Abstract
OBJECTIVE
The purpose of our pilot study was to explore the relationship between serum thyroid stimulating hormone (TSH) levels during overt hypothyroidism (OH) and hypothyroid-related neuropsychological symptoms. We hypothesized that TSH level may reflect the degree of ‘brain hypothyroidism’ such that an inverse correlation may exist between serum TSH and cognitive function in patients experiencing overt hypothyroidism (OH), and sought to explore this hypothesis.
METHODS
Eleven thyroidectomized patients underwent neuropsychological and thyroid function testing while overtly hypothyroid, and again following thyroid hormone replacement. Their test performance was compared with that of eleven healthy controls at a similarly separated two points in time, and the change over time for the patient group and the controls was likewise assessed and compared. The patients’ neuropsychological test scores were then correlated with their serum TSH levels while hypothyroid.
RESULTS
The patients’ performance while hypothyroid was worse than that of the controls in only one neurocognitive measure--Working Memory Index. The subjects improved similarly or to a greater degree than the controls, when the subjects were thyroid hormone replaced, on all but one neurocognitive measure--Thurstone Word Fluency. TSH level during hypothyroidism was inversely proportional to the patients’ performance on these same two measures, but no others.
CONCLUSION
Serum TSH level during hypothyroidism was inversely proportional to performance on the only two neurocognitive measures evidencing an adverse effect from hypothyroidism in our cohort. This suggests that serum TSH level may reflect the severity of ‘brain hypothyroidism’ during the overt stage of this condition.
Keywords: thyroid stimulating hormone, cognition, hypothyroidism, depression, working memory
INTRODUCTION
The fact that thyroid hormone plays a significant role in adult brain function is undebatable, but the precise mechanism(s) by which this occurs is not well understood (Bauer et al. 2008; Davis and Tremont, 2007). It is known that triiodothyronine (T3) binds to nuclear thyroid hormone receptors which are widely distributed throughout the brain, with the greatest densities in areas critical to mood and higher order cognition (e.g. hippocampus and frontal lobes) (Dratmen et al. 1982; Schwartz and Oppenheimer, 1978); and that in the central nervous system, thyroid hormone has neurogenic effects at the molecular level (Despuza et al. 2005).
Much of what is known about the relationship between thyroid hormone and the brain comes from assessment and treatment of patients with hypothyroidism. Grade I, or overt hypothyroidism (OH), has been known to cause cognitive impairment and depression (often accompanied by anxiety) for more than a century (Hennessey and Jackson, 1996), and improvement or complete resolution of associated neuropsychological symptoms frequently follows adequate treatment of the thyroid disorder (Whybrow et al. 1969; Jain, 1972; Boswell et al. 2002). This grade of hypothyroidism is defined by abnormally low serum concentrations of T3 and thyroxine (T4), as well as elevated basal serum thyroid stimulating hormone (TSH) concentration. When diagnosing and describing hypothyroidism, most investigators report thyroid hormone levels in the peripheral serum. It is well known, however, that significant differences exist not only between levels of T3 and T4 in the peripheral serum and those in the brain (Robbins and Lakshmanan, 1992), but that levels of T3 and T4 in the brain vary themselves between different regions (Costa et al. 1991; Joffe et al. 1994). Animal studies have firmly established that the so-called ‘brain thyroid economy’ is very tightly controlled and not dependent directly on peripheral thyroid function (Dratman et al. 1983). This, therefore, raises a question of the validity of attempts to correlate severity of hypothyroidism, as measured by peripheral T3 and/or T4 levels, with cognitive status and mood.
