Abstract
Evening bright light exposure is reported to ameliorate daytime sleepiness and age-related sleep complaints, and also delays the timing of circadian rhythms. We tested whether evening light exposure given to older adults with sleep-wake complaints would delay the timing of their circadian rhythms with respect to their sleep timing, thereby reducing evening sleepiness and improving subsequent sleep quality. We examined the impact of evening light exposure from two different light sources on subjective alertness, EEG activity during wakefulness, and sleep stages.
Ten healthy older adults with sleep complaints (mean age=63.3 yrs; 6F) participated in a 13-day study. After three baseline days, circadian phase was assessed. On the evening of days 5–8 the subjects were exposed for 2 h to either polychromatic blue-enriched white light or standard white fluorescent light, and on the following day circadian phase was re-assessed. Subjects were allowed to leave the laboratory during all but the two days when the circadian phase assessment took place. Evening assessments of subjective alertness, and wake and sleep EEG data were analyzed.
Subjective alertness and wake EEG activity in the alpha range (9.75–11.25 Hz) were significantly higher during light exposures when compared to the pre-light exposure evening (p<0.05). The light exposures produced circadian phase shifts and significantly prolonged latency to rapid eye-movement (REM) sleep for both light groups (p<0.05). The increase in wake EEG alpha activity during the light exposures was negatively correlated with REM sleep duration (p<0.05).
Evening light exposure could benefit older adults with early evening sleepiness, without negatively impacting the subsequent sleep episode.
Keywords: sleep, circadian, aging, light exposure
1. Introduction
1.2 Age-related changes in sleep and wakefulness
Sleep patterns profoundly change across the human lifespan [1], and aging is associated with impaired sleep propensity and sleep continuity, and advanced (earlier) bed- and wake times [2]. The overall reduction of deep sleep, i.e. slow wave sleep (SWS) and slow-wave activity (SWA), together with problems remaining asleep in the early morning hours, are typical age-associated changes that occur even in healthy older adults. These clinical and laboratory observations provide objective evidence which support the common sleep complaints of early morning awakening and difficulty maintaining sleep (sleep maintenance insomnia) in aged populations around the world [2, 3]. These sleep problems are in turn often associated with daytime sleepiness and napping [2–4].
1.1 Regulation of human sleep and wakefulness by homeostatic and circadian mechanisms
In humans, two major regulatory processes interact to allow for long consolidated bouts of sleep and wakefulness [5]. These processes include the circadian timing system, which promotes sleep or wakefulness at particular times across the 24-hour day, and the so-called sleep-wake homeostat, a term that refers to the accumulation of sleepiness during wakefulness and the subsequent dissipation of that sleepiness during the following sleep episode. Laboratory studies have established electroencephalographic (EEG) low frequency activity (<7 Hz) as a physiological correlate for the homeostatic accumulation of sleep pressure in the brain, even though its modulating force remains unknown [6], and the level of sleep pressure as indexed by EEG slow waves has been demonstrated to be dependent on the duration of prior wakefulness in both animals and humans [6]. Similarly, EEG theta activity (4.75–8 Hz) [7] and slow eye movements (SEM) [8] are physiological correlates of the buildup of sleep pressure during wakefulness. EEG alpha (8–12 Hz) activity during wakefulness positively correlates with subjective alertness [8,9] and negatively correlates with EEG theta activity and SEM, and can be used as a physiological index of alertness in the wake state.
The circadian timing system produces strong drives for sleep or wakefulness at particular times of day, with the strongest drive for sleep towards the end of the night and the strongest drive for wake towards the end of the day, just prior to usual bedtime [10]. Many aspects of sleep and wakefulness show a circadian oscillation, including sleep latency, the propensity for REM sleep [11], wakefulness within scheduled sleep [5], and EEG alpha activity during sleep and wake [9, 12, 13].
1.3 Age-related changes in the circadian regulation of sleep
In a previous study [14], we found that the timing of the sleep-wake cycle in healthy older adults without sleep complaints occurred significantly earlier with respect to the circadian rhythms of core body temperature and melatonin secretion compared to a group of young adults. We also found that the timing of sleep with respect to circadian phase was significantly more variable among the older adults. In other studies in which sleep was scheduled at many circadian phases, we found that in healthy older adults, sleep was more disrupted at all circadian phases than in young adults [15]. Among the older adults, a long, consolidated sleep episode was only possible when the end of the sleep episode occurred at the circadian phase near the core body temperature nadir. Together, findings from those studies suggest that one possible cause of early morning awakening, sleep maintenance insomnia, and daytime sleepiness among older adults is that their habitual sleep-wake timing does not occur at an ideal circadian phase.
1.3 Bright light exposure to alter circadian rhythms of sleep and wakefulness in older subjects
Light exposure is the primary signal from the environment that synchronizes the near-24-hour circadian timing system to the 24-hour period of the Earth’s rotation. As first described in the late 1950’s, the circadian system of most organisms (including humans) does not respond to light in the same way at all times of day, but instead has a phase-dependent response to light [16]. Light exposure in the late biological night-early biological day produces shifts in timing to an earlier hour, light exposure in the late biological day-early biological night produces shifts in timing to a later hour, and light exposure during the middle of the biological day produces little or no shift in timing [17]. Under normal conditions, this phase-dependent response to light interacts with the near-24-hour circadian timing system to produce small daily changes in rhythm timing that allow for a stable relationship between those underlying rhythms and the timing of the external light-dark cycle.
