Abstract
Igα serine 191 and 197 and threonine 203, which are located in proximity of the Igα immunoreceptor tyrosine based activation motif (ITAM), dampen Igα ITAM tyrosine phosphorylation. Here we show that mice with targeted mutations of Igα S191, 197 and T203 displayed elevated serum IgG2c and IgG2b concentrations and had elevated numbers of IgG2c and IgG2b secreting cells in the bone marrow. BCR induced Igα tyrosine phosphorylation was slightly increased in splenic B cells. Our results suggest that Igα serine/threonines limit formation of IgG2c and IgG2b secreting bone marrow plasma cells, possibly by fine-tuning Igα tyrosine mediated BCR signaling.
Introduction
Signals from the pre BCR and the BCR critically shape B cell development, survival, activation and antibody production. The signal transducing element of the pre BCR and the BCR is the associated immunoglobulin (Ig) α/β heterodimer (1, 2). The Igα and Igβcytoplasmic domains each contain one immunoreceptor tyrosine based activation motif (ITAM), a motif shared by many receptors of the immune system (3-5). The dually phosphorylated Igα/βITAMs rapidly amplify thesignal by recruiting and activating src-homology 2 domain containing src-family kinases and spleen tyrosine kinase (Syk) (3, 6). These protein tyrosine kinases phosphorylate neighboring ITAMs and downstream effectors (6, 7). Mechanisms ensuring signal termination include dephosphorylation of Igα by Src homology 2 domain phosphatase-1 (SHP-1) recruited to the transmembrane adapter CD22 (6, 8).
Many ITAM containing receptors contain evolutionarily conserved serines or threonines surrounding their ITAM tyrosines (4, 5). Some of these residues, including those in Igβ, CD3γ, CD3δ and FcεRI, are phosphorylation sites (9-14). Igα contains two evolutionarily conserved serines in position 191 and 197 and a threonine in position 203. These residues were found phosphorylated in B cell lines and in vitro (4, 9-11), and experiments in myeloma cell lines showed that Igα S191, 197 and T203 transiently decrease BCR mediated Igα ITAM tyrosine phosphorylation (15). Moreover, a recent study suggested that at least S197 is a target for phosphorylation by Syk, thereby negatively regulating Syk and src-family kinase mediated Igα ITAM tyrosine phosphorylation (16). We therefore speculated that Igα serine/threonines antagonize also the in vivo function of the Igα ITAM tyrosines. In order to test this hypothesis, we generated mice with targeted mutations of S191, 197, and T203.
Materials and Methods
Mice
Gene targeting was performed employing a strategy previously used in the laboratory (17, 18). Chimeric mice were generated through injection of targeted C57BL/6 embryonic stem cells into C57BL/6 albino blastocysts (19). Animal care and experiments were conducted according to protocols approved by the Animal Care and Use Committee of Harvard Medical School and the Immune Disease Institute. The mice were kept in a specific pathogen free facility. Homozygous mutant mice and age-matched control mice of the C57BL/6 background (Charles River Laboratories) or littermates were analyzed at 8-14 weeks unless indicated otherwise. Igα ITAM tyrosine mutant (IgαFF) mice and Igα ITAM tyrosine mutant (IgβAA) mice were previously described (17, 20). Embryonic stem cells with the IgαSATV allele are available from the authors upon request.
Flow cytometry
Spleen, bone marrow, peritoneal lavage, Peyer's patches (PPs), mesenteric, inguinal and axillary lymph nodes, as well as cultured B cells were harvested into phosphate buffered saline containing 2% fetal calf serum (FCS, Invitrogen). Single cell suspensions were stained with conjugated monoclonal and polyclonal antibodies purchased from BD Bioscience, Ebioscience and Southernbiotech or derived from hybridoma cell lines in the laboratory. IgG2c and IgG2b staining of GC B cells and lipopolysaccharide (LPS) stimulated blasts was performed with polyclonal biotinylated anti-IgG2a, recognizing both IgG2a and the homologue IgG2c present in C57BL/6 mice (21), and anti-IgG2b (Southernbiotech) after IgG subclass specificity testing by ELISA and titration on LPS stimulated B cell cultures displaying Ig class switching to IgGc and IgG2b. All samples were analyzed with a BD FACS Calibur (BD Bioscience) and FlowJo Software (Tristar Inc).
