Abstract
Age has a multifaceted impact on neural measures which are not always directly related to alterations in clinical and cognitive measures. This partial protection from the deleterious effects of age in some individuals is referred to as cognitive reserve (CR) and although linked to variations in intelligence and life experiences, its mechanism is still unclear. Within the framework of a theoretical model we tested two potential mechanistic roles of CR to maintain task performance, neural reserve and neural compensation, in young and older adults using functional and structural MRI. Neural reserve refers to increased efficiency and/or capacity of existing functional neural resources. Neural compensation refers to the increased ability to recruit new, additional functional resources. Using structural and functional measures and task performance, the roles of CR were tested using path analysis. Results supported both mechanistic theories of CR and the use of our general theoretical model.
Keywords: Aging, cognitive reserve, multi-modal neuroimaging, path analysis, function, structure
Introduction
The population is quickly growing older, making it essential that we better understand the multi-faceted impact of aging on the brain. With advancing age come a variety of neural changes (e.g. in gray-matter volume (GMV), cortical thickness, white-matter integrity, cerebral blood flow, and functional activity). The relationships between such age-related neural changes and the clinical and cognitive expression of those changes are not entirely clear. Complicating the understanding of these relationships is the fact that some individuals show evidence of severe cognitive decline with advancing brain alterations, while others are seemingly able to cope with such changes without significant clinical or cognitive expression (Roe, et al., 2008; Y. Stern, et al., 1995). The concept of some individuals being partially protected from the deleterious effects of advancing age-related neural changes has been termed cognitive reserve (CR) (Y. Stern, 2002, 2009). Cognitive reserve is a model suggesting that “individual differences in cognitive processes or neural networks underlying task performance allow some people to cope better than others with brain damage” (Y. Stern, 2009).
Variability in CR may result from differences in innate intelligence or life events and experiences, such that current and past exposures have an impact on how the brain copes with progressive pathology. This suggests that through better understanding of the mechanisms of CR one may gain better insight into which behaviors have the largest positive neural impact. Such information will be necessary to guide behavioral interventions to maximize their neural impact and subsequently maintain cognitive and clinical outcomes with increasing age.
Two potential mechanisms of CR have been termed neural reserve and neural compensation (Y. Stern, 2009). Neural reserve is the concept that CR manifests itself as inter-individual variability in levels of efficiency, or capacity of existing functional brain networks. This enables individuals with high CR to be more capable of coping with brain pathology’s negative effect. Neural compensation is the concept that CR manifests itself as the inter-individual variability in the brain’s facility to withstand interruption of standard processing networks by employing alternate brain networks. This suggests that a potential mechanism of CR is a general network of brain activity that is independent of task demands and supports task performance in the face of age-related structural and/or functional brain changes (Y. Stern, et al., 2008).
In a previous fMRI experiment conducted by our group, two functional brain networks were identified which were differentially utilized by young and older age groups during the information retention phase of a verbal delayed item recognition (DIR) cognitive task (Zarahn, Rakitin, Abela, Flynn, & Stern, 2007). While both age groups utilized the primary functional network (network 1), the elders used it to a greater extent. The second functional network (network 2) was differentially used by the two age groups. The older adults used (expressed) network 2, while the young adults, as a group, did not. As task demands increased, older adults had increased expression of network 1, resulting in increased expression of network 2 which was associated with slower performance. This suggests that aging impacted functional activity, which in turn affected task performance. The differential expression of the functional networks was later shown to be related to their underlying brain anatomy (Steffener, Brickman, Rakitin, Gazes, & Stern, 2009): increased utilization of network 2 was significantly related to the reduced GMV within the left pre-central gyral region of functional network 1. This provided support for the idea that utilization of network 2 was related to the structural integrity of network 1. These studies highlighted how age-related differences in task performance were related to functional activity and the underlying neural structure.
Our previous work did not investigate whether CR influenced the relationships between task performance, functional activity and the underlying neural structure. It is possible that CR is related to increased efficiency of functional networks, supporting the idea of neural reserve. We could also seek support for the idea of neural compensation. Neural compensation suggests that people with higher CR might be able to better recruit some compensatory functional resources that help maintain performance when the standard functional resources are impaired by pathology. In this case CR might act via the recruitment of additional resources to moderate the effect that damage to the existing/standard networks has on task performance. That is, even when the networks are damaged by pathology and individuals are performing the task with the a less than optimal network setup (high expression of both functional networks), people with higher CR may be able to recruit additional resources that allow then to maintain better performance than those with low CR.
