Abstract
Purpose
In vitro brain preparations have been used extensively to study the generation and propagation of epileptiform activity. Transverse and longitudinal slices of the rodent hippocampus have revealed various patterns of propagation. Yet intact connections between the transverse and longitudinal pathways should generate orthogonal (both transverse and longitudinal) propagation of seizures involving the entire hippocampus. This study utilizes the planar unfolded mouse hippocampus preparation to reveal simultaneous orthogonal epileptiform propagation and to test a method of arresting propagation.
Methods
This study utilized an unfolded mouse hippocampus preparation. It was chosen due to its preservation of longitudinal neuronal processes which are thought to play an important role in epileptiform hyper-excitability. 4-aminopyridine (4-AP), micro-electrodes, and voltage sensitive dye imaging were employed to investigate tissue excitability.
Key Findings
In 50 μM 4-AP, stimulation of the stratum radiatum induced transverse activation of CA3 cells but also induced a longitudinal wave of activity propagating along the CA3 region at a speed of 0.09 m/s. Without stimulation, a wave originated at the temporal CA3 and propagated in a temporal–septal direction and could be suppressed with glutamatergic antagonists. Orthogonal propagation traveled longitudinally along the CA3 pathway, secondarily invading the CA1 region at a velocity of 0.22±0.024 m/s. Moreover, a local lesion restricted to the CA3 region could arrest wave propagation.
Significance
These results reveal a complex two-dimensional epileptiform wave propagation pattern in the hippocampus that is generated by a combination of synaptic transmission and axonal propagation in the CA3 recurrent network. Epileptiform propagation block via a transverse selective CA3 lesion suggests a potential surgical technique for the treatment of temporal lobe epilepsy.
Keywords: Hippocampus, 4-AP, Propagation, Epilepsy
Introduction
Epileptiform activity arises from large numbers of neurons bursting in a synchronous fashion while disrupting the normal operation of neural activity. Several animal and pharmacological models have been developed to study the properties and the mechanisms behind generation and propagation of epileptiform activity in neuronal networks. In particular, 4-aminopyridine (4-AP), a potassium channel blocker, has been used extensively to study epileptiform activity generation and propagation. While there are numerous epileptiform models which block synaptic transmission (Bikson et al., 2002), hippocampus preparations bathed in micromolar concentrations of 4-AP can generate bursts of activity in the presence of synaptic transmission. (Perreault & Avoli, 1991 1992; Avoli, 1996; Gu et al., 2004) In this in vitro study we investigate the spatial extent of epileptiform propagation in the hyperexcitable hippocampus with intact longitudinal and transverse connections bathed in 4-AP containing solutions.
Epileptiform activity induced by the presence of 4-AP in transverse hippocampal slices is characterized by short recurrent discharges sometimes combined with slow field potential shifts and long lasting (100 ms) depolarization of cells (Perreault & Avoli, 1991). While the complete mechanism of activation is unknown, it has been shown that, upon application of 4-AP, the frequency and strength of EPSPs and IPSPs in CA3 cells increases, leading to prolonged ictal and short interictal discharges. (Gloveli, 2005) Moreover, 4-AP induces an increase in extracellular potassium levels during long-lasting field potential depressions as they propagate across the network. (Avoli, 1996) It has been shown that long term application of 35–70 mM concentrations of 4-AP in vivo generates neuronal lesions in CA1 and CA3 regions related to high glutamate levels in proximity to the synapses (Pena & Tapia, 1999). Likewise, lesions in the CA1 and CA3 regions are the primary hippocampal damage observed in mesial temporal sclerosis (MTS) epilepsy (Shinnar, 2003). The in vivo 4-AP model preserves synaptic transmission and in longer term studies mimics the cell death seen in humans (Pena & Tapia, 2000).
It is also known that the longitudinal CA3 slice can support propagation of epileptiform-like bursts along its length when GABA inhibition is suppressed (Miles et al., 1988). Similarly, epileptiform activity is known to propagate along the length of an intact neonatal hippocampus (Luhmann et al., 2000), and spontaneous bursts have been found to propagate transversely in hippocampus slice preparations. However, the effect of combined longitudinal and transverse hippocampal pathways on the propagation of 4-AP induced epileptiform activity in young and adult animals has not been studied. Previous work has shown that recurrent excitation is responsible for some longitudinal propagation of neural activity. CA3 axons innervate the entire CA1 region (Ishizuka ei al., 1990), suggesting that the combination of longitudinal and transverse pathways should generate orthogonal waves of activity in hyperexcitable tissue which travel from the CA3 region into the CA1 region and invade the entire hippocampus.
This study utilized an intact unfolded hippocampus preparation, multi-electrode recording, voltage sensitive dye imaging, and synaptic transmission antagonists to determine the nature of propagation of 4-AP induced epileptiform interictal-like bursts (ILEs) and to map the spread of this spontaneous activity in the CA3–CA1 regions of the hippocampus in both the transverse and longitudinal directions. Moreover, we applied a lesion technique to the intact hippocampus and showed that a small transverse lesion in the CA3 region is capable of preventing the propagation of these orthogonal waves.
Materials and Methods
Experiments were performed with 60 10–18 days old CD1 mice obtained from Charles River. Although there were differences in epileptiform onset delay in 4-AP solution, no significant variability in establishing orthogonal propagation was seen in this age range. All animals were housed and maintained in veterinarian-monitored animal care facility at CWRU. Health and welfare procedures complied with ALAC guidelines.
