Abstract
A key organisational feature of the cerebellum is its division into a series of cerebellar modules. Each module is defined by its climbing input originating from a well-defined region of the inferior olive, which targets one or more longitudinal zones of Purkinje cells within the cerebellar cortex. In turn, Purkinje cells within each zone project to specific regions of the cerebellar and vestibular nuclei. While much is known about the neuronal wiring of individual cerebellar modules, their behavioural significance remains poorly understood. Here, we briefly review some recent data on the functional role of three different cerebellar modules: the vermal A module, the paravermal C2 module and the lateral D2 module. The available evidence suggests that these modules have some differences in function: the A module is concerned with balance and the postural base for voluntary movements, the C2 module is concerned more with limb control and the D2 module is involved in predicting target motion in visually guided movements. However, these are not likely to be the only functions of these modules and the A and C2 modules are also both concerned with eye and head movements, suggesting that individual cerebellar modules do not necessarily have distinct functions in motor control.
Keywords: Cerebellum, Cerebellar modules, Climbing fibres, Cerebellar nuclei, Purkinje cells
Introduction
The cerebellum has long been compartmentalised in order to aid the understanding of cerebellar function (for review see [1, 2]). In particular, a longitudinal organisation was first suggested by Jansen and Brodal [3] who divided the cerebellar cortex into lateral (hemispheral), intermediate (paravermal) and vermal compartments on the basis of their corticonuclear targets. This tripartite division of the cerebellum was based upon the finding that Purkinje cells in each longitudinal division project topographically to a distinct cerebellar nucleus, with the efferent connections of each nucleus, in turn, projecting to different descending pathways, thereby controlling different aspects of movement (both acquisition and execution). In brief, the vermal cortex projects preferentially to the fastigial (medial) and vestibular nuclei, the paravermal cortex to the interpositus nucleus (anterior and posterior subdivisions) and the lateral cortex to the dentate nucleus. That these different regions are to an extent functionally distinct was first suggested by Chambers and Sprague [4, 5] who determined the classes of motor deficits that occurred after different cortical lesions. For example, lesion of the vermal cortex and the fastigius nucleus resulted in severe disturbance of axial muscle control and balance, while ablation of the paravermal cortex and the underlying interpositus nucleus resulted in the impairment of voluntary, goal-directed movements and disturbances of the postural ‘base’ for such tasks.
While this serves as a useful overview of the basic principles of functional organisation of the cerebellum, it is now recognised that these three broad longitudinal compartments can be further subdivided into a series of smaller anatomical/functional units called ‘modules’ [6–8]. Cerebellar modules are highly conserved across many species implying similar function. Structurally, each module is defined by its climbing input originating from a circumscribed subdivision of the inferior olivary complex, which targets one or more longitudinal zones of Purkinje cells within the cerebellar cortex (see Fig. 1). In turn, Purkinje cells within each zone project to specific regions of the cerebellar and vestibular nuclei. Efferent neurones from these nuclei powerfully excite motor cell groups belonging to the medial and lateral descending motor paths. Purkinje cells therefore have a rather direct influence on activity in descending motor pathways [9].
Fig. 1.

Simplified block diagram of cerebellar modules. Each module is defined by its inferior olive climbing fibre input and Purkinje corticonuclear output. From the medial to the lateral plane (right to left in the figure) are shown: the A, AX, X, B and A2 zones (in the vermis), the C1, CX, C2 and C3 zones (in the paravermis), and the D1, D0 and D2 zones (in the hemisphere). Longitudinal zones in the paraflocculus and flocculus are not shown. Note that some longitudinal zones are not necessarily present in all cerebellar lobules in the adult animal (for example, the X and B zones). cMAO (subnuc a), subnucleus a of caudal medial accessory olive; cMAO (subnuc b), subnucleus b of caudal medial accessory olive; cMAO (subnuc b 1 /c), subnucleus b1 and c of caudal medial accessory olive; dfDAO, dorsal fold of dorsal accessory olive; DLH, dorsolateral hump; DLP, dorsolateral protuberance of medial nucleus; dlPO, dorsal lamella of the principal olive; dmPO, dorsomedial subnucleus of the principal olive; ICG, interstitial cell group; iMAO (lat), lateral part of intermediate medial accessory olive; iMAO (med), medial part of intermediate medial accessory olive; LVN, lateral vestibular nucleus; MedN (lat), lateral part of medial nucleus; MedN (med), medial part of medial nucleus; NIA, nucleus interpositus anterior; NIP, nucleus interpositus posterior; NL, lateral nucleus; PML, paramedian lobule; rMAO, rostral medial accessory olive; vfDAO, ventral fold of dorsal accessory olive; vlPO, ventral lamella of the principal olive. Adapted from [7]
Many regard modules as a fundamental feature of cerebellar contributions to motor control (and indeed other functions, see for example [10, 11]), and it is now generally accepted that investigations of cerebellar function can be framed usefully in terms of their organisation [2, 6, 12]. However, despite detailed knowledge of the neuronal wiring of individual olivo-cortico-nuclear modules and their recurrent connections (for reviews see [13, 14]), the functional significance of these relationships remains far from clear. The aim therefore of this short review is to consider some of the more recent evidence that cerebellar modules have differing behavioural significance. We will focus on three modules, each located in a different cerebellar compartment: the vermal A module, the paravermal C2 module and the lateral D2 module, and with an emphasis on the olivo-cerebellar climbing fibre system.
A Module
The A module extends over the entire rostrocaudal length of the cerebellar vermis and is defined by its climbing fibre input originating from the caudal half of the medial accessory olive (caudal MAO) and its Purkinje cell corticonuclear projections to the fastigial nucleus. The A module includes what is commonly referred to as the ‘oculomotor vermis’, lobules VI and VII of the posterior lobe [15]. The caudal MAO primarily receives inputs from ascending somatic sensory pathways including direct inputs from the spinal cord, dorsal column, trigeminal, vestibular, optokinetic and tectal nuclei (see [16] for a review). Outputs from the fastigial nucleus involve connections with both ascending and descending motor pathways, with the former including terminations in the contralateral superior colliculus [17] and visual structures of the midbrain, and the latter including terminations in the vestibular nuclei and pontomedullary reticular formation [18–23]. Mossy fibre inputs to the A module include the pontine nuclei and the nucleus reticularis tegmenti pontis (NRTP) which in turn receive afferents from amongst other structures, the superior colliculus, pretectum, nucleus of the optic tract, and subcortical visual and oculomotor centres (see [19] for references, [24]).