Based on previous work in our laboratory and largely laid out in Stern (2009) we developed a conceptual path model, Figure 1, which allows us to test the role of CR in our previous findings. This simplified model merges three areas of aging research to better understand age effects on the brain and the potential role that CR has. The path arrows represent some of relationships that may exist between the variables but is not exhaustive, e.g. this diagram does include potential modulating relationships.
Figure 1.
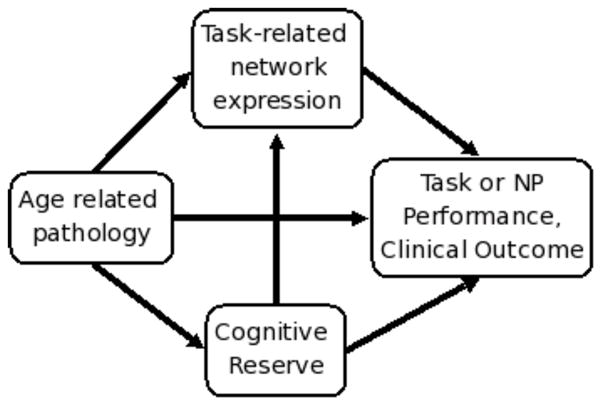
A conceptual model of potential interactions between brain structure, functional activity, their relationship to cognitive outcome and the potential roles of cognitive reserve. This simplified model is not exhaustive in showing all of the potential relationships between the variables but includes paths based on our previous findings: ‘pathology → network expression’ (Steffener, et al., 2009) and ‘network expression → task performance’ (Zarahn, et al., 2007), the model includes 'CR → network expression' supporting the idea that CR influences the expression of the functional networks; ‘pathology → CR’ supporting the concept that at some point pathology must become too severe to support the processes that underlie CR (Y. Stern, 2009). In addition, the path ‘CR → performance’ supports the idea that CR may be operating via its own common network, which is unrelated to the expression of the task-related functional networks supporting task performance (Y. Stern, et al., 2008) and the path ‘pathology → performance’ supports the idea that pathology may be affecting performance in a manner not captured by the other factors included in the model.
Using the conceptual model as a guide, we used a specific instantiation to test the role of CR. First, a path analysis model was tested that encapsulated all of the relationships identified in our previous studies, including the relationships of individual expression of the two functional MRI networks to both volumetric measures from structural MRI as well as a measure of cognitive performance (Steffener, et al., 2009; Zarahn, et al., 2007). Potential paths including CR were then included in the model. A significant relationship between the CR variable and the expression of the functional networks would provide support for the idea of neural reserve. A significant modulating effect of CR on the relationship between the expression of the functional networks and task performance would provide support for the idea of neural compensation. Based on previous findings that suggest that CR allows function to be maintained in the presence of age-related brain changes, we expected to find support for neural reserve.
Materials and Methods
The current report extends previously published findings to investigate the role of CR (Steffener, et al., 2009; Zarahn, et al., 2007). Below is a review of the methods with greater detail found in the original two papers.
Study Participants
The current study used the same participants used in the previous study (Steffener, et al., 2009), which is a subset of the original study (Zarahn, et al., 2007). This study includes data from thirty-seven healthy, young participants (out of the original 40)(29 men and 8 women; mean (± s.d.) age = 25.0 ± 3.9; mean (± s.d.) years of education = 15.6 ± 1.4; all right handed), and 15 healthy, elderly participants (out of the original 18)(7 men and 8 women; mean (± s.d.) age = 74.5 ± 6.9; mean (± s.d.) years of education = 15.5 ± 2.4; all right handed). All participants were screened with structured medical, neurological, psychiatric, and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. The screening procedure included a detailed interview that excluded individuals with a self-reported history of major or unstable medical illness, significant neurological history (e.g. epilepsy, brain tumor, stroke), history of head trauma with loss of consciousness for greater than 5 min, history of Axis I psychiatric disorder (Association, 1994). Individuals taking psychotropic medications were excluded. Global cognitive functioning was assessed with a modified version of the Folstein Mini Mental State Examination (mMMS: (Y. Stern, Sano, Paulson, & Mayeux, 1987)), which has a maximum score of 57. All participants were classified as non-demented and without clinically significant cognitive impairment, although the elder group had lower scores than the young group (young mean (± s.d.) mMMS total = 55.2 ± 1.5; elder mean (± s.d.) mMMS total = 53.3 ± 2.6, t (18.1) = 2.68, p = .015)). IQ was estimated with the American version of the New Adult Reading Test (NART: (Nelson & O'Connell, 1978)). Although the group differences for mMMS were significant it was not clinically meaningful.
