Abstract
CD93 is emerging as a novel regulator of inflammation; however, its molecular function is unknown. CD93 exists as a membrane-associated glycoprotein on the surface of cells involved in the inflammatory cascade, including endothelial and myeloid cells. A soluble form (sCD93) is detectable in blood and is elevated with inflammation. Here we demonstrate heightened susceptibility to thioglycollate-induced peritonitis in CD93−/− mice. CD93−/− mice showed a 1.6 to 1.8-fold increase in leukocyte infiltration during thioglycollate-induced peritonitis between 3 and 24 hours that returned to wildtype levels by 96 hours. Impaired vascular integrity in CD93−/− mice during peritonitis was demonstrated using fluorescence multi-photon intravital microscopy; however, no differences in cytokine or chemokine levels were detected by Luminex Multiplex or ELISA analysis. C1q-hemolytic activity in CD93−/− mice was decreased by 22% at time zero and by 46% 3 hours post thioglycollate injection suggesting a defect in the classical complement pathway. Leukocyte recruitment and C1q-hemolytic activity was restored to wildtype levels when CD93 was expressed on either hematopoietic cells or non-hematopoietic cells in bone marrow chimeric mice. However, elevated levels of sCD93 in inflammatory fluid were observed only when CD93 was expressed on non-hematopoietic cells. Since cell-associated CD93 was sufficient to restore a normal inflammatory response, these data suggest that cell-associated CD93, and not sCD93, regulates leukocyte recruitment and complement activation during murine peritonitis.
Keywords: Inflammation, complement, cell trafficking, cell surface molecules, adhesion molecules
Introduction
Acute inflammation is a normal response to injury or infection, characterized by recruitment of leukocytes to the site of injury, elimination of the pathogen or insult, and a return to normal tissue homeostasis. However, when improperly regulated, inflammation is involved in the progression of most diseases including cancer, heart disease, autoimmunity and sepsis (reviewed in 1–3). Therefore, deciphering the molecular mechanisms that regulate the inflammatory cascade has wide applicability to acute and chronic pathology. CD93, a type I transmembrane glycoprotein, is emerging as a novel regulator of inflammation.
CD93 is expressed on a variety of cells involved in the inflammatory cascade, including neutrophils, monocytes, and endothelial cells. CD93, thrombomodulin (TM) and endosialin, constitute the Group XIV family of C-type lectin-like domain (CTLD) containing proteins (reviewed in 4), characterized by a CTLD (referred to as D1), followed by a series of EGF-like repeats (D2), a highly glycosylated mucin domain, a transmembrane region and a short cytoplasmic tail. Several groups have demonstrated that TM is anti-inflammatory. For example, the CTLD of TM (TM-D1) inhibited LPS-induced proinflammatory signaling and complement activation (5–7), and cell-associated TM is also a complement regulatory protein (8). Furthermore, mice deficient in TM-D1 (TMLeD/LeD) are more susceptible to arthritis and sepsis (7). These observations led us to hypothesize that the other Group XIV family members, such as CD93, share anti-inflammatory functions with TM.
In support of this hypothesis, recent studies demonstrated that the absence of CD93 led to increased inflammation and tissue destruction in a model of cerebral ischemia reperfusion injury; however, the mechanism responsible for CD93-dependent pathology was not defined (9). We previously demonstrated that CD93 is proteolytically cleaved from activated human monocytes 4 and neutrophils (10), as well as mouse inflammatory macrophages, and the soluble form of CD93 (sCD93) is elevated during inflammation (11). Recently, Jeon et al. demonstrated that sCD93 is elevated in synovial fluid from rheumatoid arthritis patients, and that human monocytes treated with sCD93 were more adhesive, phagocytic, and responsive to TLR ligands when compared to control monocytes (12). Combined, these data support the hypothesis that CD93 regulates inflammation; however, its molecular function has remained elusive.
To investigate the molecular mechanism of CD93-dependent regulation of inflammation, we compared wildtype and CD93−/− mice during thioglycollate-induced peritonitis. We observed increased leukocyte recruitment into the peritoneal cavity, dysregulated C1q-hemolytic activity, and altered vascular integrity in CD93−/− mice post induction of peritonitis when compared to wildtype mice. Thioglycollate-induced peritonitis triggered a release of sCD93 only when CD93 was expressed on non-hematopoietic (radiation-resistant) cells. Moreover, expression of CD93 on either hematopoietic or non-hematopoietic cells was sufficient to restore both normal leukocyte infiltration into the peritoneal cavity and C1q-hemolytic activity. Therefore, in this study we further delineate the molecular function of CD93 by demonstrating that CD93 is required to maintain vascular integrity, and that the cell associated molecule, and not the soluble protein, regulates leukocyte recruitment and complement activity.
Materials and Methods
Mice
CD93-deficient mice were provided by Drs. Marina Botto and Mark Walport (Imperial College, London). CD93-deficient mice were backcrossed 11 generations on a Harlan C57BL/6 background. Genotype was confirmed by PCR using the following primers: P1 (5′AGG GAT CCC AGC GAG GAA GGG CAA CTG) and P2 (5′GGG ATC GGC AAT AAA AAG AC) for wildtype band; and P1 and P3 (5′GTC CTG GCA CTC ATC TAT ATC) for the CD93−/− band. Animals used in these studies were 8–12 weeks of age except in bone marrow chimeras where mice were 12–16 weeks of age. All studies were reviewed and approved by the University of Notre Dame Institutional Animal Care and Use Committee (IACUC).
Reagents and antibodies
All reagents were purchased from Fisher (Pittsburgh, PA) unless otherwise indicated. Annexin V-FITC Apoptosis Detection Kit was purchased from BioVision (Mountainview, CA). Rat and sheep anti-mouse CD93 antibodies were purchased from R&D (Minneapolis, MN) and reconstituted in PBS to 0.2 mg/ml. Rat anti-mouse CD11b-PE and Ig2b-PE antibodies were purchased from Beckman (Fullerton, CA). Rat anti-CD93 (AA4.1)-PE, rat anti-mouse Gr1-PC5.5, rat anti-mouse IgG2b-PC5.5, rat anti-mouse F4/80-PE, and Rat IgG2a- PE antibodies were purchased from eBioscience (San Diego, CA). HRP-conjugated donkey antisheep antibody was purchased from Jackson ImmunoResearch (West Grove, PA).
Cell culture
Stably transfected HEK293 cells were cultured in 5% CO2 in DMEM, 10% FBS, 10 mM HEPES, 0.2 units/ml of penicillin and 0.2 μg/ml of streptomycin (Pen/Strep), and 300 μg/ml zeocin (Invitrogen, Carlsbad, CA). BMDM were generated as described (13).
