Abstract
We previously demonstrated that transient stromal cell-derived factor-1 alpha (SDF-1) improved cardiac function when delivered via cell therapy in ischemic cardiomyopathy at a time remote from acute myocardial infarction (MI) rats. We hypothesized that non-viral gene transfer of naked plasmid DNA-expressing hSDF-1 could similarly improve cardiac function. To optimize plasmid delivery, we tested SDF-1 and luciferase plasmids driven by the cytomegalovirus (CMV) promoter with (pCMVe) or without (pCMV) translational enhancers or α myosin heavy chain (pMHC) promoter in a rodent model of heart failure. In vivo expression of pCMVe was 10-fold greater than pCMV and pMHC expression and continued over 30 days. We directly injected rat hearts with SDF-1 plasmid 1 month after MI and assessed heart function. At 4 weeks after plasmid injection, we observed a 35.97 and 32.65% decline in fractional shortening (FS) in control (saline) animals and pMHC-hSDF1 animals, respectively, which was sustained to 8 weeks. In contrast, we observed a significant 24.97% increase in animals injected with the pCMVe-hSDF1 vector. Immunohistochemistry of cardiac tissue revealed a significant increase in vessel density in the hSDF-1-treated animals compared with control animals. Increasing SDF-1 expression promoted angiogenesis and improved cardiac function in rats with ischemic heart failure along with evidence of scar remodeling with a trend toward decreased myocardial fibrosis. These data demonstrate that stand-alone non-viral hSDF-1 gene transfer is a strategy for improving cardiac function in ischemic cardiomyopathy.
Keywords: SDF-1, heart failure, non viral, angiogenesis, plasmid DNA
Introduction
Heart failure is one of the leading causes of morbidity and mortality in Western countries. Heart failure has a US prevalence of 5.4 million Americans and an incidence of 690 000 new cases per year.1 Chronic ischemic damage via myocardial infarction (MI) causes significant loss of cardiac function and often leads to symptomatic heart failure after treatment with current guideline-based medical therapy. There is a growing interest in the use of cell and gene transfer to prevent cardiac dysfunction and treat patients with ischemic heart disease. The potential of several different cell types for the treatment of cardiovascular disease has been studied in animal models and in clinical populations, including heterogeneous whole bone marrow preparations,2, 3 hematopoietic bone marrow stem cells,4, 5 mesenchymal stem cells,6, 7 multipotent adult progenitor cells8 and endogenous cardiac stem cells populations.9 Many of these studies have shown improvement in cardiac function and have demonstrated an increase in vascular density within the infarct zone after treatment.
We previously identified stromal cell-derived factor-1 (SDF-1, also known as, CXCL12) as a chemokine that is transiently expressed post-tissue injury to promote stem cell homing to the myocardium.10 SDF-1 enhances tissue repair by preventing cell death and recruiting blood borne and tissue-specific stem cells to the damaged region.10 SDF-1 is a naturally occurring chemokine that is rapidly increased after MI for a period of 4–5 days.10, 11 SDF-1 triggers a number of protective molecular cascades that are both anti-inflammatory12 and anti-apoptotic to preserve cardiac tissue after injury.13 Furthermore, SDF-1 is a strong chemoattractant of stem cells and progenitor cells that promote tissue preservation and blood vessel development. The tissue-preserving and reparative effects of SDF-1 led us to investigate the potential role of SDF-1 in treating ischemic cardiovascular disease.
More recently, we have demonstrated that the overexpression of SDF-1 in myocardial tissue leads to recruitment of endogenous cardiac stem cells to the infarct border zone.14 Since our original findings, several groups have expanded the molecular mechanisms by which myocardial expression of SDF-1 or its receptor CXCR4 leads to preservation of cardiac myocytes and improvement in cardiac function in an acute MI setting.15, 16 We hypothesized that reestablishing stem cell homing by increasing SDF-1 expression via non-viral gene transfer into the periinfarct tissue, late after MI, would reestablish myocardial healing through recruitment of bone marrow-derived stem cells and lead to increases in vascular density and cardiac function.10
To date, we have reestablished SDF-1 expression through a cell-based gene transfer approach using stably transfected fibroblasts,10 mesenchymal stem cells7 or skeletal myoblasts transfected with an adenovirus-encoding SDF-1 ex vivo.17, 18 Although cell-based gene transfer is a viable option, the goal of this study was to determine if reestablishing myocardial SDF-1 expression without the concomitant delivery of cells is a viable strategy for increasing myocardial vascular density and cardiac function in the setting of ischemic cardiomyopathy. SDF-1 protein, in combination with a slow release matrix, has been shown to improve cardiac function in a model of ischemic cardiomyopathy.19 Although these data would suggest that SDF-1 alone may be sufficient to improve cardiac function, such a conclusion is premature, as matrix alone has been shown to induce ventricular remodeling.20, 21
To deliver SDF-1 to the myocardium, we injected naked plasmid DNA encoding hSDF-1 into the infarct border zone. In previous studies, this strategy has proven to be safe and non-toxic, and is not associated with the toxicities and loss of function that have been observed with adenoviral injection in the infarct border zone.22 We characterized plasmid expression in cardiac tissue using a luciferase bioluminescence reporter system,23 as previous non-viral gene transfer studies demonstrated low-transfection efficiency with this approach. We tested plasmid dose, transcription enhancers and ubiquitous versus cardiac-specific promoters to increase vector expression. After identifying an optimal plasmid formulation, we injected plasmid-encoding SDF-1 into the heart 1 month after MI. This led to a significant increase in cardiac function at 4 weeks after injection that could be attributed to significant increase in vessel density and a trend toward reduction in scar tissue. The degree of functional improvement correlated with the level of vector expression.