Given the pattern of precerebellar sources and output targets of the A module, its function is likely to be involved in the control and regulation of posture and balance as well as head and eye movements (see [25] for a review). Electrophysiological studies support such a tenet: Purkinje cells in the oculomotor vermis discharge both simple spikes and complex spikes in relation to eye movements and head rotation whereas microstimulation evokes eye movements ([26–34], see [35] for a review). Chemical and mechanical lesions of the vermis and fastigial nucleus in monkeys have been shown to produce disturbances in balance and deficits in eye and head movements [36–41]. Similarly, in cats, inactivation of caudal MAO or fastigius severely impairs balance, head and trunk control with little or no deficits on voluntary limb movements such as reaching and grasping [42, 43]. In humans, focal lesions to the cerebellar vermis causes balance impairments [44, 45] and disturbances to smooth pursuit eye movements (e.g. [46]). The deficits produced by inactivation or lesion studies in both animals and humans are thus in agreement with the anatomical evidence suggesting a role for the A module in balance, head and eye movement control.
Surgical or localised delivery of pharmacological agents to induce lesions are unlikely, however, to be confined exclusively to discrete parts of a complex-shaped nucleus such as the inferior olive without concurrent disturbance of neighbouring structures or fibres of passage. An alternative approach to study the function of a whole module without affecting its neighbouring modules is by cortical injection of the retrogradely transported neurotoxin cholera toxin B conjugated to saporin [47]. A related method is a systemic treatment with neurotoxins: Llinas and co-workers [48] found that rats treated intraperitoneally with the pharmacological agent 3-acetylpyridine (3AP) resulted in destruction of the inferior olive and caused ataxic behavioural disturbances, although some recovery of motor competence was observed after the initial acute loss. Nevertheless, even 6 months after the administration of 3AP, the animals' movements remained sluggish and a distinctive gait persisted, termed ‘mud-walking’ (characterised by exaggerated flexion of the limbs and an abnormal shift of body weight from one side to the other).
Removal of the olivo-cerebellar climbing fibre projection either acutely or chronically is also known to have a profound influence on Purkinje cell activity, causing simple spikes to exhibit highly abnormal firing patterns (e.g. [49–53]), which may explain the severe motor deficits that occur after the inferior olive is damaged. Global removal of the olivo-cerebellar projection therefore demonstrates that climbing fibre inputs to cerebellar modules are critical for normal cerebellar operation. However, an important limitation of 3AP is that it has been shown to cause the degeneration of neurones and fibres in areas of the brain besides the olive, including the nucleus ambiguus, hypoglossal nuclei, substantia nigra, dorsal motor nucleus X (see [54]), even when used in conjunction with the antidote nicotinamide which, when administered 4.5 h following 3AP, limits its CNS exposure and thus restricts the extent of neuronal degeneration [54, 55]. Thus, the motor deficits observed with the use of 3AP may occur as a result not only of the removal of climbing fibre input to multiple, if not all cerebellar modules, but also due to neuronal degeneration in other brain structures that are implicated in motor function.
A modified 3AP plus nicotinamide protocol in rats, whereby nicotinamide is administered 3.5 h after 3AP treatment (3AP + 3.5 h) [56, 57] has been used in our laboratory in order to produce a subtotal lesion of the inferior olive that causes substantial degeneration in all parts of the olive but spares cells in the caudal MAO, the source of climbing fibres that target the A module, and with little or no neuronal degeneration in other CNS structures (Fig. 2a). Behavioural studies were then carried out to assess the functional responsibilities of the A module in 3AP + 3.5-h-treated animals during spontaneous motor activity and motor performance. Gait analysis of stepping movements revealed that both fore- and hindlimb stride lengths were significantly reduced in treated animals compared to control animals following treatment (Fig. 3a, b). In tests of motor performance which included a beam-walking task (which requires a high degree of balance, interlimb coordination and accuracy of foot placement), 3AP + 3.5-h-treated animals produced a greater number of foot slips and falls when compared to control animals (Fig. 3c). In contrast, in a vertical-hold test, whereby the animal had to cling to a vertical grid for a maximum period of 2 min, 3AP + 3.5-h-treated animals performed as well as control animals (Fig. 3d). Thus, a subtotal lesion of the olive which preserves climbing fibre input to the A module but deprives climbing fibre input to all other regions of the cortex indicates that modules located in the paravermis and lateral cerebellum may play an important role in interlimb coordination, but not grip strength. However, since beam walking requires a high degree of balance and interlimb coordination, further experiments will be needed to determine whether the deficits in performance are due to one or a combination of these two possibilities. Moreover, whether the A module can perform its normal function independently of other affected modules remains to be determined, and, given the predicted role of the A module in eye and head movements, it remains to be determined whether such movements are normal after 3AP + 3.5 treatment. Similarly, it will also be of interest to determine whether learning of new motor skills related to eye and head movements is retained after 3AP + 3.5 treatment, whilst motor learning related to other types of behaviour is impaired.
Fig. 2.
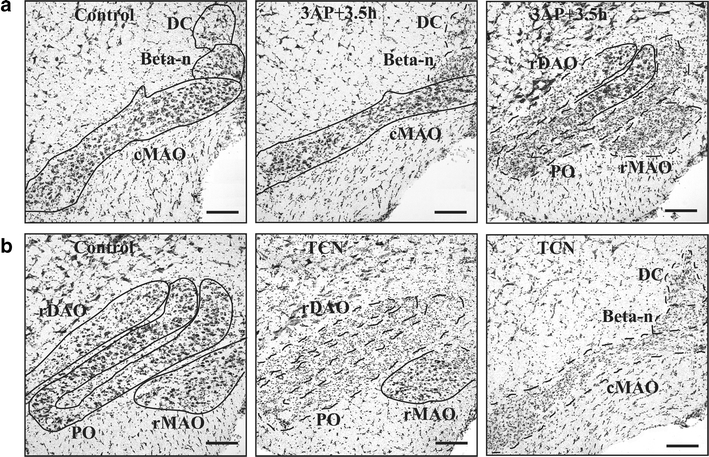
a Sites of neuronal survival identified using the Nissl stain cresyl violet in caudal interior olive in a control rat (left hand panel), cMAO of 3AP + 3.5 h animal (middle panel) and rMAO of 3AP + 3.5 h animal (right hand panel) 12 days after treatment. Olivary regions where there is an extensive loss of cells are delineated by a dashed line, while regions containing surviving cells are delineated by a solid line. b Same as a but from a TCN animal (12 days after treatment). cDAO and rDAO, caudal and rostral subdivisions of the dorsal accessory olive; cMAO and rMAO, caudal and rostral subdivisions of the medial accessory olive; PO, principal olive. Scale bars, 100 μm. Adapted from [56]
Fig. 3.