ELISAs and immunizations
Serum ELISAs were performed by coating plates with 4-hydroxy-3-nitrophenylacetic (NP) (30) conjugated to bovine serum albumin (BSA), polyclonal goat anti-mouse Igκ/τ (Southern Biotech), denatured salmon sperm DNA (Sigma), or purified mouse glucose phosphate isomerase-GST fusion protein generated in the laboratory of D. Mathis and C. Benoist. Serum was added to 96 well plates at a starting dilution of 1:25 or 1:50, followed by 3.5 or 5 fold serial dilutions. Subsequently, serum Ig was detected with polyclonal biotinylated goat anti-mouse IgM, IgG1, IgG2b, IgG3, and IgA (Southern Biotech), streptavidin-alkaline phosphatase conjugate (Roche) and chromogenic substrate 4-nitrophenyl phosphate (Sigma). Serum IgG2c was detected using cross-reactive polyclonal biotinylated goat anti-mouse IgG2a (Southern Biotech) (21). Mice were immunized by intraperitoneal injection of NP (41)-Ficoll (NP conjugated with aminoethylcarboxymethyl-FICOLL), and alum precipitated NP-CGG (chicken gammaglobulin) purchased from Biosearch Technologies. Laboratory developed purified monoclonal antibodies (S43-10, D3-13F1, B1-8μ, 18-1-16, S24/63/63) and antibodies purchased from Southern Biotech (HOPC-1, A-1, 11E10, B10, 15H6, S107) were used for the determination of the serum concentrations of NP specific antibodies, and total Ig, respectively.
ELISPOT assays
Numbers of antibody forming cells were determined by culturing spleen and bone marrow cells on polyvinylidene fluoride membranes (Pall Corporation), previously coated with polyclonal anti-Igκ and anti-Igτ (Southern Biotech), in 6 well plates for 3-4 h. Membrane bound Ig was subsequently detected with biotinylated polyclonal anti-IgM, IgG1, IgG2c, IgG2a cross-reactive with IgG2c (21), IgG2b, and IgG3 (Southern Biotech), a streptavidin horseradish peroxidase conjugate (Jackson Laboratories), electrochemiluminescent (ECL) reagent (GE/Amersham), and autoradiography film (Kodak).
Proliferation assays
Splenic B cells were MACS purified by CD43 depletion of spleen cells (Miltenyi Biotech) resulting in isolation of cellular fractions containing more than 95% CD19+ B cells. The cells were cultured in complete Dulbecco's modiαed Eagle's medium (DMEM, Invitrogen) 10% FCS (Invitrogen) with polyclonal anti-IgM F(ab)2 fragments (Jackson Immunoresearch), LPS from Escherichia coli O55:B5 (Sigma Aldrich), or synthesized 20mer of CpG DNA. B cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes) according to the manufacturer's instructions prior to culturing for three days. Following harvest of the cultures, B cells were stained with dead cell exclusion marker TO-PRO-3 (Molecular Probes) and analyzed on a FACS Calibur (BD Bioscience). The percentage of cells that had undergone one or more cell division was calculated using the proliferation platform functions of FlowJo Software (Tristar Inc).
Histology
Kidney sections were fixed in 10% formalin (Sigma) and stained with hematoxylin and eosin (H&E) or periodic acid/Schiff (PAS). Immunofluorescence staining of frozen kidney sections was performed with TRITC-conjugated goat anti-mouse IgG (Southern Biotech) and FITC-conjugated rabbit-anti-human C3d (Dako).
Immunoblotting
Purified splenic B cells in Roswell Park Memorial Institute medium (RPMI, Invitrogen) were stimulated with the indicated doses of polyclonal anti-IgM F(ab)2 fragments (Jackson Immunoresearch) and whole cell lysates prepared by immediate lysis in 1% NP-40 lysis buffer 1mM sodium orthovanadate, 5mM sodium pyrophosphate, 10mM sodium fluoride, 2mM phenylmethylsulfonyl fluoride, and further protease inhibitors (Roche). 20-40μg protein per lane was subjected to an 8 or 10% SDS-PAGE (Hoefer) under reducing conditions, and wet transferred to polyvinylidene fluoride membranes (Biorad, Millipore) according to the manufacturer's instructions. Immunoblotting antibodies raised against human pTyr525/526Syk, pThr202/Tyr204ERK, and ERK were obtained from Cell Signaling Technologies, monoclonal antibody 4G10 from Upstate, antibodies specific for Syk (N19) and B cell linker protein (H80) from Santa Cruz, and polyclonal rabbit Igα and Igβ from Y.M. Kim and H. Ploegh. Images shown were captured and quantified with a Fujifilm LAS-3000 imaging system.