Results
pCMVe demonstrates high level in vitro expression
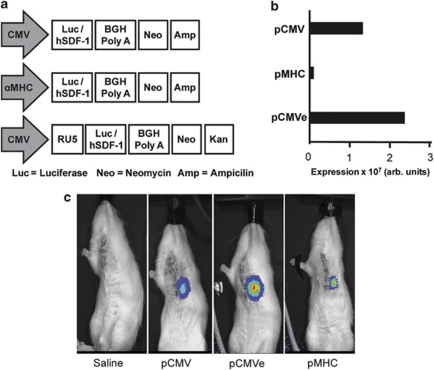
The inefficiency of physical delivery of non-viral plasmid DNA to cells has been well documented. One of the strategies used to circumvent low transfection efficiencies has been to identify vector components that provide either high levels of protein expression or enable target-specific expression using tissue-specific promoters. We tested three different plasmids in the H9C2 myoblast cell line to identify a vector that would produce sufficient cardiac cell expression over approximately 2 weeks. Plasmids were constructed with either the ubiquitous cytomegalovirus (CMV) promoter or cardiac-specific α myosin heavy chain (MHC) promoter and the luciferase reporter genes (pCMV, pMHC and pCMVe, Figure 1a). We observed that CMV-driven promoters (pCMV and pCMVe) exhibited higher luciferase expression compared with the cardiac-specific αMHC-driven plasmid (pMHC). As expected, the RU5 translational enhancer in pCMVe increased expression over pCMV and pMHC by 125 and 250%, respectively, (Figure 1b).
Figure 1.
(a) Schematic of the vector design. Vector design of pCMV, pCMVe and pMHC. (b) HEK293 cells were transfected with pCMV and pCMVe, whereas H9C2 cardiac myoblasts were transfected with pMHC. (c) Cardiac bioluminescence after direct injection. Animals were injected with vectors immediately following LAD ligation and then imaged 3 days after, following an i.p. injection of luciferin.
We assessed in vivo vector expression in adult Lewis rats infarcted via a left anterior descending artery (LAD) ligation followed by direct cardiac injection with 100 μl of 1 mg ml−1 pCMV, pMHC or pCMVe-luc in saline into the anterior wall of the normal myocardium. Control rats were injected with saline. Gene expression was quantified by measuring chemiluminescence following i.p. injection of luciferin 3 days after direct cardiac injection (Figure 1c).
Dose–response of cardiac gene transfer
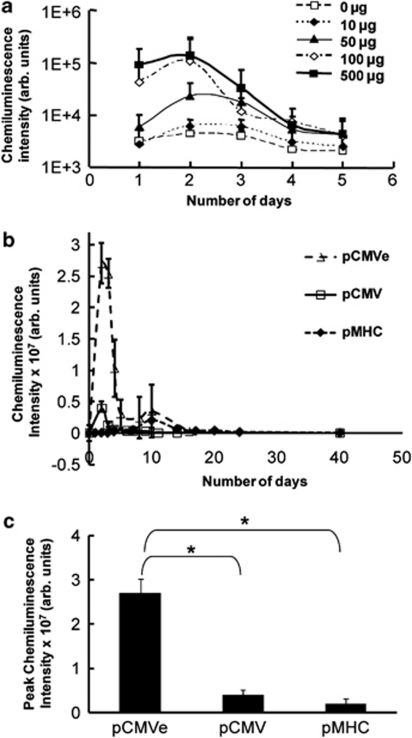
To determine the optimal plasmid dose required for efficient myocardial transfection, we injected naked plasmid encoding luciferase (pCMV-luc) into the anterior wall of Lewis rats at doses ranging from 0 to 500 μg in 100 μl phospahe-buffered saline (PBS). Importantly, the imaging data of all injected plasmids demonstrated that expression was localized to the cardiac region in all rats (Figure 1c). A dose–response curve was generated for vector expression. The peak expression increased up to a dose of 100 μg and saturated at higher doses (Figure 2a). Therefore, a dose of 100 μg was used in all subsequent experiments.
