Abstract
Background
Responses to stress vary greatly in young adolescents, and little is known about neural correlates of the stress response in youth. The purpose of this study was to examine whether variability in cortisol responsivity following a social stress test in young adolescents is associated with altered neural functional connectivity (FC) of the salience network (SN) measured during resting-state functional magnetic resonance imaging (fMRI).
Methods
Forty-nine typically developing young adolescents participated in a social stress test during which they contributed salivary cortisol samples. Following this, they underwent resting-state fMRI (rs-fMRI) scanning. We examined the association of FC of the SN (composed of anterior cingulate cortex (ACC) and bilateral anterior insula regions) with cortisol responsivity.
Results
Greater cortisol responsivity was significantly positively correlated with higher FC between subgenual anterior cingulate cortex (Cg25) and the SN, controlling for participant age. There were no regions of the brain that showed an inverse relation.
Conclusions
Brain systems that have been implicated in autonomic arousal and that influence subjective feeling states show altered FC associated with stress responsivity in early life.
Keywords: resting-state, adolescents, HPA-axis, stress, subgenual cingulate, fMRI, salience network, connectivity
When confronted with threat, humans and other animals show rapid engagement of both limbic brain circuitry (e.g., amygdala, prefrontal cortex (PFC), cingulate, anterior insula) and the hypothalamic-pituitary-adrenal (HPA) axis — a system reliably implicated in stress response. Limbic and HPA stress-response systems are mutually regulatory, in part to diminish the stress response when it is no longer necessary (Pruessner et al., 2010). In studies of rodents and in human lesion studies, medial prefrontal cortices (mPFC) have been shown to play a critical role in control over the HPA axis (Figueiredo et al., 2003, Tchiteya et al., 2003, Buchanan et al., 2010). Moreover, using high-resolution positron emission tomography (PET) with rhesus monkeys, (Jahn et al., 2010) found elevated subgenual cingulate (Cg25) activity to be associated with heightened HPA-axis activity. These results are of great interest given considerable evidence that dysregulated stress responding (see Liberzon et al., 2007) as well as elevated Cg25 activation and altered mPFC activity are involved in the pathophysiology of psychiatric disorders such as depression and anxiety (see metanalysis by Seminowicz et al., 2004). Moreover, despite strong evidence linking frontolimbic dysregulation to HPA-axis dysfunction and to difficulties in emotion regulation, a hallmark characteristic of depression (Gotlib and Hamilton, 2008), few studies have measured directly how frontolimbic circuits are related to HPA-axis responsivity.
In late childhood and early adolescence (ages 9–15), brain limbic circuitry is still developing (Cunningham et al., 2002), and protracted maturational brain changes are likely to affect the effectiveness of emotion regulation in children (Kovacs et al., 2008). A number of approaches have been used to map the ordering and timing of the development of brain circuitry, including cross-sectional studies of grey matter reduction (Giedd et al., 1999, Pfefferbaum et al., 1994), and synaptogenesis (Huttenlocher, 1979), as well as longitudinal studies mapping cortical thickness and brain growth in children (Sowell et al., 2004a). This research has demonstrated that the PFC develops more slowly than do other brain areas (reveiwed by Sowell et al., 2004b). Given that early developmental mechanisms have been posited to influence the tendency of individuals to express maladaptive behaviors in response to threatening stimuli (i.e. heightened anxiety; see Gross and Hen, 2004), it is important to examine the relation between frontolimbic function and HPA-axis reactivity in youth.
Recent advances in functional neuroimaging techniques, such as resting-state functional magnetic resonance imaging (rs-fMRI), afford new opportunities for studying in vivo the development of brain corticolimbic circuitry. By cross-correlating time-series data between regions, it is possible to determine which regions of the brain are functionally connected. Importantly, results obtained using this method are consistent with known anatomical connectivity data from post-mortem human studies and anatomical tract tracing data from studies of other mammalian species (cf. Fair et al., 2010); moreover, findings of rs-fMRI studies have revealed important principles of neural organization both in normal and atypical human development (reviewed by Uddin et al., 2010).