Induction of peritonitis and isolation of cells, fluid and serum
Peritonitis was induced as previously described (11). Briefly, 1 ml of sterile 4% Brewer’s thioglycollate solution was injected intraperitoneally (i.p.) into CD93−/− or C57BL/6 wildtype mice. Thioglycollate solution used in these studies was autoclaved and then aged for at least 6 months. Peritoneal lavage was collected with 1 ml ice cold HBSS++ 5 mM EDTA at the time points indicated. 1 × 105 peritoneal cells were cytospun at 300 × g for 10 minutes, stained with Giemsa, and coverslips were mounted with Permount. Blood was drawn via cardiac puncture, collected in borosilicate 12 × 75 mm glass tubes and allowed to clot for an initial 5 minutes at room temperature (RT) and then on ice for 45 minutes. After centrifugation of the blood sample at 3200 × g for 10 minutes, serum was recovered and stored at −80°C. In some cases 2 mM EDTA was added to blood samples to prevent clotting, red blood cells were lysed with ACK, and remaining cells were washed in FACS buffer (HBSS++ supplemented with 0.2% bovine serum albumin (BSA), and 0.2% sodium azide) for analysis by flow cytometry.
Immunohistochemistry
Tissue was fixed with formalin, embedded in paraffin, sectioned and mounted on slides at the University of Notre Dame histology core facility. Tissue was dehydrated and then cleared with xylene to remove paraffin prior to immunohistochemical analyses. Tissue was washed with tris buffered saline (TBS) and then treated with citric acid for antigen retrieval. Tissue was washed with TBS, treated with 10% methanol and 3% hydrogen peroxide to quench endogenous peroxidase activity and then blocked with TBS containing 2% BSA and donkey serum (Jackson ImmunoResearch, West Grove, PA). Tissue was incubated overnight with 10 μg/ml 1150 (anti-CD93 antibody kindly provided by Dr. Andrea Tenner, University of California Irvine, CA), washed and then incubated with the appropriate biotinylated secondary antibody (Jackson ImmunoResearch). Tissue was washed and the incubated with avidin biotin complex (ABC) (Vector Laboratories, Burlingame, CA). After washing, tissue was developed with diaminobenzidine (DAB) (Vector Laboratories), stained with hemotoxylin and coverslipped. Images were acquired with a Zeiss Axio Imager A1 microscope and AxioVision 40 V 4.6.3.0, 2006–2008 software.
Generation and purification of recombinant proteins
Mouse CD93-D1,2 was amplified from the full length cDNA using the primer set 5′ CTA GAA TTC ATT ATG GCC ATC TCA ACT GGT TTG 3′ and 5′-ATC GCG GCC GCT TGC TAC AAA AGA CCC CAT TGG G 3′. PCR products were digested using EcoR1 and Not1 and cloned into pcDNA 4 V5/His A expression vector (Invitrogen, Carlsbad, CA). Proteins were expressed in HEK293 cells following transfection with lipofectamine (Invitrogen, Carlsbad, CA) and selection with 300 μg/ml zeocin (Invitrogen, Carlsbad, CA). Stably transfected HEK293 cells were grown in 20% complete media and 80% serum-free media (293SFM II [Invitrogen, Carlsbad, CA], containing 10 mM HEPES, Pen/Strep, and 2 mM L-Alanyl-L-Glutamine [Mediatech, Inc., Manassas, VA]) for 48 hours. His-tagged proteins were purified from culture supernatants by Nickel-chromatography using the ProBond purification system (Invitrogen, Carlsbad, CA) and fractions were assayed for CD93 by ELISA as described in (11). Positive fractions containing recombinant proteins were pooled and dialyzed versus PBS. Proteins were quantified by BCA (Pierce, Rockford, IL) against a standard curve of BSA and purity was analyzed by SDS-PAGE and Western blot.
LPS-induced sepsis
Wildtype mice were injected i.p. with 10μg O111:B4 LPS/g body weight (Sigma Aldrich, St. Louis, MO). Septic mice were euthanized at 0, 6, and 18 hours post injection of LPS and sCD93 was measured by ELISA in peritoneal lavage fluid and serum.
Quantification of soluble proteins
Peritoneal lavage samples were tested using a Luminex Multiplex array according to the manufacturer’s protocol (Millipore, Billerica, MA) using a Luminex 200. CCL21 was quantified by ELISA according to the manufacturer’s instruction (R&D Systems, Minneapolis, MN). The mouse C1q ELISA was performed as described by Li et al. (14) with anti-C1q antibodies: monoclonal rat anti-mouse C1q (Hycult Biotechnology, Uden, Netherlands) and 1151 (kindly provided by Dr. Andrea Tenner, University of California, Irvine). C3a and C5a levels were quantified using commercially available kits following the manufacturer’s protocol (BD, Franklin Lakes, NJ). The mouse sCD93 ELISA was performed as described in Greenlee et al. with R&D sheep and rat anti-mouse CD93 antibodies (11).
Vascular integrity intravital assay
At 6 hours post i.p. injection of 1 ml sterile thioglycollate, animals were anesthetized with rodent cocktail anesthesia (Ketamine (50mg/Kg), Xylazine (10mg/Kg) and Acepromazine (1.7mg/Kg)), as previously described (15). The parietal peritoneum was surgically exposed by removing a lower abdominal skin flap, and visualized using an Olympus FV1000 inverted microscope equipped with a femto-second pulsed Ti:Sapphire laser with dispersion compensation, an Olympus 25X 1.05NA water emersion XLPlan N objective and 4 non-descanned detectors. The collagen surface of the parietal peritoneum was visualized via second-harmonic generation with 850 nm excitation and a 425–465 barrier filter (BF). All subsequent images were excited at 800 nm. A low molecular weight 10 kDa dextran-texas red was injected retro-orbitally and visualized with a 575–625 nm BF to highlight the vasculature and define imaging volume. Animals were then retro-orbitally injected with 150 μl Angiosense-680IVM (Perkin Elmer) and an image volume was acquired with a 675–700 nm BF in 30 second intervals. At 2 minutes post-Angiosense 680 IVM injection, images 50 microns beyond the collagen surface of the parietal peritoneum were quantified for relative fluorescence. Ten paired measurements inside and outside the blood vessel were analyzed using Imaris software (Bitplane, Inc) in each animal. Data is plotted as a ratio metric measurement to normalize for small differences in light scatter between specimens. The D’Agostino & Pearson omnibus test was used to determine whether the data points were normally distributed and a two-sided Mann-Whitney test with a 95% CI was conducted to test for significance.
