Abstract
Purpose of Review
to describe the role of FFA as a cause for insulin resistance in obese people.
Recent Findings
elevated plasma FFA levels can account for a large part of insulin resistance in obese patients with type 2 diabetes. Insulin resistance is clinically important because it is closely associated with several diseases including T2DM, hypertension, dyslipidemia and abnormalities in blood coagulation and fibrinolysis. These disorders are all independent risk factors for cardiovascular disease (heart attacks, strokes and peripheral arterial disease). The mechanism by which FFA can cause insulin resistance, although not completely known, include generation of lipid metabolites (diacylglycerol), proinflammatory cytokines (TNF-α, IL1β, IL6, MCP1) and cellular stress including oxidative and endoplasmic reticulum stress.
Summary
increased plasma FFA levels are an important cause of obesity associated insulin resistance and cardiovascular disease. Therapeutic application of this knowledge is hampered by the lack of readily accessible methods to measure FFA and by the lack of medications to lower plasma FFA levels.
Keywords: free fatty acids, insulin resistance, obesity, cardiovascular disease
INTRODUCTION
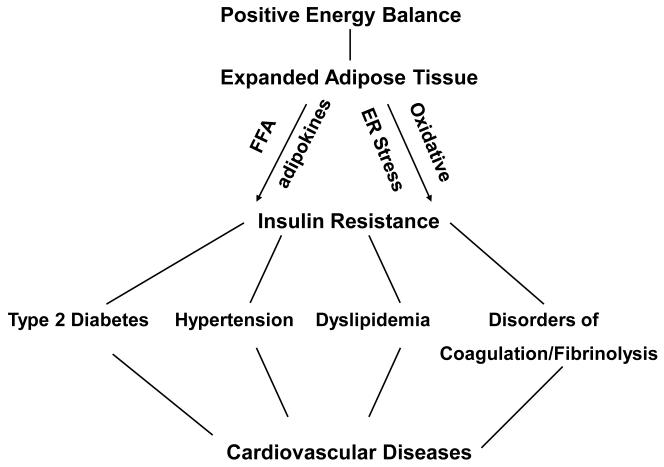
Insulin resistance, defined as inhibition of insulin stimulation of several metabolic pathways including glucose transport, glycogen synthesis and anti-lipolysis, is of considerable clinical relevance because it is pathophysiologically linked to several serious medical problems including type 2 diabetes (T2DM), hypertension, atherogenic dyslipidemia, abnormalities of blood coagulation and fibrinolysis and non-alcoholic fatty liver disease (1) (Figure 1). Some of these disorders have been collectively labeled the “metabolic or insulin resistance syndrome” (2). Importantly, they are all independent risk factors for cardiovascular diseases (CVD), which may explain why obesity is associated with a 2-4 times increased risk for heart attacks, strokes and peripheral vascular disease (1). Insulin resistance can have many causes (3), but by far the most common cause, particularly in developed countries, is obesity. In the US, obesity has reached epidemic proportions where more than 2/3 of all adults are either overweight or obese (4). Exactly why and how obesity causes insulin resistance is not completely understood. The most likely possibilities, however, are excessive nutrient intake and/or an expanded adipose tissue.
Figure 1.
Relationship between positive energy balance, an expanded adipose tissue (obesity), insulin resistance and cardiovascular disease. A chronic positive energy balance results in an expanded adipose tissue, i.e., obesity and is associated with insulin resistance and the development of serious disorders including type 2 diabetes, hypertension, dyslipidemia and disorders of coagulation and fibrinolysis which are all independent risk factors for the development of cardiovascular disease.
It is now recognized that adipose tissue is not only a storage site for excessive calories in the form of fat but is also a metabolically active tissue that synthesizes and secretes a large number of biologically active substances, such as proinflammatory cytokines, acute phase reactants, angiotensin II, leptin, resistin, adiponectin, PAI-1 and others (5). Some of these compounds, particularly, when administered in large amounts, can produce insulin resistance. Nevertheless, to be considered a physiological link between obesity and insulin resistance, an adipose tissue derived factor should meet the 3 criteria shown in Table 1.
Table 1.