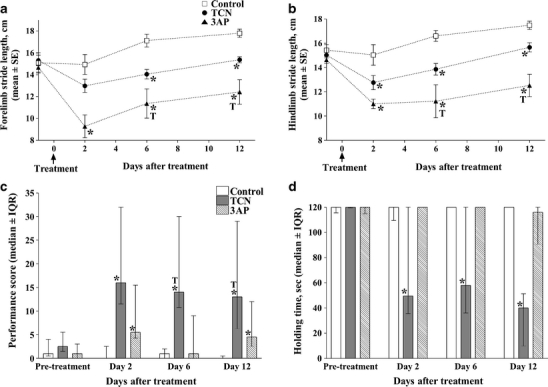
a Effects of TCN and 3AP + 3.5 h on forelimb stride length at different time points before (day 0) and after treatment. Data points represent mean±SEM. b Effects of TCN and 3AP + 3.5 h on hindlimb stride length at different time points before (day 0) and after treatment. Data points represent mean±SEM. c Effects of TCN and 3AP + 3.5 h on performance scores in the beam-walking test. Bars represent the median ± interquartile ranges. A higher score means a greater difficulty in performing the task. Animals were tested before (pretreatment) and at different time points after treatment at days 2, 6 and 12. Asterisk indicates a statistically significant difference from control (P < 0.05, Mann—Whitney U test). d Effects of TCN and 3AP + 3.5 h on holding time in the vertical grid test. Bars represent the median ± interquartile ranges of the holding time. On each day of assessment the test was carried out for a maximum time period of 120 s. Animals were tested before (pretreatment) and at different time points after treatment at days 2, 6 and 12. Asterisk indicates a statistically significant difference from control (P < 0.05, Mann—Whitney U-test). Adapted from [56]
C2 Module
The posterior division of the nucleus interpositus (NIP) is innervated by the entire rostrocaudal extent of the paravermal cerebellar cortex-designated C2 which receives its climbing input from the rostral half of MAO [8, 18, 58, 59]. The flocculus and paraflocculus, located most laterally in the cerebellar hemispheres, also contain a C2 module with climbing fibre input from rostral MAO and Purkinje cell output to NIP (see [19] for a review). The rostral MAO not only receives afferents mainly from telencephalic and diencephalic brain regions, but also indirect projections from the spinal cord (see [16] for a review), while the majority of efferents from NIP ascend to innervate the red nucleus [60, 61], superior colliculus and thalamus [41, 62]. Mossy fibre afferents to the C2 module include pontine nuclei, NRTP, lateral reticular nuclei, oculomotor and vestibular centres (see [19] for references, [63, 64]), as well as somatosensory information from the periphery by way of the spinal cord, dorsal column nuclei, trigeminal nuclei and lateral reticular nucleus (for review, see [24]).
In light of these widespread input and output connections, it is perhaps unsurprising that the functional role of the C2 module is not well understood. Stimulation of Purkinje cells within the C2 module of the flocculus produces movement of the head and eyes indicating that this module may play a role in head orientation and gaze control [65]. However, single-unit recordings from Purkinje cell simple spikes in the paravermal C2 module and their target nuclear neurones in NIP have also been shown to be related to limb movements [66–73]. Electrical stimulation of the NIP in cats [74–76] and primates [77, 78] leads to contractions most often of flexor muscles of the shoulder and limbs, as well as muscle twitches and movements of the eyes, face and neck. In cats, inactivation of the NIP affects the performance and timing (but not learning) of eyelid conditioned responses (reviewed in [79]). Chambers and Sprague (1955) showed during locomotion in cats that interpositus lesions led to limb hypoflexions while lesions of the overlying cerebellar cortex led to hyperflexions presumably, by reducing the inhibitory drive from the overlying Purkinje cells (see also [80]). Moment-to-moment control of voluntary limb movements also becomes profoundly impaired with lesions or inactivation of NIP: reaches become inaccurate and more variable [41, 42, 81, 82]. Also reversible inactivation of rostral MAO disrupts reaching and locomotion in cats [43]. Therefore, it seems that as well as a role in eye and head control, the C2 module is also likely to be involved in the control of limb movements. These data also raise the possibility that parts of the same module located in different regions of the cerebellar cortex may subserve different functions.
In the same way that 3AP is a useful tool for studying the function of the A module, intraperitoneal injection of the neurotoxic agent trans-crotononitrile (TCN) produces a subtotal lesion of the inferior olive sparing cells located in rostral MAO, the source of climbing fibres to the C2 module [56] (Fig. 2b). Behavioural studies to assess the role of the C2 module during spontaneous behaviour revealed that similar to 3AP + 3.5 h treatment, fore- and hindlimb stride lengths during gait analysis were significantly reduced when compared to control animals (Fig. 3a, b). However, TCN-treated animals produced a greater number of foot slips and falls in the beam-walking test compared to control animals and 3AP + 3.5-h-treated animals (Fig. 2c). Also, by contrast to 3AP-treated animals, TCN-treated animals displayed a reduction in holding time during the vertical-hold task for all time points tested (Fig. 2d) and a significant increase in activity in an open-field test. Thus, in a number of respects TCN- and 3-AP + 3.5-h-treated animals display significant differences in behavioural deficits but, generally speaking, TCN produces more profound motor deficits than 3AP + 3.5-h-treated animals. This is presumably because the integrity of the A module in 3AP-treated animals affords greater control of axial musculature that underpins most of the behavioural tests examined. Furthermore, the question of whether the C2 module can operate independently of other modules is unknown. Since the C2 module is implicated in the control of goal-directed reaching, it also remains to be determined whether such movements and the acquisition of related motor skills are conserved in TCN-treated animals. Similarly, given the predicted role of the C2 module in eye and head movements, to what extent are such movements preserved after TCN treatment?
D2 Module
The lateral or hemispheral compartment of the cerebellum is by far the largest part of the human cerebellum (accounting for about 90% of its size) yet the least is known about its function, particularly in relation to individual modules. It is clear however that the lateral cerebellum plays an important role in the control of complex visually guided movements, emphasised by the profound deficit in visuomotor performance that occurs when this region of the cerebellum is damaged (e.g. [83–86]). The lateral cerebellum serves as the main link between visual to motor areas of the brain (reviewed in [87]). Accordingly, visual inputs from cortical extrastriate visual areas and subcortical visual structures are routed via the pontine nuclei and terminate in the lateral cerebellum as mossy fibres, whilst information from the pretectum project via the inferior olive to terminate in the lateral cerebellar cortex as climbing fibres (e.g. [88–93]). Human functional imaging studies to identify brain areas related to the control of visually guided movements have also demonstrated that the lateral areas of the cerebellum show haemodynamic changes preferentially during visually guided movements [94, 95]. This is consistent with demonstrations that neurones in the lateral cerebellar cortex [88, 96–101] and its output nucleus, dentate [102, 103] responds to visual events such as a flash of light and to the velocity and direction of a moving target during guided limb movements. Cooling [104, 105] and pharmacological inactivation of the dentate nucleus [106–108] or overlying cerebellar cortex [109] in monkeys trained to perform goal-directed movements have also been important in demonstrating the role of the lateral cerebellum in visuomotor integration: inactivation causes a loss of control on the displacement, velocity and acceleration of ipsilateral limb movements as well as impairments in visually guided tracking associated with a prolongation of visually triggered reaction times. Given that the dentate nucleus has major connections with the parvocellular red nucleus [18, 110–112] and via the thalamus with motor and premotor areas of the cerebral cortex [113, 114], these impairments may arise because cerebellar target neurones in the motor cortex are no longer modulated by visual information regarding the direction or speed of a target or limb movement. As a consequence, the correct motor programme may not be selected [87, 115].