Hemolytic titer
Complement activity was measured by hemolytic titer. The C1q hemolytic titer was performed as described by Tenner et al. (16). Briefly, serial dilutions of mouse serum were prepared in GVB++ and then added to human C1q depleted serum. Incubations with sheep erythrocytes (E) (Colorado Serum Co., Denver CO) opsonized with anti-sheep antibodies (A’s, hemolysin) (Colorado Serum Co., Denver CO) (EA) were performed for 30 minutes at 37°C. After centrifugation at 1800 × g for 3 minutes, supernatants were split in duplicate in 96 well plates, and the OD was measured at 412 nm. Percent of C1q lytic activity, corresponding to the percentage of EA lysis was calculated using the following formula: [(sample OD)-(buffer control OD)]/[(H20 lysis OD)-(buffer control OD)]. C1q hemolytic activity, expressed as the z-value of 1 (the dilution of serum at which 63% of EA were lysed) was calculated for all samples using a logarithmic regression analysis as previously described (16, 17). Results were expressed as a percent of C1q hemolytic activity relative to uninjected wildtype control serum. Serum samples were analyzed from a minimum of 7 mice per time point. C3 hemolytic titers were performed using the same protocol with minimal modifications. Briefly, serial dilutions of mouse serum were prepared in GVB++ and then added to C3 depleted serum (C3D, Quidel, San Diego, CA). Incubation with EA was performed for 1 hour at 37°C. Results of the C3 hemolytic titer were expressed as a percent of C3 hemolytic activity relative to uninjected wildtype mouse serum. Serum samples were analyzed from a minimum of 7 animals per time point.
Bone Marrow Chimera
Bone marrow irradiation was performed on 4–8 week old recipient mice and bone marrow was collected from donor mice at 4–6 weeks of age. Mice were irradiated using the RAD SOURCE Technologies RS2000 irradiator. Mice were given 2 doses of 600 cGy at 115 cGy/min spaced three hours apart. Mice were allowed to recover for three hours after the final dose of irradiation and injected retro-orbitally with 0.2 ml bone marrow suspension (1 × 107 cells). Animals were maintained on low pH (pH 2.5–3.0) water for 8 weeks to prevent waterborne contamination of immunocompromised mice and until total replacement of bone marrow was achieved. Expression of CD93 on leukocytes of recipient mice was confirmed by flow cytometry. Blood was collected via heart puncture, 2mM EDTA was added, and red blood cells were lysed for 10 minutes in ACK buffer at room temperature.
Statistical analysis
Student’s t test were used to calculate statistical significance unless otherwise indicated.
Results
CD93 deficiency results in increased leukocyte infiltration during peritonitis
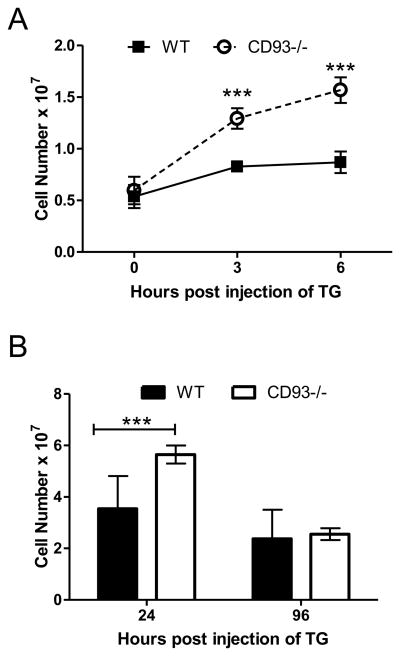
Intraperitoneal (i.p.) injection of thioglycollate has been widely used as a model to study the acute inflammatory response (18), and therefore to assess the contribution of CD93 to the acute inflammatory response, wildtype and CD93−/− mice were subjected to thioglycollate-induced peritonitis. Leukocyte numbers in the peritoneal cavity were similar in wildtype and CD93−/− mice prior to injection of thioglycollate [(0.54 ± 0.11) × 107 versus (0.60 ± 0.13) × 107, respectively], however increased leukocyte recruitment into the peritoneal cavity was observed at 3 and 6 hours post injection of thioglycollate in CD93−/− mice compared to wildtype controls (1.5 and 1.8 fold respectively, ***p < 0.0001, Figure 1A). In a separate set of experiments, the number of leukocytes was counted in the peritoneal cavity at 24 and 96 hours post injection of thioglycollate; the increase in leukocytes in the peritoneal cavity persisted 24 hours post injection of thioglycollate in CD93−/− mice [(5.6 ± 0.35) × 107 versus (3.5 ± 0.23) × 107, respectively, ***p < 0.0001], and returned to wildtype levels after 96 hours (Figure 1B).
Figure 1. CD93−/− mice show increased susceptibility to thioglycollate induced peritonitis.
Leukocytes in peritoneal lavage fluid from CD93−/− and wildtype mice were counted 0, 3 and 6 hours following i.p. injection of thioglycollate (TG). Points represent the mean values from at least 12 individual mice per genotype over four separate experiments ± SEM (A). Leukocytes in peritoneal lavage fluid were counted at 24 and 96 hours following i.p. injection of thioglycollate in CD93−/− mice (open bars) and wildtype mice (closed bars). Bars represent the mean leukocyte number for at least 7 mice per genotype ± SEM over at least three separate experiments (***p<0.001, B).
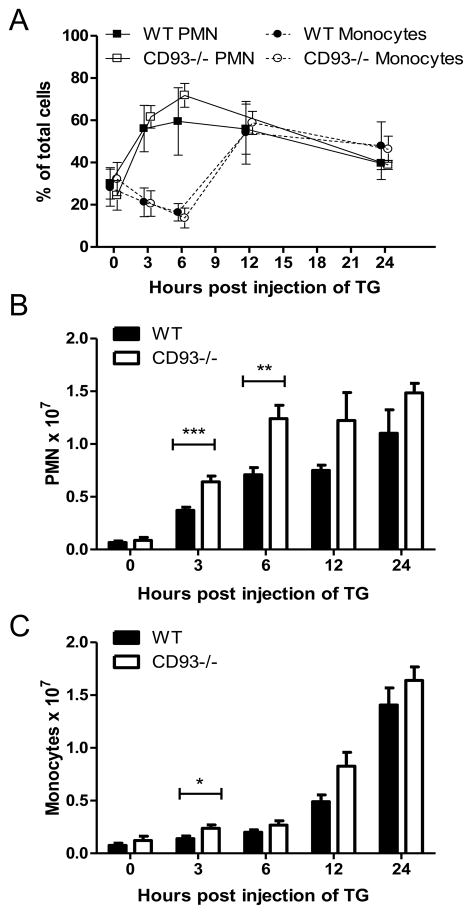
To further characterize this pro-inflammatory phenotype in the CD93−/− mice, cell populations in the peritoneal cavity were analyzed by microscopy. A normal inflammatory response was observed following thioglycollate challenge: there was an influx of neutrophils starting at 3 hours post injection, followed by an influx of monocytes starting at 12 hours post injection (Figure 2A) (19). Although the total number of neutrophils and monocytes in the peritoneal cavity of CD93−/− mice was greater than wildtype (Figure 2B and C), the relative percentage of each cell type was similar in wildtype and CD93−/− mice (Figure 2A). Flow cytometric analysis of peritoneal cells yielded similar findings. At 6 and 24 hours the relative percentages of Gr1-positive neutrophils and F4/80-positive monocyte/macrophages, as well as Annexin-V-positive apoptotic cells were equivalent between wildtype and CD93−/− mice (Supplemental Figure 1). Since there was no difference in the relative percentage of neutrophils, monocytes, or apoptotic cells, the increase in leukocyte recruitment in CD93−/− mice is not due to augmented recruitment or persistence of a single cell type.