Changes in the timing of circadian rhythms with respect to the timing of the light-dark (wake-sleep) cycle with aging have been hypothesized to be due to changes in the amount or pattern of light exposure, changes in the transmission of light through the eye, changes in the photoreceptors that subserve the circadian timing system, and/or changes in the central circadian pacemaker itself. Some evidence for each of these possibilities has been found [18, 19].
Recently, a third class of photoreceptors has been identified in the human retina. These melanopsin expressing intrinsically-photosensitive retinal ganglion cells (ipRGCs) have a maximal sensitivity in the blue range of visible light (e.g. between 460 nm–480 nm), and play an important role in non-image-forming effects of light, including the pupillary light reflex and circadian responses to light [20–24]. There is some evidence that responses mediated by these ipRGCs might change in older subjects. Older women were reported to exhibit a lower melatonin suppression during monochromatic blue light exposure than young control subjects [25]. Likewise, a recent study showed slightly shorter phase advances in older than young subjects after exposure to monochromatic blue and green light in the morning [26]. Whether these attenuated responses are due to a change in the transmission of short-wavelength light through the lens, or are due to a change in response of the circadian system to short-wavelength, light remains unclear [18]
Because of the role of light in synchronizing the circadian system, and in turn the role played by the circadian system in regulating sleep-wake propensity, light treatments for improving sleep-wake function in older adults have been attempted [27, 28]. Regular bright light exposure has been demonstrated to improve the amplitude of sleep-wake propensity in institutionalized older patients, and such treatments have even been shown to slow the progression of illness in demented patients [29, 30]. Bright light treatment was also shown to improve sleep propensity and duration in a group of older adults with insomnia [27, 31]. However, it is unclear what the ideal intensity and timing of such light treatments is, and the effects of such light treatments on wake EEG activity have not yet been shown in older adults.
We hypothesized that an evening light treatment given to older adults with sleep-wake complaints would delay the timing of their circadian rhythms with respect to their sleep timing, thereby reducing evening sleepiness and improving subsequent sleep quality.
2. Subjects and Methods
2.1 Subjects
Study participants with complaints of difficulty sleeping were recruited from the community. Ten otherwise healthy older subjects (age=63.3 ± 8.1 years; mean ± SD; 6 females, 4 men) with sleep complaints (PSQI=6.2± 1.9; mean ± SD) were included. Subjects had to report no history of head or eye injury, no history of psychiatric illness, no history of substance abuse, and no current use of any prescription or over-the-counter medication that would affect sleep, daytime alertness, or melatonin secretion. In addition, the subjects had to report a recent history of limited caffeine and nicotine use, and were asked to completely abstain from using caffeine, nicotine, and alcohol for at least 1 week prior to and throughout the study duration. All subjects underwent an all-night clinical sleep recording before the study to screen out those with clinically significant sleep apnea (AHI >15) and/or periodic limb movement disorder (PLMI with arousals >10). To ensure the circadian system of the subjects was stably entrained to their sleep-wake schedule, they had to report no history of night shift work in the 12 months prior to the study, and fewer than two time zones crossed in the three months prior to the study. They were also asked to keep a regular sleep schedule of 8 hours in bed at the same time each night for at least 2 weeks prior to the study. The study was reviewed and approved by the Human Research Committee at Partners Health Care, and written informed consent was obtained from each subject before the study.
2.2 Study Design
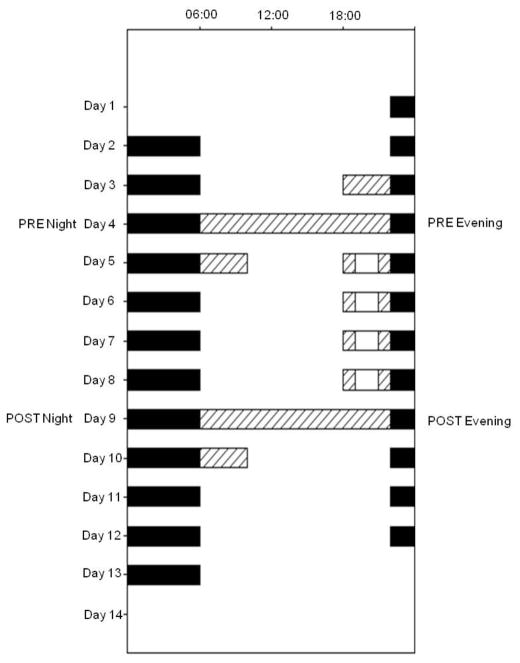
During the 2 weeks prior to admission to the laboratory, study subjects lived on a regular 8-hour sleep schedule at home, and their adherence to this was verified by ambulatory activity measurement (ActiwatchL®, Phillips Respironics, Murrysville, PA). The timing of this pre-study sleep was used to schedule the 8-hour sleep episodes during the study, and was individualized for each participant. On the afternoon of day 1, subjects entered the General Clinical Research Center of the Brigham and Women’s Hospital, where they lived for the 13 days of the study. Days 1 to 3 served as baseline days, and were followed by a constant posture protocol for circadian phase assessment on day 4–5. In the evening of days 5 to 8, subjects were exposed to the experimental light for 2 hours, beginning 3 hours before their bedtime (see below). On day 9–10, a second constant posture was performed, and days 10–12 served as recovery days with the same conditions as the 3 baseline days (see Figure 1). On all days but the constant posture days (day 4 and 9), the subjects were allowed to leave their study room following breakfast, returning prior to dinner.
