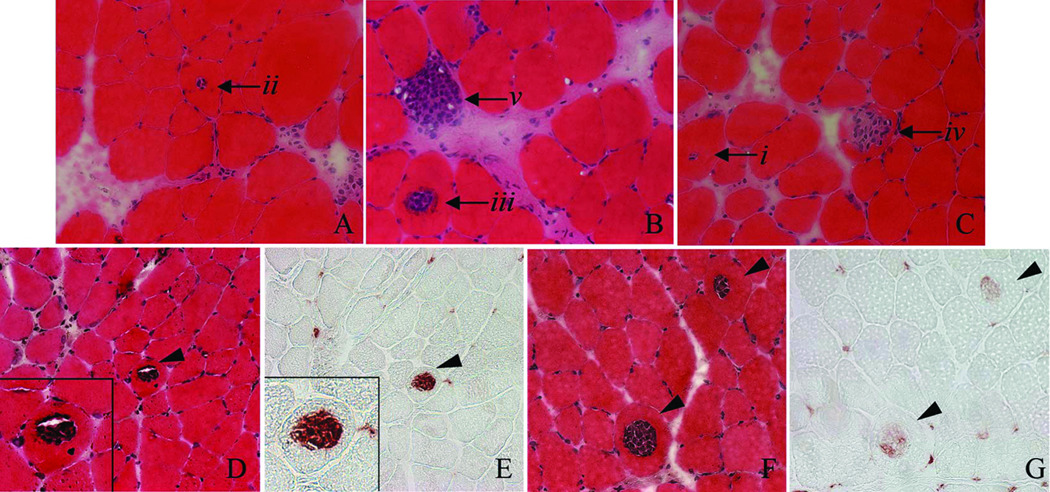
LSI and SSI produced similar losses of contractile torque (Figure 1), consistent with previous findings12, 15, 18. Most muscle cross sections had normal morphology shortly after injury, but some had small areas of localized inflammation. The LSI was characterized by a small number of centrally invaded fibers and swollen, pale fibers, likely to be necrotic (Figure 2A). Centrally invaded fibers were only observed after LSI at 1 day. To confirm that the nuclei in these fibers stained by H&E were, in fact, inflammatory cells, serial sections were labeled for macrophages (Figure 3D–G). In these examples, mostly ED1-positive macrophages were present inside the fibers. Centrally invaded fibers, also referred to as ‘central necrosis’, are thought to be downstream of an area where the same myofiber has been completely invaded19. Therefore, a centrally invaded fiber is likely to progress to a completely invaded fiber (Figure 3A–C). SSI appeared to result in more accumulation of inflammatory cells and interstitial edema than LSI (Figure 2B). Although this apparent difference was observed at 1 day post-injury, it was most evident by 3 days. These qualitative observations support previous findings in which the SSI produced more cell damage and took longer to repair than the LSI11, 18.
Figure 1.
Loss of maximal isometric torque following each injury at 0 d. Both the large strain injury (LSI) and small strain injury (SSI) protocols produced ~50% loss of contractile function. * P<0.001 compared to uninjured muscle by ANOVA applied to all three groups. N = 64 and 12, respectively.
Figure 2.
Time course of injuries, shown in muscle sections stained with hematoxylin and eosin (H&E). Panel A: LSI produced some fibers that were centrally invaded by inflammatory cells (closed arrowhead) and swollen, pale and peripherally invaded fibers (open arrowhead). Panel B: SSI produced local areas of interstitial edema with inflammatory cell accumulation, pale, swollen, and peripherally invaded fibers (open arrowheads) and fully invaded fibers (arrow). The inflammatory response was greater after the SSI.
Figure 3.
Central necrosis observed 1 d after LSI. A–C. H&E stained muscles showing the likely progression of centrally invaded fibers (starting with i and progressing to v). D, E: Serial sections of muscle stained with H&E and labeled for ED1-positive macrophages. F, G: Serial sections of muscle stained with H&E and labeled for ED2-positive macrophages. In this example, fibers were centrally invaded primarily by ED1-positive macrophages 1 d after LSI.
