Abstract
Transient thyroid function abnormalities in the new born which revert back to normal after varying periods of time are mostly identified in the neonatal screening tests for thyroid and are becoming more common because of the survival of many more premature infants. It can be due to factors primarily affecting the thyroid-like iodine deficiency or excess, maternal thyroid-stimulating hormone receptor (TSHR) antibodies, maternal use of antithyroid drugs, DUOX 2 (dual oxidase 2) mutations, and prematurity or those that affect the pituitary-like untreated maternal hyperthyroidism, prematurity, and drugs. Most of these require only observation, whereas some, such as those due to maternal TSHR antibodies may last for upto three-to-six months and may necessitate treatment. Isolated hyperthyrotropinemia (normal Tetraiodothyronine (T4) and high Thyroid Stimulating hormone (TSH)) may persist as subclinical hypothyroidism in childhood. Transient hypothyroxinemia (low T4 and normal TSH) is very common in premature babies. The recognition of these conditions will obviate the risks associated with unnecessary thyroxine supplementation in childhood and parental concerns of a life long illness in their offspring.
Keywords: Hyperthyrotropinemia, hypothyroxinemia, transient congenital hypothyroidism
INTRODUCTION
Transient congenital hypothyroidism is defined as a transient abnormality of the thyroid function in the newborn, which later reverts to normal, which may or may not require replacement therapy. Its incidence varies depending on whether the condition is defined on the basis of abnormal neonatal screening tests for congenital hypothyroidism alone or whether the diagnosis is considered only if the abnormality persists in the confirmatory tests. If it is based on abnormal screening alone, its incidence would be high and this will depend on whether the screening is done with primary TSH measurements with back up T4, or primary T4 with back up TSH. With the increasing survival of many more premature infants, transient thyroid function abnormalities have become more common than previously described.
CAUSES
Transient Primary hypothyroidism
Endemic iodine deficiency
Prenatal and postnatal iodine excess
Maternal TSHR blocking antibodies
Maternal antithyroid medications
DUOX 2 mutation
Isolated hyperthyrotropinemia (Normal T4, high TSH)
Transient Secondary or tertiary hypothyroidism
Maternal hyperthyroidism
Prematurity, very low birth weight
Drugs: Dopamine, steroids
Transient Hypothyroxinemia (Low T4, Normal TSH)
Endemic iodine deficiency
Transient congenital hypothyroidism due to iodine deficiency is more common in Europe than in North America.[1–3] India is now considered an iodine sufficient country with pockets of iodine deficiency,[4] hence, transient congenital hypothyroidism due to iodine deficiency may be seen in India also, although its exact prevalence is not known. Neonatal TSH concentrations are indicative of the iodine status of a population. The premature infants are especially at risk of hypothyroidism due to iodine deficiency because of their low iodine stores achieved in utero and immaturity of the hypothalamo-pituitary axis. These infants require more iodine to maintain a positive iodine balance in the extrauterine environment than term infants. Therefore, in areas of borderline iodine deficiency, infants show transient hypothyroidism which usually is seen in the first few weeks after birth; their cord blood thyroid concentrations may be normal. Even though transient, it may be prolonged for a few weeks, which may necessitate treatment with levothyroxine in some situations. Their urinary iodine concentrations are low and will respond to iodine replacement.