Figure 2.
(a) Dose–response of cardiac gene expression. Rodent hearts were injected with varying doses of pCMV-Luc into the anterior wall. The chemiluminescence emitted was measured everyday for 5 days after the injection. (b) Comparison of cardiac bioluminescence between vectors. Animals were injected with pCMV Luc (n=5), pCMVe Luc (n=3) and pMHC Luc (n=7) and expression monitored by quantifying the chemiluminescence emitted. (c) Duration of vector expression. The peak chemiluminescence exhibited by the vectors is shown here. pCMV-Luc (n=5), pCMVe-Luc (n=3) and pMHC-Luc (n=7). *P<0.05.
Time course of vector expression in the heart
Next we determined duration of expression after cardiac gene transfer with pCMV, pCMVe and pMHC vectors. All plasmids expressed luciferase in infarcted cardiac tissue. Expression from the CMV-based plasmids expressed for a short period of 5 days, whereas plasmid pMHC expressed for a longer period of 32 days. Adding elements to increase transcriptional and translational efficiency significantly affected the expression profile, with pCMVe expression lasting 15 days. Changing the promoter or adding elements to increase translational efficiency significantly affected the duration of expression (Table 1).
Table 1. Summary of expression profiles of vectors studied.
| Promoter | Backbone | Enhancer | Model | Peak expression | Days of expression |
|---|---|---|---|---|---|
| αMHC | pBS | None | Infarcted rats | 6.75 × 105 | 32 |
| CMV | pcDNA 3.1 | CMV | Infarcted rats | 2.39 × 106 | 5 |
| CMV | pcDNA 3.1 | CMV+RU5 | Infarcted rats | 2.75 × 107 | 16 |
Abbreviations: αMHC, α myosin heavy chain; CMV, cytomegalovirus.
Comparison of peak magnitude between the different plasmids
The magnitude of luminescent intensity was determined for the three plasmids (pCMV, pCMVe and pMHC). We observed that pCMV peaked at day 3. pMHC showed maximum expression on day 7 and continued for 30 days (Figures 2b and c). The αMHC promoter had a smaller magnitude of luciferase expression when compared with CMV promoters. Compared with pCMV, the RU5 translational enhancer in the CMV plasmid (pCMVe) increased peak expression almost 10-fold (Figure 2c).
Intra-myocardial injection of SDF-1 plasmid increased vessel density
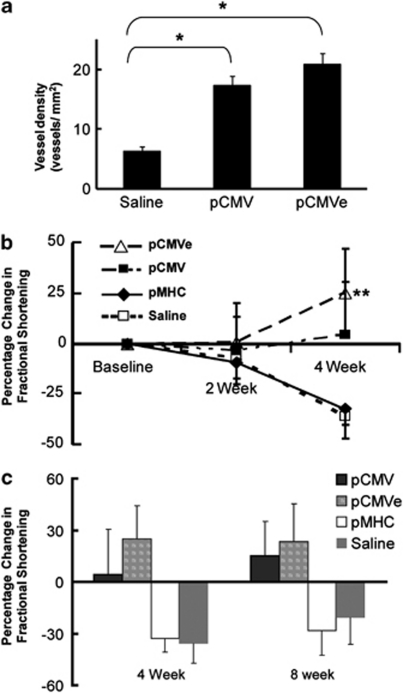
Previous studies have shown that SDF-1 expression is increased after MI and recruits stem and/or progenitor cells to promote tissue preservation and promote vasculogenesis. We tested whether non-viral gene transfer of vector expressing SDF-1 would improve vasculogenesis following MI. As described above, rodents were infarcted via LAD ligation and received direct injection of SDF-1 plasmid DNA 1 month after MI. Myocardial sections obtained from animals that received SDF-1 gene transfer, 8 weeks after injection, were stained with antibodies against von Willebrand factor to assess vasculogenesis. We observed a statistically significant increase in blood vessel density in the infarct zone in the animals that received pCMVe-hSDF-1 and pCMV-hSDF-1, as compared with saline controls (P<0.0001). The infarct border zone and infarct zone, on average, had a higher blood vessel density in the pCMVe-hSDF-1 group (21 vessels mm−2) and pCMV-hSDF-1 group (17 vessels mm−2) than those in the saline group (6 vessels mm−2) (n=7 in all groups) (Figure 3a). The vessels observed by von Willebrand factor staining were also co-stained with α-smooth muscle actin. A colocalization of endothelial cells and actin filaments was observed (data not shown), which further established the fact that the blood vessels observed were arterioles and arteries, and not capillaries.