Calcium flux
Splenic cells were labeled with Indo-1 (Molecular Probes), and BCR mediated calcium flux of B cells resuspended at 2 × 106 cells/ml RPMI 2% FCS was assayed by analysis of the 405nm/485nm emission ratio after excitation with a 350nm UV laser on a FACS Vantage flow cytometer (BD Bioscience). Data were analyzed with the Flow Jo Analysis program (Tristar Inc.).
Statistics
Averages, geometric means, standard deviations, logarithmic standard deviations and medians were calculated as indicated. P-values were determined by applying the two-tailed Student's T-test for unpaired samples to data sets.
Results
Generation of Igα serine and threonine mutant mice
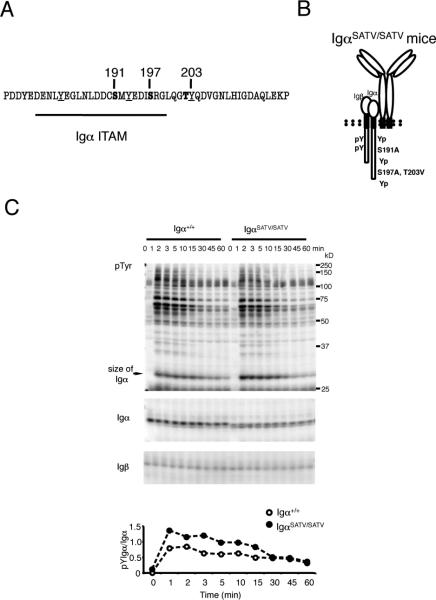
The Igα cytoplasmic domain contains serines at position 191 and 197 in close proximity of ITAM tyrosine 193 and a threonine at position 203 adjacent to non-ITAM tyrosine 204 (Fig. 1A). We generated mice with targeted mutations in the Igα encoding mb-1 gene that resulted in replacement of serines 191 and 197 with alanine and threonine 203 with valine (Fig. 1B, Supplemental Fig. 1A-C). The mutant allele was termed IgαSATV and mice homozygous for this allele were analyzed. Immunoblotting of anti-IgM stimulated splenic B cells from IgαSATV/SATV mice showed enhanced Igα tyrosine phosphorylation. Specifically, Igα tyrosine phosphorylation normalized to total Igα expression was transiently increased by 10-30% in B cells from IgαSATV/SATV mice compared to controls, while total Igα expression was slightly decreased when normalized to Igβ and extracellular signal-regulated kinase (ERK) (Fig. 1C and data not shown). These results suggest that Igα serine/threonines modulate BCR induced Igα tyrosine phosphorylation in primary splenic B cells as predicted by previous results (15, 16).
FIGURE 1. Generation of Igα serine and threonine mutant mice.
A, Amino acid sequence of the murine Igα cytoplasmic domain.
B, Scheme of mutant Igα protein expressed as part of the BCR complex in IgαSATV/SATV mice.
C, Splenic B cells were stimulated with 5μg/106 cells anti-IgM for the indicated time points, lysed with 1%NP-40 buffer, separated by SDS-PAGE, and blotted as indicated. Bands were quantified after digital chemiluminescence acquisition. Blots shown are representative of four experiments performed with lysates from two independent experiments with three to four 8-12 week old mice each.