Tissue Preparation
Mice were anesthetized with ethyl ether and decapitated using a small animal guillotine. After decapitation, the head was immediately immersed in ice cold (2°C – 4°C) oxygenated (O2 95%, CO2 5%), and sucrose-rich artificial cerebrospinal fluid (ACSF) with following composition (in mM): Sucrose 220, KCl 3.75, NaH2PO4 1.25, MgSO4 2.0, NaHCO2 26, CaCl2 2.0, and Dextrose 10. Substitution of NaCl with sucrose in ACSF has been shown to reduce the potential effects of cutting-induced trauma and consistently yields more viable tissue (Aghajanian, 1989).
The planar hippocampus was prepared via our hippocampus unfolding procedure, detailed in the supplemental materials of this manuscript, and then transferred into a recovery chamber filled with ACSF with the following composition (in mM): NaCl 124, KCl 3.75, KH2PO4 1.25, CaCl2 2.0, MgSO4 2.0, NaHCO3 26, Dextrose 10.0. Solution was bubbled with 95% O2 / 5% CO2 and maintained at a room temperature (25° C). The preparation was stored in this chamber for 1–5 hours before recording.
Multisite Extracellular Recording and Data Analysis
The hippocampus was placed in a submersion brain/tissue chamber system model PH1 (Warner Instruments, Hamden, CT) to provide oxygenation (O2 95%, CO2 5%), temperature (25–32°C) and rapid exchange of superfusing fluids (normal (n) ACSF, 50 μM 4-AP ACSF solution, or ‘blocker’ solution ACSF with 50μM NMDA receptor antagonist D-2-amino-5-phosphonovaleric acid (DAPV), 40μM 6,7-dinitroquinoxaline-2,3-dione non-NMDA glutamate receptor antagonist DNQX, and 100μM picrotoxin GABAA receptor antagonist PTX where indicated) during recording. In the case of normal ACSF recording and 4-AP ACSF, the drug solution was switched in to circulation after nACSF tests were complete. Field recordings were made using pulled glass micropipettes (1mm outside diameter, 0.5mm inside diameter, 2–6 MΩ) filled with 150 mM NaCl. Population spikes were evoked using a cathodic 100 μsec, 50–150 μA, antidromic and/or orthodromic stimulus pulse with a period of 2.5 s. Current was injected into the Schaffer collateral pathway (orthodromic to CA1), alveus (antidromic to CA1), or CA3 pyramidal layer and recordings were obtained from the CA1 and CA3 pyramidal cell layers. Only preparations yielding a population spike with an amplitude of ≥ one mV were used for data analysis. Signals were amplified with an Axoprobe-2B microelectrode amplifier (Axon Instruments, Inc., Union City, CA) and filtered with a Cygnus FLA-01 or Princeton Applied Research model 113 amplifier. Data was digitized and stored with a Microdata Instruments DT-200 digital recorder. Data processing was performed in Matlab or Spike2. Student’s t-tests were used for statistical analysis with P < 0.05. Amplitudes were measured with a normalized baseline voltage of zero. Transmission latency was calculated using a cross correlation algorithm between two recorded channels. Results are shown as mean ± SD unless otherwise noted.
Voltage sensitive dye recording
The planar preparation was stained with the fast voltage-sensitive styryl dye RH-414 (N-(3-(triethylammonium)propyl)-4-(4-(p-diethylaminophenyl)-butadienyl)-pyridium dibromide; Molecular Probes) at 200 μM concentration in normal ACSF solution in a micro superfusion chamber for 15 minutes in a darkened room. Excess stain was washed away with dye-free ACSF before recording. The planar preparation was then transferred to a submerged recording chamber with alveus side down, superfused with nACSF or ACSF containing 50 μM 4-AP where indicated. A tungsten stimulating electrode was inserted through the back of the hippocampus tissue into the stratum radiatum using a dissecting microscope. Extracellular field potential recordings were simultaneously performed during the imaging to assess the viability of the preparation and to monitor the amplitude of evoked responses. Further details on the equipment and methods used to record the fluorescent signal can be found in this manuscripts supplemental materials. A 0.1 to 0.4% change in fluorescence was seen during neuronal firing. In addition, 2× spatial interpolative subsampling was employed to improve image visualization.
Results
Orthogonal Widespread Activation of CA1–CA3 from a Single Stimulus in CA1
It is well known that 4-AP increases neuronal excitability, which can lead to epileptiform activity in the hippocampus. Using the planar hippocampus preparation bathed in ACSF containing 50μM 4-AP, we investigated in two dimensions the excitability of the CA1 to CA3 regions by recording the preparations’ response to a single apical stimulus in central (longitudinally) CA1. Membrane-binding voltage sensitive dye RH-414 was applied to the preparation, and fluorescent imaging was used to reveal the extent of propagation of evoked potentials across the entire CA1–CA3 hippocampus with and without 4-AP in solution. Stratum radiatum (SR) axons were stimulated with a 200 uA, 120 μs pulse to generate a transverse antidromic activation potential in CA3 neurons with a control solution of normal ACSF (Fig. 1A) . In 4-AP ACSF, field potentials continued to propagate longitudinally along the entire length of CA3 (Fig. 1C). Recovery time from stimulus to return to baseline fluorescence (resting potential) in the preparation required 33–40 ms (Fig. 1E) which was significantly longer than the 15–20ms recovery time in normal ACSF, each tested in the six preparations analyzed with RH-414 that met the required orthodromic response threshold of one mV. This response was compared to a control CA1 stimulus in normal ACSF solution (Fig. 1A). In the absence of 4-AP, the evoked activity propagates transversely into CA3 but does not spread longitudinally. This noticeable difference in activation pattern with and without 4-AP indicates that an orthogonal propagation from CA1 to CA3 can take place in hyper-excitable tissue but is not seen in normal solution.