Figure 1.
Raster Plot of the 13-day study design. Time of day is presented across the horizontal axis and day of the experiment is presented from top to bottom along the vertical axis. Black bars indicate scheduled 8-hour sleep episodes, hatched bars indicate dim-light Constant Posture circadian phase assessment procedures and white bars indicate the 2-hour LEs.
2.3 Constant Posture Protocol
On two occasions (days 4–5 and 9–10), an assessment of circadian phase was conducted using a constant posture protocol (see hatched areas in Figure 1). This protocol was designed to allow determination of the timing of the circadian rhythm of melatonin secretion while minimizing sleep loss. Each constant posture lasted approximately 28 hours, beginning at usual wake time on the first day and continuing until approximately 4 hours after wake time on the following day. Throughout the constant posture protocol, the subject was required to remain in a semi-recumbent posture in bed. During the 16-hour wake episode on the first day, and the first 4 wake hours on the second day, the light levels were kept dim (~1 μW/cm2). During the 8 hour sleep episode, the lights were turned off and the subject was allowed to sleep.
2.4 Methods
2.4.1 Experimental Light Exposures
The 2-hour light exposures (LE) took place in the evenings of days 5 to 8 beginning 3 hours before bedtime (see Figure 1). During the LE, the subject was required to remain seated approximately 30–50 cm directly facing the front of a 62 × 30 cm light box. Throughout LE, the subject was required to keep his/her eyes open and to face towards the light box. Compliance was verified by a trained study team member who remained in the room throughout the LE. Irradiance and illuminance levels throughout the LE were regularly assessed using a research radiometer/power meter (Model IL 1400, International Light, Peabody, MA). Each subject was randomly assigned to one of two different LE sources: polychromatic white fluorescent light (4100 K) or blue-enriched polychromatic white fluorescent light (Philips Lighting B.V, The Netherlands). The photon density of the two sources was designed to be equal, at a target level of 1.E+15photons/cm2/s, corresponding to 370 μW/cm2 (white polychromatic light source) and 320 μW/cm2 (blue enriched polychromatic light source). The blue-enriched lamps were prototypes that were provided by Philips Lighting (Eindhoven, The Netherlands) for use in this study.
2.4.2 EEG Recordings
The EEG was recorded using an ambulatory recording system (Vitaport 3, Temec, The Netherlands). The montage included 6 scalp recording sites during wakefulness (C3, C4, Fz, Cz, Pz, Oz) and 8 scalp recording sites during sleep (F3, F4, C3, C4, P3, P4, O1, O2), two electrooculograms, and a submental electromyogram (during sleep only). The EEG signals were high-pass filtered at a time constant of 1 second and low-pass filtered at 70 Hz (Bessel fourth-order antialiasing; > 80 dB). The signals were digitized with a resolution of 12 bit (range 500 μV; sampling rate 256 Hz, storage rate 128 Hz), stored on a Flash RAM card, and downloaded offline after wake time. The EEG recordings were visually scored in 30-sec epochs according to standard criteria [32] using a central derivation (C3 for sleep, Cz for wakefulness) referenced against mastoids.
2.4.3 Wake EEG
During the constant postures and the LEs, 3-minute Karolinska Drowsiness Tests (KDT) were administered at pre-determined intervals (see below) in order to better obtain artefact-free wake EEG segments. During KDT testing, subjects were asked to fix their gaze and remain still without moving, blinking, or talking. For the constant posture, the KDTs were scheduled hourly. On the LE evenings, a baseline KDT was scheduled 20 minutes prior to the LE start, and then every 30 minutes throughout the LE. Prior to analysis of the KDTs, all artefacts (due to blinking or movements) were manually removed, and the remaining artefact-free EEG recordings were then subjected to spectral analysis by using a Fast Fourier Transform (Vitascore, Temec, The Netherlands). Because of blinks, artefacts and other technical problems, 13 KDT recordings (out of 199 of total) were excluded from analysis. For data reduction, 30-second epochs of wake EEG were averaged using a 2-second window, which resulted in a 0.5 Hz resolution.
2.4.4 Subjective Alertness
Subjective alertness was assessed every 30 minutes throughout every study evening and each constant posture using a computer administered 9-item Karolinska Sleepiness Scale (KSS).