Figure 2. Analysis of monocyte/macrophage and neutrophil (PMN) populations in the peritoneal cavity of CD93−/− and wildtype mice.
Peritoneal cells were stained with Giemsa and differential cell counts were performed. 200 cells per mouse were scored blinded by three separate individuals and the percentage of PMNs and monocytes (A) and total PMN and monocyte numbers (B and C) were plotted. Bars represent the mean of at least three mice per genotype ± SD (* p < 0.05, **p < 0.01, ***p < 0.001).
Cell-associated and sCD93 are elevated with inflammation
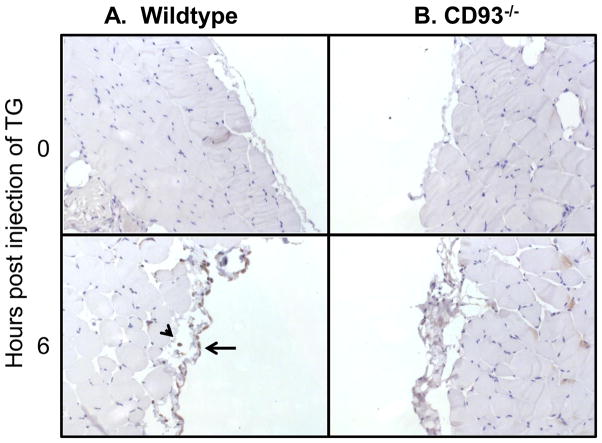
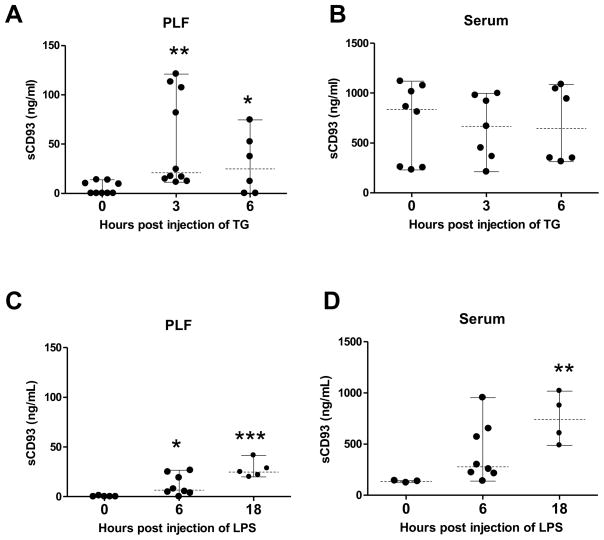
CD93 exists in two forms – a cell-associated full length form and a truncated soluble form. Cell-associated CD93 was measured by immunostaining for CD93 on tissue from the peritoneal cavity at 0 and 6 hours post injection of thioglycollate. CD93-positive cells were detected at 6 hours post injection of thioglycollate in wildtype mice. As expected CD93 was absent from tissue in CD93−/− mice (Figure 3). We previously demonstrated an increase in sCD93 concentration at 24 and 96 hours post injection of thioglycollate (11). To further characterize the kinetics of sCD93 production, sCD93 was quantified at 0, 3, and 6 hours post injection of thioglycollate using purified recombinant protein as a standard (Figure 4A and B). sCD93 was low to undetectable at time zero and was elevated in peritoneal lavage fluid (PLF) 3 and 6 hours after thioglycollate challenge (20.9 ng/ml and 24.8 ng/ml [median value], Figure 4A). sCD93 was also elevated in peritoneal lavage fluid of wildtype mice 6 and 18 hours following LPS-induced sepsis (6.5 ng/ml and 24.8 ng/ml [median], respectively, Figure 4C). sCD93 was elevated in serum during LPS-induced sepsis (3.1 – 5.6 times greater post injection of LPS) but was not elevated in the serum of thioglycollate injected mice. These data demonstrate that sCD93 concentration is elevated with inflammation in two independent mouse models, and that sCD93 is preferentially elevated at the site of inflammation compared to the concentration of sCD93 detected systemically during thioglycollate-induced peritonitis.
Figure 3. CD93 is upregulated during peritonitis.
Peritoneal tissue was fixed from wildtype (A) and CD93−/− mice (B) and stained for CD93 at 0 (upper row) and 6 (lower row) hours post injection of TG. The arrow head points to a CD93-positive leukocyte and the arrow points to CD93 staining associated with the vasculature. 25
Figure 4. CD93 is shed during inflammation.
sCD93 was measured by ELISA in peritoneal fluid and serum at indicated time points from mice with thioglycollate (TG)-induced peritonitis (A and B) and LPS-induced sepsis (C and D). Each point represents the mean of duplicate wells containing fluid from an individual mouse, dashed horizontal line represents the median value, solid vertical line represents the range, and points at baseline are below the limit of detection (* p < 0.05, **p < 0.01, ***p < 0.001 compared to time zero).
Detection of cytokines and chemokines in the peritoneal fluid
Increased leukocyte chemotaxis is influenced by changes in the cytokine and chemokine milieu, thus cytokine (IL-1, IL-4, IL-5, IL-6, IL-10, and TNFα) and chemokine (MCP-1, RANTES, GMCSF, KC, and MIP1α) levels were measured in the peritoneal lavage fluid of CD93−/− and wildtype mice using Luminex Multiplex array. The majority of cytokines and chemokines measured in peritoneal lavage fluid (IL-1, IL-5, IL-6, IL-10, TNFα, MCP-1, RANTES, GMCSF, KC, and MIP1α) were detectable at 3 and 6 hours post injection of thioglycollate, while IL-4 was detectable only at 6 hours post injection of thioglycollate. However, there were no statistically significant differences in the cytokine and chemokine levels between CD93−/− and wildtype mice (p > 0.05, Table 1). IL-12 (p40), IL-13 and IFNγ were also measured but the concentration in the fluid was below the limit of detection. Elevated levels of the chemokine CCL21 has been implicated in mediating leukocyte recruitment in CD93−/− mice during cerebral ischemia and reperfusion injury (9), where CCL21 was elevated in CD93−/− mice both prior to injury and during injury. In this model, CCL21 levels were equivalent in peritoneal lavage fluid from CD93−/− and wildtype mice under both baseline and inflammatory conditions (Supplemental Figure 2). Therefore heightened susceptibility to thioglycollate-induced peritonitis in CD93-deficient mice did not correlate with an alteration in proinflammatory cytokines and chemokines measured.