Rs-fMRI data have reliably indicated that there is a canonical coherent network consisting of ACC and bilateral anterior insula (Seeley et al., 2007, Taylor et al., 2008). Because this network comprises regions of the brain that are critical for interoceptive and emotional awareness, it has been labeled the Salience Network (SN) (Seeley et al., 2007, White et al., 2010). Regions of the SN have been shown to respond to pain, uncertainty, and other homeostatic challenges (Grinband et al., 2006, Peyron et al., 2000). In addition, the SN has been found to be robust and reliable across repeat rs-fMRI measurements in children and young adolescents (Thomason et al., 2011). The SN is of particular interest for the present study because it involves regions known to modulate physiological responses to stress (i.e. insula, see Critchley, 2005),
The present study was designed to examine the association between functional interrelations of SN brain circuitry and HPA-axis reactivity in children and adolescents. connectivity of the SN has not been examined in the context of HPA-axis reactivity. We applied a model-free independent component analysis (ICA) to resting-state data to define the brain salience network in 49 children and young adolescents. The participants also underwent a social stress interview during which cortisol was sampled to measure HPA-axis function. We hypothesized that the strength of the association between prefrontal cortical and SN functional connectivity would be influenced by HPA-axis activity. Because the mPFC expresses high levels of glucocorticoid receptors and is therefore involved in negative feedback control of the HPA axis, we hypothesized that significant group differences in connectivity would be observed in midline, prefrontal cortical regions. We further predicted that the Cg25, a structure that has been found to be involved in the experience of emotion in healthy individuals (Maddock et al., 2003) and that is both structurally and functionally anomalous in depression, may be more strongly coupled to SN function in individuals with higher levels of HPA system stress response.
Methods
Participants
Participants were 49 children and young adolescents (22 females) between the ages of 9 and 15 years (M = 11.8, SD = 1.9). They were recruited through their mothers via online forums, advertisements, and parent networks; each mother-child pair was compensated $25/hour. All participants had no reported history of brain injury, no behavioral indications of possible mental impairment, no past or present Axis I disorder, were right-handed, were fluent in English, and had no learning disorder. Parents and participants gave informed consent and assent, respectively, as approved by the Stanford University Institutional Review Board.
Laboratory assessments
Interviews
Participants were administered structured interviews to assess current and lifetime psychopathology. Trained interviewers assessed the diagnostic status of the young adolescents by administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (Geller et al., 1996, Geller et al., 2001), which has been shown to generate reliable and valid psychiatric diagnoses (Kaufman et al., 1997). During this session, participants and parents also viewed a video to prepare them for the magnetic resonance imaging (MRI) scan session.
Cortisol collection and stress task
Participants underwent a 15-min, controlled, laboratory stress interview that consisted of a 3-min serial subtraction task and the 12-min Ewart Social Competence Interview (Ewart et al., 2002), a semistructured interview developed to induce emotional stress and arousal in adolescents by discussing details of stressful life situations. Salivary cortisol was collected at regular intervals during and following the interview. During the serial subtraction task, children/adolescents were instructed to begin at 400 and count backward by sevens as quickly and accurately possible. If they made a mistake, they were interrupted by the experimenter and were told to start over. Further details on administration and analysis of the cortisol measurements and stress test are provided in supplemental material on line.
Physiological responses during scanning
We wanted to examine whether physiological responses measured during fMRI scanning (i.e. heart rate and breathing rate) are related to measures of stress reactivity measured outside of the scanner, particularly given that previous work has shown that the scanning environment can induce anxiety in youth (Eatough et al., 2009), and that physiological responses are altered in response to anxiety provocation. If these measures were related, it would be necessary to test the relative contributions of physiological response during scanning versus stress responsivity (HPA-axis activation) to observed differences in brain FC. Thus, it was critical to test the relation between these parameters in the present study and to correct fMRI data for physiological contributions to signal during data reconstruction (as described above). Bivariate correlation statistics were used to test whether physiological measures were related to cortisol responsivity. In addition, given that HR and respiration rates decrease with age, we also tested the relations between participant age and physiological measures; based on our past work (Thomason et al., 2005), we predicted a negative relation between age and HR, and between age and respiration rate. In an exploratory analysis, we examined whether participant gender was related to physiological parameters, given that recent studies have reported differences between women and men in both neural (Koch et al., 2007) and autonomic (Buchanan et al., 2010) responses to emotional material.