Onset and Cessation of the RBE
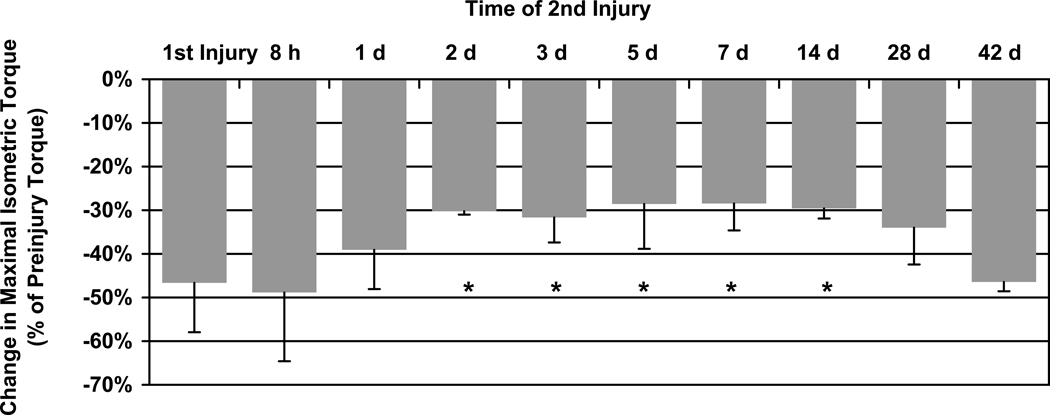
To determine the times of onset and cessation of the RBE, we performed a second LSI at various times after the initial LSI. Maximal isometric torque was measured before and after each injury. If the second injury was performed 2 days later, when the mean torque completely recovered from the first injury (our unpublished results), the second injury was less severe (Figure 4). This RBE persisted for at least 14 days after the initial injury, diminished at 28 days and disappeared by 42 days.
Figure 4.
The loss of maximal isometric torque following a second LSI injury. If the second injury was performed 2, 3, 5, 7, or 14 d following the first injury the muscle was partially protected from torque loss, indicating a repeated bout effect (RBE). * P < 0.001 significantly different than first injury by ANOVA. N = 63 for the first injury and N ≥ 4 for all other groups.
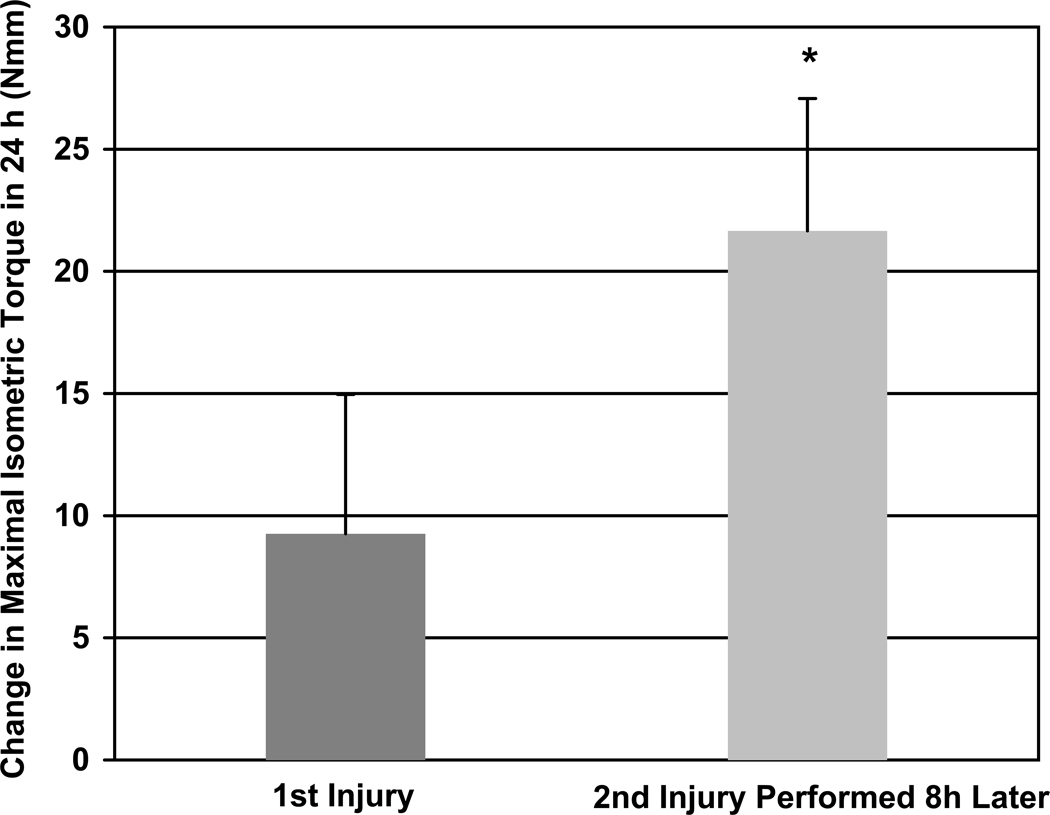
We also examined the how soon muscles recovered their contractile torque following the first and second injuries. The RBE was not observed following a second LSI 8 hours later; however, the early recovery following a second large strain injury performed 8 h after the first was two-fold greater than following a single injury (Figure 5).