FMRI Behavioral Task
Working memory was examined in all participants using a delayed-item-recognition (DIR) task with 1, 3 or 6 (study) letters visually presented for 3s followed by a 7s unfilled delay period (Rypma & D'Esposito, 1999; Sternberg, 1966; Zarahn, et al., 2007). Following the delay period, a single (probe) letter was presented and participants decided whether or not it was included in the initial set of letters. Based on previous findings, the cognitive performance measure of slope of reaction times with respect to memory load was used (sRT). The sRT is the variable indicating the speed with which the probe is compared to each member of the study set in a serial search (Sternberg, 1966).
Functional Image Analysis
Group-level analysis of BOLD image data used multivariate linear modeling (MLM: (Worsley, Poline, Friston, & Evans, 1997)) to identify significant load-dependent networks, or covariance patterns, comprising latent spatial variables engaged by the young and older groups. Once calculated, the spatial patterns were multiplied voxel-wise by the participant specific load dependent maps that were entered into the MLM analysis, then summed to calculate each participant’s network expression (Zarahn, et al., 2007). These network expression scores served as independent measures describing the degree to which each participant used, or expressed, a significant spatial pattern. The measures of task-related functional activity were the individual expression of the two functional networks during the information retention phase of a verbal DIR cognitive task.
Structural Image Analysis
Modulated, spatially normalized gray-matter probability maps, (gray-matter volume maps) (Good, et al., 2001), were intensity thresholded at 10% (Busatto, et al., 2003). The images were spatially smoothed and used for voxel based statistical parametric mapping using SPM5 (Wellcome Department of Cognitive Neurology). General linear modeling tested whether the expression of network 2 was associated with regional gray-matter volume within network 1 (see Steffener, et al., 2009). It was found that only a region within the left pre-central gyrus demonstrated a significant relationship between expression of network 2 and GMV. This cluster of 91 significant voxels was averaged over all voxels to create the structural measures of region of gray-matter volume (rGMV). In addition normalized whole brain volume (nWBV) was used as a structural measure and was the volume of gray- and white-matter divided by the summed volume of gray-matter, white-matter and cerebrospinal fluid (Fotenos, Snyder, Girton, Morris, & Buckner, 2005).
Cognitive Reserve Factor
Cognitive reserve was represented as a factor score summarizing years of education and scores on two IQ indices, the NART (Nelson & O'Connell, 1978) and WAIS-R vocabulary score (Wechsler, 1987). The factor weightings were determined from a set of 168 young and 97 elder participants from other studies conducted in our laboratory.
Path Models
Initially, a model of the relationships between the structural, functional and task performance measures without CR was tested, Model A in Figure 2. After establishing the significant relationships in the absence of CR, two models of CR were tested. Model B tested whether CR took on the role of neural reserve by testing whether CR influenced the expression of the functional networks which led to changes in task performance; therefore, the indirect effect of CR on task performance via the functional networks was tested. Model C tested whether CR took the role of neural compensation by testing whether the previously identified relationship between functional network expression and task performance was modulated by an individual's level of CR. Both models B and C included the direct path between CR and task performance. The models included the two age groups; separate parameter estimates for each group were calculated and compared.
Figure 2. Testing the mechanism of CR in the current instantiation of the theoretical model.