Figure 3.
SDF-1 gene transfer improves cardiac function through increased angiogenesis. At 8 weeks after plasmid injection, animals were killed and the hearts processed for immunohistochemistry. Slides were stained for von Willebrand factor and ventricular myosin for blood vessels and myocytes, respectively, in the infarct zone after injection with SDF-1 plasmid in the pCMV- and pCMVe-treated animals. (a) Vessel density was significantly increased in the SDF-1-treated animals when compared with control animals. pCMVe-SDF1, 21±1.82 vessels mm−2; pCMV-SDF1, 17±1.48 vessels mm−2 and saline controls 6±0.73 vessels mm−2. *P<0.001, n=7 in all groups. (b) Echocardiographic assessment following plasmid injection. Pre-injection (1 month after infarct), all rats had a fractional shortening (FS) <30%. At 4 weeks after injection, the control group (n=10) decreased in FS by 35.97±11.08 CMV-driven plasmids showed a statistically significant improvement of 4.32±26.10% (pCMV-SDF1) (n=9) and 24.97±28.87% (pCMVe-SDF1) (n=10), respectively, **P<0.05. The cardiac-specific promoter plasmid (pMHC-SDF1) (n=9) did not improve function compared with control. (c) Comparative analysis between the percentage of FS at 4 and 8 weeks. The improvement in cardiac function, as observed at 4 weeks was sustained at 8 weeks after injections.
Interestingly, the pCMVe vector that had the highest peak expression did not induce significantly greater vasculogenesis than the lesser expressing unenhanced pCMV vector. This result suggests that delivering a minimum threshold amount of SDF-1 was necessary to induce vasculogenesis but that more SDF-1 did not induce more.
SDF-1 plasmid therapy increases left ventricular function in ischemic rats
We hypothesized that the SDF-1 plasmids that induced vasculogenesis would also improve cardiac function after MI. To test this hypothesis, ischemic hearts were injected with hSDF-1 pDNA vectors. Pre-injection (1 month after infarct), all rats had fractional shortening (FS) <30%. At 4 weeks after injection, control animals had a decrease in FS by 35.9%. In contrast, the pCMV-SDF1 and pCMVe-SDF1 groups showed statistically significant improvements of 4.62 and 24.97%, respectively, by 4 weeks, (P<0.01) compared with control. In contrast, a cardiac-specific promoter, αMHC-driven plasmid (pMHC-hSDF1)-treated animals did not improve function compared with control animals exhibiting a decline in FS of 32.65% (Figure 3b). These changes in cardiac function, as determined by FS were sustained to 8 weeks after injection of the respective plasmids (Figure 3c). Importantly, the improvement in cardiac function correlated with an increase in blood vessel density, strongly suggesting a link between vasculogenesis and cardiac functional improvement. However, the greater benefit seen with the pCMVe compared with pCMV suggests that there are other mechanisms in action as well, as pCMVe did not induce greater vascular density compared with pCMV. SDF-1 gene transfer induced cardiac benefit was sustained at 8 weeks, as the animals that received the CMV-driven plasmids continued to demonstrate improved cardiac function compared with saline control and αMHC vector groups.
Other parameters of cardiac function, such as the thickening of the anterior and posterior walls, trended toward significant improvement (i.e., an increase in wall thickness) in the pCMVe-hSDF1-treated animals. No significant change was observed in the pCMV-hSDF1-treated animals, the pMHC-SDF1-treated animals or the saline control animals. There was no change in left ventricular (LV) mass in any group by echocardiograrphy.
As the animals treated with the plasmid producing the highest peak expression had the largest cardiac benefit, these data suggest the importance of the magnitude of hSDF-1 vector expression rather than the length of plasmid expression in determining improvement in cardiac function.