Figure 5.
Change in functional recovery over the first 24 h after LSI. The recovery following a second LSI performed 8 h after the first was two-fold greater than following a single LSI. * P<0.001 significantly different than first injury, as determined by t-test. N = 8 and 7, respectively.
Specificity of the RBE
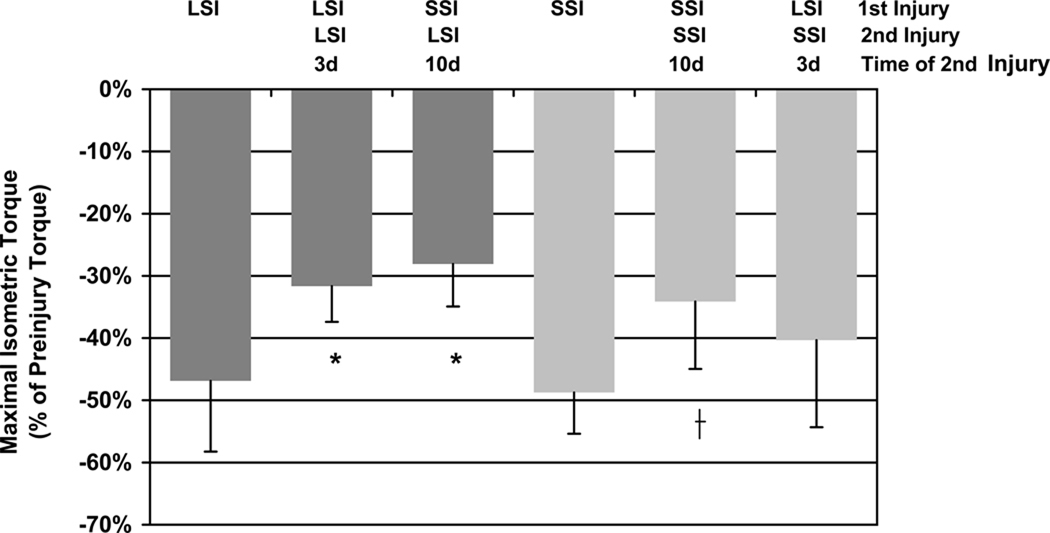
To determine the specificity of the RBE, we performed a LSI followed by a SSI, and vice-versa, with the second injury induced only after full functional recovery from the initial injury. Results indicated an initial SSI protected against both subsequent LSI and SSI. The initial SSI was no better at protecting against damage caused by a subsequent LSI than SSI. By contrast, LSI did not protect against a subsequent SSI, although it provided a substantial RBE to subsequent LSI (Figure 6).
Figure 6.
Torque loss following either a single injury or a second injury, showing the specificity of the RBE. SSI protected against a subsequent LSI, while LSI did not protect against a subsequent SSI. SSI afforded the same level of protection against a subsequent LSI as an initial bout of LSI. * P < 0.001 significantly different than a single SSI by ANOVA. † P = 0.027 significantly different than a single LSI by ANOVA. N = 63 for the single LSI and n ≥ 5 for all other groups.
DISCUSSION
Lengthening contractions are known to produce muscle injury, but they can also induce an adaptation that renders the muscle more resistant to subsequent injury – the repeated bout effect (RBE). Little is known about the timing of the onset and cessation of the RBE, or the specificity of the adaptation. Consequently, we sought to determine the onset of the RBE, which we hypothesized would be very rapid, and its cessation, which we hypothesized would be many weeks later. We found that a large strain injury (LSI) conferred a RBE, or an attenuated loss of torque, within 2 days of the initial injury. The RBE lasted for at least 14 days, well after functional recovery, and was lost by 42 days after the initial LSI. Furthermore, the RBE was specific to the conditions of the small strain injury (SSI), but not specific to the LSI. Both LSI and SSI protected against a subsequent LSI, though only SSI protected against a subsequent SSI. These results suggest that some features of the RBE are specific for the kinds of injuries studied, and that some aspects of the RBE are not directly linked to the time required for complete functional recovery from the initial injury.