Enlarged early B cell fractions
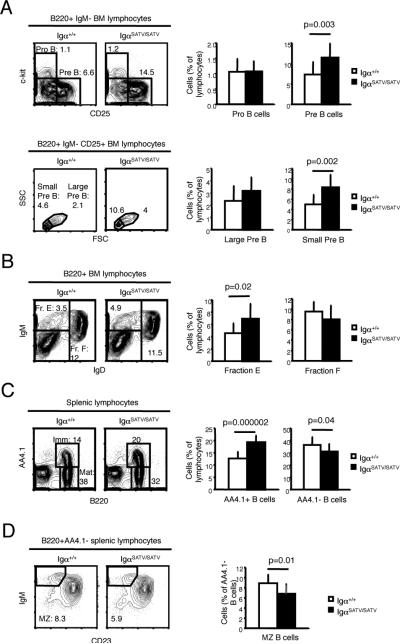
Hypothesizing that the Igα serine/threonines antagonize the in vivo Igα ITAM tyrosine function, we speculated that IgαSATV/SATV mice might display features opposite to those of Igα ITAM tyrosine (IgαFF/FF) mutant mice. While IgαSATV/SATV mice did not show phenotypes opposite to those observed in IgαFF/FF mice, the mutant mice had some features that were opposite to those observed in IgαFF/FF mice also carrying a heterozygous or homozygous Igβ ITAM tyrosine mutation (IgβAA). Thus, the percentage of pre-B cells among lymphocytes was increased by approximately half, with the greatest increase in the percentage of cells detected at the small pre-B cell stage (68%)(Fig. 2A, Supplemental Fig. 2A), and without differences in bone marrow cellularity (IgαSATV/SATV: 20.4+/-10.7 × 106 cells/femur; controls: 16.1 +/- 7.5 × 106 cells/femur in 7-12 week old mice; n=18, p=0.159). Similarly, immature B cell fractions in the bone marrow and transitional B cells in the spleen were expanded by approximately half in IgαSATV/SATV mice compared to controls (Fig. 2B-C, Supplemental Fig. 2B), while total B cell numbers in the spleen were normal (Supplemental Fig. 2C). In contrast, IgαFF and IgβAA compound mutants, but not IgαFF/FF mice or IgβAA/AA showed impaired pre B cell and later stage B cell development (Supplemental Fig. 2D)(20, 22). These results suggest that Igα serine/threonines negatively regulate the size of the pre, immature and transitional B cell compartments. These findings are in line with a previous study suggesting that Igα serine/threonines antagonize the in vivo function of Igα/β ITAM tyrosine signaling at the pre B cell stage as indicated by increased pre BCR mediated calcium responses in pre B cells expressing serine and threonine mutant Igα (16).
FIGURE 2. B cell development.
A-D The pro (Fr. A-C’) and pre (Fr. D) (A), immature and recirculating (B), transitional and mature (C) and marginal zone B cell (D) compartments were analyzed by flow cytometry of bone marrow and spleen from 8-12 week old mice (n=8-14). Histograms display average numbers of cells, standard deviations, and p-values as determined by Student's T-test.
Minor roles in B cell subset development and BCR mediated in vitro and in vivo responses
The fraction of MZ B cells in Igα SATV/SATV mice was reduced by 25-30% compared to controls (Fig. 2D, Supplemental Fig. 2E). The size of the mature B cell compartment was also slightly reduced (Fig. 2C). The size of the peritoneal B1 cell compartment and the ratio of Igκ to Igτ expressing cells in the spleen and bone marrow were normal (Supplemental Fig. 2F-G). Following immunization with the TD antigen NP-CGG, IgαSATV/SATV mice had normal NP (4-hydroxy-3-nitrophenylacetyl) specific IgM and IgG1 responses compared to controls (Supplemental Fig. 2H). The anti-IgM induced calcium response, as well as surface expression of BCR components on mature B cells from IgαSATV/SATV mice was largely unchanged (Supplemental Fig. 3A-B). Further, no major differences in BCR induced in vitro B cell responses were detected as suggested by largely normal anti-IgM mediated Syk and ERK phosphorylation, CD69 expression and proliferation of splenic B cells from the mutant animals (Supplemental Fig. 3C-D). These results suggest that Igα serine/threonines modulate marginal zone B cell numbers, but do not play a critical role in regulating Igτ usage, surface BCR expression, B1 cell development and antigen specific IgG1 responses.