The D2 module is one of the cerebellar modules located in the lateral cerebellum, and like the A and C2 modules extends over the entire rostrocaudal extent of the cerebellum. The D2 module receives its climbing fibre input from the dorsal lamella/lateral bend of the principal olive and its target nucleus is the rostromedial subdivision of dentate [116, 117]. At present, there is no neurotoxin available that selectively preserves climbing fibre input to the D2 module, so it is not possible to study the behavioural deficits that arise from such a subtotal lesion of the olive. Instead, we have investigated the function of this module by recording the spike trains of individual Purkinje cells during motor performance in chronically instrumented cats (for details of the methods see [118, 119]). The findings from our initial studies support the view that the lateral cerebellum is intimately involved in visually guided movement, since Purkinje cell simple spike activity was found to precisely signal visual events, and encode target motion during visually guided reaching [119].
However, the tonically altered simple spike activity that occurred during a persistent visual stimulus that moved in a predictable way across the animal's field of view may reflect either direct sensory activation or the operation of an ‘internal model’. The latter because an increasing body of evidence [115, 120–127] suggests that an important aspect of cerebellar contributions to motor control is through the operation of internal simulations of movements. Internal models are a neural representation of one's body and the external world and are thought to help perform movement smoothly and accurately through prediction without the need for continuous sensory feedback [109, 128–131].
To identify whether the neuronal activity in the lateral cerebellum previously found to be related to target movement represents the active operation of an internal model closely simulating target motion, single-unit recordings were made from Purkinje cells in the D2 module in cats trained to perform a predictable visually guided reaching task [118]. The localization of the recording sites in the D2 module was determined at the end of the experiment by injecting retrograde tracer into the cortical area where the Purkinje-cell recordings were made and mapping (post-mortem) the location of labelled cells in the contralateral inferior olive- labelled cells were located within the dorsal lamella/lateral bend of the principal olive, thus confirming that the Purkinje-cell recordings were mainly within the D2 module (Fig. 4a).
Fig. 4.
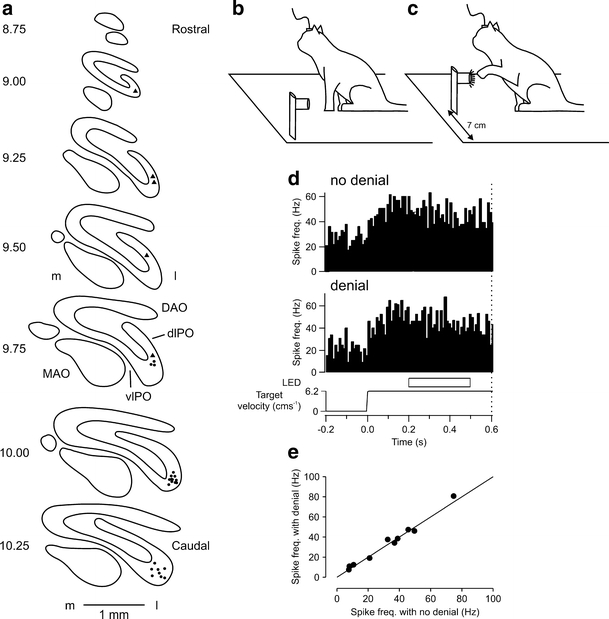
a Distribution of retrogradely labelled olive cells after a tracer injection of red latex microspheres was made into crus I coinciding with the parts of the cerebellar cortex where most of the microelectrode tracks were made. Equally spaced standard transverse outlines of the inferior olive between AP levels 10.25 and 8.75. Each circle and triangle corresponds to one retrogradely labelled cell in Cat P and Cat F, respectively. DAO, dorsal accessory olive; dLPO, dorsal lamella of the principal olive; l, lateral; m, medial; MAO, medial accessory olive; PF, primary fissure; vlPO, ventral lamella of the principal olive. b Schematic diagram of behavioural task. Cats were trained to perform a visually guided reaching task in the dark in which a tube, dimly lit by a ring of LEDs and containing a food reward was initially stationary 7 cm to the left of centre. c In a ‘go’ trial, the tube started to move horizontally in the rightwards direction at a constant velocity of 6.2 cm s−1. After an interval of approximately 600 ms after the commencement of target motion, the LEDs brightened to cue the animal to make a reach with its left forelimb, ipsilateral to the cerebellar recording, to retrieve a food reward from the tube. d Peri-event time histogram showing an example Purkinje cell which displayed a tonic increase in simple spike activity in relation to target motion. In one half of the trials, the moving target disappeared for 300 ms during target motion. Target denial occurred 200 ms after the onset of target motion. Dotted vertical line at 0.6 s represents ‘go’ signal. e Comparison of responses during target denial with no denial control. No significant change (paired t test, p > 0.05, n = 10). Line represents unity. Adapted from [118]
In the Purkinje-cell recording stage of the experiment, cats were trained to reach (after receipt of a ‘go’ signal) into a moving visual target travelling in a predictable fashion. The target for reach consisted of a hollow Perspex tube dimly lit by a ring of LEDs. Experiments were conducted without ambient illumination in a light-proof room. Thus, the only source of visual information available to the cat was from the target LEDs. The tube was initially stationary to the left of centre (as viewed by the cat) at a comfortable height for reaching (Fig. 4b). The tube then moved at a constant velocity rightwards across the cat's visual field (Fig. 4c). At various stages of the target's motion, illumination of the ring of LEDs around the tube was temporarily extinguished during which time the animal was in total darkness.
Purkinje cells that displayed tonic simple spike activity during movement of the target maintained their tonic activity when the cat's view of the target was occluded during the transient extinction of the target LEDs (Fig. 4d). Since the simple spike activity of the same Purkinje cells could not be correlated to eye or limb movements, and the target was familiar and moved in a predictable fashion, it was concluded that a model of target movement had been constructed which predicts the target's velocity and position and thereby maintains neural activity in the absence of sensory inputs. Such a mechanism is likely to be important for movement planning and control during the interception of a moving object, and may explain the profound deficit in visuomotor performance that results from cerebellar injury of the lateral cerebellum [83, 84, 109, 132, 133]. Outstanding questions include: to what extent is the D2 module dedicated to specific internal models? For example, are internal models of other types of predictable movement also present in the same module (e.g. an object falling under gravity, [134]), and is the same internal model present in more than one cerebellar module? Do the output neurones in dentate also show activity consistent with an internal model? And given the role of the cerebellum in learning, how is an internal model acquired and can it be modified?