2.4.5 Circadian Phase Assessments
On the evening prior to the first of each constant posture day (i.e. evening of days 3 and 8), an intravenous catheter was inserted into a forearm vein of the subject so that blood samples could be collected. The catheter was connected to a 12-foot IV line and manifold system so that blood sampling could take place from outside the room during the night without disturbing the subject. Samples were collected approximately hourly, and the plasma frozen and stored for later analysis. For 2 subjects, there were problems with IV sampling system, and hourly saliva samples collected only during wakefulness were used. The plasma or saliva samples were frozen and later assayed for melatonin by Solidphase, Inc. (Portland, ME) using a commercially-available radioimmunoassay kit (Melatonin Direct RIA, Bühlmann Laboratories AG, US Distributor ALPCO Diagnostics, Salem NH). The melatonin assay results were used to provide information about the subject’s circadian phase before and following the LE. The circadian phase was determined using the dim light melatonin onset (DLMO), which was defined as the time at which melatonin concentrations rose to a fixed threshold of 10 pg/ml (for blood) or 1 pg/ml (for saliva).
2.4.6 Statistical Analyses
Statistical analyses were performed with the software SAS (SAS Institute Inc, Cary, NC, version 9.1) and Statistica (Stat Soft Inc., 1984–2004, STATISTICA for Windows, Tulsa, OK). Mixed regression models with the factors ‘light group’ (blue-enriched polychromatic white light or white polychromatic light); the repeated factors ‘condition’ (PRE=pre-LE evening/night, POST=post-LE evening/night; ‘light exposure’ (LE=average of light exposure evenings 1–4) and/or ‘time interval’ were applied. Post-hoc tests were performed with Duncan’s multiple rank test or t-tests, and p-values were adjusted for multiple comparisons. Analysis of wake EEG spectra, KSS scores, and sleep latencies were done on log-transformed data to better-approximate a normal distribution. For correlation analyses, a Spearman-correlation was applied.
3. Results
3.1 Light intensity and circadian phase shifts
As designed, both light exposure groups were exposed to similar irradiances (p=0.46; t-test; see Table 1a). Illuminance was significantly brighter for the polychromatic white light group than for the polychromatic blue-enriched light group (p<0.05). As previously reported [33], we observed equivalent circadian phase shifts in response to the two light sources (p=0.58; see Table 1b). One subject who received blue-enriched polychromatic white light had to be excluded from the phase shift calculation because a post-LE circadian phase could not be determined. In both light groups, the phase delay produced by the LE resulted in a significantly shorter phase angle of entrainment (i.e. the interval between the circadian DLMO phase and habitual bedtime/lights off time) (2-way rANOVA; F1,7=64.7; p<0.05) than at baseline, and this did not differ between the two groups (p=0.5; see Table 1b).
Table 1a.
Average light irradiance, photon density, and illuminance measured at vertical eye level (mean±SD) for the two light groups during the LE’s. (N=10)
| Light source | Irradiance (mean±SD) p=ns |
Photon Density (mean±SD) p=ns |
Illuminance (mean±SD) P<0.05 |
|---|---|---|---|
| Blue-enriched polychromatic white | 370.8 ± 49 μW/cm2 | 1.11E+15 ± 0.15E+15 photons/cm2/s | 654 ± 78 lx |
| Polychromatic white | 352.0 ± 23 μW/cm2 | 1.02E+15 ± 0.07E+15 photons/cm2/s | 1313 ± 103 lx |
Table 1b.
Phase shifts and phase angles of entrainment (interval between circadian phase of the dim light melatonin onset and bedtime/lights out) for the two light groups (mean±SD).
| Light source | Phase Shift (mean±SD) p=ns |
Baseline Phase Angle of Entrainment before LE (mean±SD) p=ns |
Phase Angle of Entrainment after LE (mean±SD) p=ns |
|---|---|---|---|
| Blue-enriched polychromatic white | 116.0 ± 48 min | 1.82± 1.1 h | −0.04 ± 1.0 h |
| Polychromatic white | 100.4 ± 33 min | 2.32 ± 1.1 h | 0.65 ± 1.2 h |
3.2 Subjective sleepiness
Both light groups reported similar sleepiness levels on the evening before the LEs (F1,8=0.6; p=0.5) and there was no significant change of average subjective sleepiness across the four LE evenings (2-way rANOVA: F3,20=1.0; p=0.4). When we compared subjective sleepiness ratings from the Karolinska Sleepiness Scale (KSS) between the conditions PRE, POST, and LE evenings (average of LE 1–4), there was a significant main effect of condition (3-way rANOVA; F2,16 = 17.6; p < 0.05), such that subjects rated themselves as significantly more alert on the LE evenings compared to the PRE evening (p<0.05), and slightly more alert compared to the POST evening (p=0.07). We observed no difference between the PRE and POST evening (p>0.1; Duncan’s multiple range test) or between the two light groups (one-way rANOVA F1,8=0.02; p>0.8).