SDF-1 gene transfer reduced fibrotic tissue in the infarct zone
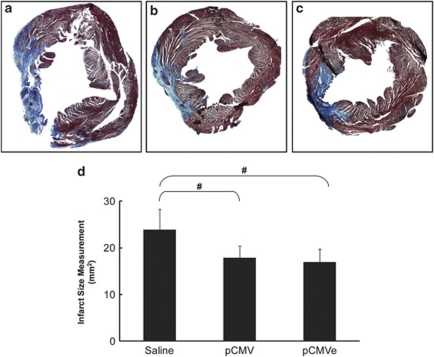
We performed hematoxylin and eosin and Masson's Trichrome staining to determine the infarct size, represented by fibrotic tissue, 8 weeks after treatment with plasmids. The infarct size was reduced in the animals that received SDF-1 plasmid when compared with the saline animals. The fibrotic area was smaller in the pCMVe-SDF1 group (16.92%) and pCMV-SDF1 group (17.81%), than in the saline group (23.82%) (n=7 in all groups). These results exhibit a strong trend toward reduction in cardiac fibrosis following SDF-1 treatment (P=0.08) (Figures 4a and b).
Figure 4.
Massons Trichrome staining for collagen content. Left ventricular fibrosis declined in the pCMVe-SDF1 group (a) and pCMV-SDF1 group (b) compared to the saline group (c) (n=7 in all groups). SDF-1 gene therapy reduced infarct size 8 weeks after treatment in chronic heart failure. (d) Decrease in fibrotic scar following SDF-1 treatment. This correlates to a–c. The left ventricular fibrotic area was smaller in the pCMVe-SDF1 (16.92±2.82%) and pCMV-SDF1 groups (17.81±2.59%), compared to the saline group (23.82±4.47%) (n=7 in all groups), #P=0.08, demonstrating a trend towards reduced scar tissue following plasmid injections.
Discussion
Regenerative medicine has significant potential for the treatment of ischemic cardiac disease because, unlike current treatments that focus on either alleviating symptoms or reducing cardiac workload, regenerative medicines provide an opportunity to repair and retain function in degenerating organs. Non-viral gene delivery, or the application of naked plasmid DNA to express a therapeutic protein at a specific site, is a simple delivery method that has been tested clinically in ischemic patients for over 15 years. A substantial body of literature, both preclinical and clinical, has demonstrated that non-viral vector delivery of therapeutic genes is safe and effective. The safety profile of non-viral gene delivery is attractive when compared with viral vector therapy delivery because neither it produces a significant inflammatory response that viral vector delivery can cause nor is there concern for antibody response to the vector due to previous viral exposure in the patient.
Cardiovascular gene therapy has been found to be a safe strategy to obtain transient protein expression in the heart. Skeletal and cardiac muscles have been shown to take up and express plasmid-encoded genes as well as transgenes incorporated into viral vectors.24, 25 Although viral vectors provide increased transfection efficiencies compared with non-viral strategies, they induce inflammatory responses that may result in unwanted side effects and preclude repeat administrations.26 In vitro and in vivo studies have also shown that despite the lower gene-transfer efficiency with non-viral methods, site-specific administration of the gene still results in physiological effects within the local tissue.27, 28 Some advantages of non-viral gene transfer are the ability for repeat administration of therapy and the potential for transient protein expression. Several clinical trials have demonstrated safety and efficacy for non-viral gene therapy in ischemic cardiovascular disease.29
We previously identified SDF-1 as a naturally occurring protein that is rapidly produced in response to ischemic tissue injury.11 SDF-1 induction stimulates a number of protective anti-inflammatory pathways, is increased in the myocardium after a heart attack, but only lasts for a matter of days, and therefore the protective response quickly fades. This short duration of SDF-1 action reduces the potential for tissue repair. A non-viral plasmid-producing SDF-1 for treatment of heart failure provides a potentially safe means through which to obtain longer, although transient (<15 day), therapeutic protein production in the heart, whereas allowing for the possibility of repeat injections.
In this study, we have demonstrated that the delivery of SDF-1 plasmid to the infarct border zone, 1 month after MI, led to an increase in vascular density and improvement in cardiac function that was sustained 8–10 weeks after gene transfer. Our expression studies demonstrated that expression of our optimized plasmid peaked at day 2 after gene transfer and decreased over the following 2 weeks. These findings are consistent with previous literature showing a peak in CMV-driven gene expression, 2 days after injection in the rat heart.30, 31 Peak expression in the presence of the RU5 element (pCMVe) was 10-fold greater than that seen in the non-enhanced CMV plasmid (pCMV).
It is noteworthy that direct injection of plasmids into the anterior wall led to the transfection of cardiac myocytes. In our studies with plasmid delivery restricted to cardiac myocytes, using the cardiac-specific αMHC promoter, there was a delay of 4 days after plasmid injection, before any evidence of significant expression. Furthermore, the magnitude of expression was 10-fold less than that observed with the pCMV. Although the signal was low, expression persisted for 32 days. This suggests that combining the αMHC promoter with the RU5 translational enhancer may result in significant plasmid expression for up to 1 month after injection.