In addition to measurements of T3 and T4, a typical thyroid function test battery includes a TSH level. Because pituitary TSH is considered the most sensitive test for the early diagnosis of hypothyroidism, has been considered by some the putative best overall marker of at least peripheral thyroid hormone availability (Marangell et al. 1997), and has been suggested by others as a possible index of the degree of hypothyroidism (Meier et al. 2003), the relationship between serum TSH and clinical severity of the hypothyroid condition has been explored. The results of these investigations have been inconsistent (Davis and Tremont, 2007). In one of the few studies in which a correlation was found, Cleare et al. (1995) reported that TSH levels were positively correlated with level of depression. This particular study was different from many other generally similar neuropsychological investigations in that a significant percentage of the subjects had OH, with markedly elevated TSH levels and associated undetectable peripheral thyroid hormone concentrations. This thyroid function test (TFT) profile is similar to that of thyroidectomized thyroid cancer patients presenting for diagnostic radioiodine surveys, who have had exogenous thyroid hormone discontinued, and are in a state of marked, albeit relatively brief, grade I hypothyroidism. These patients have significantly elevated TSH levels and exhibit neuropsychological symptomatology typically associated with the more slowly developing, non-iatrogenically-induced form of the disorder (Denicoff et al. 1990; Constant et al. 2001; Nagamachi et al. 2004; Regalbuto et al. 2006). Burmeister et al. (2001) found that a cohort of such patients had a specific decrement in delayed recall of verbal information and Constant et al. (2005) found decrements in executive functioning not present when their respective subjects were thyroid-hormone-replaced. While the subjects in both of these studies were also more depressed when hypothyroid, and mood is frequently alluded to as a potentially confounding factor in the assessment of cognition during hypothyroidism, neither of these investigators found depression to be confounding.
These well done studies certainly shed additional light on cognitive aspects of hypothyroidism. However, neither of these investigators, nor others specifically examining overtly hypothyroid patients, have, to our knowledge, attempted to correlate neurocognitive status with serum TSH. We performed such an analysis as a pilot study in a small cohort of thyroidectomized patients presenting for metastatic surveys in a state of marked hypothyroidism. To what extent an elevated serum TSH level reflects the degree of ‘brain hypothyroidism’ in OH is uncertain, but we hypothesized that just as serum TSH was found to be directly proportional to degree of depression by Cleare et al. (1995) in overt hypothyroidism, serum TSH would be inversely proportional to performance in cognitive domains apparently affected by this condition.
Materials and methods
Experimental subjects and controls
Eleven subjects who had undergone a thyroidectomy for treatment of thyroid carcinoma and were presenting to the Nuclear Medicine Service for a metastatic thyroid cancer survey participated in the study. This included six woman and five men with a mean age (± s.d.) of 33.0 (± 8.8) years. Each subject was free of significant current or past medical problems with the exception of thyroid cancer. This included no current or past history of neurologic or psychiatric disease; and no subject was taking psychotropic medication. Radioiodine scintigraphy was negative for brain metastases in all subjects, and brain magnetic resonance imaging (MRI) was negative for any significant morphological abnormalities or evidence of metastatic disease. Each of the subjects was right handed and, educationally, each had at least a high school diploma. Each subject had been on a low iodine diet for approximately two weeks prior to the ‘hypothyroid evaluation’. There were otherwise no dietary restrictions. The control group consisted of four women and seven men with a mean age (± s.d.) of 32.9 (±8.5) years who, in addition to being matched in terms of age, education, handedness (i.e. all were right handed) and freedom from current or past neurologic and psychiatric illness, also had no evidence of current or past thyroid disease. Likewise, no one in the control group was taking psychotropic medication. Subject and control demographics are displayed in Table 1.
Table I.
Sample Characteristics for Subjects and Controls
| Demographics | Thyroid cancer subjects | Normal controls | p value |
|---|---|---|---|
| Mean (±SD) | Mean (±SD) | ||
| Age1 | 33.00 (8.8) | 32.9 (8.5) | 0.98 |
| Education1 (in years) | 16.5 (2.4) | 15.9 (2.5) | 0.52 |
| Gender2 (number) | 0.39 | ||
| Female | 6 | 4 | |
| Male | 5 | 7 |
t-test comparisons, equal variances
chi-square
The study was approved by the Institutional Review Board of the National Naval Medical Center, the procedures followed were in accordance with the ethical standards of the Institutional Review Board, and all of the subjects and controls provided written informed consent.