Criteria for a physiologic link between obesity and insulin resistance.
| 1. | The factor should be elevated in the blood of obese people |
| 2. | Physiologic elevations of it’s blood levels should increase insulin resistance |
| 3. | Lowering of elevated blood levels should decrease insulin resistance |
So far, only FFAs meet the 3 criteria in human subjects. Therefore, this review focuses primarily on the effects of FFA on insulin action. This does not rule out that some of the other adipose tissue derived factors may eventually be recognized as physiologic causes for obesity associated insulin resistance.
FFA levels are increased in obese people
Plasma FFA levels are elevated in most obese subjects (6) because 1) the enlarged and stressed adipose tissue releases more FFA and 2) FFA clearance may be reduced (7). Moreover, once elevated, FFA will inhibit insulin’s antilipolytic action which will further increase FFA release into the circulation (8).
Raising FFA levels causes acute insulin resistance
Acute elevations of plasma FFA levels, for instance by infusion of heparinized triglyceride emulsions, decreases whole body insulin stimulated glucose uptake. This FFA induced insulin resistance develops after a delay of approximately 2 hours and disappears approximately 4 hours after normalization of plasma FFA, is dose dependent and affects men and women equally, regardless of whether or not they are diabetic (9-11). Because insulin stimulated glucose uptake under these euglycemic-hyperinsulinemic clamp conditions occurs mostly (greater than 80%) in skeletal muscle (12), it follows that FFA causes acute insulin resistance in skeletal muscle. Acute elevations of plasma FFA to levels frequently seen in obese individuals, also inhibit insulin suppression of hepatic glucose production. This acute FFA induced hepatic insulin resistance is primarily due to inhibition of insulin mediated suppression of glycogenolysis with little or no acute effect on gluconeogenesis (13). On the other hand, longer lasting FFA elevations are likely to also increase gluconeogenesis.
In endothelial cells, IV infusion of insulin has been shown to increase nitric acid production resulting in increased peripheral vascular blood flow (14). Physiologically elevated FFA levels produce insulin resistance in endothelial cells by inhibiting this insulin induced increase in nitric oxide and blood flow (15).
Acute lowering of plasma FFA reduces chronic insulin resistance
That the chronically elevated FFA levels seen in most obese individuals were responsible for a large part of their insulin resistance was demonstrated by acutely normalizing the chronically elevated plasma FFA levels in obese, diabetic and non-diabetic subjects. This resulted in normalization of insulin sensitivity in obese non-diabetic individuals and improved insulin sensitivity from ~ 25% to ~ 50% of normal in obese patients with T2DM (16). Similar results have been reported in subjects genetically predisposed to develop T2DM (17).
Moreover, a substantial part of the insulin resistance lowering activity of thiazolidinediones (TZDs) can be attributed to their lowering of plasma FFA levels (18), which they do by increasing FFA oxidation (19).
Mechanisms of FFA mediated insulin resistance
Despite much work, the mechanisms by which obesity and FFA cause insulin resistance are still not completely understood. Almost 50 years ago, Randle et al. proposed that FFA inhibited insulin stimulated glucose uptake in rat heart and diaphragm muscle by increasing fat and decreasing carbohydrate oxidation (20). Later studies in human skeletal muscle revealed, however, that the FFA induced increased fat and decreased carbohydrate oxidation was not a likely cause for the ensuing insulin resistance. Rather, FFAs were found to inhibit insulin action at the level of insulin stimulated glucose transport and/or phosphorylation by inhibiting insulin signaling (11,21). Recently, several mechanisms have been proposed to explain how obesity and/or FFA can interfere with insulin signaling.
The lipid metabolite hypothesis
This hypothesis is based on the finding that an increase in plasma FFA concentration results in intramyocellular and intrahepatic accumulation of triglycerides as well as several metabolites of the FFA reesterification pathway including longchain Acyl-CoAs and diacylglycerol (DAG) (22). DAG is a potent activator of conventional and novel protein kinase C (PKC) isoforms (23). In human skeletal muscle, elevation of plasma FFA levels has been shown to increase DAG and to activate PKC β2 and δ (24). PKC, a serine/threonine kinase, has been shown in rodents to cause insulin resistance by decreasing tyrosine phosphorylation of the insulin receptor substrate (IRS) 1/2 (25). Complicating the issue is that there are more than 20 serine/threonine consensus sites in IRS1 that can be phosphorylated. Not surprisingly, therefore, other serine/threonine kinases have been postulated to be involved in obesity/FFA induced inhibition of insulin signaling and action (see below).