Figure 1.
A) Fluorescent dye imaging of evoked population potential in the Intact Planar Hippocampus in NACSF, two ms/frame, eight trials time-averaged. Simulus location is indicated by circle in the first frame. Response is confined mostly to a transverse lamella 2.8×1.1mm B) Diagram of hippocampus positioning and propagation pathways for A. C) Fluorescent dye imaging of evoked potentials in 50μM 4-AP ACSF, two ms/frame, eight trials time-averaged. Response travels down across CA1 then orthogonally along CA3. D) Diagram of hippocampus positioning and propagation pathways for C. E) Data from the center channel of optical recording in C. F) Photomicrograph of hippocampus preparation with overlaid view of voltage sensitive dye acquisition window.
Five contour plots of the excitation were generated to analyze propagation across the tissue with a representative contour plot presented in Fig. 2. Initially, the stimulus response travelled transversely into CA3, as indicated by the 4–5 ms contours of Fig. 2A. This initial antidromic propagation followed CA3 projections into CA1. From central CA3, activation spread longitudinally along CA3 in both directions (5–10 ms post-stimulus). In CA1, longitudinal propagation is seen to slow, (3–6 ms post-stimulus Fig. 2A) until the time CA3 has been activated, indicating that neural activation may have traveled back into the temporal and septal CA1, most likely along the Schaffer collaterals (SC). CA1 activation area peaks 12–14 ms post stimulus. See Supplemental material for additional contour plots. While the data displayed are gathered from individual optical experiments, the results are confirmed in the following sections. These data show that a single stimulus in the presence of 4-AP could generate widespread activation of the hippocampus.
Figure 2.
Representative figure of contours of activation over time from a stimulus in CA1 of five preparations analyzed with this technique. Contour labels are in ms. Time average of four recordings in one preparation. A) Stimulus occurs at contour time 0 in the CA1 region and activation propagates down to CA3 in 4ms. From there, activation spreads longitudinally along CA3 and into CA1, following the diagonal contours. B) Tissue orientation for A), contour labels are in ms.
Orthogonal propagation from CA1 into CA3
This wide-spread propagation of neural activity in 4-AP solution could be generated by either a broad spatial arborization of CA1 axons into CA3 or by longitudinal propagation of the activity along CA3 from the plane of stimulus. We first tested the arborization hypothesis by applying bath solution containing either normal ACSF (nACSF) or ACSF with 50 μM 4-AP over the unfolded hippocampus preparation while 100 μs, 100 μA stimulus (ST) was applied at 0.3 Hz to the stratum radiatum (SR) (Fig. 3A). Recording electrodes were placed along CA3 spanning a distance of approximately 2.8 mm. The longitudinal extent of CA3 activation from a single central antidromic stimulus was recorded with synaptic transmission antagonists, and compared to the extent of activation in a normal ACSF bath following normalization. Examples of waveforms recorded at points 1–2 are shown in Fig. 3B. Stimulation in the CA1 SR in normal ACSF generated an antidromic evoked potential of 1.5 ±0.3 mV recorded in the CA3 region. However, in control solution (normal ACSF), longitudinal activation of the CA3 was mostly confined to a transverse lamella of ± 500μm defined as the region of at least 60% maximum amplitude recorded (Fig. 3C) where the amplitude is shown as a function of distance. This result confirms the fact that the axonal arborization of CA3 axons in the SR primarily maintains a lamellar organization. In 4-AP ACSF, evoked potentials showed little attenuation along the entire length of the CA3. Therefore, these data show that 4-AP enhances longitudinal propagation along the CA3 layer either by increasing the number of activated axons, or by inducing a wave of activation within the CA3 region, or both.
Figure 3.
Extent of evoked response in CA3 from a stimulus in CA1. A) Location of stimulus and recording sites in the unfolded hippocampus preparation. B) Examples of extra cellular field recordings at two locations in normal and 4-AP solution. C) Evoked responses in 4-AP spread across the entire CA3 region while the control (Normal ACSF) responses are only seen in a 1–1.5 mm lamella. (n=6) With 4-AP and synaptic transmission antagonists, evoked response envelope is similar to NACSF control, indicating the broadened response envelope seen in 4-AP alone is caused by synaptic transmission.
Synaptic contribution to widespread activation was tested by adding a synaptic blocker cocktail of 50μM DAPV, 40μM DNQX, and 100μM PTX to the 4-AP bath (Fig. 3C). The enhanced longitudinal propagation was reduced to control levels by these synaptic transmission antagonists (Fig. 3C). These data show that the increase in the longitudinal extent of propagation in CA3 requires synaptic transmission and cannot be attributed to the increase in the number of activated axons. Further examination of a synaptic requirement for orthogonal propagation can be found in the supplemental literature for this manuscript.