Procedures
Thyroid function testing was performed at two points in time: following at least 6 weeks without thyroxine supplementation (with a range of approximately 6–8 weeks) when each subject was markedly hypothyroid-- just prior to the metastatic survey, and after each subject had received thyroxine replacement for approximately 8 weeks. The mean (±s.d.) number of days between thyroid function testing was 59 (± 6.0) days. In addition to thyroid function testing for the subjects, both the ‘hypothyroid evaluation’ and the ‘thyroid-hormone-replaced evaluation’ consisted of an assessment of mood, anxiety level, psychomotor performance and neurocognitive status. This neuropsychological testing was consistently performed between 08:00 h and 12:00 h by an experienced psychometrician supervised by a Ph.D. neuropsychologist. The testing was performed an average of 3.5 (± 2.3) days following the serum measurements. The controls underwent the same battery of tests/measures under identical conditions to the subjects, also at two points in time separated by approximately 8 weeks. The mean (± s.d.) number of days between testing sessions for the controls was 57.5(± 7.8) days.
Thyroid function measurements
The following thyroid function tests (TFT’s) were performed as part of both the hypothyroid and thyroid hormone-replaced evaluations: TSH, measured by microparticle enzyme immunoassay (Abbot Laboratories) with a lower limit of detectability of 0.08 uIU/mL, an interassay coefficient of variation of 0.7% to 3.6%, an intraassay coefficient of variation of 3.1% to 4.4%, and a normal range of 0.49 to 4.67 uIU/mL; free T4, measured by microparticle enzyme immunoassay (Abbot Laboratories) with a lower limit of detectability of 0.4 ng/dL, an interassay coefficient of variation of 0.8% to 4.6%, an intraassay coefficient of variation of 2.6% to 4.5%, and a normal range of 0.71 to 1.85 ng/dL; and free T3 (Quest Diagnostic, Inc.) with a lower limit of detectability of 160 pg/dL, an interassay coefficient of variation of 2.35%, an intraassay coefficient of variation of 2.87%, and a normal range of 230 to 420 pg/dL.
Neuropsychological assessment measurements
Neurocognitive and motor tasks that were included in this study were selected to sample abilities broadly across major neurobehavioral domains of functioning using well-validated standardized measures. The neuropsychological tests selected for use in this study are all published measures and the standardized administration and scoring procedures were utilized. The specific tests/measures and the respective neuropsychological domains examined by each are noted in Table 2. Particular tasks were selected to include domains that have demonstrated sensitivity to the hypothyroid condition in previous research (Burmeister et al. 2001; Constant et al. 2005), as well as domains that have not been specifically noted in controlled studies to be affected during hypothyroidism. In addition to the neurocognitive and psychomotor tests, mood and anxiety were assessed with the Beck Depression Inventory-2 (BDI)-2 (Beck et al. 1961) and the Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger, 1983), respectively.
Table 2.
Neuropsychological Tests/Measures and Neuropsychological Domains Examined
| Test/Measure | Neuropsychological Domain |
|---|---|
| Grooved Pegboard Test (both hands) | Psychomotor Speed |
|
| |
| Trail Making Test Part A | Attention |
| Trail Making Test Part B | |
|
| |
| Thurstone Word Fluency | Language |
|
| |
| Rey Osterreith Complex Figure Test | Visuospatial Construction |
|
| |
| Auditory Consonant Trigrams | Learning/Memory |
| Buschke Selective Reminding Test | |
| WMS-III* Logical Memory I, II | |
| WMS-III* Visual Reproduction I, II | |
|
| |
| Working Memory Index ** | Working Memory |
| Paced Auditory Addition Test | |
|
| |
| Wisconsin Card Sorting Test | Problem Solving/Set Shifting |
|
| |
| Beck Depression Inventory-2 (BDI 2) | Depression |
|
| |
| Speilberger State-Trait Anxiety Inventory | Anxiety |
| State Anxiety | |
| Trait Aniety | |
WMS-III:Wechsler Memory Scale-III
Composite of Letter Number Sequencing and Spatial Span
Statistical analyses
Paired t-tests were computed for subjects evaluating changes in TFT’s from the hypothyroid to thyroid-hormone-replaced states. Group differences in cognitive and psychomotor performance, mood, and anxiety between the subjects and controls at each of the two testing sessions were also assessed using t-tests. Additionally, changes in each of these neuropsychological measures were evaluated across the hypothyroid to thyroid-hormone-replaced states for the subjects and across the two similarly separated points in time for controls; and the mean change in each of these measures was compared between subjects and controls using t-tests.