Iodine excess
Just like iodine deficiency, iodine excess can also lead to transient hypothyroidism, especially in premature infants. This is because the infant's thyroid gland is unable to decrease the thyroidal iodine uptake when exposed to an iodine load. In addition, the renal clearance of iodine may be decreased and skin absorption may be increased in infants. Iodine exposure occurs because of the use of iodine containing antiseptics, drugs like amiodarone or radiocontrast agents,[5–7] in either the baby or the mother. The amount of iodine required to cause iodine-induced hypothyroidism in infants varies from 50 – 100 mcg / kg. Typically the urinary iodine concentrations may be high; goiter may or may not be present. The avoidance of iodine containing antiseptics from new born nurseries have shown to decrease the recall rates of abnormal thyroid function tests.[8]
Maternal Thyroid-Stimulating Hormone Receptor Blocking Antibodies
Transplacental passage of maternal TSHR blocking antibodies can cause transient blockage of neonatal thyroid function and transient hypothyroidism.[9–11] The half life of these antibodies is around three-to-four weeks and it may take three-to-six months for its complete disappearance. Thus, in all children with congenital hypothyroidism, it is important to enquire about the history of maternal thyroid disease, either Graves and/or its treatment or atrophic hypothyroidism, which are associated with TSHR-blocking antibodies. Since the neonatal hypothyroidism may last for three-to-six months, a short period of treatment may be warranted in most of these children. As it blocks the binding and action of TSH, this type of hypothyroidism is not associated with a goiter. The thyroid gland may be visualized on ultrasonography, but will be absent in a thyroid scintigraphy, since uptake of Technetium as well as iodine is dependent on TSH.[11,12] Therefore, this type of hypothyroidism closely resembles thyroid agenesis, even though it is transient. If these antibodies had caused fetal hypothyroidism in utero it would result in permanent intellectual defect in the baby. There is a risk of recurrence of transient congenital hypothyroidism in future pregnancies as well since the maternal antibodies persist for a long time in maternal circulation.[10]
Maternal antithyroid drugs
If the mother has been on antithyroid drugs like propylthiouracil or methimazole, it can result in the formation of fetal and neonatal goiter and hypothyroidism. In the fetus this may be severe enough to cause fetal respiratory distress, which may warrant intrauterine thyroid replacement. This can occur even if the mother is on low-dose antithyroid drugs, because of the exquisite sensitivity of the fetus to antithyroid drugs. The neonatal goiter and hypothyroidism normalizes in a few days time so that most often the confirmatory tests will come back normal even if TSH is high on screening.[13,14]
DOUX 2 mutations
DUOX 2 is a gene involved in production of hydrogen peroxide (H2O2) needed for thyroid peroxidation. Previously it was thought that biallelic mutations result in permanent hypothyroidism, whereas, monoallelic mutations result in transient hypothyroidism.[15] However, even biallelic mutations in DOUX 2 have been reported to cause transient congenital hypothyroidism in Japanese patients.[16] The presence of coexistent iodine deficiency may alter the phenotype of such congenital defects.
Isolated hyperthyrotropinemia
An elevated TSH in the face of normal T4 and FreeT4 (FT4) concentrations have been initially described in Japanese children, but now it is a better characterized entity. It can be associated with both permanent and transient hypothyroidism. Inactivating mutations of TSHR can result in isolated, mild subclinical hypothyroidism in the neonate. Such isolated abnormalities are also more common in children with Down's syndrome. In some children it is due to transient immaturity of the hypothalamo-pituitary thyroid axis, but in some others it may last up to 10 years of age.[17] Studies have shown that morphological defects of the thyroid gland are more common in these children.[18,19] Whether to treat this condition and the long-term cognitive outcome is not well-studied. Most physicians will treat the isolated high TSH if it persists above 10 after the first two weeks of life, considering that normal TSH is up to 9.1 in < 20-week-old infants. If treatment is not started, repeat thyroid function tests should be done at two and four weeks, and probably throughout childhood, since up to 30% of such children can develop subclinical hypothyroidism in childhood.[17] If treatment is started, the therapy should be reassessed at two-to-three years of age, when brain development is completed.
Transient central hypothyroidism
This commonly occurs with low FT4 concentrations and a low or normal TSH. This may be due to the effect of uncontrolled maternal hyperthyroidism, which suppresses fetal TSH[20] or due to the effect of drugs used in critically ill babies, like steroids and dopamine. This is also common in premature infants because of the significant contributions from the withdrawal of maternal-placental thyroxine transfer, hypothalamic-pituitary-thyroid immaturity, developmental constraints on the synthesis and peripheral metabolism of iodothyronines, and iodine deficiency. In the absence of other pituitary hormone deficiencies, it is prudent to reassess the need for continuation of levothyroxine therapy in a case of isolated central hypothyroidism when brain development is complete
Isolated hypothyroxinemia
Low FT4 concentrations in the face of normal TSH and a normal response of TSH to Thyrotropin-releasing hormone (TRH) stimulation indicating a normally responsive pituitary, is called isolated hypothyroxinemia. This is very common in premature babies and very low birth weight babies.[21] It is not possible to distinguish clinically or from laboratory measurements, whether transient hypothyroxinemia is an independent condition or simply a consequence of non-thyroidal illness and / or drug usage.[21] Studies are conflicting as to whether to treat this condition in premature babies.[22] A low total T4 with relatively normal FT4 and TSH can be seen in the thyroxine-binding globulin (TBG) deficiency, which can be transient in prematurity due to undernutrition, liver dysfunction or recovery from sick euthyroid syndrome.[1]
Clinical clues to differentiate between transient and permanent congenital hypothyroidism
It is not very easy to clinically differentiate between permanent and transient forms of congenital hypothyroidism in the newborn period. The few clinical points that confirm the permanent nature of congenital hypothyroidism are the presence of an ectopic thyroid gland, absent thyroid gland by ultrasound, an initial TSH of more than 100 mIU/L and multiple pituitary hormone deficiencies.[12,13] In the absence of such clinical clues the possibility of transient congenital hypothyroidism should be entertained, especially if there are risk factors for it, like iodine deficiency, perinatal iodine exposure, history of maternal hyperthyroidism or its treatment, prematurity, low birth weight, malnutrition, and use of drugs like steroids and dopamine.