Spontaneous Self-propagating Longitudinal Wave
To investigate the mechanisms of the strong longitudinal activation in CA3 in the presence of 4-AP, the propagation of spontaneous events was analyzed. In 50μM 4-AP solution, spontaneously occurring potentials were observed in the CA3 and CA1 regions of the preparation. Fig. 4 shows a typical set of spontaneous events recorded from the CA3 region. These spontaneous potentials had an amplitude of 0.5–5 mV and lasted 50–200 ms. These potentials are generally referred to as interictal-like events (ILEs) similar to potentials recorded in the transverse slice (Avoli, 1991). These 4-AP induced ILEs were recorded from multiple simultaneous locations in order to determine their focus, propagation path, and extent of coverage of the hippocampus.
Figure 4.
Spontaneous 4-AP induced activity in the CA3 region of the hippocampus. A) Spontaneous activity recorded over a period of two minutes showing repeated bursting pattern. Bursts occurred at a frequency of 1.3 ± 0.8s. B) Field potentials recorded at far sides of CA3. Potentials recorded at location two (Rec2) are delayed on average 13.9 ms from Rec1. Cross correlation analysis for a sample of waveforms shows a propagation delay of 13.9 ms, the recording distance was 1.5mm for this set, giving a propagation velocity of 0.11 m/s. C) Cross-correlation of potentials at Rec1 and Rec2, showing group delay (n=8).
Recording electrodes were placed at a depth of 200–300 micrometers for quantifying wave activity in the somatic region of CA3 in the experiments illustrated in Figures six through nine. Interictal-like events consisting of tonic activity commenced two to five minutes after application and continued for five to six hours until washout or the preparation was removed. Spontaneous activity was characterized by frequent (one to three second interval) depolarizing bursts recorded in the CA3 region (Fig. 4A) and synchronous potentials in the CA1 region of the intact hippocampus. While in normal ACSF, evoked activity was confined to transverse lamellae, spontaneous activity in 4-AP ACSF was quite different. As in the case of the voltage sensitive dye recording shown previously, the entire CA3–CA1 region of the hippocampus was found to be active. Recordings of spontaneous ILE potentials at opposite ends of CA3 (Fig. 4B) were analyzed via cross-correlation to determine the propagation delay between the recording sites. Initiation site was determined by observing the timing latency between the recording locations of the initial negative peaks in the ILE waveform. ILEs initiated at the temporal pole of the hippocampus and propagated quickly (amplitude 1.6±0.9 mV, speed 0.09±0.03 m/s (Fig. 4B,C). Typical longitudinal propagation delay lasted 19.7 ± 6.8 ms (n=8) along the CA3 region from the temporal to septal pole. Axonal conduction velocity was measured by applying synaptic transmission antagonists and measuring the latency from a stimulus response recorded in septal CA3 to a recording electrode in temporal CA3. Compared to axonal propagation along CA3 which was recorded at 0.31±0.08m/s (n=3), the 4-AP induced wave traveled approximately three times more slowly. In this hyper-excitable state, neurons in the entire CA3 region were spontaneously active with bursts that propagated as non-attenuating waves across the entire preparation (Fig. 5). An evoked traveling response wave similar in shape and duration to the ILE could be induced by stimulation at either end of CA3, creating a wave traveling in a direction leading away from the site of stimulus (data not shown). Immediately following septal stimulation, occasionally the spontaneous wave would initiate at the same pole, so even though the ILEs were typically generated at the temporal pole, the propagation path is bidirectional, which confirms other reports (Derchansky et al., 2006).
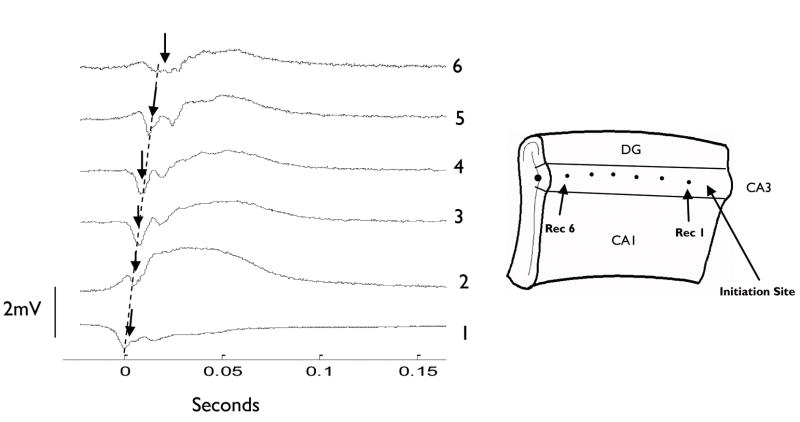
Figure 5.
Spontaneously generated wave propagating along the CA3 region in the unfolded mouse hippocampus in 50 μM 4-AP. Dashed line indicates propagation speed of 0.11m/s. Trace one is a recording from the temporal pole of the CA3, the trace at location six is recorded from the septal pole of the unfolded hippocampus preparation, and the traces in between are recorded along the longitudinal axis. Peak initial wave amplitude is indicated by arrows.
Synaptic dependence of spontaneous self-propagating longitudinal wave in the CA3 region
A synaptic blocker cocktail consisting of 50μM DAPV, 40μM DNQX, and 100μM PTX was applied to the bath in addition to 50μM 4-AP to determine the role of synaptic transmission in the longitudinal wave propagation in the hippocampus. Self-propagating waves were induced by applying a single stimulus to the temporal CA3 (see Fig. 6A). Recording electrodes were positioned in three locations along CA3 to measure the response field potential to stimulus before and after the synaptic transmission antagonists were added. Fig. 6B shows field potential recordings from the center of CA3 before, during and following washout of the blocker cocktail. In 4-AP alone, the evoked wave traveled the length of hippocampus without significantly decreasing in amplitude (1.7 ± 0.8 mV at the stimulus site to 1.6 ± 0.5 mV at the edge). In the presence of synaptic transmission antagonists, the stimulus response amplitude, while ± 0.8 mV at the site of stimulation, decreased to background noise levels as measured in the longitudinal direction in CA3 away from the site of stimulus to 0.1 ± 0.1 mV at the far end of the CA3 region (Fig 6C). These data show that the self-propagating wave can occurr spontaneously or via orthogonally located stimulation and that it is synaptically mediated.