The degree of functional changes with any given plasmid-encoding hSDF-1 correlated with the level of luciferase expression achieved with that plasmid construct. We observed continued decline in cardiac function in the animals that received saline or the low-expressing αMHC plasmid (pMHC). At 4 weeks after plasmid injection (8 weeks after MI), there was a decline in cardiac function in both these groups, which correlated with low-expression profiles. Both CMV vectors resulted in an increase in cardiac vascular density. However, the animals that received the CMV plasmid with enhancer elements (pCMVe) increased cardiac function to a greater extent than those animals that received the CMV plasmid alone (pCMV).
Increased SDF-1 expression in the infarct border zone also led to a decrease in myocardial fibrosis at 8–10 weeks after plasmid injection. Similar to the change in vascular density, there was an inverse correlation between the degree of myocardial fibrosis and SDF-1 expression, suggesting that the mechanisms behind scar remodeling may be crucial to the understanding of the therapeutic response to SDF-1. These data suggest that SDF-1 treatment attenuates the progression of chronic ischemic heart failure and may partially reverse manifestations of the disease by increasing vasculogenesis, reducing scar formation and attenuating pathological cardiac remodeling after MI.
Materials and methods
Vector preparation
pCMV-Luc was created by inserting a luciferase cDNA generated by PCR from a pGL3 vector (Promega, Madison, WI, USA) using primers, forward: 5′-GAATTCGTCGACTATGGAAGACGCCAAAAA CATAAAGAAAGGC-3′ reverse: 5′-TCTAGAAAGCTTTTACACGGCGATCTTTCCGCCCTTC-3′, at the EcoRI and XbaI sites and ligated it pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA). The luciferase gene from pCMV was digested with SalI/HindIII and ligated into a pBSK-αMHC vector (a kind gift from Jeffery Robbins Lab at the University of Cincinnati Medical Center, Children's Hospital Medical Center) to generate the pMHC-Luc vector. The αMHC promoter is a mouse αMHC promoter in pBluescript SK(+) vector (Stratagene, Agilent Technologies, La Jolla, CA, USA) with a 23 bp linker region and a human growth hormone poly A tail. The pCMVe Luc plasmid was generated by inserting the Luc cDNA into the pCMVe vector, which contains a CMV enhancer region, CMV promoter, CMV exon/intron/A region and an RU5 enhancer sequence, followed by a multi-cloning site and a BGH polyA tail. The human SDF-1 gene was cloned from the mRNA of human foreskin fibroblasts using the primers, forward: 5′-GCTAGCGTCGACATGAACGCCAAGGTCGTGGTCGTGCTGGTC-3′ reverse: 5′-AAGCTTTTACTTGTTTAAGGCTTTCTCCAGGTACTCCTGAAT-3′. The Luc gene was subcloned out of the pcDNA 3.1(+) vector and the SDF-1 cDNA was inserted in by using a NheI/HindII digest, to generate the pCMV SDF-1; and used SalI/HindIII to generate the pMHC SDF-1. The pCMVe SDF-1 vectors were derived by performing PCR of the SDF using the primers. Forward: 5′-GCTAGCGTCGACAAGCTTGCCACCACCATGAACGCCAAGGTCGTGGTC-3′ reverse: 5′-TCTAGATTACTTGTTTAAGGCTTTCTCCAGGTACTCCTGAAT-3′, digesting with HindIII/XbaI and ligating into the pCMVe. Plasmid DNA was prepared using the PureLink HiPure Plasmid Filter Maxiprep Kit (Invitrogen) as per the manufacturer's instructions.
Cell transfection and luciferase expression
Plasmids were transfected into HEK293A cells or H9C2 cardiomyoblasts at 50% confluence at 300 000 cells per well in a six-well plate using the FUGENE Transfection kit (Roche, Basel, Switzerland) as per the manufacturer's instructions. The cells were exposed to luciferin at 10 μg per ml of PBS per well and the chemiluminescence emitted was quantified using a cooled couple device camera from the Xenogen Imaging Systems (Alameda, CA, USA).
Experimental animals
All animal experiments were approved by the Institutional Animal Care and Use Committee and followed the guidelines provided by them. All animals were 8 weeks old, weighing 200–225 grams, Lewis rats (Jackson Laboratories, Bar Harbor, ME, USA) for the chronic heart failure study. DNA (500 μg) was diluted into PBS for a total volume of 500 μl. Sample (400 μl) was used for each animal with 100 μl per injection site, for four injection sites, at the border zone.