Whenever a decision cannot be made regarding the nature of congenital hypothyroidism it is always prudent to repeat the thyroid function tests at two weeks and one month of life. If the TSH remains persistently high and / or the FT4 is persistently low, treatment should be started with levothyroxine in a dose of 10 – 15 mcg / kg, similar to that for permanent hypothyroidism. However, the need to continue treatment should be reassessed at three years of age, when brain development is complete.[13] If the suspicion of TSHR antibody-induced transient congenital hypothyroidism is high, the treatment can be withdrawn at three-to-six months of age. A recommended flow chart for follow up of abnormal thyroid functions detected at birth is given in Figure 1.
Figure 1.
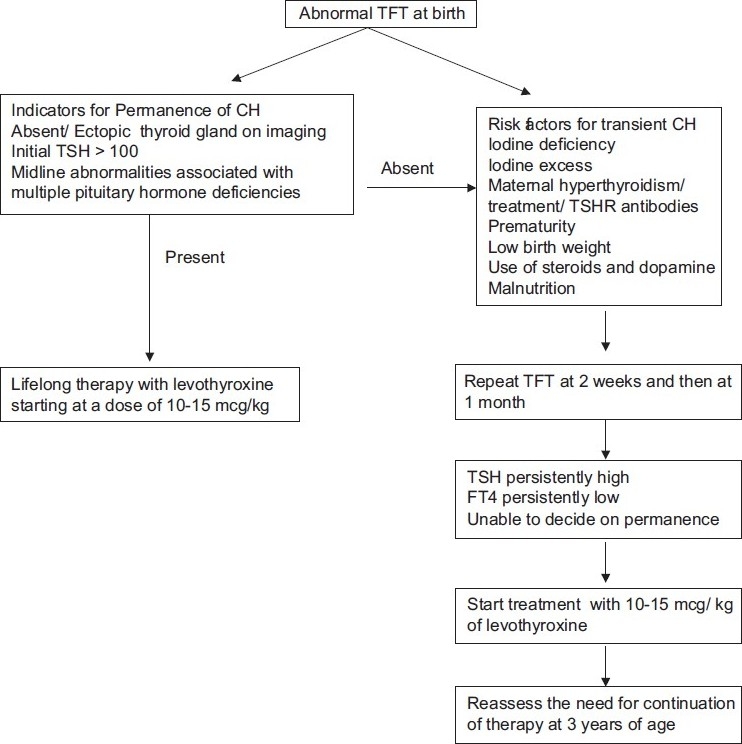
Flow chart for follow up of abnormal thyroid function test at birth
CONCLUSION
Thyroid function abnormalities should be interpreted with caution in neonates. The risks of undertreatment on brain development should be the primary concern of the treating physician when encountering a neonate with abnormal thyroid function tests. However, given the myriad causes of transient thyroid function abnormalities in neonates and the not so insignificant risks of unnecessary treatment with levothyroxine, such as, hyperactivity, advancement of bone age, and craniosynostosis, caution should always be exercised before committing a child to life-long treatment with thyroxine. If treatment is started on grounds of suspicion, in the absence of a firm evidence of permanent congenital hypothyroidism, the need for continuation of levothyroxine replacement therapy should always be reassessed in children being treated for congenital hypothyroidism at three years of age.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Brown Rosalind S. 6th edition. UK: Wiley Blackwell; 2009. The Thyroid in Brook's Clinical Pediatric Endocrinology; p. 264. [Google Scholar]
- 2.Gaudino R, Garel C, Czernichow P, Leger J. Proportion of various types of thyroid disorders among newborns with congenital hypothyroidism and normally located gland: A regional cohort study. Clin Endocrinol (Oxf) 2005;62:444–8. doi: 10.1111/j.1365-2265.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 3.Delange F. Neonatal screening for congenital hypothyroidism: Results and perspectives. Horm Res. 1997;48:51–61. doi: 10.1159/000185485. [DOI] [PubMed] [Google Scholar]
- 4.Toteja GS, Singh P, Dhillon BS, Saxena BN. Iodine deficiency disorders in 15 districts of India. Indian J Pediatr. 2004;71:25–8. doi: 10.1007/BF02725651. [DOI] [PubMed] [Google Scholar]
- 5.Glinoer D. Pregnancy and Iodine. Thyroid. 2001;11:471–81. doi: 10.1089/105072501300176426. [DOI] [PubMed] [Google Scholar]
- 6.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001;11:501–10. doi: 10.1089/105072501300176462. [DOI] [PubMed] [Google Scholar]
- 7.Bartalena L, Bogazzi F, Braverman LE, Martino E. Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinol Invest. 2001;24:116–30. doi: 10.1007/BF03343825. [DOI] [PubMed] [Google Scholar]