Hypergammaglobulinemia and increased bone marrow plasma cell formation
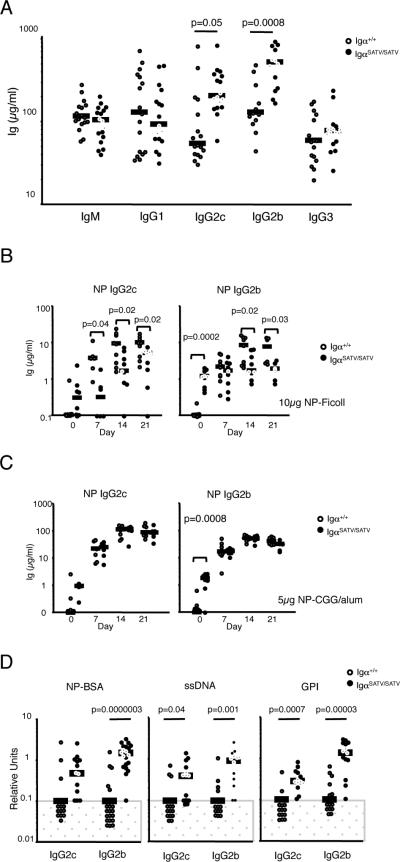
Unlike IgαFF/FF mice, which showed normal IgG2c and IgG2b concentrations in the serum (22), serum concentrations of IgG2c and IgG2b in unimmunized, 8-10 week old IgαSATV/SATV mice were fourfold increased compared to controls, with no such increase seen in the case of IgM, IgG1, IgG3 and IgA (Fig. 3A; and data not shown). These findings contrasted decreased NP specific IgG2c and IgG2b responses to NP-Ficoll, while NP specific IgM and IgG3 responses were only slightly reduced or normal (Fig. 3B, Supplementary Fig. 4A). Further, NP specific IgG2c and IgG2b responses to NPCGG were normal in the mutant mice (Fig. 3C). However, unimmunized, 8-10 week old IgαSATV/SATV mice displayed low concentrations of IgG2c and IgG2b antibodies recognizing NP, single strand (ss) DNA and the metabolic enzyme glucose phosphate isomerase (GPI), whereas such antibodies were below or at the detection limit in controls (Fig. 3D). Anti-ssDNA, GPI and NP IgM serum concentrations were normal in IgαSATV/SATV mice, while IgG1 and IgG3 with these specificities were undetectable in both wildtype and mutant mice (Supplemental Fig. 4B). Autoantibody production in the human and mouse can be associated with spontaneous germinal center formation, the development of autoimmune disorder including glomerulonephritis, and high autoantibody titers as the disease progresses. No evidence of spontaneous GCs in lymph nodes, spleen and bone marrow or enhanced GC formation in the Peyer's patches and mesenteric lymph nodes was found in 8-12 week old mutant mice (Supplemental Fig. 4C). Nine month old IgαSATV/SATV mice did not show evidence of kidney inflammation, nor IgG or complement deposition in the kidneys compared to age-matched controls (Supplemental Fig. 4D). Serum ssDNA and GPI IgG2c and IgG2b were readily detectable in 9 month old wildtype as well as the mutant mice, but differences between mutants and controls, which were seen in 8-10 week old mice, had largely disappeared (Supplemental Fig. 4E). These results suggest that Igα serine/threonines limit the production of serum total IgG2c and IgG2b as well as serum NP, ssDNA and GPI specific IgG2c and IgG2b in unimmunized, 8-10 week old mice. The results further suggest that Igα serine/threonines are required for an efficient IgG2c and IgG2b response to a TI-II antigen, but do not play a critical role in regulating the T cell dependent IgG2c and IgG2b response.
FIGURE 3. Total serum Ig, antibody responses and spontaneous autoantibody production.
A, Serum total Ig in unimmunized mice was determined in 8-10 week old mice by ELISA (n=12-17). Dots and bars represent values from individual mice, and medians, respectively. P-values were calculated by Student's T-test.
B-C, NP specific serum IgG2c and IgG2b after intraperitoneal immunization with 10μg NP (41) Ficoll (B) and 5μg NP-CGG (C) were analyzed in 8-10 week old mice by ELISA (n=6-9). Graphs show values from individual mice, medians and p-values as calculated by Student's T-test. Undetectable values were depicted at the detection limit.
D, Anti- NP, GPI and ssDNA Ig were determined in unimmunized 8-10 week old mice by ELISA (n=13-22). ELISAs were performed using a starting dilution of 1:25 and 3.5 fold serial dilutions. Graphs show values from individual mice, medians and p-values as calculated by Student's T-test. Undetectable values were depicted at and below the detection limit (dotted area). Graphs for anti-NP Ig depict all results obtained, which included those shown in Figure 3B-C.