We now know that, despite an almost identical injury as defined by the amount of force loss, the SSI and LSI do not provide reciprocal protection from subsequent injury. The reasons why only the SSI protects against future bouts of SSI and LSI are unclear. The possibilities are numerous and could include contributions from neural, mechanical, and/or cellular adaptations already mentioned. The most likely explanation is that the myogenesis that occurs only after the SSI is integral to this protection against both types of future injury.
Although little is known about the changes in muscle that lead to a RBE, we observed the effect when the second bout of exercise was imposed just 2 days after the first. In humans performing lengthening contractions with the quadriceps muscles, testing immediately after a second bout of the same exercise performed 13 days following the first bout resulted in a decrease in force loss20. Subjects performing lengthening contractions also showed attenuated creatine kinase and soreness, when the second bout was performed 7 days after the initial injury6, 21. Studies of earlier time points show conflicting results, however. A RBE is observed after 3 days22 and 5 days23 prior to the muscle regaining full contractile function after the first bout. Based on measurements of edema 4 days after exercise, rats do not have a RBE from the first inflammatory response6. Such findings make it difficult to understand the nature of the RBE, likely because the criteria for injury and RBE differ from study to study.
Our results indicated that a second injury performed just 8 hours after the initial injury, prior to full recovery, showed an increase in functional recovery over the following 24 hours (Figure 5). While this latter finding agrees with others23, ours is the first study to demonstrate a relative improvement in contractile function within hours of the initial injury, well before recovery of function was complete. Although this improvement in early recovery does not necessarily constitute a RBE, such findings suggest that the RBE may be even more rapid than previously thought.
Studies regarding persistence of the RBE in human subjects have yielded conflicting results. While several studies show a RBE, as measured by contractile function immediately following the injury and at 6 weeks later5, 23, most studies of humans report no RBE when the second bout was performed any time between 4 weeks to 1 year after the initial injury3, 5, 24. However, consistent with our observation (Figure 5), some investigators found improved recovery at these later time points in the days following the second injury3, 23, 25 with the exception of one study that did not find an improved recovery at 10 weeks5. Nosaka et al3 have shown in human studies that the recovery of contractile function is still evident when a second bout is performed 1 year later, although it was less than that observed at 9 months, suggesting that the adaptation was subsiding3. The disadvantage of using human subjects in studies of the RBE is the lack of control over exposure to lengthening contractions in the period between bouts. Moreover, maximal voluntary contraction in people can be limited by soreness and is subject to changes in motivation. Consequently, the duration of the RBE determined in these studies may be more variable than that which is measured in non-volitional animal studies.
Our results with young adult rats suggest that the LSI affords some protection against a subsequent LSI for at least 14 d. The RBE began to diminish by 28 days and was lost by 42 days. Previous studies with laboratory animals have routinely waited 2 weeks between bouts to test for the RBE, but there is little information on its cessation. Indeed, only one study showed that the RBE is attenuated sometime between 28 and 84 days in mice26.
As shown in this study, although two different injuries produced a similar loss of contractile function, the amount of cell damage induced by each was markedly different. Following the LSI, the architecture of most of the muscle fibers was comparable to uninjured muscle, although we observed occasional centrally-infiltrated or swollen cells. The inflammatory response following the SSI appeared to be more robust, and was characterized by local areas of interstitial edema, fully invaded fibers, accumulation of inflammatory cells and swollen fibers, as we previously reported11, 18. The two injuries produced different cellular responses, and, as such, adaptations are likely to be specific to the conditions of the initial injury. As suggested above, the fact that muscles regenerate after SSI, but not after LSI, may help to explain our observation that SSI, but not LSI, partially protects against a second bout of SSI.
Biomechanical considerations, which we did not examine, could contribute selectively to the RBE after one form of injury but not the other, however. For example, a rightward shift in the length-tension curve of muscles after injury (longitudinal addition of sarcomeres) might be present in only one of the injury models. Initial muscle length can affect the degree of damage and might have confounding effects that we did not detect. A second, and more practical consideration, is that we do not know how readily our current findings apply to humans. Although the rat provides obvious advantages (age-matched, gender-matched, controlled environment and history, ability to recruit all motor units, etc), there are some inherent disadvantages to any animal model. For example, the orderly recruitment of motor units from small to large (the “size principal”) may not apply in experiments with anesthetized rodents. The assumption that all mammalian skeletal muscles are identical is also not true, despite the remarkable similarities. Thus, one must be careful when extrapolating these findings to humans.