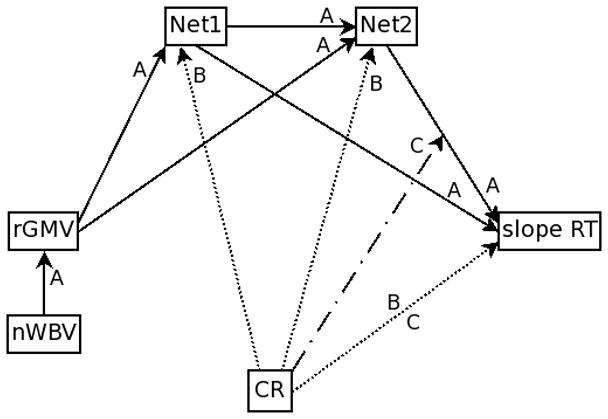
Full path diagram showing the models tested. Solid lines (labeled A) are those paths tested in Model A and are used to confirm the relationships between the structural, functional and performance measures. The dashed line (labeled B) is the included path to test model B, which tests for the neural reserve mechanism of CR. Dash-dotted lines (labeled C) represent the testing of model C, which tests for the neural compensation mechanism of CR.
Several statistics determined the fit of the path models: chi-square (χ2), root mean square error of approximation (RSMEA) and the comparative fit index (CFI) (Hu & Bentler, 1998; MacCallum, Browne, & Sugawara, 1996). Significance of path coefficients were assessed using 1000 bootstrap permutations and the bias-corrected percentile method (MacKinnon, Lockwood, & Williams, 2004). Model comparisons were made using the Bayes Information Criteria (BIC) and the Akaike Information Criteria (AIC). Path analyses were performed using AMOS software (Arbuckle, 2007) as implemented in SPSS release 17 (SPSS Inc. Chicago, Illinois).
Results
Summaries of means, standard deviations and bivariate correlations for all variables used in this analysis are in Tables 1 and 2. The older adults had decreased regional and whole brain gray matter volume. Older adults also had slightly lower mean NART and vocabulary scores, but these IQ measures were above average in both age groups and the difference posed no clinical significance. Expression of both functional networks differed significantly between the two age groups, with increased expression in the older group. Task performance was significantly poorer in the older group.
Table 1.
Descriptive data for variables used in path model.
| Mean (S.D.)
|
||
|---|---|---|
| (n = 37) | (n = 15) | |
| Y | E | |
| Age | 25.0(3.93)**a | 74.5(6.95) |
| rGMV | 0.46(0.052)** | 0.33(0.068) |
| nWBV | 0.80(0.040)** | 0.64(0.041) |
| CR | 0.19(0.83)* | −0.46(1.05) |
| NART | 120.38(6.14)* | 116.13(6.79) |
| Vocab. | 14.51(2.78)**b | 12.27(1.94) |
| Edu. | 15.62(1.42) | 15.53(2.44) |
| Net1 | 0.10(0.081)* | 0.17(0.080) |
| Net2 | −0.050(0.12)** | 0.16(0.13) |
| s RT | 56.6(29.5)** | 90.0(35.6) |
Note:
p < 0.05;
p < 0.01; df = 50 except for
df = 17.8 and
df = 36.89.
Table 2.
Correlations between variables used in path model; Young: Above midline, Elders: Below midline.
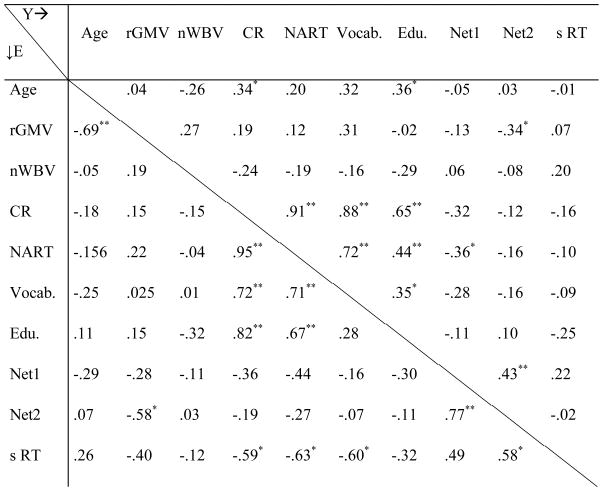
|
Note:
p < .05;
p < .01
Model A: The relationships between structure, function and performance
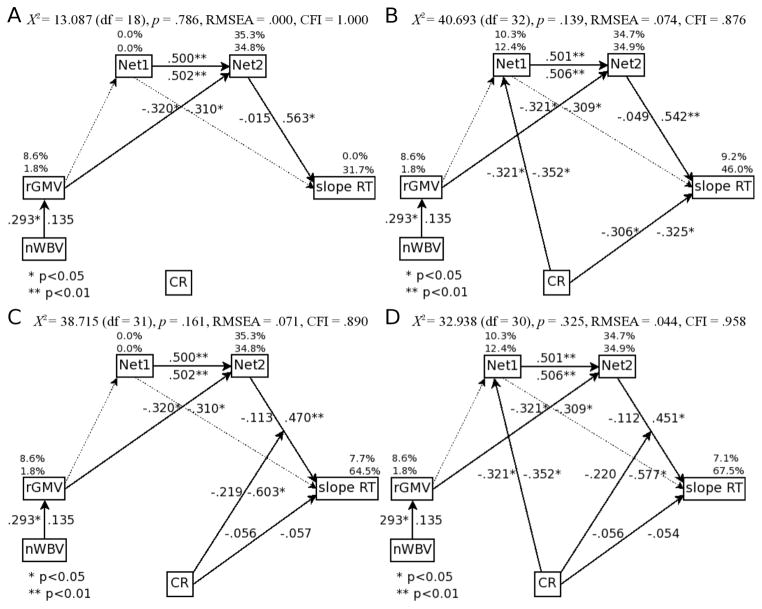
The first path model tested (model A), included structural MRI measures, expression of both functional MRI networks and the task performance variable, see Figure 3A. As determined from previous work, this model constrained the relationship between rGMV and Network 1 to be zero and the relationship between rGMV and Network 2 to be equal across age groups (Steffener, et al., 2009). The path from network 1 to the performance measure was non-significant and constrained to zero. This model significantly fit the data and resulted in no non-significant paths (χ2=13.087 (df=18), p=.786; RMSEA=.000; CFI=1.000; AIC = 37.087; BIC = 48.952). This reduced model is summarized as follows: nWBV had a positive