Concluding Comment
This short review summarises some recent studies that have sought to gain further insight into the behavioural significance of individual cerebellar modules. Whilst such studies are still in their infancy, the use of new pharmacological tools has provided evidence that the A and C2 modules located in the vermis and paravermis, respectively, have some differences in function, e.g. the A module is concerned with balance and the postural base for voluntary movements, while the C2 module is concerned more with limb coordination. This is not to imply that these are the only functions of these modules. Indeed, the available evidence suggests that they may also have some responsibilities in common—notably a shared or perhaps complementary role in the control of eye and head movements. In addition, Purkinje-cell recordings in the lateral cerebellar D2 module have provided evidence of the operation of an internal model associated with visuomotor control. But it remains to be determined how specific such a function is to this particular module. Thus, while individual modules play a role in particular aspects of motor control, their functions may be overlapping rather than entirely distinct. Caution is therefore needed when devising experiments which seek to study the role of a particular cerebellar module in relation to a specific behaviour, since more than one module is likely to be involved.
Acknowledgements
NLC is supported by a Wellcome grant held jointly by NLC and RA.
Conflict of interest
We declare that there are no conflicts of interest with this submission.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21(9):370–5. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. The cerebellum and neural control. New York: Raven; 1984. [Google Scholar]
- 3.Jansen J, Brodal A. Experimental studies on the intrinsic fibres of the cerebellum: II. The corticonuclear projection. J Comp Neurol. 1940;73:267–321. [Google Scholar]
- 4.Chambers WW, Sprague JM. Functional localization in the cerebellum II. Somatotopic organization in the cortex and nuclei. Arch Neurol Psychiat. 1955;74:653–80. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- 5.Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955;103(1):105–29. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- 6.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 7.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10(9):670–81. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 8.Voogd J, Bigare F. Topographical distribution of olivary and cortico nuclear fibers in the cerebellum: a review. In: Courville J, editor. The inferior olivary nuclues. New York: Raven; 1980. pp. 207–37. [Google Scholar]
- 9.Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26(4):273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 11.Schmahmann JD. Rediscovery of an early concept. International review of neurobiology. San Diego: Academic; 1997. pp. 2–35. [DOI] [PubMed] [Google Scholar]
- 12.Garwicz M, Ekerot CF, Jorntell H. Organizational principles of cerebellar neuronal circuitry. News Physiol Sci. 1998;13:26–32. doi: 10.1152/physiologyonline.1998.13.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Ruigrok TJ. Cerebellar nuclei: the olivary connection. Prog Brain Res. 1997;114:167–92. doi: 10.1016/s0079-6123(08)63364-6. [DOI] [PubMed] [Google Scholar]
- 14.Ruigrok TJH. Ins and outs of cerebellar modules. Cerebellum. 2010. doi:10.1007/s12311-010-0164-y. [DOI] [PMC free article] [PubMed]
- 15.Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2009. doi:10.1016/j.cortex.2009.09.001. [DOI] [PubMed]
- 16.Brodal A, Kawamura K. Olivocerebellar projection: a review. Adv Anat Embryol Cell Biol. 1980;64:1–140. [PubMed] [Google Scholar]
- 17.Angaut P, Bowsher D. Ascending projections of the medial cerebellar (fastigial) nucleus: an experimental study in the cat. Brain Res. 1970;24(1):49–68. doi: 10.1016/0006-8993(70)90273-8. [DOI] [PubMed] [Google Scholar]
- 18.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–72. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- 19.Voogd J, Barmack NH. Oculomotor cerebellum. Prog Brain Res. 2006;151:231–68. doi: 10.1016/S0079-6123(05)51008-2. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto T, Mizuno N, Nomura S, Nakamura Y. Fastigio-olivary fibers in the cat as revealed by the autoradiographic tracing method. Brain Res. 1980;199(2):443–6. doi: 10.1016/0006-8993(80)90701-5. [DOI] [PubMed] [Google Scholar]
- 21.Dietrichs E, Walberg F. The cerebellar nucleo-olivary projection in the cat. Anat Embryol. 1981;162(1):51–67. doi: 10.1007/BF00318094. [DOI] [PubMed] [Google Scholar]
- 22.Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res. 1983;286(3):299–322. doi: 10.1016/0165-0173(83)90017-6. [DOI] [PubMed] [Google Scholar]
- 23.Voogd J. The cerebellum of the cat. Structure and fibre connexions. Assen: Van Gorcum; 1964. [Google Scholar]
- 24.Ruigrok TJ. Precerebellar nuclei and red nucleus. In: Paxinos G, editor. The Rat Nervous System. 3. San Diego: Elsevier Academic; 2004. pp. 167–204. [Google Scholar]
- 25.Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- 26.Krauzlis RJ, Miles FA. Role of the oculomotor vermis in generating pursuit and saccades: effects of microstimulation. J Neurophysiol. 1998;80(4):2046–62. doi: 10.1152/jn.1998.80.4.2046. [DOI] [PubMed] [Google Scholar]
- 27.Noda H, Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol. 1987;57(5):1247–61. doi: 10.1152/jn.1987.57.5.1247. [DOI] [PubMed] [Google Scholar]
- 28.Ron S, Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973;36(6):1004–22. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- 29.Kase M, Noda H, Suzuki DA, Miller DC. Target velocity signals of visual tracking in vermal Purkinje cells of the monkey. Science. 1979;205(4407):717–20. doi: 10.1126/science.111350. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis [corrected] Exp Brain Res. 1992;88(2):455–8. doi: 10.1007/BF02259122. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki DA, Keller EL. Vestibular signals in the posterior vermis of the alert monkey cerebellum. Exp Brain Res. 1982;47(1):145–7. doi: 10.1007/BF00235896. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. II. Target velocity-related Purkinje cell activity. J Neurophysiol. 1988;59(1):19–40. doi: 10.1152/jn.1988.59.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. J Neurophysiol. 1988;59(1):1–18. doi: 10.1152/jn.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki DA, Noda H, Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol. 1981;46(5):1120–39. doi: 10.1152/jn.1981.46.5.1120. [DOI] [PubMed] [Google Scholar]