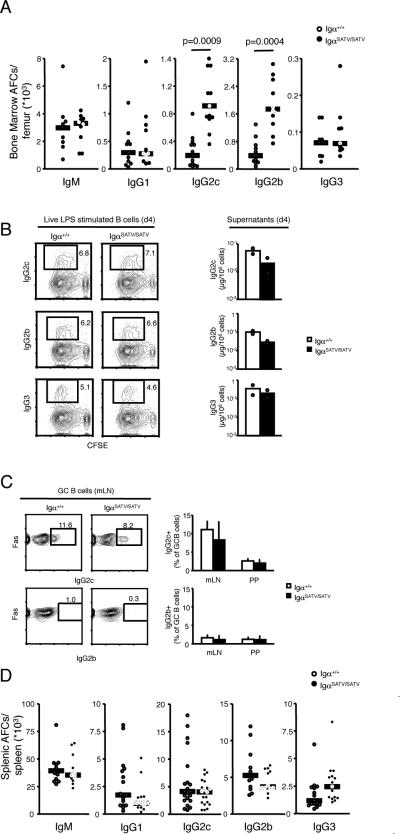
Increased serum Ig concentrations may be the result of an expanded bone marrow plasma cell compartment, as plasma cells residing in the bone marrow are a major source of serum Ig (23, 24). Indeed, quantification of antibody secreting cells in the bone marrow revealed that the number of cells secreting IgG2c and IgGb were increased four to five fold in IgαSATV/SATV mice compared to controls, whereas the number of cells secreting IgM, IgG1 or IgG3 were normal (Fig. 4A). B cell stimulation through the BCR and other receptors results in B cell activation, proliferation, Ig class switching and differentiation to plasmablasts found in secondary lymphoid organs such as the spleen, some of which go on to become long-lived resting plasma cells homing to the bone marrow (23, 24). No gross enlargement of the proportion of IgαSATV/SATV B cells switching to IgG2c and IgG2b in response to lipopolysaccharide (LPS) in vitro, nor any major changes in the percentage of IgG2c and IgG2b positive cells in germinal centers (GCs) of Peyer's patches (PPs) and mesenteric lymph nodes of the mutant mice were detected (Fig. 4B-C). Further, the number of splenic cells secreting IgG2c and IgG2b as well as LPS induced IgG2c and IgG2b secretion in vitro were not increased (Fig. 4C-D). These findings suggest that Igα serine/threonines critically control formation of IgG2c and IgG2b secreting cells in the bone marrow and serum IgG2c and IgG2b concentrations, but not Ig class switching to or production of cells secreting these isotypes in the spleen.
FIGURE 4. Plasma cell formation and Ig isotype switching.
A, Antibody forming cells in the bone marrow of one femur were quantified in 7-10 week old mice (n=9-13) in 5 independent experiments by ELISPOT analysis. Graphs show values from individual mice, medians and p-values as calculated by Student's T-test.
B, Ig class switching to IgG2c and IgG2b was determined by culture of splenic B cells from three 8 week old mice with 20μg/ml LPS for 4 days, staining with anti-IgG2c and IgG2b Ig and loss of CFSE by flow cytometry. Numbers shown represent the percentage of gated cells. IgG2c and IgG2b secretion by LPS stimulated B cells into the supernatants was determined by ELISA, and depicted as ratio of Ig to number of B cells plated on day 0. Results were comparable in a second independent experiment.
C, The fraction of IgG2c and IgG2b expressing GC cells in mesenteric lymph nodes and PPs were determined in 8-12 week old mice by flow cytometry (n=5-6) in two independent experiments. Histograms display averages and standard deviations.
D, Antibody forming cells in the spleen were quantified in 7-10 week old mice (n=8-18) by ELISPOT analysis. Graphs show values from individual mice, medians and p-values as calculated by Student's T-test.
Discussion
The Igα cytoplasmic domain contains two serines and one threonine in addition to the well characterized ITAM and non-ITAM tyrosine phosphorylation sites (3, 4, 17, 18). In line with previous studies (15, 16), the present analysis of mice with targeted mutations of the cytoplasmic Igα serine/threonines suggests that Igα serine and threonine phosphorylation dampens Igα tyrosine phosphorylation in anti-IgM stimulated splenic B cells. One might thus expect that the Igα serine/threonines antagonize the in vivo function of the Igα ITAM tyrosines as well. The abnormalities observed in early B cell development of IgαSATV/SATV mice were indeed compatible with the idea that Igα serine/threonines negatively regulate BCR signaling. The decreased marginal zone B cell numbers and TI-II responses in the mutants, although not opposing the phenotype of Igα ITAM tyrosine deficient mice, which also had decreased marginal zone B cell numbers and normal TI-II responses (22), support this notion as well, given that mice deficient in negative regulators such as CD22 also exhibit impaired marginal zone B cell development and TI-II responses (8). CD22 deficient mice and IgαSATV/SATV mice further resembled each other in regard to production of low concentrations of autoantibodies, although autoreactive IgG accumulates only at older age in CD22 deficient mice (25), while differences to wild type were found only in 8-10 week old, but not aged IgαSATV/SATV mice. However, any of these changes in IgαSATV/SATV mice were of modest extent. Furthermore, no abnormalities in B1 cell development, Igτ usage and the T cell dependent IgG1 response were observed in the mutants, whereas IgαFF/FF mice exhibit a phenotype in these respects (22).