- 8.Chanoine JP, Pardou A, Bourdoux P, Delange F. Withdrawal of iodinated disinfectants at delivery decreases the recall arte at neonatal screening for congenital hypothyroidism. Arch Dis Child. 1988;63:1297–8. doi: 10.1136/adc.63.10.1297-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RS, Bellisario RL, Botero D, Fournier L, Abrams CA, Cowger ML, et al. Incidence of transient congenital hypothyroidism due to maternal TRAB in over one million babies. J Clin Endocrinol Metab. 1996;81:1147–51. doi: 10.1210/jcem.81.3.8772590. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura N, Yamada Y, Nohara Y, Konishi J, Kasagi K, Endo K, et al. Familial Neonatal transient hypothyroidism due to maternal THS binding inhibitor immunoglobulins. N Engl J Med. 1980;303:738–41. doi: 10.1056/NEJM198009253031306. [DOI] [PubMed] [Google Scholar]
- 11.Yang RL, Zhu ZW, Zhou XL, Zhao ZY. Treatment and follow-up of children with transient congenital hypothyroidism. J Zhejiang Univ Sci B. 2005;6:1206–9. doi: 10.1631/jzus.2005.B1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoen EJ, Clapp W, To TT, Fireman BH. The key role of newborn thyroid scintigraphy with isotopic iodide in defining and managing congenital hypothyroidism. Pediatrics. 2004;114:e683–8. doi: 10.1542/peds.2004-0803. [DOI] [PubMed] [Google Scholar]
- 13.Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–303. doi: 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]
- 14.Chan GW, Mandel SJ. Therapy insight: Management of Graves’ disease during pregnancy. Nat Clin Pract Endocrinol Metab. 2007;3:470–8. doi: 10.1038/ncpendmet0508. [DOI] [PubMed] [Google Scholar]
- 15.Hoste C, Rigutto S, Van Vliet G, Miot F, De Deken X. Compound heterozygozity for a novel missense mutation and a partial deletion affecting the catalytic core of the H2O2 generating enzyme DUOX 2 associated with transient congenital hypothyroidism. Hum Mutat. 2010;31:E1304–19. doi: 10.1002/humu.21227. [DOI] [PubMed] [Google Scholar]
- 16.Maruo Y, Takahashi H, Soeda I, Nishikura N, Matsui K, Ota Y, et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J Clin Endocrinol Metab. 2008;93:4261–7. doi: 10.1210/jc.2008-0856. [DOI] [PubMed] [Google Scholar]
- 17.Leonardi D, Polizzotti N, Carta A, Gelsomino R, Sava L, Vigneri R, et al. Longitudinal study of thyroid function in children with mild hyperthyrotropinemia at neonatal screening for congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:2679–85. doi: 10.1210/jc.2007-2612. [DOI] [PubMed] [Google Scholar]
- 18.Zung A, Tenenbaum-Rakover Y, Barkan S, Hanukoglu A, Hershkovitz E, Pinhas-Hamiel O, et al. Neonatal hyperthyrotropinemia: Population characteristics, diagnosis, management and outcome after cessation of therapy. Clin Endocrinol (Oxf) 2010;72:264–71. doi: 10.1111/j.1365-2265.2009.03634.x. [DOI] [PubMed] [Google Scholar]
- 19.Daliva AL, Linder B, DiMartino-Nardi J, Saenger P. Three-year follow-up of borderline congenital hypothyroidism. J Pediatr. 2000;136:53–6. doi: 10.1016/s0022-3476(00)90049-0. [DOI] [PubMed] [Google Scholar]
- 20.Liebrand CA, de Mol AC, Kempers MJ, Noordam C. Central congenital hypothyroidism due to Graves’ disease in the mother. Ned Tijdschr Geneeskd. 2006;150:2229–32. [PubMed] [Google Scholar]
- 21.Williams F, Hume R. The measurement, definition, aetiology and clinical consequences of neonatal transient hypothyroxinaemia. Ann Clin Biochem. 2011;48:7–22. doi: 10.1258/acb.2010.010174. [DOI] [PubMed] [Google Scholar]
- 22.Osborn DA. Thyroid hormones for preventing neurodevelopmental impairment in preterm infants. Cochrane Database Syst Rev. 2001:CD001070. doi: 10.1002/14651858.CD001070. [DOI] [PubMed] [Google Scholar]