LAD ligation
Briefly, LAD ligation was performed in rats anesthetized with sodium pentobarbital, 50 mg kg−1. Animals were intubated and ventilated with room air at 100 breaths per minute using a rodent ventilator (Harvard Apparatus, Holliston, MA, USA). The chest was sterilized with ethanol, and a sternotomy was performed. The left atrium was retracted and the proximal LAD was identified visually. The proximal LAD was ligated using 6-0 prolene (Ethicon, Somerville, NJ, USA). We were able to verify ligation of the LAD by blanching and dysfunction of the anterior wall. The sternum and then skin was closed with 4-0 prolene (Ethicon) with interrupted sutures. Any residual pneumothrorax was reduced using negative pressure generated by a 20-G needle placed in the closed chest, attached to a 10 ml syringe. The rat was then weaned from the ventilator over the next 10–15 min.
Intramyocardial gene delivery
To inject the DNA, the chest was reopened 4 weeks after MI, applying the same procedure as above. DNA (100 μg) solution in 100 μl of saline per injection site was injected using a 30-gauge needle, for a total of four injection sites around the border zone. The border zone was identified by the blanched region around the LAD identified by dyskinetic motion of the anterior wall. The DNA was injected into the wall and distention of the tissue was observed when the DNA was injected. The chest was closed as mentioned above for the LAD ligation.
Animal imaging
To determine the optimal plasmid dose required for efficient myocardial transfection, we injected naked plasmid DNA-encoding luciferase (pCMV-luc) immediately after ligation of the LAD in Lewis rats. Plasmid injections in various doses ranging from 0 to 500 μg of plasmid in a total volume of 100 μl in saline were administered in the infarct border zone, which was identified by the blanching of the tissue. The luciferin substrate for chemiluminescent imaging was administered intraperitoneally. The animals were routinely measured for luciferase expression using a cooled couple device camera from the Xenogen Imaging Systems. The animals were anesthetized using 2% isofluorane and luciferin was injected i.p. at a concentration of 125 mg kg−1 of the animal. After 10 min, real-time images were obtained to determine the whole-body chemiluminescence of luciferase expression.
Physiological analysis of left ventricular function
We routinely perform 2-D echocardiography on rats using a 15-MHz linear array transducer interfaced with an Acuson Sequoia C256 (Siemens, Munich, Germany). The animals were sedated with ketamine, the chest was shaved and the sedated animal maintained in a supine position. For quantification of LV dimensions and wall thickness, we digitally record 2-D clips and m-mode images in a short-axis view from the mid-LV just below the papillary muscles. This anatomical location was chosen to consistently obtain measurements from the same anatomical location in different mice. Measurements were taken offline by using ProSolv (Indianapolis, IN, USA). Each measurement in each animal was made six times, from three randomly chosen m-mode clips out of five. As a measure of LV functions, shortening fractions was calculated from m-mode studies.
Shortening fraction: (LVEDD-LVESD)/LVEDD × 100 where,
LVEDD, LV end-diastolic dimension;
LVESD, LV end-systolic dimension.
Dimensions were measured between the anterior and the posterior walls from short-axis view just below the papillary muscle. LV mass was calculated by
LV mass=1.05 × ((anterior wall thickness+LVEDD+posterior wall thickness)3−LVEDD3).
Immunohistochemistry
At the time of necropsy, 8 weeks after the injection of the plasmid, the animals were perfused with saline and histochoice (AMRESCO, Solon, OH, USA) and the hearts were collected. The hearts were fixed in histochoice for 7 days and then embedded in paraffin. Hearts were then sectioned and stained with hemotoxylin and eosin staining for identifying the infarct. Mason's trichrome was used to quantitate the collagen content. Fibrosis size or scar tissue was deduced by the percentage of area with collagen as compared with the total area. Tissues were fixed in formalin and embedded in paraffin blocks according to established protocols. Antigen retrieval was performed using 10 m sodium citrate buffer (pH 6.0) and heat at 95 °C for 5 min. The buffer was replaced with fresh buffer and reheated for an additional 5 min, then cooled for 20 min. The slides were washed in deionized water three times for 2 min each. Specimens were then incubated with 1% normal blocking serum with goat and donkey serum in PBS for 60 min to suppress nonspecific binding of immunoglobulin G. Slides were then incubated for 60 min with rabbit polyclonal von Willebrand factor (Abcam, Cambridge, MA, USA) at 1:100 dilutions in blocking buffer with serum. Myosin was stained with mouse monoclonal LV myosin (Millipore, Billerica, MA, USA) at a dilution of 1:10 in blocking buffer with serum. Optimal antibody concentration was determined by titration. Slides were then washed with PBS and incubated for 45 min, with immunoglobulin G donkey anti-rabbit Alexa fluor 594 (Molecular Probes, Invitrogen) at 1:800 dilution in blocking buffer with serum and immunoglobulin G goat anti-mouse AlexaFluor 488 (Molecular Probes, Invitrogen) at 1:800 dilution in blocking buffer with serum, respectively, in a dark chamber. After washing extensively with PBS, coverslips were mounted with aqueous mounting medium. (Vectashield Mounting Medium with DAPI, H-1200; Vector Laboratories, Burlingame, CA, USA). For arteriole imaging, a mouse monoclonal α-sarcomeric actin antibody was used at a 1:200 dilution (Sigma, St Louis, MO, USA).