Nevertheless, the factors underlying the specificity of the RBE demand study, both in animal models and in man, for their potential beneficial effects. Studies of the volume and intensity of exercise, type of contraction, and magnitude of muscle strain have provided evidence that the RBE can be induced by a brief exposure to lengthening contractions that result in an immediate and significant (~50%) loss of contractile function. While training with concentric contractions prior to a bout of lengthening contractions makes the muscle more susceptible to contraction-induced injury in humans13, it produces a RBE in old and young rats27, 28. Both passive stretches and isometric contractions offer some protection against later injury with little risk of muscle damage. Therefore, extensive muscle damage may not be necessary to induce a RBE. Likewise, a low volume or intensity of contractions produces an adaptation that partially protects against a larger volume or intensity of exercise14, 22, 29–31. Conversely, small strain lengthening contractions are reported to provide little to no protection against injury sustained from large strain contractions32, 33, observations that are not in keeping with our results with rats subjected to SSI followed by LSI. These earlier studies with human subjects modified one variable of the injury protocol; however, it is a rare occurrence, if at all, that a person would only change one element of the contraction, keeping all others exactly the same, in a daily movement. So, it may be more functional to compare injury models that alter both strain and number or repetitions, for example.
Results from human studies, in which subjects altered their stride length during an initial bout of downhill running and then performed a second bout at their preferred stride or vice-versa, suggest that the RBE is more dependent on strain than volume of exercise, and that the RBE effect is not always specific to the initial conditioning bout34, 35. Our results show that the RBE can be specific in some injuries but not others. This type of information will be useful to sports medicine professionals in designing fitness programs to minimize contract-induced injury on the field. Our findings that the RBE is not necessarily specific to one type of injury also suggest that as we collect this type of data, patients that are more susceptible to eccentric injury, such as the elderly and those with muscle diseases, might be able to use one protocol that is safe to protect them from injuries that might otherwise occur with lengthening contractions. Future studies will use the parameters established here to examine the molecular mechanisms underlying the RBE.
Acknowledgments
Disclosures:
This work was supported by grants to RML and RJB from the National Institutes of Health (K01AR053235 and 1R01AR059179 to RML; RO1 AR 55928 to RJB), the Muscular Dystrophy Association (#4278 to RML; # 3771 to RJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med. 1989;7:207–234. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hunter KD, Faulkner JA. Pliometric contraction-induced injury of mouse skeletal muscle: effect of initial length. J Appl Physiol. 1997;82:278–283. doi: 10.1152/jappl.1997.82.1.278. [DOI] [PubMed] [Google Scholar]
- 3.Nosaka K, Newton MJ, Sacco P. Attenuation of protective effect against eccentric exercise-induced muscle damage. Can J Appl Physiol. 2005;30:529–542. doi: 10.1139/h05-139. [DOI] [PubMed] [Google Scholar]
- 4.Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- 5.Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol. 1991;63:70–76. doi: 10.1007/BF00760804. [DOI] [PubMed] [Google Scholar]
- 6.Marqueste T, Giannesini B, Fur YL, Cozzone PJ, Bendahan D. Comparative MRI analysis of T2 changes associated with single and repeated bouts of downhill running leading to eccentric-induced muscle damage. J Appl Physiol. 2008;105:299–307. doi: 10.1152/japplphysiol.00738.2007. [DOI] [PubMed] [Google Scholar]
- 7.Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281:R155–R161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- 8.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995;488(Pt 2):459–469. doi: 10.1113/jphysiol.1995.sp020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauritzen F, Paulsen G, Raastad T, Bergersen LH, Owe SG. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J Appl Physiol. 2009;107:1923–1934. doi: 10.1152/japplphysiol.00148.2009. [DOI] [PubMed] [Google Scholar]