- 35.Leigh RJ, Zee DS. The neurology of eye movements. New York: Oxford University Press; 1999. [Google Scholar]
- 36.Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol. 2004;92(6):3351–67. doi: 10.1152/jn.01199.2003. [DOI] [PubMed] [Google Scholar]
- 37.Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70(5):1741–58. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- 38.Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiol. 1997;78(2):848–59. doi: 10.1152/jn.1997.78.2.848. [DOI] [PubMed] [Google Scholar]
- 39.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80(4):1911–31. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- 40.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83(4):2047–62. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 41.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 42.Martin JH, Cooper SE, Hacking A, Ghez C. Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol. 2000;83(4):1886–99. doi: 10.1152/jn.2000.83.4.1886. [DOI] [PubMed] [Google Scholar]
- 43.Horn KM, Pong M, Gibson AR. Functional relations of cerebellar modules of the cat. J Neurosci. 2010;30(28):9411–23. doi: 10.1523/JNEUROSCI.0440-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Ann Neurol. 1998;44(4):601–10. doi: 10.1002/ana.410440405. [DOI] [PubMed] [Google Scholar]
- 45.Dichgans J, Diener HC. Clinical evidence for functional compartmentalization of the cerebellum. In: Bloedel JR, Dichgans J, Precht W, editors. Cerebellar functions. Berlin: Springer; 1984. pp. 126–47. [Google Scholar]
- 46.Baier B, Stoeter P, Dieterich M. Anatomical correlates of ocular motor deficits in cerebellar lesions. Brain. 2009;132(Pt 8):2114–24. doi: 10.1093/brain/awp165. [DOI] [PubMed] [Google Scholar]
- 47.Pijpers A, Winkelman BH, Bronsing R, Ruigrok TJ. Selective impairment of the cerebellar C1 module involved in rat hind limb control reduces step-dependent modulation of cutaneous reflexes. J Neurosci. 2008;28(9):2179–89. doi: 10.1523/JNEUROSCI.4668-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llinas R, Walton K, Hillman DE, Sotelo C. Inferior olive: its role in motor learing. Science. 1975;190(4220):1230–1. doi: 10.1126/science.128123. [DOI] [PubMed] [Google Scholar]
- 49.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci. 2004;24(19):4510–7. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187(1):3–27. doi: 10.1016/0006-8993(80)90491-6. [DOI] [PubMed] [Google Scholar]
- 51.Montarolo PG, Raschi F, Strata P. On the origin of the climbing fibres of the cerebellar cortex. Pflugers Arch. 1980;383(2):137–42. doi: 10.1007/BF00581874. [DOI] [PubMed] [Google Scholar]
- 52.Savio T, Tempia F. On the Purkinje cell activity increase induced by suppression of inferior olive activity. Exp Brain Res. 1985;57(3):456–63. doi: 10.1007/BF00237832. [DOI] [PubMed] [Google Scholar]
- 53.Demer JL, Echelman DA, Robinson DA. Effects of electrical stimulation and reversible lesions of the olivocerebellar pathway on Purkinje cell activity in the flocculus of the cat. Brain Res. 1985;346(1):22–31. doi: 10.1016/0006-8993(85)91090-x. [DOI] [PubMed] [Google Scholar]
- 54.Balaban CD. Central neurotoxic effects of intraperitoneally administered 3-acetylpyridine, harmaline and niacinamide in Sprague-Dawley and Long-Evans rats: a critical review of central 3-acetylpyridine neurotoxicity. Brain Res. 1985;356(1):21–42. doi: 10.1016/0165-0173(85)90017-7. [DOI] [PubMed] [Google Scholar]
- 55.Desclin JC, Escubi J. Effects of 3-acetylpyridine on the central nervous system of the rat, as demonstrated by silver methods. Brain Res. 1974;77(3):349–64. doi: 10.1016/0006-8993(74)90627-1. [DOI] [PubMed] [Google Scholar]
- 56.Seoane A, Apps R, Balbuena E, Herrero L, Llorens J. Differential effects of trans-crotononitrile and 3-acetylpyridine on inferior olive integrity and behavioural performance in the rat. Eur J Neurosci. 2005;22(4):880–94. doi: 10.1111/j.1460-9568.2005.04230.x. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe Y, Kinoshita K, Koguchi A, Yamamura M. A new method for evaluation of motor deficits in 3-acetylpyridine-treated rats. J Neurosci Methods. 1997;77(1):25–9. doi: 10.1016/s0165-0270(97)00104-0. [DOI] [PubMed] [Google Scholar]
- 58.Pardoe J, Apps R. Structure-function relations of two somatotopically corresponding regions of the rat cerebellar cortex: olivo-cortico-nuclear connections. Cerebellum. 2002;1(3):165–84. doi: 10.1080/14734220260418402. [DOI] [PubMed] [Google Scholar]
- 59.Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum. 2. the C2 zone. Exp Brain Res. 1998;118(3):316–30. doi: 10.1007/s002210050286. [DOI] [PubMed] [Google Scholar]
- 60.Faull RL, Carmen JB. The cerebellofugal projections in the brachium conjuntivum of the rat. I. The contralateral ascending pathway. J Comp Neurol. 1978;178(3):495–518. [PubMed] [Google Scholar]
- 61.Teune TM, van der Burg J, Ruigrok TJ. Cerebellar projections to the red nucleus and inferior olive originate from separate populations of neurons in the rat: a non-fluorescent double labeling study. Brain Res. 1995;673(2):313–9. doi: 10.1016/0006-8993(94)01431-g. [DOI] [PubMed] [Google Scholar]
- 62.Horne MK, Butler EG. The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog Neurobiol. 1995;46(2–3):199–213. [PubMed] [Google Scholar]
- 63.Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJ. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26(46):12067–80. doi: 10.1523/JNEUROSCI.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pijpers A, Ruigrok TJ. Organization of pontocerebellar projections to identified climbing fiber zones in the rat. J Comp Neurol. 2006;496(4):513–28. doi: 10.1002/cne.20940. [DOI] [PubMed] [Google Scholar]
- 65.De Zeeuw CI, Koekkoek SK. Signal processing in the C2 module of the flocculus and its role in head movement control. Prog Brain Res. 1997;114:299–320. doi: 10.1016/s0079-6123(08)63371-3. [DOI] [PubMed] [Google Scholar]
- 66.Apps R, Lidierth M, Armstrong DM. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. J Physiol. 1990;424:487–512. doi: 10.1113/jphysiol.1990.sp018079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong DM, Edgley SA. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984;351:411–32. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibson AR, Horn KM, Stein JF, Van Kan PL. Activity of interpositus neurons during a visually guided reach. Can J Physiol Pharmacol. 1996;74(4):499–512. [PubMed] [Google Scholar]