fMRI preprocessing
Participant data were preprocessed using Statistical Parametric Mapping software (SPM8; www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing included image realignment and co-registration of functional and anatomical images. Functional images were normalized to the Montreal Neurological Institute (MNI) template, using the participant-specific transformation parameters created by fitting mean functional images to the single reference EPI standard SPM template. The default setting in SPM is to resample image data to 2 × 2 × 2 mm during the normalization step of preprocessing; for normalization of this sample, however, data were not resampled, and thus retained the native resolution (3.44 × 3.44 × 4 mm). Following normalization, all participant images were visually inspected, and we determined that all normalizations had proceeded satisfactorily. Images were smoothed with a 6-mm Gaussian kernel to decrease spatial noise.
Independent component analysis (ICA)
Data from all participants were concatenated and submitted to a group ICA procedure (http://icatb.sourceforge.net) implemented in Matlab (www.mathworks.com) using GIFT (Calhoun et al., 2001). Infomax was used to estimate 26 components. Our decision to derive 26 components was made by taking the mean number from three published reports that used similar analysis approaches (Stevens et al., 2009, Thomason et al., 2011, White et al., 2010). Then, a spatial template-matching technique was used (as described in Greicius et al., 2007) to automatically identify the component corresponding to the salience network. The SN template used in the present study was derived from an independent set of data (Seeley et al., 2007). Finally, using the GIFT processing toolbox, SN maps were back reconstructed for each participant. These ICA-derived individual participant SN maps were then carried into second-level random effects and regression analyses. Subject specific SN maps derived by this method are therefore similar in overall spatial features but each has variance in spatial topography that reflects unique temporal features. The ICA-derived individual participant SN maps can be regarded as functional connectivity maps, in which the highest values are recorded in voxels with time-courses that are highly correlated with the mean temporal trace for the SN for that participant, and thus areas of high functional connectivity to key regions within that network.
We used the ICA-based analytic procedure in this study because, unlike univariate methods (e.g. regression analysis), ICA does not rely on drawing inferences based on a priori regions of interest (ROIs). Selecting ROI seed points for children and adolescents based on previous work with adult samples is problematic. Although recent studies have examined properties of the salience network in young populations (Fair et al., 2007, Stevens et al., 2009, Thomason et al., 2011), none of these studies provides an independent published set of salience network peak coordinates that we could use to run a seed-based analysis in our youth sample. We have, however, recently published salience network peak coordinates in a young sample (Thomason et al., 2011), and these coordinates may be useful in future work in which a seed-region-based analysis is more appropriate.
Random effects analysis
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) was used to create a groupwise statistical parametric map of the SN network. Back-reconstructed single subject spatial maps that corresponded to the SN were subjected to a one-sample t-test; a method that has been validated in previous studies (Stevens et al., 2009). Results were used to derive maximally significant peaks comprising nodes of the SN; these results are summarized in Table 2. Results reported are significant at a family wise error (FWE)-corrected level of p < .001.
Table 2.
Regions for which FC was significantly positively correlated with cortisol response
| X | Y | Z | n voxels | T val | Z val | p val | ||
|---|---|---|---|---|---|---|---|---|
| Limbic | ||||||||
| Cingulate | L24 | −13 | 3 | 39 | 16 | 3.37 | 3.17 | 0.001 |
| Cingulate | R25 | 8 | 18 | −9 | 2 | 2.87 | 2.78 | 0.003 |
| Frontal | ||||||||
| Medial | R9 | 15 | 36 | 26 | 15 | 3.03 | 2.88 | 0.002 |
| Medial | L25 | −2 | 27 | −17 | 4 | 3.16 | 3.2 | 0.001 |
| Medial | R11 | 8 | 28 | −13 | 3 | 2.69 | 2.58 | 0.005 |
| Superior | R9/10 | 11 | 60 | 28 | 3 | 2.93 | 2.74 | 0.003 |
| Middle | L11 | −43 | 44 | −11 | 2 | 2.92 | 2.79 | 0.003 |
| Subcortex | ||||||||
| Thalamus - Ventral | −13 | −5 | 6 | 2 | 2.85 | 2.72 | 0.003 | |
| Anterior Nucleus |
Coordinates are given in Talairach and Tournoux convention.
BA = Bromann’s area.