Confocal microscopy
Tissues were analyzed using an upright spectral laser scanning confocal microscope (Model TCS-SP; Leica Microsystems, Heidelberg, Germany) equipped with blue argon (for DAPI), green argon (for Alexa Fluor 488) and red krypton (for Alexa Fluor 594) lasers. Data were collected by sequential excitation to minimize ‘bleed-through.' Image processing, analysis and the extent of colocalization were evaluated using the Leica Confocal software (Leica). Optical sectioning was averaged over four frames, and the image size was set at 1024 × 1024 pixels. No digital adjustments were made to the images.
Statistical analysis
All results are expressed as mean±s.e.m. Statistical analysis was performed by using Student's t-test.
Acknowledgments
This work was funded by the Skirball Foundation and Juventas Therapeutics, Inc.
Matthew Kiedroski and Marc S Penn are listed as inventors on patent applications filed by the Cleveland Clinic for the use of SDF-1 for the prevention and treatment of cardiac dysfunction. Each has equity and consults for Juventas Therapeutics, Inc., which has licensed these patent applications from the Cleveland Clinic. Dr Miller, Dr Aras and Dr Pastore are employees of Juventas Therapeutics, Inc. and receive salary and equity from the company.
References
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7 (Suppl 3:86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal-cell-derived factor-1alpha (SDF-1alpha) treatment. Regul Pept. 2005;125:1–8. doi: 10.1016/j.regpep.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- Agbulut O, Mazo M, Bressolle C, Gutierrez M, Azarnoush K, Sabbah L, et al. Can bone marrow-derived multipotent adult progenitor cells regenerate infarcted myocardium. Cardiovasc Res. 2006;72:175–183. doi: 10.1016/j.cardiores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, et al. Stromal cell-derived factor-1alpha in unstable angina: potential anti-inflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, et al. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis. 2008;201:36–42. doi: 10.1016/j.atherosclerosis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Deglurkar I, Mal N, Mills WR, Popovic ZB, McCarthy P, Blackstone EH, et al. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Hum Gene Ther. 2006;17:1144–1151. doi: 10.1089/hum.2006.17.1144. [DOI] [PubMed] [Google Scholar]
- Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Wang T, Jiang XJ, Tang QZ, Li XY, Lin T, Wu DQ, et al. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5:2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Thai HM, Juneman E, Lancaster J, Hagerty T, Do R, Castellano L, et al. Implantation of a three-dimensional fibroblast matrix improves left ventricular function and blood flow after acute myocardial infarction. Cell Transplant. 2009;18:283–295. doi: 10.3727/096368909788535004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- Muller OJ, Katus HA, Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc Res. 2007;73:453–462. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering JG, Jekanowski J, Weir L, Takeshita S, Losordo DW, Isner JM. Liposome-mediated gene transfer into human vascular smooth muscle cells. Circulation. 1994;89:13–21. doi: 10.1161/01.cir.89.1.13. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Tsurumi Y, Couffinahl T, Asahara T, Bauters C, Symes J, et al. Gene transfer of naked DNA encoding for three isoforms of vascular endothelial growth factor stimulates collateral development in vivo. Lab Invest. 1996;75:487–501. [PubMed] [Google Scholar]
- Shah PB, Losordo DW. Non-viral vectors for gene therapy: clinical trials in cardiovascular disease. Adv Genet. 2005;54:339–361. doi: 10.1016/S0065-2660(05)54014-8. [DOI] [PubMed] [Google Scholar]
- Herweijer H, Zhang G, Subbotin VM, Budker V, Williams P, Wolff JA. Time course of gene expression after plasmid DNA gene transfer to the liver. J Gene Med. 2001;3:280–291. doi: 10.1002/jgm.178. [DOI] [PubMed] [Google Scholar]
- Inubushi M, Wu JC, Gambhir SS, Sundaresan G, Satyamurthy N, Namavari M, et al. Positron-emission tomography reporter gene expression imaging in rat myocardium. Circulation. 2003;107:326–332. doi: 10.1161/01.cir.0000044385.60972.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]