- 69.Harvey RJ, Porter R, Rawson JA. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979;297:559–80. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JH, Wang JJ, Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987;57(3):787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- 71.Thach WT, Perry JG, Kane SA, Goodkin HP. Cerebellar nuclei: rapid alternating movement, motor somatotopy, and a mechanism for the control of muscle synergy. Rev Neurol (Paris) 1993;149(11):607–28. [PubMed] [Google Scholar]
- 72.van Kan PL, Horn KM, Gibson AR. The importance of hand use to discharge of interpositus neurones of the monkey. J Physiol. 1994;480(Pt 1):171–90. doi: 10.1113/jphysiol.1994.sp020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Kan PL, Houk JC, Gibson AR. Output organization of intermediate cerebellum of the monkey. J Neurophysiol. 1993;69(1):57–73. doi: 10.1152/jn.1993.69.1.57. [DOI] [PubMed] [Google Scholar]
- 74.Asanuma H, Hunsperger RW. Functional significance of projection from the cerebellar nuclei to the motor cortex in the cat. Brain Res. 1975;98(1):73–92. doi: 10.1016/0006-8993(75)90510-7. [DOI] [PubMed] [Google Scholar]
- 75.Giuffrida R, Li Volsi G, Panto MR, Perciavalle V, Sapienza S, Urbano A. Single muscle organization of interposito-rubral projections. Exp Brain Res. 1980;39(3):261–7. doi: 10.1007/BF00237115. [DOI] [PubMed] [Google Scholar]
- 76.Jimenez-Diaz L, Navarro-Lopez Jde D, Gruart A, Delgado-Garcia JM. Role of cerebellar interpositus nucleus in the genesis and control of reflex and conditioned eyelid responses. J Neurosci. 2004;24(41):9138–45. doi: 10.1523/JNEUROSCI.2025-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rispal-Padel L, Cicirata F, Pons C. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio) Exp Brain Res. 1982;47(3):365–80. doi: 10.1007/BF00239355. [DOI] [PubMed] [Google Scholar]
- 78.Schultz W, Montgomery EB, Jr, Marini R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain. 1979;102(1):127–46. doi: 10.1093/brain/102.1.127. [DOI] [PubMed] [Google Scholar]
- 79.Delgado-Garcia JM, Gruart A. Building new motor responses: eyelid conditioning revisited. Trends Neurosci. 2006;29(6):330–8. doi: 10.1016/j.tins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Udo M, Matsukawa K, Kamei H, Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980;44(1):119–34. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]
- 81.Milak MS, Shimansky Y, Bracha V, Bloedel JR. Effects of inactivating individual cerebellar nuclei on the performance and retention of an operantly conditioned forelimb movement. J Neurophysiol. 1997;78(2):939–59. doi: 10.1152/jn.1997.78.2.939. [DOI] [PubMed] [Google Scholar]
- 82.Uno M, Kozlovskaya IB, Brooks VB. Effects of cooling interposed nuclei on tracking-task performance in monkeys. J Neurophysiol. 1973;36(6):996–1003. doi: 10.1152/jn.1973.36.6.996. [DOI] [PubMed] [Google Scholar]
- 83.Liu X, Ingram HA, Palace JA, Miall RC. Dissociation of ‘on-line’ and ‘off-line’ visuomotor control of the arm by focal lesions in the cerebellum and brainstem. Neurosci Lett. 1999;264(1–3):121–4. doi: 10.1016/s0304-3940(99)00165-2. [DOI] [PubMed] [Google Scholar]
- 84.Classen J, Kunesch E, Binkofski F, Hilperath F, Schlaug G, Seitz RJ, et al. Subcortical origin of visuomotor apraxia. Brain. 1995;118(Pt 6):1365–74. doi: 10.1093/brain/118.6.1365. [DOI] [PubMed] [Google Scholar]
- 85.Becker WJ, Morrice BL, Clark AW, Lee RG. Multi-joint reaching movements and eye-hand tracking in cerebellar incoordination: investigation of a patient with complete loss of Purkinje cells. Can J Neurol Sci. 1991;18(4):476–87. doi: 10.1017/s0317167100032194. [DOI] [PubMed] [Google Scholar]
- 86.Beppu H, Nagaoka M, Tanaka R. Analysis of cerebellar motor disorders by visually-guided elbow tracking movement. 2. Contribution of the visual cues on slow ramp pursuit. Brain. 1987;110(Pt 1):1–18. doi: 10.1093/brain/110.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72(4):967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- 88.Edge AL, Marple-Horvat DE, Apps R. Lateral cerebellum: functional localization within crus I and correspondence to cortical zones. Eur J Neurosci. 2003;18(6):1468–85. doi: 10.1046/j.1460-9568.2003.02873.x. [DOI] [PubMed] [Google Scholar]
- 89.Glickstein M, May JG, 3rd, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235(3):343–59. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- 90.Kawamura K, Onodera S. Olivary projections from the pretectal region in the cat studied with horseradish peroxidase and tritiated amino acids axonal transport. Arch Ital Biol. 1984;122(2):155–68. [PubMed] [Google Scholar]
- 91.Robinson FR, Cohen JL, May J, Sestokas AK, Glickstein M. Cerebellar targets of visual pontine cells in the cat. J Comp Neurol. 1984;223(4):471–82. doi: 10.1002/cne.902230402. [DOI] [PubMed] [Google Scholar]
- 92.Walberg F, Nordby T, Hoffmann KP, Hollander H. Olivary afferents from the pretectal nuclei in the cat. Anat Embryol (Berl) 1981;161(3):291–304. doi: 10.1007/BF00301827. [DOI] [PubMed] [Google Scholar]
- 93.Mower G, Gibson A, Robinson F, Stein J, Glickstein M. Visual pontocerebellar projections in the cat. J Neurophysiol. 1980;43(2):355–66. doi: 10.1152/jn.1980.43.2.355. [DOI] [PubMed] [Google Scholar]
- 94.Ellermann JM, Siegal JD, Strupp JP, Ebner TJ, Ugurbil K. Activation of visuomotor systems during visually guided movements: a functional MRI study. J Magn Reson. 1998;131(2):272–85. doi: 10.1006/jmre.1998.1379. [DOI] [PubMed] [Google Scholar]
- 95.Jueptner M, Jenkins IH, Brooks DJ, Frackowiak RS, Passingham RE. The sensory guidance of movement: a comparison of the cerebellum and basal ganglia. Exp Brain Res. 1996;112(3):462–74. doi: 10.1007/BF00227952. [DOI] [PubMed] [Google Scholar]
- 96.Akaike T. Electrophysiological analysis of the tecto-olivo-cerebellar (crus II) projection in the rat. Brain Res. 1986;378(1):186–90. doi: 10.1016/0006-8993(86)90304-5. [DOI] [PubMed] [Google Scholar]
- 97.Akaike T. Differential localization of inferior olivary neurons projecting to the tecto-olivo-recipient zones of lobule VII or crus II in the rat cerebellum. Brain Res. 1986;386(1–2):400–4. doi: 10.1016/0006-8993(86)90180-0. [DOI] [PubMed] [Google Scholar]
- 98.Akaike T. Spatial distribution of evoked potentials in the inferior olivary nucleus by stimulation of the visual afferents in the rat. Brain Res. 1986;368(1):183–7. doi: 10.1016/0006-8993(86)91060-7. [DOI] [PubMed] [Google Scholar]