The reason for the increase in DAG following lipid infusions is not entirely clear. A reasonable assumption is that the fatty acid reesterification pathway can be overwhelmed in high fatty acid flux situations. Supporting this notion is the demonstration that overexpression of DGAT (the enzyme which catalyzes the formation of triacylglycerol from DAG) in skeletal muscle of mice, protected against high fat diet induced insulin resistance, whereas DGAT deficiency increased FFA induced insulin resistance in isolated mouse muscles (26). Moreover, one single 90 minute session of moderate intensity exercise completely reversed FFA induced insulin resistance in healthy volunteers one day later (27). This was accompanied by increases in skeletal muscle triglyceride, GPAT, DGAT and IκB-α proteins and decreases in skeletal muscle DAG, glycogen and phosphorylated JNK levels (27). Nevertheless, so far, the DAG/PKC hypothesis in human tissue is based on correlative data and requires more direct evidence.
The inflammation hypothesis
It is now recognized that obesity is an inflammatory state associated with increased levels of proinflammatory cytokines and chemokines in the circulation and in tissues (28). Recent studies have shed some light on possible causes. For instance, FFA have been shown in vivo and in vitro to activate the canonical proinflammatory NF-κB pathway. In vivo, acutely increasing plasma FFA levels activated NF-κB in human skeletal muscle (24) and resulted in increased expression of several proinflammatory cytokines including TNF-α, IL1β and IL6 in rat liver as well as an increase in circulating MCP1 levels (29). The rise in plasma MCP1 is particularly interesting because MCP1 is well established to regulate macrophage recruitment to sites of inflammation (30). The rise in plasma MCP levels, therefore, may be involved in the recently reported monocyte recruitment into adipose tissue of obese animals where they differentiate into macrophages and produce proinflammatory cytokines (31,32). In vitro, linoleic acid (C18:2) activated NF-κB and increased IL6 and TNF-α in cultured human adipocytes and stomal vascular cells (33). The concept that emerges from these observations is that elevated plasma FFA levels, either as result of obesity or high fat feeding, can produce a state of insulin resistance as well as low grade inflammation. The early events leading from a rise of circulating FFA to activation of NF-κB are not clear but include several possibilities. First, Gao et al. have recently shown that the FFA mediated activation of IKK (a kinase involved in the activation of NF-κB) in fat cells was PKC dependent (34). Thus, DAG mediated PKC activation may be an upstream effector of NF-κB activation connecting the lipid metabolite with the inflammation hypothesis. Second, there is evidence to suggest that FFA mediated activation of IKK and NF-κB may be, at least in part, mediated by the Toll like receptor 4 (TLR-4) (35). The TLR-4 pathway, which is essential for the development of innate immunity to pathogens, triggers production of inflammatory cytokines (36). Thus it appears, that the sensing of excess nutrients such as FFA and the sensing of infectious pathogens may use the same signaling pathway.
Possible mechanisms through which FFA mediated NF-B activation and cytokine production can result in insulin resistance includes activation of JNK by cytokines (37,38) and induction of suppressor of cytokine signaling (SOCS) which can interfere with binding of IRS1/2 to the insulin receptor (39).
However, whether FFA induced inflammation actually causes insulin resistance in vivo is not certain. On one hand, there is little doubt that the very high concentration of proinflammatory cytokines observed during severe bacterial infection or burns or increasing TNF-α blood levels 10-fold by infusion of rh TNF-α, can result in insulin resistance (40). Also, Salicylate, a non-specific inhibitor of IKK, given in high doses (4-8 g/day), increased insulin sensitivity, supporting a role of NF-κB activation in the pathogenesis of insulin resistance (41,42). On the other hand, the evidence that the cytokine levels typically observed in response to lipid infusions or in obese people contribute to the insulin resistance, is less convincing (reviewed in ref. 43). For instance, TNF-α and IL-6 are considered important inhibitors of insulin action. Yet, administration of TNF-α neutralizing antibodies to obese diabetic patients did not reduce their insulin resistance (44,45), nor did infusion of IL-6 into normal volunteers produce insulin resistance (40) and IL-6 deficient mice are insulin resistant (46,47). This, however, does not exclude the possibility that even modest elevations of several of these cytokines together, may produce insulin resistance.