Regression analysis
We conducted a voxelwise regression analysis with the aim of identifying regions in which SN connectivity correlated significantly with stress-induced cortisol response. Cortisol response was computed using the incremental area under the curve (AUCi), an index of time-dependent change measuring the increase in cortisol from baseline across a series of measurements (as described by Pruessner et al., 2003). Before calculating AUCi we excluded outlying cortisol responses (>2 standard deviations) from the analysis, as has been done in previous cortisol studies (Gotlib et al., 2008). Following this, SPM8 was used to conduct a whole-brain regression analysis examining the relation between HPA-axis cortisol reactivity and FC of the salience network of the brain. We controlled for age by including this variable as a regressor of non-interest in the regression model. Results for the whole brain are given for p < .005, uncorrected.
Results
Participant characteristics
Participants were 34 Caucasians (69%), 3 Hispanic Americans (6%), and 12 participants of multi- or bi-racial descent (25%). Participants were 9 to 15 years of age, covering a range over which the processes of brain maturation are still occurring (Sowell et al., 1999).
Stress reactivity
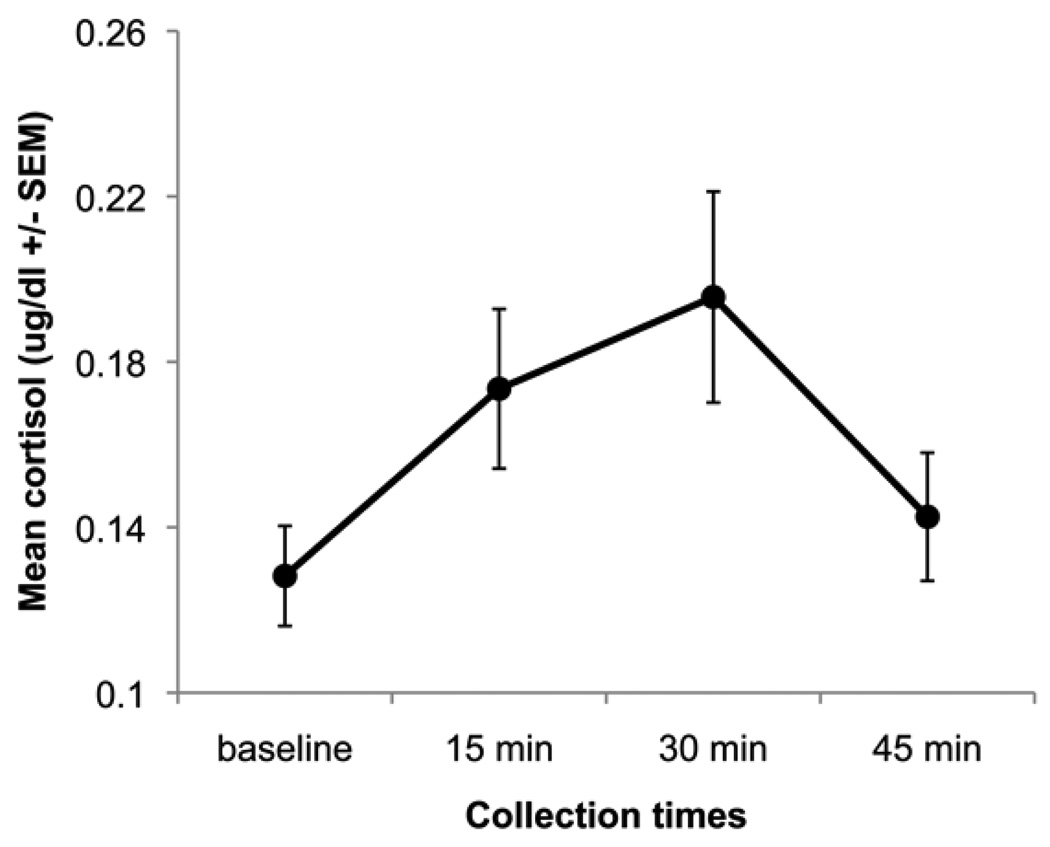
Participants showed a characteristic gradual and incremental rise in cortisol at 15 and 30 minutes following baseline and onset of the behavioral stressor, followed by a decrease at the 4th measurement time point, 45 minutes after baseline (see Fig. 1). The elevated cortisol production in response to stress observed in this sample is similar to what we have measured in our previous work with other samples of children (Gotlib et al., 2008). Inter-assay CV%s were 11.46, 7.76, and 6.19 for low, medium, and high cortisol concentrations, respectively; and intra-assay CV%s were 7.73, 6.08, and 4.48 for low, medium, and high cortisol concentrations, respectively.