direct effect on rGMV for the young adults but not the elders (higher nWBV was associated with higher rGMV); rGMV had a significant negative direct effect on network 2 in both age groups (more rGMV was related to lower expression of network 2); network 1 had a significant positive direct effect on network 2 for both age groups (greater expression of network 1 was associated with greater expression network 2); network 2 had a significant positive direct effect on slope RT in the elders but not the young adults (greater expression of network 2 resulted in longer RT with increasing memory load).
Figure 3. Path model results for cognitive reserve.
A) Confirmatory model of the relationships between the structural, functional and performance measures. B) Model results for testing of neural reserve as a mechanism of CR. C) Model results for testing of neural compensation as a mechanism of CR. D) Combination of neural reserve and neural compensation as joint models of CR. Values on path arrows are standardized regression coefficients of direct relationships. Percentage values above variable boxes are the variance accounted for. In all cases the left or upper number is for the young age group and the right/lower for the older age group. Solid lines represent paths in the final model, light, dotted lines represent paths that were initially included in the models but dropped in the final model due to non-significant path weights. Abbreviations: Net1 and Net2: individual expression of the two functional networks; RT: reaction times; slope: increase in measure with increasing memory load demands; inter: intercept of measure across memory load demands; CR: cognitive reserve; rGMV: regional gray-matter volume within the left pre-central gyrus. nWBV: normalized whole brain volume.
Model B: The neural reserve model of cognitive reserve
Starting with the reduced model A path diagram, the neural reserve model of CR was tested within the same path diagram. This tested whether there was an indirect effect of CR on task performance via the functional networks. This indirect effect was the same between age groups and resulted in a significant model fit, see Figure 3B (χ2=40.693 (df=32), p=.139; RMSEA=.074; CFI=.876; AIC = 88.693; BIC = 128.880). To summarize, higher CR resulted in decreased expression of network 1 (i.e. greater efficiency) in both age groups; decreased expression of network 1 led to decreased expression of network 2 in both age groups; decreased expression of network 2 led to improved task performance (i.e. less increase in RT as memory load increased) in the older adults. That is, cognitive reserve had a significant indirect effect in the older adults on task performance due to it lowering the expressions of networks 1 and 2 which resulted in a lower negative impact on task performance.
In addition, the previously reported negative indirect effect of rGMV on task performance in the older adults (greater rGMV was related to a smaller impact of memory load on RT via network 2) was still significant in this model, although its indirect effect on sRT is now shown to be influenced by CR. Thus, at any level of local atrophy, individuals with higher CR can sustain reduced expression of network 2 and therefore preserve task performance.