Figure 6.
A) CA3 recording locations for evoked wave synaptic propagation blocking experiment. B) Synaptic dependence of longitudinal wave propagation: Evoked CA3 wave in 4-AP (bottom trace), 4-AP with 50 μM DAPV, 40 μM DNQX, 100 μM PTX (center trace), and wash with 4-AP (top trace) all recordings from location 2. The presence of synaptic transmission antagonists reversibly eliminates the evoked wave in the presence of 4-AP. C) CA3–CA3 wave propagation amplitude from lateral to septal poles. The 4-AP induced wave does not significantly decrease in amplitude along CA3, whereas the evoked potential in blocker solution does not propagate to the opposite (septal) end of CA3.
Effect of CA3 lesion on longitudinal propagation
The previous data show that the wave can propagate along the CA3 layer at a lower velocity than that of axonal propagation. We hypothesize that this wave uses the synaptic CA3 recurrent excitation pathways for which the axons are located within or close to the CA3 layer. To test this hypothesis, a lesion technique was applied to five preparations displaying spontaneous ILEs in 50 μM 4-AP ACSF. Using a small cutting blade, an area limited to the CA3 region was lesioned (Fig. 7A) and pyramidal cell spontaneous field potentials were recorded on each side of the lesion in CA3 and CA1 before and following the procedure. The spontaneous orthogonal wave traveled in the longitudinal direction (1→ 2) and then transversely (2 → 3) (Fig 7B). However, propagation in the longitudinal direction ceased following the lesion. The recorded field potentials were statistically analyzed for cross-correlation timing between temporal and septal lobes and the resulting correlations before and after the lesion were normalized for each experiment (Fig. 7C). In all cases, the signal correlation between the septal and temporal lobes after the lesion was drastically reduced across both CA1 and CA3, from 0.87 ± 0.07 to 0.17 ± 0.06 across CA3 and 0.95 ± 0.05 to 0.12 ±0.02 across CA1. The data show that wave propagation from temporal to septal poles was completely arrested in both CA1 and CA3 post-lesion. Likewise, in all cases the amplitude of the spontaneous septal pole activity decreased significantly after the lesion was made. No significant decrease in spontaneous temporal pole amplitude was seen, however a decrease in frequency was observed ranging from 1/3 to 1/4 of the pre-lesion cadence. In three out of five cases, the septal hippocampus became completely silent while in the other two cases, the septal pole began generating its own low amplitude spontaneous potentials, although at a lower frequency and amplitude than the temporal pole (every 2.6 ± 1.4 s vs. 1.3 ± 0.8s). These data show that 1) the 4-AP induced wave propagation path lies exclusively in the CA3 region, 2) a CA3 cut can arrest both longitudinal and orthogonal propagation, and 3) post-lesion CA3 can generate desynchronized spontaneous activity on each side in the presence of 4-AP.
Figure 7.
Transverse lesion of the CA3 region blocks longitudinal wave propagation in the hippocampus and reduces spontaneous amplitude at the septal pole. A) location of lesion and recording sites. For CA3–CA3 recordings, locations one and two were used. For CA3 to CA1 recordings, locations one and three were used. B) Top traces (Control): Synchronous activity observed at the poles of the CA3 region in 50μM 4-AP. Top traces (Lesion): Unsynchronized activity observed at the poles of the CA3 region after a selective CA3 lesion (marked in red). Note the septal pole still shows spontaneous bursts, however at a lower amplitude than before the lesion. Bottom traces: Septal CA1 activity recorded before and after selective lesion of the CA3. Note the amplitude decrease along with de-synchronization from the temporal CA3, indicating that a selective lesion of the mid-CA3 blocks wave propagation along the preparation. C) Bar graph: normalized cross correlation of temporal and septal CA3 recordings before and after CA3 lesioning. Before the lesion, there existed strong correlation between recordings at locations one and two, and one and three. After lesion there was a significant decrease in correlation in both recording sets (P<0.01, bars MSE), indicating the CA3 lesion severed the 4-AP induced wave pathway from the initiation site to the septal pole CA3 and CA1. (n=5)
Discussion
The planar unfolded hippocampus preparation was used to investigate transverse and longitudinal propagation of spontaneous and evoked 4-AP induced interical-like epileptiform activity in the mouse hippocampus. This experimental study has six major conclusions: 1) An orthogonal wave can be generated traveling first transversely along CA1 and then longitudinally into CA3 in the unfolded hippocampus in the presence of 4-AP. 2) A regenerating wave propagates along the CA3 region of the hippocampus. 3) This wave-like propagating activity is synaptically mediated along the longitudinal recurrent excitatory pathway in the CA3. 4) The wave-like activity propagates at a speed 1/2–1/3 of axonal propagation speed. 5) This longitudinal wave spreads orthogonally into CA1, affecting much of the hippocampus. 6) Transverse lesioning of the CA3 region can inhibit the propagation of epileptiform ILEs to longitudinal lamellae while preserving the CA3–CA1 network. Discussion on the background and benefits of utilizing the unfolded hippocampus preparation is contained in the supplemental material for this manuscript.