Figure 1.
Stress reactivity measured across all participants, N = 49, ages 9–15.
Cortisol AUCi was not significantly related to participants’ age, r(49) = .21, p = .15, nor did male and female participants differ with respect to cortisol AUCi, t(47) = .74, p = .46. Furthermore, the four ethnic groups did not differ in AUCi, F(2,46) = .69, p = .5. In addition, AUCi was not significantly correlated with physiological parameters (heart rate and respiration rate) measured during scanning, all p > 0.5.
Movement and physiological responses during rs-fMRI scans
Participant age was not significantly related to translational or rotational mean movement (all p > .4), max excursion (all p > .2), mean jitter (all p > .3). In addition, males and females did not differ in movement for any of the six motion parameters, all p > .05. Across the participant sample, none of the 6 motion parameters were correlated with AUCi measurements, all p > .15. Finally, the number of frames retained for each participant after removing frames corresponding to movement spikes (described above) was not correlated with AUCi, r(49) = .1, p =.58.
As predicted, participant age was significantly negatively correlated both with HR (mean = 74 beats/min), r(48) = −.39, p = .007, and with respiration (mean = 21 breaths/min), r(49) = .39, p = .006. The relation between movement and physiological data was not significant for 11 of 12 correlations tested (6 motion parameters, 2 physiological measures), all p > .05; there was, however, a significant positive correlation between average HR and mean translational movement, r(48) = .3, p = .04. There was not a significant relation between the number of frames retained for the rs-fMRI analysis (after removing movement spikes) and physiological measurements, all p > .05. Finally, male and female participants did not differ in HR or respiration measures, or in number of frames removed for motion correction, all p > .4.
Salience network
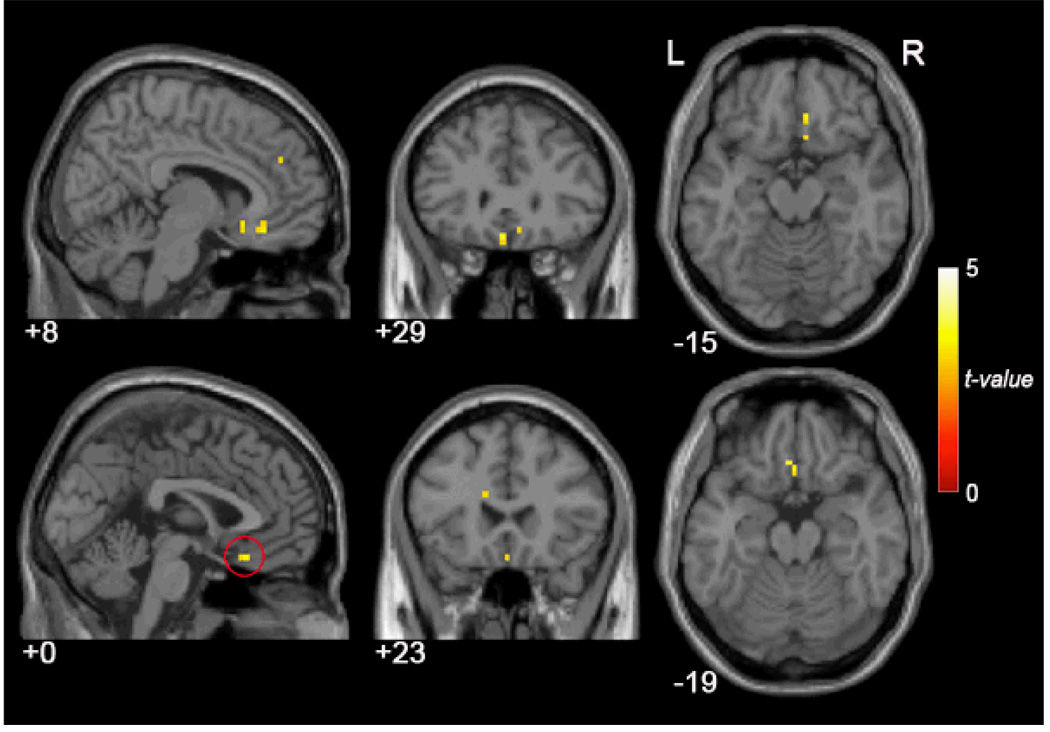
Brain regions in which HPA-axis reactivity was significantly correlated with FC to the SN are listed in Table 2. Consistent with our hypothesis, greater stress reactivity was associated with greater temporal coupling between time-series data from the subgenual anterior cingulate cortex (Cg25) and from areas of the mPFC and the resting time course of the SN. Regions showing significant HPA response-by-SN FC correlation are shown in Fig. 3.