Table I. CD93−/− mice have similar levels of cytokines compared to wildtype mice following induction of peritonitis.
Chemokines and cytokines in peritoneal lavage fluid from wildtype and CD93−/− mice were measured by Luminex at 3 and 6 hours post injection of thioglycollate. Each value represents the mean from at least three individual mice per genotype (pg/ml) ± SD (p > 0.05).
| Conditions | wildtype 3 hours | CD93−/− 3 hours | wildtype 6 hours | CD93−/− 6 hours |
|---|---|---|---|---|
| IL-1 | 49 ± 19 | 61 ± 16 | 37 ± 26 | 61 ± 27 |
| IL-6 | (5.1 ± 0.43) × 104 | (4.6 ± 0.58) × 104 | (2.1 ± 1.7) × 103 | (1.7 ± 1.6) × 103 |
| TNFα | 79 ± 6.9 | 80. ± 35 | 2.8 ± 1.0 | 5.5 ± 3.3 |
| MCP1 | (6.5 ± 2.0) × 103 | (6.4 ± 0.82) × 103 | (8.3 ± 5.8) × 102 | (4.6 ± 3.6) × 102 |
| IL-4 | nd | nd | 55 ± 28 | 66 ± 99 |
| IL-5 | 42 ± 10. | 33 ± 11 | 55 ± 22 | 40. ± 17 |
| RANTES | 6.9 ± 1.1 | 12 ± 6 | 14 ± 6 | 12 ± 6 |
| GMCSF | 46 ± 12 | 39 ± 25 | 18 ± 9 | 22 ± 16 |
| IL-10 | (5.9 ± 1.4) × 102 | (4.3 ± 1.0) × 102 | 43 ± 18 | 37 ± 12 |
| KC | (7.3 ± 3.8) × 103 | (10. ± 1.6) × 103 | (1.0 ± 0.5) × 102 | 91 ± 75 |
| MIP1α | (7.5 ± 1.6) × 102 | (7.6 ± 2.5) × 102 | (1.4 ± 0.3) × 102 | (1.6 ± 0.7) × 102 |
CD93-deficient mice have altered vascular integrity
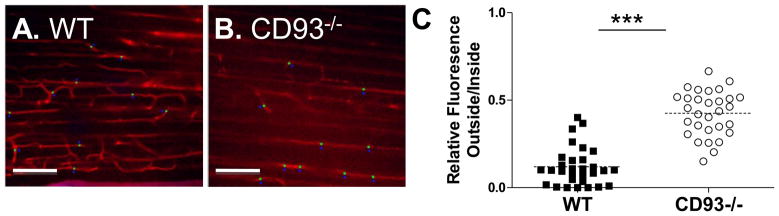
Since CD93 deficiency led to increased cell recruitment, but did not affect cytokine or chemokine levels, we therefore hypothesized that particularities of the endothelium in the mice may correspond to increased inflammation. Using fluorescence multi-photon intravital microscopy, wildtype and CD93−/− mice were examined for changes in vascular integrity 6 hours post injection of thioglycollate (Figure 5). Using the indicator dye Angiosense 680 IVM, a ratiometric analysis of the relative fluorescence inside and outside of the blood vessels of the parietal peritoneum during thioglycollate-induced peritonitis revealed a significant increase in Angiosense 680 IVM permeability in CD93−/− mice (***p < 0.0001, Figure 5C). Although an increase in the permeability of Angiosense 680 IVM was observed, total protein concentration was equivalent in the peritoneal lavage fluid of CD93−/− mice and wildtype controls at this time point (1.8 ± 0.28 mg/ml and 1.9 ± 0.91 mg/ml, respectively). Similarly, no changes in total protein were observed at 3, 12, and 24 hours post injection (Table II). These results demonstrate that vascular integrity is altered in CD93−/− mice during sterile peritonitis.
Figure 5. CD93−/− mice have altered vascular integrity during peritonitis.
Wildtype (A) and CD93−/− mice (B) were examined by intravital microscopy for the permeability of Angiosense-680 IVM at 6 hours post injection with thioglycollate. The relative fluorescence of tracer dye was quantified inside and outside blood vessels at 50 microns past the collagen surface of the parietal peritoneum. Data is plotted as the ratio of fluorescence outside/inside the blood vessel. The increased fluorescence in CD93−/− mice indicates changes in vascular integrity (***p < 0.0001. n = 3 mice per genotype, C).
Table II. Analysis of protein concentration in peritoneal lavage fluid of CD93−/− and wildtype mice.
Peritoneal lavage fluid was collected and subjected to BCA to determine the total protein concentration. Each value represents the mean of at least three mice per genotype ± SD.