Pearson correlations were performed to explore the relationship of TSH with measures of neuropsychological functioning of the subjects during the hypothyroid state. Correlations were not performed between TSH and subject performance during thyroid hormone replacement because of the undetectability of TSH in the majority of subjects when thyroid hormone-replaced. Because of the potentially confounding effect of hypothyroidism-related depression and anxiety on cognitive and psychomotor functions during hypothyroidism (Constant et al. 2005), additional Pearson correlations were performed relating the BDI-2 and STAI scores with each of the neurocognitive (and psychomotor) measures.
Results
Thyroid function tests
Descriptive information on TFT’s during the hypothyroid and thyroid-hormone-replaced states is shown in Table 3. As expected, for all subjects, T3 and T4 were undetectable and the TSH levels were markedly elevated during iatrogenically-induced hypothyroidism. Also as expected, the TSH decreased significantly (p < 0.0001) during thyroid hormone replacement.
Table 3.
Subject’s thyroid function test levels during hypothyroidism and thyroid hormone-replaced conditions
| Test | Hypothyroid | Thyroid Hormone-replaced |
|---|---|---|
| Mean (± SD) | Mean (± SD) | |
| TSH (uIU/mL) | 119.8 (77.7) | 0.7(1.3)* |
| Free T4 (ng/dL) | <0.4 (NA)a | 1.5 (0.4)* |
| Free T3 (pg/dL) | <160.0 (NA)a | 354.4 (89.2)* |
All measured values represent the lowest level of assay detectability.
p<0.0001 change from hypothyroid level.
Neuropsychological measures
At the initial assessment, there was a statistically significant difference (p<0.01) in performance between the hypothyroid subjects and the controls on only one cognitive test--The Working Memory Index, in which the controls significantly outperformed the subjects. The only other measure showing a significant difference (p<0.01) between the subjects and controls during the hypothyroid state was the BDI-2 (p<0.01), which demonstrated that the subjects were more depressed than the controls. There were no differences in levels of anxiety.
Although there was some improvement in the performance of both groups on all of the neurocognitive and psychomotor tests when the subjects were thyroid-hormone-replaced and they, like the controls, were being tested/measured for the second time, there was no significant difference in any neuropsychological measurement score between the two groups (i.e. p≥ 0.05). Assessment for degree or amount of change from session 1 to session 2 revealed that the subjects improved to an extent equal to or greater than the controls on all measures except for one: the Thurstone Word Fluency test. On this particular cognitive evaluation, there was a significantly greater improvement for the controls than the subjects (p < 0.05).
Correlational analysis of TSH and neuropsychological functioning during hypothyroidism
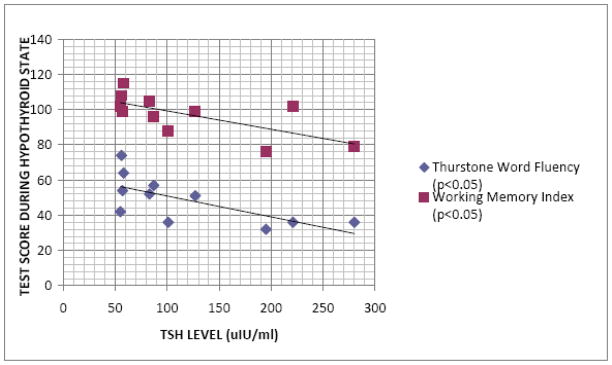
Significant correlations (p<0.05) were obtained between serum TSH concentration during the hypothyroid state and performance of the subjects on the Working Memory Index (p=0.02) and the Thurstone Word Fluency test (p=0.02). A graph depicting these relationships is shown in Figure 1. There were no other statistically significant correlations of TSH with measures of neuropsychological functioning during hypothyroidism, including measures of depression and anxiety.
Fig. 1.
Relationship of Selected Test Scores during the Hypothyroid State to Thyroid Stimulating Hormone (TSH) Levels
Correlational analysis of anxiety and depression and neuropsychological functioning
None of the neurocognitive (or psychomotor) test scores were found to be significantly correlated (p≥ 0.10) with the measures of depression or anxiety represented by the BDI-2 and STAI scores, respectively.