In contrast, 8-10 week old IgαSATV/SATV mice displayed strikingly elevated total serum IgG2c and IgG2b concentrations with a corresponding increase in IgG2c and IgG2b secreting bone marrow plasma cells, but normal in vitro Ig class switching to these isotypes, no accumulation of surface IgG2b and IgG2c positive germinal center cells in spontaneous germinal centers and normal numbers of IgG2c and IgG2b secreting cells in the spleen, a major site of plasmablast production (26). Since Igα is not expressed at the plasma cell stage (27), it appears that Igα serine/threonines promote selection of cells into the bone marrow plasma cell compartment rather than survival of plasma cells. It is also possible that Igα serine/threonines limit the formation of bone marrow plasma cells by controlling the numbers of IgG2c and IgG2b expressing memory cells, which we did not analyze due to technical limitations. How can these observations be explained? Given the similarities between the IgM and the IgG BCR in structure and utilization of Igα for signal transduction (28), it is conceivable that the IgαSATV/SATV mutation increases Igα tyrosine phosphorylation not only in IgM but also in IgG2c and IgG2b expressing cells and thereby enhances selection of the latter cells into the Igα negative bone marrow plasma cell pool. Support for this idea comes from observations that strong antigen-BCR interactions, shown to correlate with increased Igα tyrosine phosphorylation, promote plasma cell differentiation in vitro, in T cell independent, and in extrafollicular T cell dependent responses (29-33). The restriction of the phenotype to the IgG2c and IgG2b isotypes in IgαSATV/SATV mice could reflect an as yet undefined difference in signal transduction through BCRs of the various IgG isotypes. In this context, the presence of low levels of self-reactive antibodies of these isotypes in the mutant mice may suggest a link to the activation of cells expressing such specificities, given the similar isotype distribution of autoantibodies in mouse models of autoimmunity (34-36).
Supplementary Material
Acknowledgments
We thank J. Xia, S. Willms, and K. Jenssen for help with ELISAs, D. Ghitza, V. Smith, L. Du, C. Aristoff, A. Monti, A. Tetreault, C. Xiao and A. Pellerin for mouse work and blastocyst injection, M. Nussenzweig, Y.M. Kim, H. Ploegh, D. Mathis and C. Benoist for reagents and all Rajewsky lab members and P. Schur for discussion and advice.
This work was supported by the National Institute of Health (NIH R37 AI054636) and a research fellowship from the Deutsche Forschungsgemeinschaft and T32 training grant (transfusion medicine) to H.C.P.
Footnotes
Abbreviations used in this paper: ITAM, immunoreceptor tyrosine based activation motif; Syk, spleen tyrosine kinase; ERK, extracellular signal-regulated kinase; PPs, Peyer's patches; GC, germinal center; LPS, lipopolysaccharide; NP, 4-hydroxy-3-nitrophenylacetyl.