- 10.Sudo M, Kano Y. Myofiber apoptosis occurs in the inflammation and regeneration phase following eccentric contractions in rats. J Physiol Sci. 2009;59:405–412. doi: 10.1007/s12576-009-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson N, Eston R, Marginson V, McHugh M. Effects of prior concentric training on eccentric exercise induced muscle damage. Br J Sports Med. 2003;37:119–125. doi: 10.1136/bjsm.37.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37:1135–1142. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovering RM, McMillan AB, Gullapalli RP. Location of myofiber damage in skeletal muscle after lengthening contractions. Muscle Nerve. 2009;40:589–594. doi: 10.1002/mus.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche JA, Lovering RM, Bloch RJ. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport. 2008;19:1579–1584. doi: 10.1097/WNR.0b013e328311ca35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathology of Skeletal Muscle. Oxford University Press, Inc.; 2001. [Google Scholar]
- 20.Mair J, Mayr M, Muller E, Koller A, Haid C, rtner-Dworzak E, Calzolari C, Larue C, Puschendorf B. Rapid adaptation to eccentric exercise-induced muscle damage. Int J Sports Med. 1995;16:352–356. doi: 10.1055/s-2007-973019. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson PM, Byrnes WC, Gillisson E, Harper E. Adaptation to exercise-induced muscle damage. Clin Sci (Lond) 1987;73:383–386. doi: 10.1042/cs0730383. [DOI] [PubMed] [Google Scholar]
- 22.Chen TC. Effects of a second bout of maximal eccentric exercise on muscle damage and electromyographic activity. Eur J Appl Physiol. 2003;89:115–121. doi: 10.1007/s00421-002-0791-1. [DOI] [PubMed] [Google Scholar]
- 23.Ebbeling CB, Clarkson PM. Muscle adaptation prior to recovery following eccentric exercise. Eur J Appl Physiol Occup Physiol. 1990;60:26–31. doi: 10.1007/BF00572181. [DOI] [PubMed] [Google Scholar]
- 24.Nosaka K, Sakamoto K, Newton M, Sacco P. How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc. 2001;33:1490–1495. doi: 10.1097/00005768-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Nosaka K, Sakamoto K, Newton M, Sacco P. How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc. 2001;33:1490–1495. doi: 10.1097/00005768-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Sacco P, Jones DA. The protective effect of damaging eccentric exercise against repeated bouts of exercise in the mouse tibialis anterior muscle. Exp Physiol. 1992;77:757–760. doi: 10.1113/expphysiol.1992.sp003642. [DOI] [PubMed] [Google Scholar]
- 27.Hughes W, Gosselin LE. Impact of endurance concentric contraction training on acute force deficit following in vitro lengthening contractions. Eur J Appl Physiol. 2002;87:283–289. doi: 10.1007/s00421-002-0635-z. [DOI] [PubMed] [Google Scholar]
- 28.Gosselin LE. Attenuation of force deficit after lengthening contractions in soleus muscle from trained rats. J Appl Physiol. 2000;88:1254–1258. doi: 10.1152/jappl.2000.88.4.1254. [DOI] [PubMed] [Google Scholar]
- 29.Nosaka K, Sakamoto K, Newton M, Sacco P. The repeated bout effect of reduced-load eccentric exercise on elbow flexor muscle damage. Eur J Appl Physiol. 2001;85:34–40. doi: 10.1007/s004210100430. [DOI] [PubMed] [Google Scholar]
- 30.Chen TC, Nosaka K, Sacco P. Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J Appl Physiol. 2007;102:992–999. doi: 10.1152/japplphysiol.00425.2006. [DOI] [PubMed] [Google Scholar]
- 31.Paddon-Jones D, Abernethy PJ. Acute adaptation to low volume eccentric exercise. Med Sci Sports Exerc. 2001;33:1213–1219. doi: 10.1097/00005768-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 32.McHugh MP, Pasiakos S. The role of exercising muscle length in the protective adaptation to a single bout of eccentric exercise. Eur J Appl Physiol. 2004;93:286–293. doi: 10.1007/s00421-004-1196-0. [DOI] [PubMed] [Google Scholar]
- 33.Nosaka K, Newton M, Sacco P, Chapman D, Lavender A. Partial protection against muscle damage by eccentric actions at short muscle lengths. Med Sci Sports Exerc. 2005;37:746–753. doi: 10.1249/01.mss.0000162691.66162.00. [DOI] [PubMed] [Google Scholar]
- 34.Eston RG, Lemmey AB, McHugh P, Byrne C, Walsh SE. Effect of stride length on symptoms of exercise-induced muscle damage during a repeated bout of downhill running. Scand J Med Sci Sports. 2000;10:199–204. doi: 10.1034/j.1600-0838.2000.010004199.x. [DOI] [PubMed] [Google Scholar]
- 35.Rowlands AV, Eston RG, Tilzey C. Effect of stride length manipulation on symptoms of exercise-induced muscle damage and the repeated bout effect. J Sports Sci. 2001;19:333–340. doi: 10.1080/02640410152006108. [DOI] [PubMed] [Google Scholar]