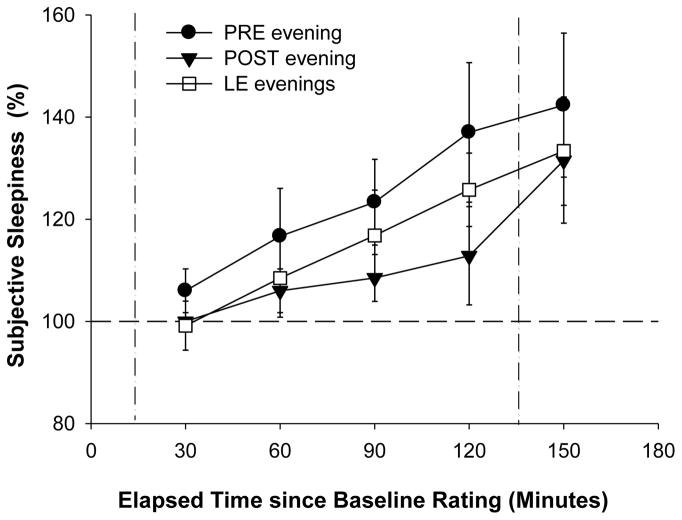
In order to examine the relative increase of subjective sleepiness during the LE, KSS ratings during the LEs were expressed as percentage of the first rating that evening (called ‘baseline rating’), which occurred approximately 20 min prior to the start of the LE (and 3.3 h prior to habitual bedtime). This was also done for KSS ratings collected at the same clock times during PRE and POST evenings. We found that subjective sleepiness increased across all evenings for both light groups (main effect ‘time interval’; 3-way rANOVA; F4,96=13.8; p<0.05). Compared to the baseline rating each evening, subjects became significantly sleepier at an earlier time on the PRE evening than on the LE evening (i.e. 1.8 h before bedtime vs. 1.3 h before bedtime), and during the POST evening subjective sleepiness did not increase significantly above baseline levels until the end of the evening (i.e. 50 min before bedtime; t-test to mean; p-values adjusted for multiple comparisons; p<0.03; see Figure 2).
Figure 2.
Time course of subjective sleepiness ratings expressed relative to the first rating that evening (defined as BL rating and set equal to 100%) for PRE evening (black circles), average of the four LE evenings (white squares), and POST evening (black triangles). Vertical dotted lines show the timing of the LE. The horizontal line indicates the BL value, set equal to 100% (n=10; mean ± SEM).
3.3 Objective alertness
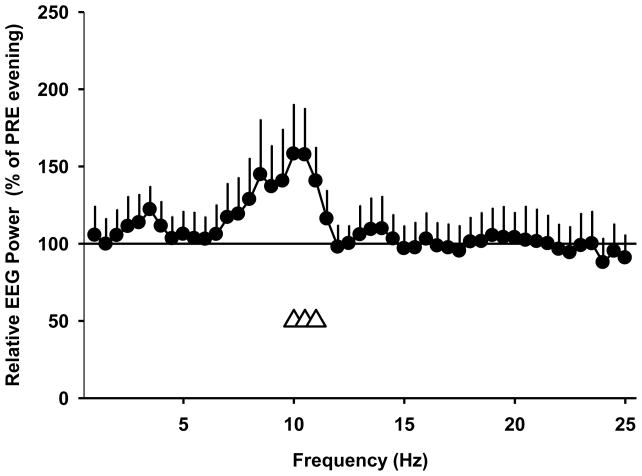
There was no difference between the two light groups during the LE (one-way rANOVA: main effect of light: F1,8<3.8; p >0.09). When we compared PRE and POST evenings, we found a significantly higher EEG power density in some frequency bins in the EEG alpha (10.25–11.75 Hz) and EEG beta (12.75–19.75; 20.25–22.25; and 22.75–25.25 Hz) ranges during the POST evening. These differences were present for both light groups with the exception of 15.75–16.25 Hz, where only the polychromatic white light group showed higher EEG activity during POST evenings (2-way rANOVA: F1,8>5.8; p<0.05). There was no difference between the two light groups during the LE (2-way rANOVA: main effect of light: F1,8<1.5; p>0.25). Because we found only very minor changes between the two light groups, we combined the wake EEG data from the two light groups across the LEs to compare them to the PRE evening. We found a significantly higher EEG power density between 9.75–11.25 Hz during the LEs than during the dim light PRE evening (p<0.05; F1,8>5.3; one-way rANOVA; see Figure 3).
Figure 3.
Relative EEG power density (% of PRE evening) from subjects in both light groups across all LEs (average of LE 1–4); mean + SEM; n=10). Open triangles at the bottom indicate frequency bins in which a significant difference between the LE evening and the dim light conditions on the PRE evening prior to LE 1 was observed (one-way rANOVA; p < 0.05).
3.4 Sleep stages
When we compared sleep stages from the PRE and POST nights (nights 3 and 8), we found no significant differences in total sleep time, amount of wakefulness during scheduled sleep, amount of any sleep stage, or in sleep efficiency (see Table 2). We did find that on the POST night, the latencies to stage 1 sleep (SL1) and stage 2 sleep (SL2) were slightly longer (2-way rANOVA; p=0.09; F1,8=3.66 and p=0.054; F1,8=5.11), and latency to REM sleep (RL) was significantly prolonged (p<0.05; F1,8=6.42) compared to the PRE night (see Table 2). There was a significant interaction between ‘condition’ and ‘light group’ for slow wave sleep (SWS; p<0.05 2-way rANOVA; F1,8=5.7), such that there tended to be more SWS on the POST night in the polychromatic white light group (p=0.09). There were no significant correlations between the change of phase angle (or the magnitude of the phase shift) and changes in sleep. The change in RL (between PRE and POST evenings) was negatively correlated with the change in sleep efficiency (r= −0.84) and the change in RL was positively correlated with the change in the amount of wakefulness (r=0.78) and the change in SL2 (r= 0.73; p<0.05) between the PRE and POST nights.