Discussion
We found that the state of overt, iatrogenically induced hypothyroidism in our subjects was associated with cognitive dysfunction limited to memory, specifically working memory, compared to a control group. We also found that Working Memory Index performance was inversely proportional to TSH during this condition. Working memory is considered a limited-capacity store for short term retention and mental manipulation of information--both verbal and visual (Strauss et al. 2006), and the Working Memory Index scores for our study participants reflected both of these dimensions. Our findings corroborated those of other investigators regarding the specificity of hypothyroid-related cognitive impairment (Burmeister et al. 2001; Miller et al. 2007), but also suggest that the more elevated the hypothyroid subject’s TSH level, the worse their performance in this particular cognitive domain.
At the follow-up evaluation (i.e. the second testing session for each group and following approximately 8 weeks of thyroid hormone replacement for the initially hypothyroid cohort), there were no statistically significant differences between the subjects and controls on any of the neuropsychological measures. Consistent with some degree of ‘practice effect’ being operative (Strauss et al. 2006), both the subjects and the controls improved on all of the neurocognitive measures at the second testing session, and the degree of improvement for the subjects was either statistically similar to or greater than the controls on all but one measure--Thurstone Word Fluency. This is a test of written verbal fluency, a cognitive domain shown to be adversely affected by hypothyroidism in a controlled study by Osterweil et al. (1992). Although our subjects did not manifest a significant difference in performance on this measure when hypothyroid compared to the controls, their relative lack of improvement when thyroid hormone replaced implies an inability to benefit to the same extent from the ‘practice effect’ apparent for the controls. This suggests that the subject’s hypothyroid state at the time of initial testing affected his or her implicit or explicit learning ability in this cognitive domain (Reber et al. 1999). Remarkably, performance on this written verbal fluency test was the only other neuropsychological measure (i.e. other than Working Memory Index) with which there was a significant correlation between subject performance while hypothyroid and TSH (i.e. the higher the TSH, the worse the performance).
The etiology and significance of the inverse correlations between TSH and subject performance on these neurocognitive measures during hypothyroidism are uncertain. While other investigators have looked for a relationship between symptoms and signs of hypothyroidism and TSH level, most of these studies have either not included (or not specifically examined) neuropsychological manifestations of hypothyroidism; or have examined subjects exclusively with subclinical rather than overt hypothyroidism--different conditions with presumably different effects on the brain (Davis and Tremont, 2007; Saravanan et al. 2006) (with normal peripheral thyroid hormone concentrations in the case of the former versus below normal concentrations in the case of the latter). In an examination of patients similar to our subjects, Meier and colleagues found no significant correlation between TSH level and various clinical and metabolic markers of so-called ‘tissue’ hypothyroidism (e.g. ankle reflex time, total cholesterol). These authors concluded that the secretion of TSH is driven by maximal stimulation with no further increase occurring with greater severity of hypothyroidism (at least in the case of OH), and therefore that the biological effects of thyroid hormones at the peripheraltissues (neither mood nor cognition were examined)—and not TSH concentration—reflect the clinical severityof the condition (Meier et al. 2003). Ridgeway et al.’s (1980) findings of discordant responses between the pituitary andperipheral target tissues in hypothyroid patients treated with T3 appear to support a similar conclusion.
While we know that at the time of our subject’s hypothyroid evaluation, peripheral thyroid hormone levels were undetectable by our laboratories’ serum assays, we do not know the level of thyroid hormone in our subject’s brains. We can, of course, assume that the brain levels of T3 and T4 were also very low, but in that the so-called ‘brain thyroid economy’ is very tightly controlled and not dependent directly on peripheral thyroid function (Dratman et al. 1983), and central nervous system measurements were not taken, the levels are, in fact, unknown. Although TSH as a surrogate for severity of hypothyroidism would seem to assume that all patients’ hypothalami are working normally and similarly, our data do suggest that out of a battery of neuropsychological measurements assessing a broad range of cognitive domains, performance on only the particular cognitive tests evidencing a significant effect from hypothyroidism, in our cohort, correlate (inversely) with TSH level during the hypothyroid state. Unlike Cleare et al. (1995), with a somewhat similar cohort of hypothyroid subjects, we did not find that degree of depression during hypothyroidism correlated with TSH level. This could be attributable to differences in our respective subjects in terms of duration and abruptness of onset of hypothyroidism (i.e. relatively prolonged, gradually developing hypothyroidism without treatment in the case of Cleare’s subjects; compared to abrupt, short term, severe hypothyroidism in subjects recently not hypothyroid, for our subjects).