Figure 3.
HPA-axis reactivity was significantly correlated with SN activity during rs-fMRI. Participants with higher HPA-axis response (measured using cortisol AUCi) showed significantly higher (p < .005) temporal coupling in regions of the medial frontal cortices and subgenual cingulate (Cg25; encircled by red rings).
Discussion
The present study was designed to examine the relation between activation of the HPA axis in response to stress and neural network brain connectivity in young adolescents. We found that those adolescents who exhibited higher HPA-axis reactivity to a structured social stress interview administered in the laboratory had higher functional coupling between the subgenual anterior cingulate (Cg25) and the SN. Cg25 has been identified as a key component in studies of self-referential processing and emotional monitoring (Zald et al., 2002). There is considerable evidence to suggest that aberrant Cg25 neural activation is associated with disturbed emotional (Gotlib et al., 2005) and physiological function (Wager et al., 2009). Aberrant metabolism in Cg25 has been reported in sad mood induction paradigms in both normal and depressed subjects (Liotti et al., 2002, Damasio et al., 2000, Mayberg et al., 1999). Furthermore, resting-state fMRI has revealed increased Default-mode Network (DMN) FC in Cg25 in adults with major depression (Greicius et al., 2007). In fact, the Cg25 region has been the successful target of deep brain stimulation for treatment-resistant depression (Mayberg et al., 2005), further corroborating the unique centrality of this region in the neuropathophysiology of mood disorders. The subgenual cortex has recently been associated with HPA system activity in rhesus monkeys (Jahn et al., 2010), but to our knowledge this is the first demonstration of an association between HPA response magnitude and Cg25 neural network function in humans.
To identify patterns of brain network connectivity that may correspond to heightened stress responsivity, we took an approach that was both theory- and data-driven. While a more extensive analysis may have included several brain rs-fMRI networks accumulated from group ICA analysis, we chose to limit our analysis to the SN, with special emphasis on the theoretical construct of physical response to psychological stress (i.e., HPA activation). SN circuitry has been implicated in self-generated emotional processing (Damasio et al., 2000), homeostatic processing (Seeley et al., 2007, Taylor et al., 2008), resolving uncertainty (Grinband et al., 2006), anticipatory anxiety (Chua et al., 1999), and error-detection (Menon et al., 2001). In particular, the ACC component of the SN may be specialized in generating autonomic changes, whereas the anterior insular component may be specialized for monitoring visceral sensory inputs (reviewed by Critchley, 2005). Research using rs-fMRI has begun to establish a critical role for insular components of the SN in switching between activity in the central executive network (also known as the ‘task-positive network’ (TPN)) and the DMN (also called the ‘task-negative network’) (Sridharan et al., 2008). Work by our group has demonstrated that individuals with major depression who adopt positive, adaptive rumination strategies engaged the TPN more often, and that engagement of TPN was associated with increased activation of the anterior insula (Hamilton et al., 2011). Our previous results, therefore, implicated an adaptive relation between the TPN and DMN in depression that may be instigated by insula-mediated awareness of negative emotional states. In the present study, we have extended this formulation by demonstrating that the SN, a system that is critical to awareness and that may drive action in other brain networks, is characterized by increased functional coupling with medial prefrontal circuitry that is implicated in key aspects of emotional (Mayberg, 2003) and physiological function (Wager et al., 2009) in children and adolescents with greater HPA-axis response to psychological stress. It is noteworthy that the results obtained for this unmasked, whole-brain analysis demonstrate specificity, in that significant results reside almost exclusively in medial frontal brain areas. Importantly, there were no regions of the brain in which there was a negative correlation between FC and cortisol reactivity.