Table 2.
Visually scored sleep stages from the combined light groups (n=10) on the night before (night 3, PRE) and the night following (night 8, POST) the LE.
| Sleep Stage | PRE LE Mean (n=10) |
± SD | POST LE Mean (n=10) |
± SD | P-Value |
|---|---|---|---|---|---|
| NREM (min) | 279.1 | 28.7 | 265.7 | 43.7 | ns |
| REM (min) | 74.1 | 13.7 | 77.7 | 28.1 | ns |
| SWS (min) | 31.4 | 25.5 | 32.3 | 25.9 | ns |
| Stage 1 (min) | 46.6 | 16.8 | 45.8 | 17.2 | ns |
| Stage 2 (min) | 247.7 | 37.3 | 233.4 | 52.5 | ns |
| Wake (min) | 74.3 | 31.1 | 82.1 | 54.4 | ns |
| TST (min) | 399.7 | 27.5 | 389.1 | 55.2 | ns |
| SE % | 83.6 | 6.2 | 81.2 | 11.5 | ns |
| SL1 (min) | 4.1 | 2.4 | 13.4 | 11.3 | 0.092 |
| SL2 (min) | 9.8 | 5.4 | 19.4 | 11.3 | 0.054 |
| RL (min) | 65.0 | 11.0 | 80.7 | 21.2 | 0.035 |
| SOFF (min) | 7.6 | 14.7 | 1.7 | 1.4 | ns |
NREM: non REM sleep (stages 2–4); SWS: slow wave sleep (stages 3 and 4); TST: total sleep time (stages 1–4 and REM); SE: sleep efficiency (TST/scheduled sleep episode); SL: sleep latency (number of minutes from lights out to first three consecutive epochs of this stage of sleep); SOFF: time from last epoch of sleep (stages 1–4 or REM) to lights on; ns=P>0.2.
3.5. Correlation between changes in wake EEG and changes in sleep stages
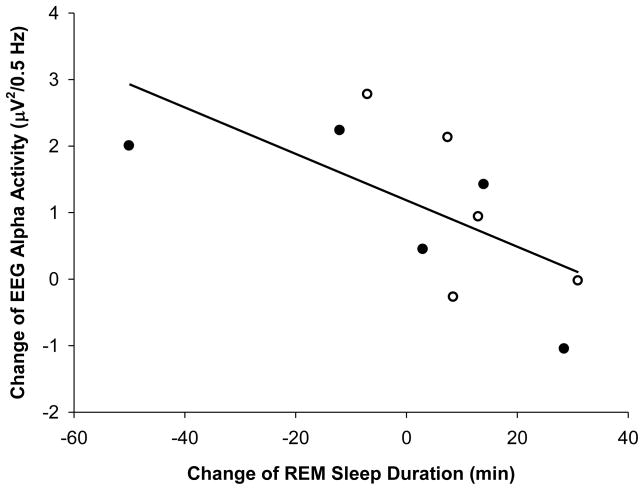
As described above, we observed significantly greater EEG power in the alpha range (between 9.75–11.25 Hz) on the LE evening than during the PRE evening. To determine whether that acute response to the LE was associated with any change in sleep, we performed correlation analysis to look at the association between the change in wake EEG alpha activity in response to the LE and the change in sleep stages (between the PRE and the POST night). We found a significant negative correlation between the change in EEG alpha activity and the amount of REM sleep (r= −0.70; p<0.05), such that the greater the increase in EEG alpha activity in response to the LE the less REM sleep the subject had following the LE treatment (Figure 4). However, we did not find a significant correlation between the change of EEG alpha activity during wakefulness with any other sleep stage, nor with sleep latencies, phase shift, or change of phase angle of entrainment.
Figure 4.
Correlation between the change in REM sleep duration from nights before and after the series of light exposures (PRE to POST nights), and the change of wake EEG alpha activity during the LEs. Open circles: polychromatic white light subjects (n=5); filled circles: blue-enriched polychromatic white light subjects (n=5). Spearman correlation coefficient R= −0.70 (p<0.05).
Discussion
We tested the effects of exposure to two different polychromatic white light sources on objective and subjective alertness and changes in subsequent sleep in a group of healthy older adults with sleep complaints. We found similarly increased subjective and objective alerting effects with the two light sources. We also found that both light sources produced similar shifts of the timing of the circadian rhythm of melatonin onset. Comparing sleep on the night after the series of LE to the night before the LE began, sleep latencies were slightly and REM sleep latency was significantly prolonged, although there was no association between that change in sleep or REM latency and the magnitude of the phase shift. A longer REM sleep latency after the light intervention was also associated with a longer sleep latency and a shorter phase angle of entrainment, suggesting that the phase relationship between the circadian timing system and the timing of sleep had been altered by the light intervention. When we compared the changes in wake EEG that we observed during the light exposures with changes in sleep stages after the light exposures, we found a negative association between higher wake EEG alpha activity during the light exposures and a shorter REM sleep duration, indicating an acute response to LE in the evening with repercussions during the following sleep episode, most presumably via the endogenous circadian timing system.