The potassium channel blocker 4-AP was chosen in this study as a model of epilepsy due to its ability to generate spontaneous epileptiform activity and maintain synaptic activity at low concentrations. Furthermore, the prolongation of action potential duration enhanced recording of spontaneous activity using voltage sensitive dyes. Previous transverse slice-based studies have shown foci in the CA3–CA2 region of the hippocampus with voltage sensitive dye recordings (Colom & Saggau, 1994). This work expands on previous work to include the entire CA3–CA1 region for network observations in two dimensions. While the focus of 4-AP induced spontaneous activity in CA3 was found to be the temporal pole, the septal pole can act as an oscillator when removed from the driving temporal oscillator. It has been shown that removal of inhibition in the hippocampus can lead to the propagation of longitudinal wave-like activity (Miles et al., 1988). Propagation velocities similar to those reported above were found in the presence of GABAergic IPSPs (0.15 m/s vs 0.1 m/s noted here), a 2–3× decrease in conduction velocity compared to that of axons in the area (Soleng et al., 2003). 4-AP increases the excitability of CA3 cells, and at high concentrations (200μM) can have a depressing effect on interneuronal inhibition (Perreault & Avoli 1991). Depressed inhibition can lead to an excitable state where group activation on one side of CA3 can quickly travel the length of the hippocampus. For this reason, the concentration of 50μM was chosen as it has been shown to maintain inhibition in the networks (Traub et al., 2001). Under these conditions, a self-regenerating, orthogonally propagating wave was generated along the CA3 throughout the recurrent excitatory synapses on groups of local CA3 cells. 4-AP additionally causes a prolongation of the EPSP (Avoli, 1996) seen in CA3 neurons that is likely to come (at least partially) from other excitable CA3 pyramidal cells. The strengthening of these synapses generates a positive feedback effect that, when combined with the hyper-excitable state of the cells and the increased occurrence of spontaneous neurotransmitter release, induces spontaneous ILE generation and subsequent propagation to the entire CA3. It has been shown that CA3–CA3 recurrent excitatory synapses exist in rodents, (MacVicar & Dudek, 1981; Ichizuka et al., 1990) and computer modelling has suggested a well developed network in CA3 could play a role in epileptiform burst propagation (Traub et al. 1987, Miles et al. 1988). As such, it is reasonable to suggest that the longitudinal extent of CA3–CA3 recurrent arborization plays a significant role in the hyperexcitability and ILE propagation capability of the hippocampus network.
It has been noted that spontaneous ILEs occur in slices also, and these experiments demonstrate that halving the hippocampus can result in two spontaneously firing halves, each containing an oscillator operating at different frequencies. Similarly, spontaneous oscillations were observed in longitudinal hippocampal slices (Gloveli et al., 2005) and multi-foci seizure activity was seen in an intact hippocampus preparation by Derchansky et al. (2006). As the septal pole oscillator tends to drive the whole CA3, and that location has a higher density of connected axons (Amaral & Witter, 1989; Luhmann et al., 2000), it is possible that the frequency of the local oscillator is affected by the local density of axons and recurrent synapses. Whereas bidirectional propagation of epileptiform activity in the planar hippocampus was observed with low Mg++ (Derchansky et al. 2006), the propagation seen with 4-AP could be entrained to propagate in the septal-temporal direction, but spontaneous activity originated from the temporal pole. The relatively slow activation of the whole CA3 region in 4-AP from an antidromic stimulus is likely related to the synaptic link between persistent bursting (ILEs) in the CA3 region and the recurrent excitation pathway (Stoop et al., 2003). The frequency stability of the spontaneous ILEs is thought to be related to NMDA receptor interactions at CA3 recurrent collateral synapses (Hellier et al., 2007), and is a likely explanation for septal lobe network oscillation when it is removed from the temporal hippocampal lobe.
Longitudinal self-generating waves similar to those observed in the intact hippocampus preparation have been previously recorded in ACSF with picrotoxin in longitudinal slices (Miles et al., 1988). These waves travel longitudinally along CA3 in slices, and as shown above they also invade the CA1 region, creating orthogonal propagation. Therefore, in these hyperexcitable models, the whole hippocampus can be excited by a (single) input into CA3. This activity is most likely mediated by the recurrent excitation network, since small cuts through the CA3 region can eliminate longitudinal propagation through the recurrent pathway while preserving the lamellar transverse circuits and longitudinal propagation through axons outside the CA3 region. A surgical technique of multiple subpial transection (MST) for the treatment of temporal lobe epilepsy has been proposed as a treatment for intractable epilepsy in order to preserve the formation of verbal memory (Shimizu et al., 2006). The MST technique transects the longitudinal interneuronal connections of the hippocampus while preserving transverse connection fibers. Lesions of sections of the hippocampus have been shown to block synchronous propagation of epileptiform activity (Derchansky et al., 2006). Our experiments have shown that a selective transection of the CA3 alone is sufficient to significantly decrease or eliminate trans-hippocampal epileptiform (ILE) propagation not only in the CA3 region but also orthogonal propagation to the CA1 region as well, while at the same time decreasing spontaneous activity in the temporal hippocampal lobe and maintaining transverse functional pathways.