- 99.Marple-Horvat DE, Criado JM, Armstrong DM. Neuronal activity in the lateral cerebellum of the cat related to visual stimuli at rest, visually guided step modification, and saccadic eye movements. J Physiol. 1998;506(Pt 2):489–514. doi: 10.1111/j.1469-7793.1998.489bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marple-Horvat DE, Stein JF. Neuronal activity in the lateral cerebellum of trained monkeys, related to visual stimuli or to eye movements. J Physiol. 1990;428:595–614. doi: 10.1113/jphysiol.1990.sp018230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fadiga E, Pupilli GC. Teleceptive components of the cerebellar function. Physiol Rev. 1964;44:432–86. doi: 10.1152/physrev.1964.44.3.432. [DOI] [PubMed] [Google Scholar]
- 102.Chapman CE, Spidalieri G, Lamarre Y. Activity of dentate neurons during arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. J Neurophysiol. 1986;55(2):203–26. doi: 10.1152/jn.1986.55.2.203. [DOI] [PubMed] [Google Scholar]
- 103.Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol. 1993;70(6):2660–4. doi: 10.1152/jn.1993.70.6.2660. [DOI] [PubMed] [Google Scholar]
- 104.Brooks VB, Kozlovskaya IB, Atkin A, Horvath FE, Uno M. Effects of cooling dentate nucleus on tracking-task performance in monkeys. J Neurophysiol. 1973;36(6):974–95. doi: 10.1152/jn.1973.36.6.974. [DOI] [PubMed] [Google Scholar]
- 105.Vilis T, Hore J, Meyer-Lohmann J, Brooks VB. Dual nature of the precentral responses to limb perturbations revealed by cerebellar cooling. Brain Res. 1976;117(2):336–40. doi: 10.1016/0006-8993(76)90743-5. [DOI] [PubMed] [Google Scholar]
- 106.Spidalieri G, Busby L, Lamarre Y. Fast ballistic arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. II. Effects of unilateral dentate lesion on discharge of precentral cortical neurons and reaction time. J Neurophysiol. 1983;50(6):1359–79. doi: 10.1152/jn.1983.50.6.1359. [DOI] [PubMed] [Google Scholar]
- 107.Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. NeuroReport. 1999;10(5):1029–34. doi: 10.1097/00001756-199904060-00025. [DOI] [PubMed] [Google Scholar]
- 108.Goodkin HP, Thach WT. Cerebellar control of constrained and unconstrained movements. I. Nuclear inactivation. J Neurophysiol. 2003;89(2):884–95. doi: 10.1152/jn.00114.2002. [DOI] [PubMed] [Google Scholar]
- 109.Miall RC, Weir DJ, Stein JF. Visuo-motor tracking during reversible inactivation of the cerebellum. Exp Brain Res. 1987;65(2):455–64. doi: 10.1007/BF00236319. [DOI] [PubMed] [Google Scholar]
- 110.Pong M, Horn KM, Gibson AR. Spinal projections of the cat parvicellular red nucleus. J Neurophysiol. 2002;87(1):453–68. doi: 10.1152/jn.00950.2000. [DOI] [PubMed] [Google Scholar]
- 111.Ruigrok TJ, Cella F. Precerebellar nuclei and red nucleus. In: Paxinos G, editor. The Rat Nervous System. Sydney: Academic; 1995. pp. 277–308. [Google Scholar]
- 112.Angaut P, Cicirata F. The dentatorubral projection in the rat: an autoradiographic study. Behav Brain Res. 1988;28(1–2):71–3. doi: 10.1016/0166-4328(88)90079-4. [DOI] [PubMed] [Google Scholar]
- 113.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19(4):1446–63. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu X, Robertson E, Miall RC. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol. 2003;89(3):1223–37. doi: 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- 116.Herrero L, Yu M, Walker F, Armstrong DM, Apps R. Olivo-cortico-nuclear localizations within crus I of the cerebellum. J Comp Neurol. 2006;497(2):287–308. doi: 10.1002/cne.20976. [DOI] [PubMed] [Google Scholar]
- 117.Voogd J, Pardoe J, Ruigrok TJ, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23(11):4645–56. doi: 10.1523/JNEUROSCI.23-11-04645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol. 2009;587(Pt 2):429–42. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miles OB, Cerminara NL, Marple-Horvat DE. Purkinje cells in the lateral cerebellum of the cat encode visual events and target motion during visually guided reaching. J Physiol. 2006;571(Pt 3):619–37. doi: 10.1113/jphysiol.2005.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miall RC. The cerebellum, predictive control and motor coordination. Sensory Guidance of Movement Novartis Foundation Symposium. 1998;218:272–90. doi: 10.1002/9780470515563.ch15. [DOI] [PubMed] [Google Scholar]
- 121.Suh M, Leung HC, Kettner RE. Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. J Neurophysiol. 2000;84(4):1835–50. doi: 10.1152/jn.2000.84.4.1835. [DOI] [PubMed] [Google Scholar]
- 122.Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol. 1998;80(2):818–31. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- 123.Higuchi S, Imamizu H, Kawato M. Cerebellar activity evoked by common tool-use execution and imagery tasks: an fMRI study. Cortex. 2007;43(3):350–8. doi: 10.1016/s0010-9452(08)70460-x. [DOI] [PubMed] [Google Scholar]
- 124.Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci USA. 2003;100(9):5461–6. doi: 10.1073/pnas.0835746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Imamizu H, Kuroda T, Yoshioka T, Kawato M. Functional magnetic resonance imaging examination of two modular architectures for switching multiple internal models. J Neurosci. 2004;24(5):1173–81. doi: 10.1523/JNEUROSCI.4011-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403(6766):192–5. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 127.Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365(6441):50–2. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 128.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4(11):423–31. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 129.Glasauer S. Cerebellar contribution to saccades and gaze holding: a modeling approach. Ann NY Acad Sci. 2003;1004:206–19. doi: 10.1196/annals.1303.018. [DOI] [PubMed] [Google Scholar]
- 130.Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11(18):R729–32. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 131.Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269(5232):1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 132.Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- 133.Vercher JL, Gauthier GM. Cerebellar involvement in the coordination control of the oculo-manual tracking system: effects of cerebellar dentate nucleus lesion. Exp Brain Res. 1988;73(1):155–66. doi: 10.1007/BF00279669. [DOI] [PubMed] [Google Scholar]
- 134.Zago M, Lacquaniti F. Visual perception and interception of falling objects: a review of evidence for an internal model of gravity. J Neural Eng. 2005;2(3):S198–208. doi: 10.1088/1741-2560/2/3/S04. [DOI] [PubMed] [Google Scholar]