In young subjects, nocturnal exposure to bright light has been reported to exert a dose-dependent alerting effect on both subjective and objective alertness measures (and melatonin suppression), when compared to dim light conditions [34, 35]. Functional magnetic resonance imaging studies (fMRI) have shown that these acute alerting effects of nocturnal light are conveyed via thalamo-cortical projections as well as via brainstem and other ascending reticular arousal system (ARAS) related neurons [36]. In the present study in older subjects, we also observed direct alerting effects of evening light on both subjective and objective measures. When we examined subjective alertness on the evening following the last light exposure, subjects were still subjectively more alert than they had been at baseline, presumably reflecting a shift in the underlying circadian rhythm of sleepiness, similar to what has been found after evening bright light exposure in young subjects [37].
Overall, we did not find a difference in the acute alerting effects produced by the blue enriched white light source compared to the polychromatic white light source. As described in the methods, the irradiance and photon density of the two light sources were designed to be similar. While the illuminance of the blue enriched polychromatic white light source was only about half that of the polychromatic white light source, in both cases the light was significantly brighter than typical indoor light. Thus, the similar changes in circadian phase and alertness produced by the two light sources may reflect a ceiling effect [38]. In designing the study, we selected a level of light brighter than typical indoor light for the LE because subjects in the study were allowed to leave the laboratory during the daytime hours, and we wanted to ensure that despite that uncontrolled light exposure (both in terms of timing and intensity), the evening LE could still produce significant circadian phase shifts.
The light treatment regimen used in our study produced significant shifts in circadian phase, but that was associated with only modest changes in sleep on the night following the series of light exposures. This may be due in part to the fact that these subjects, despite their subjective sleep complaints, showed fairly good baseline night sleep quality (sleep efficiencies >80%). In retrospect, our pre-study instructions (to maintain a regular, 8-h time in bed and to abstain from any caffeine, alcohol, and nicotine use for the two weeks prior to study) may have already improved the subjects’ baseline sleep quality. The slightly prolonged latencies to stage 1 and stage 2 sleep that we observed following the series of light exposures are consistent with earlier reports in young subjects [39, 40]. The significantly prolonged REM sleep latency after the light exposure treatment in our study is not surprising given that REM sleep timing is under strong circadian control and that the light treatment produced a phase delay shift (shift to a later hour) of the circadian rhythm of melatonin secretion. However, we did not find a significant correlation between the magnitude of the phase shifts and the magnitude of change in REM sleep latency.
The (negative) association between changes in EEG alpha activity during wakefulness and changes in REM sleep duration suggests that a common underlying sleep-wake regulatory mechanism may have been impacted by the light treatment. Both the alerting effects and the timing of REM sleep are controlled in part by the endogenous circadian timing system [41]. It is well established that in normal adults, most REM sleep occurs in the latter part of the night [42], and that the timing of REM sleep can be shifted by bright light or exogenous melatonin interventions [37]. Thus, the modulations in wake EEG alpha activity and REM sleep we observed in response to the LE would have occurred via interactions between the SCN and sleep-wake regulatory neurons of other brain areas [43, 44]. If the modulations of wake EEG alpha activity and REM sleep observed in the present study reflect a change in a common underlying mechanism, then we would have expected the magnitude of the acute alerting effects during wakefulness and the change in REM sleep latency (or REM sleep duration) to show a significant relationship with the light induced phase shift, which was not the case. Instead, our results suggest that any interaction between wake EEG alpha activity and REM sleep is complex, and the magnitude of acute light effects is not directly linked to the magnitude of phase shifting effects or to the timing of subsequent REM sleep.
Taken together, our study demonstrated that a series of evening light exposures can acutely increase subjective and objective alertness in older subjects and shift the timing of their circadian rhythms. These alerting and phase-shifting effects were seen in subjects who were not restricted to a controlled laboratory environment, but who were allowed to spend several hours each day outdoors. However, while the light exposure regimen did produce significant circadian phase shifts and therefore did alter the circadian phase at which the sleep episode occurred, we did not observe any improvements in sleep quality. Together with other recent studies [45], these results suggest that evening light treatments may not be effective in improving sleep quality in older adults with sleep problems. However, our findings do indicate that such treatments may be useful in addressing problems related to evening sleepiness.
Supplementary Material
Research Highlights.