| Hours post injection of thioglycollate | Protein concentration in wildtype peritoneal lavage fluid | Protein concentration in CD93−/− peritoneal lavage fluid |
|---|---|---|
| 3 | 5.5 ± 1.5 mg/ml | 4.0 ± 2.7 mg/ml |
| 6 | 1.8 ± 0.28 mg/ml | 1.9 ± 0.91 mg/ml |
| 12 | 1.5 ± 0.73 mg/ml | 2.3 ± 0.36 mg/ml |
| 24 | 2.9 ± 1.4 mg/ml | 2.3 ± 0.36 mg/ml |
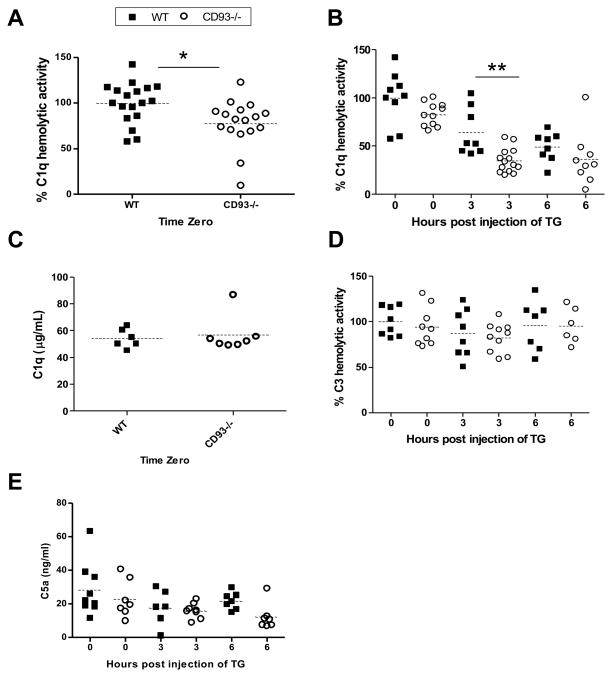
CD93-deficient mice have dysregulated C1q hemolytic activity
A significant decrease in C1q hemolytic activity was observed at time zero in CD93−/− mice compared to wildtype controls (22 ± 26%, *p < 0.05, Figure 6A). At 3 hours post injection of thioglycollate the difference was more pronounced. C1q hemolytic activity was 46 ± 20% lower in serum from CD93−/− mice compared to wildtype mice (**p < 0.01, Figure 6B). There was no defect in C1q production in CD93−/− mice since equivalent C1q protein concentration was detected in serum from CD93−/− and wildtype mice at time zero (Figure 6C). The defect in C1q hemolytic activity in CD93−/− mice did not reflect a general defect in complement activity because C3 hemolytic activity was equivalent between wildtype and CD93−/− mice (Figure 6D). To determine if dysregulated C1q hemolytic activity resulted in increased production of anaphylatoxins, as would be expected if the components of the classical pathway were more readily consumed in CD93−/− mice, C5a protein concentration was measured by ELISA. C5a was not elevated in serum (Figure 6E) or peritoneal lavage fluid (Supplemental Figure 3B) from CD93−/− at 0, 3 or 6 hours post injection of thioglycollate. These data demonstrate that C1q-hemolytic activity, but not C3-hemolytic activity, is reduced in CD93−/− mice compared to wildtype controls. Furthermore, increased production of analphylatoxins C3a or C5a was not detected in CD93−/− mice and does not contribute to the pro-inflammatory phenotype in null mice (Figure 6 and Supplemental Figure 3).
Figure 6. C1q hemolytic activity is dysregulated in CD93−/− mice.
C1q hemolytic activity was measured in serum from wildtype (closed squares) and CD93−/− mice (open circles) by hemolytic titer at time zero (n = 5 experiments, A) and between 0 and 6 hours post injection of thioglycollate (n = 3 experiments, B). The concentration of C1q in serum was measured at time zero by ELISA (n = 6 mice per genotype, C), C3 hemolytic activity in serum was measured by C3 hemolytic titer (D), and concentration of C5a in serum was measured by ELISA (E) in wildtype and CD93−/− mice. For hemolytic titers, each point represents the mean of duplicate wells from an individual mouse, and for ELISAs each point represents the mean of triplicate wells from an individual mouse. The dotted line represents the mean of each group.
Cell-associated CD93 regulates leukocyte recruitment and complement activation
To begin to address the mechanism by which CD93 regulates myeloid cell recruitment in peritonitis, we sought to identify the bioactive form of CD93 (soluble or full-length), and the cellular source of bioactive CD93 (hematopoietic or non-hematopoietic cells). Reconstitution of CD93−/− mice with recombinant mouse sCD93 failed to restore leukocyte migration to wildtype levels (data not shown) and therefore bone marrow chimeras were generated to determine the role of hematopoietic and non-hematopoietic cell-expressed CD93 during leukocyte recruitment.
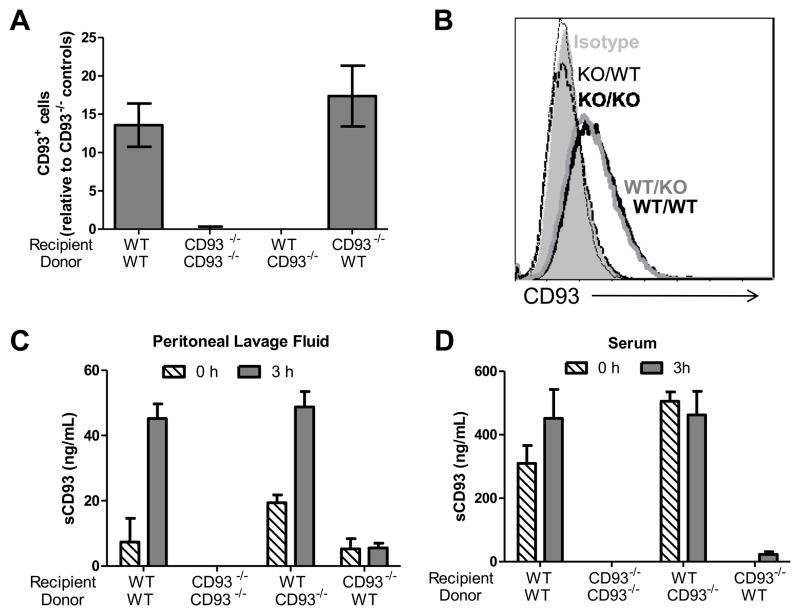
Eight weeks after bone marrow reconstitution, CD93−/− mice that received wildtype donor bone marrow expressed CD93 on leukocytes in blood and on bone marrow derived macrophages (BMDM, Figure 7A and B). CD93 expression on blood leukocytes and BMDM from wildtype mice that received CD93-deficient donor bone marrow was indistinguishable from CD93 expression from CD93−/− mice that received CD93−/− donor bone marrow (Figure 7A and B). sCD93 concentration in peritoneal lavage fluid and serum from chimeric mice, was dependent on CD93 expression on the non-hematopoietic cells. Mice that expressed CD93 on non-hematopoietic cells (wildtype recipients) had elevated levels of sCD93 in the peritoneal lavage fluid three hours following injection of thioglycollate (45.2 ± 10.1 ng/ml [wildtype donor and wildtype recipient] and 48.8 ± 11.5 ng/ml [wildtype donor and CD93−/− recipient]). CD93−/− mice that received wildtype bone marrow had low (or undetectable) sCD93 in peritoneal lavage fluid, which was not elevated with inflammation (Figure 7C). Similar findings were observed in serum: sCD93 was present in serum when CD93 was expressed on non-hematopoietic cells, while low (or undetectable) levels of sCD93 were present in CD93−/− recipient mice receiving wildtype donor bone marrow (Figure 7D).
Figure 7. sCD93 is produced from non-hematopoietic cells in vivo.
Peripheral blood leukocytes were stained for CD93 and analyzed by flow cytometry. The bars represent the average percentage of CD93-positive cells relative to the CD93−/− controls from at least seven mice per group ± SEM (A). CD93 expression was also measured on BMDM by flow cytometry. Shown is an overlay of representative staining from an individual mouse in each group (isotype control filled gray; KO/KO [CD93−/− recipients with CD93−/− donors] dashed black line, KO/WT [wildtype recipients with CD93−/− donors] dashed gray line, WT/KO [CD93−/− recipients with wildtype donors] solid gray line; WT/WT [wildtype recipients with wildtype donors] solid black line, B). sCD93 was measured from peritoneal lavage fluid (C) and serum (D) from chimeric mice 0 (dashed bars) and 3 hours (gray bars) post injection of thioglycollate. Bars represent the mean of at least four mice per group (two mice for CD93−/− controls) ± SD.