The oxidative and endoplasmic reticulum (ER) stress hypotheses
In response to converting excessive amounts of FFAs and other nutrients into fat, the adipocyte can develop signs of oxidative and ER stress. In healthy volunteers, a lipid infusion induced a rise in plasma FFA levels and produced an increase in plasma free radical concentrations (indicating oxidative stress) (48). In cultured adipocytes, Furukawa et al. have shown that FFAs activated NADPH oxidase and induced reactive oxygen species (ROS) production and that the ROS induced oxidative stress resulted in dysregulated production of proinflammatory cytokines from white adipose tissue (49). These proinflammatory cytokines may then be able to produce insulin resistance (see above).
In vitro, FFA have been shown to produce ER stress in cultured adipocytes (50), liver cells (51) and pancreatic β-cells (52,53). In vivo, heparinized lipid infusions increased plasma FFA and produced ER stress in rat liver (Boden et al., unpublished). ER stress can produce insulin resistance via activation of JNK (54,55), whereas relieving ER stress by overexpression of ORP150, a molecular chaperone that protects cells from ER stress, reduces insulin resistance in diabetic (dbdb) mice (56). Thus, there is evidence supporting the notion that FFA induced oxidative and/or ER stress may contribute to the FFA mediated insulin resistance.
CONCLUSIONS
The pivotal role of elevated plasma FFA levels as a cause for insulin resistance and as a pathogenetic factor in the development of T2DM has been established. In addition, it has recently been recognized that FFAs simultaneously cause insulin resistance and activate the proinflammatory NF-κB pathway resulting in secretion of many proinflammatory and proatherogenic cytokines and chemokines. Thus, elevated plasma FFA levels in obese subjects can produce a low grade inflammatory state which may contribute to the accelerated atherosclerosis and non-alcoholic fatty liver disease, conditions whose prevalence is several-fold increased in obesity.
Challenges for the future include the prevention or correction of obesity and elevated plasma FFA levels mainly through decreased caloric intake and increased caloric expenditure, development of easy, fast and reliable methods to measure FFA in small blood samples and the development of efficient pharmacological approaches to normalize increased plasma FFA levels.
 Acutely increasing plasma FFA levels produces insulin resistance
Acutely increasing plasma FFA levels produces insulin resistance Lowering of chronically elevated plasma FFA levels improves insulin resistance
Lowering of chronically elevated plasma FFA levels improves insulin resistance Elevated plasma FFA levels can account for up to 50% of the insulin resistance in obese patients with type 2 diabetes
Elevated plasma FFA levels can account for up to 50% of the insulin resistance in obese patients with type 2 diabetes Acutely increasing plasma FFA levels is proinflammatory
Acutely increasing plasma FFA levels is proinflammatory
ACKNOWLEDGEMENTS
I thank Maria Mozzoli, BS for excellent technical assistance and Constance Harris Crews for typing the manuscript.
Supported by a grant from the National Institutes of Health (R01-DK58895) and a grant from the American Diabetes Association (1-10-CT-06)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bray GA. Medical consequences of obesity. J Clin Endocrinology Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Boden G. Pathogenesis of Type 2 Diabetes: Insulin Resistance. Endocrinology and Metabolism Clinics of North America. 2001;30:801–815. doi: 10.1016/s0889-8529(05)70216-4. [DOI] [PubMed] [Google Scholar]
- ** 4.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents. 2007-2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. Provides updated information on recent changes in the prevalence of obesity in children and adults in the US.