Test of the impact of evening light exposure on subjective alertness, EEG wake activity and sleep in older human subjects
Subjective alertness and wake EEG activity in the alpha range were higher during light exposures, compared to baseline
Light exposures produced circadian phase shifts and significantly prolonged latency to REM sleep
The increase in wake EEG alpha activity was negatively correlated with REM sleep duration
Evening light exposure could benefit older adults with early evening sleepiness, without negatively impacting sleep
Acknowledgments
4. Acknowledgements and Financial Support
We wish to thank the subjects; Ms. Jennifer Row for subject recruitment; Mr. C.F. Dennison and the staff of the Division of Sleep Medicine Chronobiology Core for assistance with data collection, subject monitoring, and data processing; and the Brigham & Women’s Hospital General Clinical Research Center staff for assistance in conducting the study. This study was supported by NIH grant R01 AG06072 (to JFD), and was conducted in the Brigham & Women’s Hospital General Clinical Research Center, supported by NIH grant M01 RR02635; MM was supported by fellowships from the La-Roche and Novartis Foundations (Switzerland) and Jazz Pharmaceuticals (USA); KS was supported by NIH fellowships T32 HL07901 and F32 AG031690. The polychromatic white and blue enriched lamps were provided by Philips Lighting B.V. for use in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaudreau H, Carrier, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. Journal of Sleep Research. 2001;10(3):165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 2.Bliwise DL. Normal aging. Philadelphia: W.B. Saunders Company; 2000. [Google Scholar]
- 3.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3(2):1–220. [PubMed] [Google Scholar]
- 4.Buysse DJ, Browman KE, Monk TH, Reynolds CF, 3rd, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. Journal of the American Geriatrics Society. 1992;40(8):779–86. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 5.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 6.Borbély AA. A two process model of sleep regulation. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 7.Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. European Journal of Neuroscience. 2001;13(12):2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 1999;277(3):R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 9.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neuroscience Letters. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience Letters. 1994;166(1):63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 11.Weitzman ED, Czeisler CA, Zimmermann JC, Ronda JM. Timing of REM and stages 3+4 sleep during temporal isolation in man. Sleep. 1980;2:391–407. [PubMed] [Google Scholar]
- 12.Cajochen C, Wyatt JK, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114(4):1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 13.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. The Journal of Neuroscience. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:R1478–R87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 15.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of Non REM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Hastings J, Sweeney B. A persistent diurnal rhythm of luminescence in Gonyaulax polyedra. Bioll Bull. 1958;115:440–58. [Google Scholar]
- 17.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. Journal of Physiology. 2003;549.3:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. British Journal of Ophthalmology. 2008;92(11):1439–44. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman MA, Swaab DF. Living by the clock: The circadian pacemaker in older people. Ageing Research Reviews. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neuroscience. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. Journal of Physiology. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. TheJournal of Clinical Endocrinology & Metabolism. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 23.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation and heart rate to short wavelength light. TheJournal of Clinical Endocrinology & Metabolism. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 24.Münch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2006;290(5):R1421–8. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 25.Herljevic M, MIddleton B, Thapan K, Skene DJ. Light - induced melatonin suppression: age - related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–42. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. Journal of Biological Rhythms. 2009;24(73):73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 27.Lack L, Wright H. The effect of evening bright light therapy in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep. 1993;16(5):436–43. doi: 10.1093/sleep/16.5.436. [DOI] [PubMed] [Google Scholar]
- 28.Campbell SS, Dawson D, Anderson MC. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41(8):829–36. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biological Psychiatry. 1997;41:955–63. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 30.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Van Someren EJW. Effect of Bright Light and Melatonin on Cognitive and Noncognitive Function in Elderly Residents of Group Care Facilities: A Randomized Controlled Trial. Journal of the American Medical Association. 2008 June 11;299(22):2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 31.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of alzheimer’s type. Chronobiol Int. 1998;15:647–54. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: US Dept of Health, Education and Welfare, Public Health Service; 1968. [Google Scholar]
- 33.Scheuermaier K, Münch M, Guzik A, Silva EJ, Ronda JM, Duffy JF. Phase delay shifts to blue-enriched vs. standard polychromatic white light in healthy older people in a semi-ambulatory setting. Sleep. 2008;31(Suppl 8):A44-A. [Google Scholar]
- 34.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. Journal of Physiology. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose- response relationship for light intensity and ocular and electroencephalographic correlates of human-alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 36.Vandewalle G, Maquet P, Dijk D-J. Light as a modulator of cognitive brain function. Trends in cognitive sciences. 2009;13(10):429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Cajochen C, Kräuchi K, Danilenko KV, Wirz-Justice A. Evening administration of melatonin and bright light: interactions on the EEG during sleep and wakefulness. Journal of Sleep Research. 1998;7:145–57. doi: 10.1046/j.1365-2869.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 38.Duffy JF, Zeitzer JM, Czeisler C. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiology of Aging. 2007;28(5):799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dijk DJ, Cajochen C, Borbély AA. Effect of a single 3- hour exposure to bright light on core body temperature and sleep in humans. Neuroscience Letters. 1991;121:59–62. doi: 10.1016/0304-3940(91)90649-e. [DOI] [PubMed] [Google Scholar]
- 40.Cajochen C, Dijk DJ, Borbély AA. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 1992;15:337–43. [PubMed] [Google Scholar]
- 41.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–7. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depends on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 43.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 44.Deboer T, Vansteensel MJ, Détari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–90. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 45.Friedman L, Zeitzer J, Kushida C, Zhdanova I, Noda A, Lee T, et al. Scheduled bright light for treatment of insomnia in older adults. Journal of the American Geriatrics Society. 2009;57(3):441–52. doi: 10.1111/j.1532-5415.2008.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.