Model C: The neural compensation model of cognitive reserve
Model C tested whether the relationship between functional network expression and task performance was modulated by an individual's levels of CR. Cognitive reserve moderated the effect of network 2 on task performance in the elder group but not the young and resulted in a significant model fit, see Figure 3C (χ2=38.715 (df=31), p=.161; RMSEA=.071; CFI=.890; AIC = 88.715; BIC = 130.576). Thus, increased CR led to a decreased impact of expression of network 2 on task performance. To summarize, higher CR led to a decreased negative impact of increased expression of the functional networks on task performance in the older adults. Once this CR modulating effect was included in the model there was no longer any direct effects of CR on task performance in either age group. Therefore, older adults with high CR were better able to maintain task performance even when increased local atrophy is decreasing the efficiency of their functional networks.
Model D: Combined neural reserve and neural compensation as a model of cognitive reserve
In order to determine if CR could be playing a role of maintaining efficiency of functional networks and modulating the effects of functional network expression on task performance, Models B and C were combined to form Model D. In the overall combined model the indirect effect of CR on task performance via the functional networks and the modulating effect of CR on the relationship between functional network expression and task performance both remained significant in the older adults and resulted in a significant model fit, see Figure 3D (χ2= 32.938 (df=30), p=.325; RMSEA=.044; CFI=.958; AIC = 84.938; BIC = 128.474). Standardized indirect effect sizes and significance for both age groups are in Table 3. To summarize, increased CR in the older adults maintained task performance due to it lowering the expressions of networks 1 and 2 which resulted in a lower negative impact of local atrophy on task performance. CR additionally allowed older adults to better maintain task performance even when increased local atrophy is decreasing the efficiency of their functional networks.
Table 3.
Standardized Indirect effects for model D
| E | Y | ||
|---|---|---|---|
| Net1 | sRT | 0.228** | −.056 |
| CR | sRT | −0.080** | .018 |
| rGMV | sRT | −0.139* | .036 |
| nWMV | sRT | −0.019 | .011 |
| CR | Net2 | −0.178** | −.161* |
| nWBV | Net2 | −0.042 | −.094* |
Note:
p < .05;
p < .01
Discussion
Cognitive reserve had significant effects on memory load-related speeded task performance (sRT). Two proposed mechanisms of CR (neural reserve and neural compensation) were supported in the older adults and only the neural reserve mechanism in the young adults.
In the final path model, the measure of age-related atrophy (rGMV) in network 1 was related to the expression of network 2. We have posited that decreased structural integrity within the primary functional network (network 1) resulted in the reliance on less-efficient functional resources to maintain task performance (network 2 (Steffener, et al., 2009)). As we reported, expression of network 2 increased as network 1 became more inefficient due to higher gray matter atrophy. Cognitive reserve acted as a mechanism to maintain the efficiency of network 1 in the presence of the atrophy: those with low CR used their functional resources to a greater extent (higher expression of the networks) for equal performance as compared to those with high CR, whose functional networks maintained efficiency and were expressed to a lower degree. Thus CR acted to maintain efficiency of the functional networks in the presence of atrophy. Therefore, in the older adults CR had an indirect effect on task performance by altering the efficiency of the functional networks. This finding is concordant with the concept of neural reserve, which posits that CR could maintain performance in the face of brain changes via the differential efficiency or capacity of existing networks.
We also found that within the older adults the relationship between functional network expression and task performance was modulated by an individual's level of CR. Those older adults with greater CR demonstrated less of a negative impact of the expression of network 2 on task performance. The underlying neural source of this moderating effect of CR is not clear, but by definition (of moderation) it cannot be differential activation of network 2. Instead CR must be acting via some other, unidentified, functional neural mechanism that helps support performance even as expression of network 2 increases. This finding could be consistent with the concept of neural compensation (Y. Stern, 2009) in that CR may be associated with the activation of a new network that allows performance to be maintained in the face of alterations to the networks that typically support performance (in this particular case networks 1 and 2). Such a CR related compensatory network could be particular to performance of the present task or CR might be acting here through a more generalized network that would support performance of many different tasks in the face of brain pathology.