Conclusion
While the hippocampus does have an orderly lamellar organization and as shown above, a preferentially transverse activation direction in normal solution, the often-overlooked longitudinal pathways of the CA3 region can have a large effect on neuronal activity in in vitro models of epileptiform discharge or synchronization induced by 4-AP. In particular, these results show that 4-AP can induce orthogonal waves that propagate longitudinally along the CA3 layer and transversely into the CA1 region. In a hyperexcitable state such as the one generated by 4-AP, the hippocampus is transformed from a primarily lamellar organization of activity into a broadly activated structure through orthogonal propagation into the whole hippocampus. This propagation is slower than axonal propagation and is synaptically mediated. Furthermore, it is possible to reduce or arrest epileptiform propagation along CA3 via a selective transverse lesion. It is expected that the unfolded planar hippocampus preparation will enable further study of the importance of orthogonally active hippocampus networks in seizure propagation and in vitro testing of novel surgical techniques.
Supplementary Material
Figure 1: Procedure for preparing the unfolded planar hippocampus. The ends are trimmed (1,2) next the perforant path and vessels are cut (3,4) finally the DG is folded back (5,6) to form a planar preparation.
Figure. 2: Wave delay from axonal field potential seen in 4-AP + blocker cocktail. Top trace: Response field potential in 4-AP with no synaptic blockers to a stimulus in the CA1 which is 1.5mm lateral to the lamella of the recording electrode. The stimulus elicits a strong response at the recording electrode 1.5mm lateral to the plane of stimulation. Bottom trace: Null field potential response in the same location in the presence of synaptic blockers. These data show that the wave of activity in 4-AP is not merely due to enhanced axonal response to stimulus, and that the response lasts longer than a typical orthodromic field potential.
Figure. 3: A) Exemplary time-wise activation contours shown in B and C out of five preparations analyzed. B) Levels of activation as a function of time along a transverse plane (B) in the hippocampus from a stimulus in CA1. This cross section is in the plane of stimulation. Stimulus occurs at contour 0ms, distance 0mm. At 4ms, the CA1 stimulus location is activated while CA3 remains near resting potential. At 8ms, CA3 is becoming active and at 12ms the CA3 response reaches its peak. This figure shows transverse antidromic propagation of the stimulus response into CA3 (propagation a to b). Contour labels are in ms. C): Levels of activation as a function of time along a transverse plane (C) in the hippocampus from a stimulus in CA1. In this cross section, activation in CA3 precedes same in CA1 in a cross-sectional lamella lateral from the stimulus. At contour time 4ms, CA3 begins to respond, and during contours 6–12ms, CA3 and CA1 activate, with CA1 following CA3. This figure shows the septal CA1 being activated after orthogonal propagation of the stimulus response along CA3 (propagation B to C). Contour labels are in ms.
Acknowledgments
Funded By NIH Grant# 5R01NS040785-04 and a department of education GANN fellowship.
Abbreviations
- EC
Entorhinal Cortex
- 4-AP
4-aminopyridine
- ILE
Interictal-like event
- SR
Stratum Radiatum
Footnotes
None of the authors has any conflict of interest to disclose.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–8. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The Three-Dimensional Organization of the Hippocampal Formation: A Review of Aanatomical Data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Avoli M. GABA-mediated Synchronous Potentials and Seizure Generation. Epilepsia. 1996;37(11):1035–42. doi: 10.1111/j.1528-1157.1996.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Bikson M, Baraban SC, Durand DM. Conditions sufficient for nonsynaptic epileptogenesis in the CA1 region of hippocampal slices. J Neurophysiol. 2002;87(1):62–71. doi: 10.1152/jn.00196.2001. [DOI] [PubMed] [Google Scholar]
- Colom LV, Saggau P. Spontaneous Interictal-Like Activity Originates in Multiple Areas of the CA2-CA3 Region of Hippocampal Slices. J Neurophys. 1994;71(4):1574–1585. doi: 10.1152/jn.1994.71.4.1574. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Rokni D, Rick JT, Wennberg R, Bardakjian BL, Zhang L, Yarom Y, Carlen PL. Bidirectional multisite seizure propagation in the intact isolated hippocampus: The multifocality of the seizure “focus”. Neurobiology of Disease. 2006;23:312–328. doi: 10.1016/j.nbd.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Gajda Z, Hermesz E, Gyengési E, Szupera Z, Szente M. The Functional Significance of Gap Junction Channels in the Epileptogenicity and Seizure Susceptibility of Juvenile Rats. Epilepsia. 2006;47(6):1009–1022. doi: 10.1111/j.1528-1167.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, Whittington MA, Kopell NJ. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(37):13295–13300. doi: 10.1073/pnas.0506259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Shao-Yu GE, Di-Yun R. Effect of 4-aminopyridine on synaptic transmission in rat hippocampal slices. Brain Res. 2004;1006:225–232. doi: 10.1016/j.brainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. NMDA Receptor Traffiking at Recurrent Synapses Stabilizes the State of the CA3 Network. J Neurophysiol. 2007;98:2818–2826. doi: 10.1152/jn.00346.2007. [DOI] [PubMed] [Google Scholar]