References
- 1.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annual review of immunology. 1997;15:453–79. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 2.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nature reviews. Immunology. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 3.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). Journal of immunology (Baltimore, Md. : 1950) 1995;155:3281–5. [PubMed] [Google Scholar]
- 4.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. [PubMed] [Google Scholar]
- 5.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunological reviews. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 6.Reth M, Brummer T. Feedback regulation of lymphocyte signalling. Nature reviews. Immunology. 2004;4:269–77. doi: 10.1038/nri1335. [DOI] [PubMed] [Google Scholar]
- 7.Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annual review of immunology. 1999;17:555–92. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 8.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunological reviews. 2009;230:128–43. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Noesel CJ, Borst J, De Vries EF, Van Lier RA. Identification of two distinct phosphoproteins as components of the human B cell antigen receptor complex. European journal of immunology. 1990;20:2789–93. doi: 10.1002/eji.1830201238. [DOI] [PubMed] [Google Scholar]
- 10.Leprince C, Draves KE, Ledbetter JA, Torres RM, Clark EA. Characterization of molecular components associated with surface immunoglobulin M in human B lymphocytes: presence of tyrosine and serine/threonine protein kinases. European journal of immunology. 1992;22:2093–9. doi: 10.1002/eji.1830220820. [DOI] [PubMed] [Google Scholar]
- 11.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science (New York, N.Y.) 1992;258:123–6. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 12.Pribluda VS, Pribluda C, Metzger H. Biochemical evidence that the phosphorylated tyrosines, serines, and threonines on the aggregated high affinity receptor for IgE are in the immunoreceptor tyrosine-based activation motifs. The Journal of biological chemistry. 1997;272:11185–92. doi: 10.1074/jbc.272.17.11185. [DOI] [PubMed] [Google Scholar]
- 13.Luton F, Legendre V, Gorvel JP, Schmitt-Verhulst AM, Boyer C. Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. Journal of immunology (Baltimore, Md. : 1950) 1997;158:3140–7. [PubMed] [Google Scholar]
- 14.Germano P, Gomez J, Kazanietz MG, Blumberg PM, Rivera J. Phosphorylation of the gamma chain of the high affinity receptor for immunoglobulin E by receptor-associated protein kinase C-delta. The Journal of biological chemistry. 1994;269:23102–7. [PubMed] [Google Scholar]
- 15.Müller R, Wienands J, Reth M. The serine and threonine residues in the Ig-alpha cytoplasmic tail negatively regulate immunoreceptor tyrosine-based activation motif-mediated signal transduction; Proceedings of the National Academy of Sciences of the United States of America; 2000. pp. 8451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heizmann B, Reth M, Infantino S. Syk is a dual-specificity kinase that self-regulates the signal output from the B-cell antigen receptor; Proceedings of the National Academy of Sciences of the United States of America; 2010. pp. 18563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus M, Pao LI, Reichlin A, Hu Y, Canono B, Cambier JC, Nussenzweig MC, Rajewsky K. Interference with immunoglobulin (Ig)alpha immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igbeta cytoplasmic tail. The Journal of experimental medicine. 2001;194:455–69. doi: 10.1084/jem.194.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson HC, Kraus M, Kim Y-M, Ploegh H, Rajewsky K. The B cell receptor promotes B cell activation and proliferation through a non-ITAM tyrosine in the Igalpha cytoplasmic domain. Immunity. 2006;25:55–65. doi: 10.1016/j.immuni.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Köntgen F, Süss G, Stewart C, Steinmetz M, Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. International immunology. 1993;5:957–64. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- 20.Gazumyan A, Reichlin A, Nussenzweig MC. Ig beta tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. The Journal of experimental medicine. 2006;203:1785–94. doi: 10.1084/jem.20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. Journal of immunological methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 22.Kraus M, Pao LI, a Reichlin, Hu Y, Canono B, Cambier JC, Nussenzweig MC, Rajewsky K. Interference with immunoglobulin (Ig)alpha immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igbeta cytoplasmic tail. The Journal of experimental medicine. 2001;194:455–69. doi: 10.1084/jem.194.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature reviews. Immunology. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 24.Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Current opinion in immunology. 2008;20:162–9. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 25.O□Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. The Journal of experimental medicine. 1999;189:1307–13. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García de Vinuesa C, O□Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. European journal of immunology. 1999;29:1314–23. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi N, Kashiwamura S, Kimoto M, Thalmann P, Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. The EMBO journal. 1988;7:3457–64. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777–81. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 29.Kouskoff V, Famiglietti S, Lacaud G, Lang P, Rider JE, Kay BK, Cambier JC, Nemazee D. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. The Journal of experimental medicine. 1998;188:1453–64. doi: 10.1084/jem.188.8.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih T-AY, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nature immunology. 2002;3:570–5. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 31.Shih T-AY, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nature immunology. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 32.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–9. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 33.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of experimental medicine. 2006;203:1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert D, Margaritte C, Payelle-Brogart B, Tron F. Development of the B cell anti-DNA repertoire in (NZB x NZW)F1 mice. Relationship with the natural autoimmune repertoire. Journal of immunology (Baltimore, Md. : 1950) 1992;149:1795–801. [PubMed] [Google Scholar]
- 35.Steward MW, Hay FC. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clinical and experimental immunology. 1976;26:363–70. [PMC free article] [PubMed] [Google Scholar]
- 36.Park CL, Balderas RS, Fieser TM, Slack JH, Prud□Homme GJ, Dixon FJ, Theofilopoulos AN. Isotypic profiles and other fine characteristics of immune responses to exogenous thymus-dependent and -independent antigens by mice with lupus syndromes. Journal of immunology (Baltimore, Md. : 1950) 1983;130:2161–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.