By identifying neural processes that are associated with maladaptive stress responses in late childhood and early adolescence, our results elucidate differences in brain connectivity that may influence the development of adaptive emotional responses. Investigators have demonstrated that maladaptive behaviors that have been found to be associated with emotional disorders are not simply correlates of the disorder, but instead may play a critical role in the etiology and maintenance of psychopathology (e.g., Joormann et al., 2007). We complement these findings by focusing on neural developmental processes and, in this context, document the significance of limbic and prefrontal circuitry that is continuing to develop across the age range studied here (Cunningham et al., 2002, Sowell et al., 2004b). Brain white-matter tracts continue to mature through adolescence (reviewed by Thomason and Thompson, 2011) and, with this maturation there is a strengthening of long-range, and a weakening of short-range, functional connections (Fair et al., 2007). We suggest that altered functional connectivity influences individuals to experience and express maladaptive responses to stress, and that repeated experiences of a heightened stress response will, in turn, strengthen the activation of brain regions that will further exacerbate these effects.
While the present results highlight neural circuitry that may underlie the association between psychological experience and visceral reactions of the body, we should note a limitation of this research and an important difference between this study and previous work. First, our measure of HPA-axis reactivity was obtained within one week of the scan session, but on a different day. Although we found an association between HPA-axis reactivity and neural FC, it will be important in future research to assess neural connectivity and cortisol reactivity concurrently. Recent empirical studies have documented that participants in MRI studies experience a range of stress in response to the scanner environment (Muehlhan et al., 2010), and it would be of interest to distinguish transient versus trait-like HPA-axis activation effects on FC.
Conclusion
In the present study we used an FC neuroimaging strategy to identify neural-systems level mechanisms corresponding to heightened HPA-axis activity in response to stress. Whole-brain analysis implicated significant FC differences that occurred almost exclusively in midline frontal cortical brain regions (e.g., Cg25, BA11; see Table 2) that have been found in previous studies to be associated both with mood and anxiety disorders and with physiological responses to stress. While investigations of functional abnormalities during task-provocation-based BOLD fMRI studies have provided insights about heightened HPA-axis responsivity, our data underscore the importance of using rs-fMRI to examine dynamic relations in neural systems that are not constrained by a particular type of stress-induced thought or activity. We think that this analysis captures unique aspects of the altered neurodevelopmental processes that can lead to maladaptive behaviors or represent a vulnerability to developing a psychiatric disorder. This result provides evidence that in early life, greater stress reactivity is associated with network-level neural patterns that may hinder the development of healthy emotional responsivity.
Supplementary Material
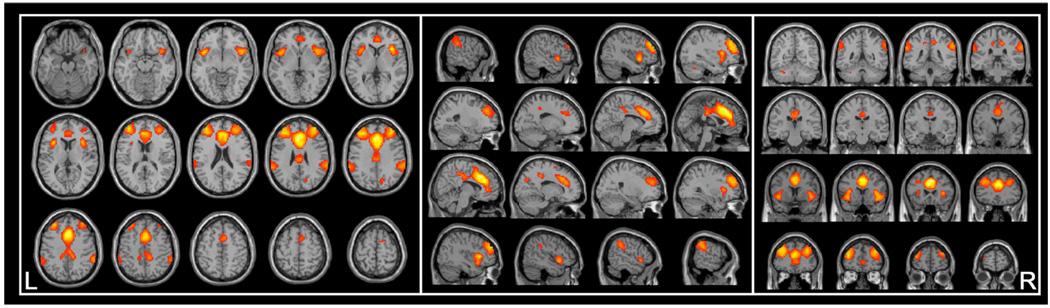
Figure 2.
Salience network (SN) functional connectivity across all study participants. Results of one-sample t-test are presented for values p < .001, FWE-corrected. Transverse, sagittal, and coronal slices of the SN are projected over a template brain to provide anatomical reference. L = left; R = right. Corresponding network peak coordinates are provided in Table 1.
Table 1.