Returning to our initial conceptual model, our data support the notion that in the current case CR had indirect effects on task performance through the expression of functional networks and minimal direct effects on task performance. This conceptual model, and the presented results, incorporates three diverse modalities of aging research, cognitive testing, structural neuroimaging and functional neuroimaging, into one unified conceptual framework, garnering a more complete view of the aging process. With more complete understanding of the aging mechanisms and the role of CR, targets of intervention for forestalling cognitive decline may be developed. One example of a possible intervention is aerobic exercise. The beneficial effects of exercise in older adults are apparently ubiquitous ,having been related to increased cognitive performance (Fabre, Chamari, Mucci, Masse-Biron, & Prefaut, 2002), decreased rates of dementia (Laurin, Verreault, Lindsay, MacPherson, & Rockwood, 2001), increased neural functioning (Colcombe, et al., 2004), decreased rates of atrophy (Colcombe, et al., 2003) and increased CR (Scarmeas & Stern, 2003). Within the conceptual model, the role of an exercise intervention may be tested to identify how the diverse effects of exercise affect the brain and cognitive performance leading to potentially more efficient means of intervention.
The presented conceptual model theorizes the relationships between measurable quantities making it very general and easily used with multiple data sets and across laboratories. We provide supportive evidence with a single instantiation of the model and continue to use this model as a starting point for development of ideas related to the role of CR. The greatest benefit of the conceptual model is its generalizability, in that each of its nodes can be operationalized differently without altering the overall structure or the theoretical underpinnings of the model. The structural measures are expandable to include measures of white matter integrity, blood flow, cortical thickness, or amyloid burden and additional measures of functional activity may be included (e.g. measures from other tasks or activity within regions of interest). Although our measure of CR was based on education and IQ scores, this could be expanded to include other factors (e.g. lifetime occupation (Y. Stern, et al., 1995), or life time cognitive activities (Wilson, et al., 2002). In addition, it could include expression of task non-specific networks related to CR (Y. Stern, et al., 2008). Finally, we used a direct measure of activation-task performance as our clinical outcome variable which might be represented with other indices of current cognitive/functional capacity or clinical outcomes (such as cognitive decline or incident dementia).
A limitation in this study is the small number of participants, especially in the older age group. To minimize the impact this has on our findings, path significance were assessed using the bias-corrected percentile method with 1000 bootstrap estimates (MacKinnon, et al., 2004). This approach accurately assessed statistical significance even in the face of potential non-normal distributions resulting from the small sample sizes. Future works aims to replicate these findings in larger samples.
The implementation of the model incorporated previous findings from neuroimaging studies conducted in our laboratory, where consecutive studies independently tested one or two pathways of the model. To test the model as a whole, the current path analyses incorporated many of the findings in a single analysis with the additional inclusion of CR. Ongoing work in our group continues to use this conceptual model to integrate information across these different domains. We offer this approach as particularly useful for multi-modal studies of the aging phenomenon. This approach provides a straightforward way of assimilating multi-modal imaging data with clinical and performance measures. Its implementation can provide greater insight into the age-related neural alterations that lead to cognition changes and illuminate how CR mediates their effects.
Acknowledgments
This study was supported by National Institute of Aging grant 5R01AG026158-5 awarded to Y.S. and National Institute of Aging grant 1K01AG035061 awarded to J.S.
References
- Arbuckle JL. AMOS 16.0. Chicago, IL: SPSS; 2007. [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Busatto GF, Garrido GEJ, Almeida OP, Castro CC, Camargo CHP, Cid CG, et al. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiology of Aging. 2003;24(2):221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23(6):415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Fit Indices in Covariance Structure Modeling: Sensitivity to Underparameterized Model Misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods. 1996;1(2):130–149. [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14(2):234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer Disease and Cognitive Reserve: Variation of Education Effect With Carbon 11-Labeled Pittsburgh Compound B Uptake. Arch Neurol. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A. 1999;96(11):6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Brickman A, Rakitin B, Gazes Y, Stern Y. The Impact of Age-Related Changes on Working Memory Functional Activity. Brain Imaging and Behavior. 2009;3(2):142–153. doi: 10.1007/s11682-008-9056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, et al. Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45(1):55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability. Neurology 1987 [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18(4):959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the Response of PET and fMRI Data Using Multivariate Linear Models. NeuroImage. 1997;6(4):305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28(5):784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]