- 5.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 6.Opie LH, Walfish PG. Plasma free fatty acid concentration in obesity. N Engl J Med. 1963;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover in obesity. Acta Med Scand. 1969;185:351–356. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MD, Haymond MW, Rizza RA, et al. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989:1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boden G, Jadali F, White J, et al. Effects of fat on insulin stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88:960–966. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Ruiz J, et al. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Jacot E, Jequier E, et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 13.Boden G, Cheung P, Stein TP, et al. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol. 2002;283:E12–E19. doi: 10.1152/ajpendo.00429.2001. [DOI] [PubMed] [Google Scholar]
- 14.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santomauro ATMG, Boden G, Silva M, et al. Overnight lowering of free fatty acids with acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 17.Cusi K, Kashyap S, Gastaldelli A, et al. Effect on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Metab. 2007;292:E1775–E1781. doi: 10.1152/ajpendo.00624.2006. [DOI] [PubMed] [Google Scholar]
- 18.Boden G, Zhang M. Recent findings concerning thiazolidinediones in the treatment of diabetes. Expert Opin Investig Drugs. 2006;15:243–250. doi: 10.1517/13543784.15.3.243. [DOI] [PubMed] [Google Scholar]
- 19.Boden G, Homko C, Mozzoli M, et al. Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue in diabetic patients. Diabetes. 2005;54:880–885. doi: 10.2337/diabetes.54.3.880. [DOI] [PubMed] [Google Scholar]
- 20.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose-fatty acid cycle, its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 21.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1 associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boden G, Lebed B, Schatz M, et al. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 23.Farese R. In: Diabetes Mellitus: a Fundamental and Clinical Text. LeRoith D, Taylor SI, Olefsky JM, editors. Lippincott; Phiadelphia: 2000. pp. 239–251. [Google Scholar]
- 24.Itani SI, Ruderman NB, Schmieder, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Zhang Y, Chen N, et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tataranni PA, Ortega E. A burning questions: does an adipokines-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 29.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 30.Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–1116. [PubMed] [Google Scholar]
- 31.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 32.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. First demonstration that adipose tissue lipolysis and plasma FFA levels coincide with changes in the number of adipose tissue macrophages. These results suggest that plasma FFA levels and local lipid fluxes regulate adipose tissue macrophage recruitment.
- 33.Chung S, Brown JM, Provo JN, et al. Conjugated linoleic acid promotes human adipocyte insulin resistance through NF kappa B-dependent cytokine production. J Biol Chem. 2005;280:38445–38456. doi: 10.1074/jbc.M508159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, Zhang X, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Molecular Endocrinology. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 35.Shi A, Kokoeva V, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medzhitov R. Toll-like-receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 37.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 38.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKKB and NF-κB. Nature Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebrun P. SOCS proteins causing trouble in insulin action. Acta Physiol. 2008;192:29. doi: 10.1111/j.1748-1716.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- 40.Krogh-Madsen R, Plomgaard P, Moller K, et al. Influence of TNF-α and IL-6 infusions on insulin sensitivity and expression of IL-8 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108–E114. doi: 10.1152/ajpendo.00471.2005. [DOI] [PubMed] [Google Scholar]
- 41.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 42.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 43.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nature Med. 2010;16:400–402. doi: 10.1038/nm0410-400. Recent review of the lipid metabolite/DAG hypothesis.
- 44.Ofei F, Hurel S, Newkirk J, et al. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez H, Storgaard H, Rask-Madsen C, et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 46.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- * 47.Matthews VB, Allen TL, Risis S, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. In vivo demonstration in transgenic, IL-6 deficient mice that IL-6 deficiency did not prevent but actually exacerbated diet induced hepatic insulin resistance and inflammation.
- 48.Paolisso G, Gambardella A, Tagliamonte MR, et al. Does free fatty acid infusion impair insulin action also through an increase in oxidative stress? J Clin Endocrinol Metab. 1996;81:4244–4248. doi: 10.1210/jcem.81.12.8954022. [DOI] [PubMed] [Google Scholar]
- 49.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, Wong S, Xie W, et al. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–E586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 52.Karaskov E, Scott C, Zhang L, et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cells apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 53.Kharroubi I, Ladriere L, Cardozo AK, et al. JNK and tumor necrosis factor-α mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. Endocrinology. 2004;145:5087–5096. [Google Scholar]
- 54.Nguyen MTA, Satoh H, Favelyukis S, et al. Free fatty acids and cytokines induced pancreatic β-cell apoptosis by different mechanisms: Role of nuclear factor-κB and endoplasmic reticulum stress. J Biol Chem. 2005;280:35361–35371. [Google Scholar]
- 55.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 56.Nakatani Y, Kaneto H, Kawamori D, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]