Summary of regions that comprise the salience network of the brain in youth
| X | Y | Z | n voxels | T val | Z val | p val | ||
|---|---|---|---|---|---|---|---|---|
| Limbic/Frontal | ||||||||
| * Cingulate/MFG | L32/9 | −2 | 26 | 26 | 1906 | 28.25 | >7.5 | <0.001 |
| Subcortex/Frontal | ||||||||
| Insula/IFG | R13/47 | 39 | 18 | 5 | 250 | 19.5 | >7.5 | <0.001 |
| Insula/IFG | L13/47 | −40 | 11 | −6 | 192 | 18.94 | >7.5 | <0.001 |
| Parietal | ||||||||
| Inferior | R40 | 62 | −34 | 29 | 178 | 14.61 | >7.5 | <0.001 |
| Inferior | L40 | −64 | −37 | 33 | 145 | 13.72 | >7.5 | <0.001 |
| Precuneus | R7 | 15 | −70 | 35 | 15 | 9.71 | 7.19 | <0.001 |
| Cerebellum | ||||||||
| Culmen/Anterior Lobe | −30 | −60 | −26 | 15 | 8.8 | 6.76 | <0.001 |
secondary peaks with euclidian distance >30mm from the peak of this cluster were observed at −34, 43, 34 and 36, 43, 34, bilaeral BA9 MFG.
Coordinates are given in Talairach and Tournoux convention.
BA = Bromann’s area.
Acknowledgements
This project was supported by awards from the National Institute of Mental Health [MH081583 to MET, MH079651 to JPH, and MH074849 to IHG], and by NARSAD Young Investigator Awards to MET and to JPH. The authors thank Melissa Henry, Emily Dennis, and Rebecca Johnson, for their assistance in acquiring the scan data, and Yamanda Wright, and Hannah Burley for their assistance in participant recruitment, screening, and conducting structured behavioral interviews. The authors also thank Jutta Joormann, Ben Varasteh, and Michael Chen for consultation on cortisol analyses.
Abbreviations
- Cg25
subgenual cingulate cortex
- FC
functional connectivity
- fMRI
functional magnetic resonance imaging
- rs-fMRI
resting state functional magnetic resonance imaging
- HPA
hypothalamic-pituitary-adrenal
- ROI
region of interest
- ICA
independent components analysis
- SN
salience network
References
- Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, 3rd, Thayer JF, Kirschbaum C, Tranel D. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal of Comparative Neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart C, Jorgensen R, Suchday S, Chen E, Matthews K. Measuring stress resilience and coping in vulnerable youth: The social competence interview. Psychological Assessment. 2002;14:339–352. doi: 10.1037//1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Frontiers in Systems Neuroscience. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. European Journal of Neuroscience. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Geller B, Williams M, Zimerman B, Frazier J. WASH-U-KSADS (Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia) St. Louis (Missouri): Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, Delbello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science. 2008;17:159–163. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in Major Depressive Disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P. Synaptic Density in Human Frontal-Cortex - Developmental Changes and Effects of Aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biological Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Stocker T, Shah NJ, Amunts K, Kircher T, Schneider F, Habel U. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Joormann J, Gotlib IH. Emotion (Dys)regulation and Links to Depressive Disorders. Child Dev Perspect. 2008;2:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, Taylor SF. Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. The American Journal of Psychiatry. 2007;164:1250–1258. doi: 10.1176/appi.ajp.2007.06081367. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Mcginnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. The American Journal of Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clinics of North America. 2003;13:805–+. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, Mcginnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. The American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, Mcneely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C. The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol. 2010 doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Clinical Neurophysiology. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, Mcintosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004a;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004b;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchiteya BM, Lecours AR, Elie R, Lupien SJ. Impact of a unilateral brain lesion on cortisol secretion and emotional state: anterior/posterior dissociation in humans. Psychoneuroendocrinology. 2003;28:674–686. doi: 10.1016/s0306-4530(02)00050-1. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JD, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage. 2005;25:824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, Henry ML, Johnson RF, Thompson PM, Toga AW, Glover GH, Van Horn JD, Gotlib IH. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion Imaging, White Matter and Psychopathology. Palo Alto: 2011. [DOI] [PubMed] [Google Scholar]
- Uddin L, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Frontiers in Systems Neuroscience. 2010 doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophrenia Research. 2010;123:105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.