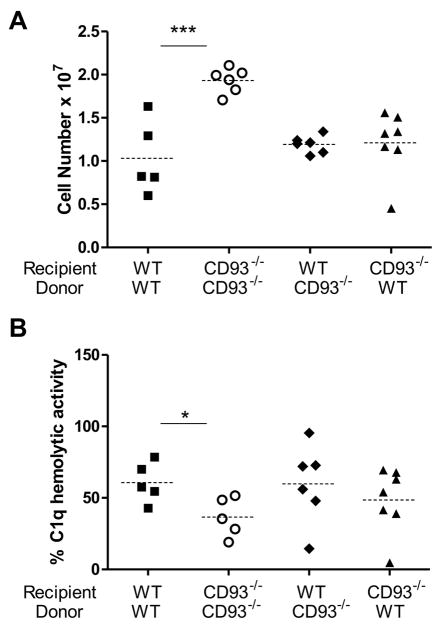
Expression of CD93 on either hematopoietic cells or non-hematopoietic cells restored leukocyte recruitment to wildtype levels. CD93−/− mice reconstituted with wildtype bone marrow, and wildtype mice reconstituted with CD93−/− bone marrow both recruited only 62% of the leukocytes found in the inflamed peritoneal cavity of CD93−/− controls (Figure 8A). Expression of CD93 hematopoietic or non-hematopoietic cells alone also reconstituted C1q-hemolytic activity to wildtype levels. There was no significant difference in C1q-hemolytic activity in serum from mice that expressed CD93 on hematopoietically derived or nonhematopoietically derived cells when compared to wildtype controls. Irradiation and reconstitution did not alter the phenotype in the CD93−/− mice as a significant decrease in C1q hemolytic activity was detected in CD93−/− controls (*p < 0.05, Figure 8B). Increased leukocyte migration in CD93−/− mice directly correlated with a decrease in C1q hemolytic activity at three hours post injection of thioglycollate suggesting that alterations in complement activity may regulate the recruitment phenotype (Figure 8 and Supplemental Figure 4, *p < 0.05, one-tailed Pearson’s correlation test).
Figure 8. Hematopoietic or non-hematopoietic cell-associated CD93 regulates leukocyte recruitment and complement activity.
Leukocytes were counted (A) and C1q-hemolytic activity was measured (B) 3 hours post injection of thioglycollate in chimeric mice. Each point represents a single mouse and dotted lines represent the average (* p < 0.05 and *** p < 0.001).
Discussion
This study demonstrates that CD93 is required for the regulation of acute inflammation in thioglycollate-induced peritonitis. In mice deficient in CD93, more leukocytes were recruited into the peritoneal cavity and vascular integrity was altered, while total protein, cytokine and chemokine levels remained the same. This study also identifies a novel relationship between CD93 and classical complement activity in vivo as mice deficient in CD93 show dysregulated C1q hemolytic activity that is correlated with leukocyte infiltration. sCD93 is elevated with inflammation and the source of sCD93 in vivo is non-hematopoietic cells; however, these data suggest that cell-associated CD93, and not the soluble form, regulates leukocyte recruitment and complement activity.
Using the same model of thioglycollate-induced peritonitis shown here, Norsworthy et al. showed that CD93-deficient mice had a defect in the engulfment of apoptotic cells when exogenous apoptotic cells were introduced into the inflamed peritoneal cavity. Therefore, the increase in leukocytes in the CD93−/− peritoneal cavity could result from a failure to efficiently clear apoptotic cells (20). However, there was no difference in the relative percentage of Annexin-V-positive cells in the inflamed peritoneum of wildtype and CD93−/− mice (Supplemental Figure 1), suggesting that failure to clear apoptotic cells was not responsible for the increase in leukocytes in the CD93−/− peritoneal cavity during peritonitis. Norsworthy et al. also assessed leukocyte recruitment into the peritoneal cavity, but did not observe enhanced neutrophil recruitment four hours following thioglycollate challenge (20). Extended kinetics, batch variability of thioglycollate, and differences in genetic background of the mice, could account for the discrepancies between these two studies.
Consistent with our observation that CD93−/− mice show increased cellular recruitment into the peritoneal cavity following thioglycollate challenge, Harhausen et al. found that CD93 deficiency led to increased infiltration of CD11b+ cells following cerebral ischemia reperfusion injury (9). Harhausen et al. attributed enhanced leukocyte migration to CCL21, which was upregulated in brain tissue of non-ischemic CD93-deficeint mice and further upregulated ischemic CD93-deficient mice compared to wildtype controls. Although no difference in CCL21 was detected in peritoneal lavage fluid from CD93−/− and wildtype mice under baseline and inflammatory conditions (Supplemental Figure 2), CCL21 cannot be discounted as an important factor in driving increased leukocyte recruitment; for example tissue-specific elevation in CCL21 or the ratio of CCL21 in peritoneal lavage fluid to serum may contribute to enhanced migration.
In addition to the production of chemokines and cytokines, the complement system plays an important role in propagating the inflammatory response, and as expected, activation of the complement system is a tightly regulated process. In the present study, thioglycollate-induced peritonitis led to C1q consumption as detected by a decrease in C1q-hemolytic activity in mouse serum. C1q hemolytic activity was further decreased in CD93−/− mouse serum compared to wildtype serum, suggesting that there is a defect in classical pathway activation in CD93−/− mice. Despite dysregulated C1q-hemolytic activity, increased C3a and C5a did not accompany increased leukcocyte recruitment in CD93−/− mice. In addition, consumption of C3 hemolytic activity with inflammation was not detected. C3 is the most abundant complement component in serum, therefore moderate changes in C3 consumption may not have been detected in this assay.
In addition to a defect in C1q hemolytic activity, altered vascular integrity was observed in CD93-deficient mice compared to wildtype controls 6 hours post injection of thioglycollate. Integrity of the vasculature was measured using Angiosense 680 IVM indicator dye. This 250 kDa dye has been used previously to quantify vascular integrity in a model of collagen-induced arthritis (21). Specific leakage of Angiosense 680 IVM into the extravascular space indicates a change in vascular integrity since smaller molecules (e.g. 40 kDa dextrans) are usually confined to the vasculature (22). Although these changes could explain dysregulation of cellular recruitment into the peritoneum, total protein- and chemokine and cytokine levels- were comparable between wildtype and CD93−/− mice, indicating that CD93 plays a specific role in regulating the integrity of the vasculature. It is possible that excessive complement activation in a CD93 deficient environment contributes to the loss of vascular integrity, although C3 deposition was not detected in the peritoneum of wildtype or CD93−/− mice (data not shown).