- Ichizuka N, Weber J, Amaral D. Organization of Intrahippocampal Projections Originating From CA3 Pyramidal Cells In the Rat. J Comp Neur. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Dzhala VI, Ben-Ari Y. Generation and propagation of 4-AP-induced epileptiform activity in neonatal intact limbic structures in vitro. Eur J Neurosci. 2000;12:2757–2768. doi: 10.1046/j.1460-9568.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Electronic Coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981;213:782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronisation of Electrically Coupled Pairs of Inhibitroy Interneurons in Neocortex. J Neurosci. 2007;27(8):2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Traub R, Wong R. Spread of Synchronous Firing in Longitudinal Slices From the CA3 Region of the Hippocampus. J Neurophysiol. 1988;60(4):1481–1496. doi: 10.1152/jn.1988.60.4.1481. [DOI] [PubMed] [Google Scholar]
- Pais I, Hormuzdi SG, Monyer H, Traub RD, Wood IC, Buhl EH, Whittington MA, LeBeau FE. Sharp Wave-Like Activity in the Hippocampus In Vitro I Mice Lacking the Gap Junction Protein Connexin 36. J Neurophysiol. 2003;89:2046–2054. doi: 10.1152/jn.00549.2002. [DOI] [PubMed] [Google Scholar]
- Pena F, Tapia R. Relationships Among Seizures, Etracellular Amino Acid Changes, and Neurodegeneration Induces by 4-Aminopyridine in Rat Hippocampus. J Neurochem. 1999;72:2006–2014. doi: 10.1046/j.1471-4159.1999.0722006.x. [DOI] [PubMed] [Google Scholar]
- Pena F, Tapia R. Seizures and Neurodegeneration Induced by 4-Aminopyridine in Rat Hippocampus In Vivo: Role of Glutamate- and GABA-Mediated Neurotransmission and of Ion Channels. Neuroscience. 2000;101(3):547–561. doi: 10.1016/s0306-4522(00)00400-0. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and Pharmacology of Epileptiform Activity Indiced by 4-Aminopyridine in Rat Hippocampal Slices. J Neurophysiol. 1991;65(4):771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-Aminopyridine-induced Epileptiform Activity and a GABA-mediated Long-lasting Depolarization In the Rat Hippocampus. J Neurosci. 1992;12(1):104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu HK, Kensuke S, Sugano H, Yamada T. Hippocampal transaction for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13:322–328. doi: 10.1016/j.jocn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Shinnar S. Febrile Seizures and Mesial Temporal Sclerosis. Epilepsy Curr. 2003;3(4):115–118. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Raastad M, Andersen P. Conduction Latency Along CA3 Hippocampal Axons From Rat. Hippocampus. 2003;13:953–961. doi: 10.1002/hipo.10141. [DOI] [PubMed] [Google Scholar]
- Stoop R, Conquet F, Zuber B, Voronin LL, Pralong E. Activation of Metabotropic Glutamate 5 and NMDA Receptors Underlies the Induction of Persistent bursting and Associated Long-lasting Changes in CA3 Recurrent Connections. J Neurosci. 2003;23(13):5634–5644. doi: 10.1523/JNEUROSCI.23-13-05634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Knowles WD, Miles R, Wong RKS. Models of the Cellular Mechanism Underlying Propagaiont of Epileptiform Activity in the CA2-CA3 Region of the Hippocampal Slice. Neuroscience. 1987;21(2):457–470. doi: 10.1016/0306-4522(87)90135-7. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig R, Piechotta A, Draguhn R, Schmitz D. Synaptic and Nonsynaptic Contributions to Giant IPSPs and Ectopic Spikes Induced by 4-Aminopyridine in the Hippocampus In Vitro. J Neurophys. 2001;85:1246–1256. doi: 10.1152/jn.2001.85.3.1246. [DOI] [PubMed] [Google Scholar]
- Velazquez J, Carlen P. Gap junctions, synchrony, and seizures. Neurosci Trends. 2000;23:68–74. doi: 10.1016/s0166-2236(99)01497-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Procedure for preparing the unfolded planar hippocampus. The ends are trimmed (1,2) next the perforant path and vessels are cut (3,4) finally the DG is folded back (5,6) to form a planar preparation.
Figure. 2: Wave delay from axonal field potential seen in 4-AP + blocker cocktail. Top trace: Response field potential in 4-AP with no synaptic blockers to a stimulus in the CA1 which is 1.5mm lateral to the lamella of the recording electrode. The stimulus elicits a strong response at the recording electrode 1.5mm lateral to the plane of stimulation. Bottom trace: Null field potential response in the same location in the presence of synaptic blockers. These data show that the wave of activity in 4-AP is not merely due to enhanced axonal response to stimulus, and that the response lasts longer than a typical orthodromic field potential.
Figure. 3: A) Exemplary time-wise activation contours shown in B and C out of five preparations analyzed. B) Levels of activation as a function of time along a transverse plane (B) in the hippocampus from a stimulus in CA1. This cross section is in the plane of stimulation. Stimulus occurs at contour 0ms, distance 0mm. At 4ms, the CA1 stimulus location is activated while CA3 remains near resting potential. At 8ms, CA3 is becoming active and at 12ms the CA3 response reaches its peak. This figure shows transverse antidromic propagation of the stimulus response into CA3 (propagation a to b). Contour labels are in ms. C): Levels of activation as a function of time along a transverse plane (C) in the hippocampus from a stimulus in CA1. In this cross section, activation in CA3 precedes same in CA1 in a cross-sectional lamella lateral from the stimulus. At contour time 4ms, CA3 begins to respond, and during contours 6–12ms, CA3 and CA1 activate, with CA1 following CA3. This figure shows the septal CA1 being activated after orthogonal propagation of the stimulus response along CA3 (propagation B to C). Contour labels are in ms.