Several recent studies have highlighted the role of CD93 as a regulator of inflammation; however, the source of active CD93 is unknown. Jeon et al. recently showed that sCD93 was elevated in synovial fluid from rheumatoid arthritis patients. Moreover, recombinant sCD93 induced the adhesion and phagocytic capacity of monocytes (11,12). Here we identified radiation-resistant, non-hematopoietic cells as the source of sCD93 in vivo since sCD93 is produced and elevated only when CD93 is expressed on radiation-resistant cells in chimeric mice. These data suggest that the endothelium, the major non-hematopoietic site of CD93 expression, is the source of sCD93. Therefore, this protein may be a useful biomarker to assess endothelial cell damage during inflammation. In support of this hypothesis, Mälarstig et al. recently demonstrated that sCD93 concentration in plasma was predictive of risk for myocardial infarction and associated coronary artery disease (23).
In addition, the data presented suggest that sCD93, produced from endothelium, is not required to regulate leukocyte recruitment in vivo, since leukocyte recruitment at 3 hours post injection of thioglycollate was comparable to wildtype controls when sCD93 concentration were negligible (CD93−/− recipient mice with wildtype donor bone marrow) (Figures 7 and 8). Moreover, these data demonstrate that the CD93 null mutation does not lead to a global developmental defect that leads to dysregulated inflammation, since the inflammatory phenotype was reversed with wildtype bone marrow. Similarly, reconstitution of membrane-associated CD93 restored C1q hemolytic activity to wildtype levels (Figure 8B). Therefore, this study demonstrates that cell-associated CD93 regulates leukocyte recruitment that correlates with C1q hemolytic activity during thioglycollate-induced peritonitis. Future studies are aimed at determining the mechanism by which complement activity is regulated in the CD93-deficient mice.
Supplementary Material
Acknowledgments
The authors thank Deborah Foreman and Dr. Deborah Donahue for excellent technical assistance, and Drs. Marina Botto, Deborah Fraser, Marilyn Slater, and Rebecca Haberstroh for thoughtful review of the manuscript.
Footnotes
NIH NIAID R56AI080877, American Heart Association 0630068N and the Research Support Funds Grant from Indiana University School of Medicine to SSB.
Reference List
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenlee MC, Sullivan SA, Bohlson SS. CD93 and related family members: their role in innate immunity. Curr Drug Targets. 2008;9:130–138. doi: 10.2174/138945008783502421. [DOI] [PubMed] [Google Scholar]
- 5.Shi CS, Shi GY, Hsiao SM, Kao YC, Kuo KL, Ma CY, Kuo CH, Chang BI, Chang CF, Lin CH, Wong CH, Wu HL. Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood. 2008;9:3661–3670. doi: 10.1182/blood-2008-03-142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van de Wouwer M, Plaisance S, De VA, Waelkens E, Collen D, Persson J, Daha MR, Conway EM. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4:1813–1824. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 7.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van AH, De VA, Weitz JI, Weiler H, Hellings PW, Schaeffer P, Herbert JM, Collen D, Theilmeier G. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delvaeye M, Noris M, De VA, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harhausen D, Prinz V, Ziegler G, Gertz K, Endres M, Lehrach H, Gasque P, Botto M, Stahel PF, Dirnagl U, Nietfeld W, Trendelenburg G. CD93/AA4.1: a novel regulator of inflammation in murine focal cerebral ischemia. J Immunol. 2010;184:6407–6417. doi: 10.4049/jimmunol.0902342. [DOI] [PubMed] [Google Scholar]
- 10.Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol. 2005;175:1239–1247. doi: 10.4049/jimmunol.175.2.1239. [DOI] [PubMed] [Google Scholar]
- 11.Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res. 2009;58:909–919. doi: 10.1007/s00011-009-0064-0. [DOI] [PubMed] [Google Scholar]
- 12.Jeon JW, Jung JG, Shin EC, Choi HI, Kim HY, Cho ML, Kim SW, Jang YS, Sohn MH, Moon JH, Cho YH, Hoe KL, Seo YS, Park YW. Soluble CD93 Induces Differentiation of Monocytes and Enhances TLR Responses. J Immunol. 2010;185:4921–4927. doi: 10.4049/jimmunol.0904011. [DOI] [PubMed] [Google Scholar]
- 13.Roach T, Slater S, Koval M, White L, Cahir McFarland ED, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Ager RR, Fraser DA, Tjokro NO, Tenner AJ. Development of a humanized C1q A chain knock-in mouse: assessment of antibody independent beta-amyloid induced complement activation. Mol Immunol. 2008;45:3244–3252. doi: 10.1016/j.molimm.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez P, Wei B, McPherson M, Mendoza LM, Nguyen SL, Turovskaya O, Kronenberg M, Huang TT, Schrage M, Lobato LN, Fujiwara D, Brewer S, Arditi M, Cheng G, Sartor RB, Newberry RD, Braun J. Villous B cells of the small intestine are specialized for invariant NK T cell dependence. J Immunol. 2008;180:4629–4638. doi: 10.4049/jimmunol.180.7.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J Immunol. 1981;127:648–653. [PubMed] [Google Scholar]
- 17.Whaley K, North J. Haemolytic assays for whole complement activity and individual components. In: Dodds AW, Sim RB, editors. Complement: a practical approach. Oxford University Press; Oxford, UK: 1997. pp. 19–47. [Google Scholar]
- 18.Current Protocols in Immunology. Vol. 4. John Wiley & Son’s, Inc; Hoboken: 2006. pp. 14.1.1–14.1.3. [Google Scholar]
- 19.Qureshi R, Jakschik BA. The role of mast cells in thioglycollate-induced inflammation. J Immunol. 1988;141:2090–2096. [PubMed] [Google Scholar]
- 20.Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, Nourshargh S, Walport MJ, Botto M. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 21.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weissleder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 22.Dunn K, Sutton T, Sandoval R. Current Protocols in Cytometry. Vol. 41. John Wiely & Sons, inc; Hoboken: 2007. Live-Animal Imaging of Renal Function by Multiphoton Microscopy; pp. 12.9.1–12.9.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malarstig A, Silveira A, Wagsater D, Ohrvik J, Backlund A, Samnegard A, Khademi M, Hellenius ML, Leander K, Olsson T, Uhlen M, de FU, Eriksson P, Hamsten A. Plasma CD93 Concentration is a Potential Novel Biomarker for Coronary Artery Disease. J Intern Med. 2011 doi: 10.1111/j.1365-2769.2011.02364.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.