Our findings therefore raise the possibility that contrary to the case with ‘peripheral tissue’ hypothyroidism, serum TSH does, in fact, reflect central thyroid hormone availability and, thus, the severity of ‘brain hypothyroidism’ as it relates to cognition (i.e. the more severe the condition, the worse the performance on cognitive measures affected by the condition), in overt hypothyroidism. An alternative explanation is that thyroid stimulating hormone itself has an adverse effect on particular aspects of cognition, at least at very high levels; and, the higher the level, the more significant the effect. This possibility of a direct pathologic effect of TSH on the brain obviously requires further exploration, and may, for example, be at least partially involved in the relationship between thyroid hypofunction and rapid cycling bipolar disorder (Gyulai et al. 2003).
Although we believe this apparent relationship between TSH and cognition in overt hypothyroidism to be interesting and deserving of further investigation, we recognize that inferences from our data are certainly preliminary in that our study is exploratory, with a number of limitations which we intend to overcome in future research. These limitations include a small number of subjects, measurements at only two points in time, and a cohort of subjects with relatively abrupt, iatrogenically induced changes in thyroid status with a history of thyroid cancer. Another potential shortcoming of our study relates to the fact that, despite no historical or clinical data to suggest thyroid dysfunction in our controls, thyroid dysfunction was not biochemically ruled-out in these individuals. In that our subjects were thyroid cancer patients, it might also be argued that the control subjects should have also been thyroid cancer patients, but in a persistent state of thyroid hormone replacement. Additionally, our decision to not control for multiple comparisons in the setting of our small sample size and multiplicity of tests raises the issue of statistical validity. We understand that this decision is open to criticism; but believe that our rationale is sound: Adjustment for multiple comparisons controls for Type I errors, but inflates Type II errors. As this is an exploratory study, we were more concerned about Type II errors leading to erroneous results so we did not elect to perform a Bonferroni adjustment. Despite the aforementioned limitations, the fact that the only two neurocognitive measures evidencing an adverse effect from hypothyroidism were the same (and the only) measures demonstrating a correlation between performance and TSH during hypothyroidism would seem to militate against occurrence by chance.
We look forward to exploring these and other brain-thyroid interactions without the above-noted limitations, and hope that our preliminary findings will encourage others to do likewise.
Acknowledgments
Dr. Beason-Held’s work was supported by the Intramural Research Program of the NIH, National Institute on Aging. The opinions expressed in this work are those of the authors, and do not reflect the official policy or the position of the U.S. Department of the Navy, U.S. Department of Defense, or the U.S. government.
References
- 1.Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 2.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 3.Boswell BB, Anfinson TJ, Nemeroff CB. Neuropsychiatric aspects of endocrine disorders. In: Yudofsky SC, Hales RE, editors. The American Psychiatric Publishing Textbook of Neuropsychiatry and Clinical Neurosciences. 4. Washington, D.C.: American Psychiatric Publishing, Inc.; 2002. pp. 851–875. [Google Scholar]
- 4.Burmeister L, Ganguli M, Dodge HH, Toczek T, DeKosty ST, Nebes RD. Hypothyroidism and cognition: preliminary evidence for a specific defect in memory. Thyroid. 2001;11(12):1177–1185. doi: 10.1089/10507250152741037. [DOI] [PubMed] [Google Scholar]
- 5.Cleare AJ, McGregor A, O’Keane V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin Endocrinol. 1995;43(6):713–9. doi: 10.1111/j.1365-2265.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 6.Constant EL, de Volder AG, Ivanoiu A, Bol A, Labar D, Seghers A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab. 2001;86:3864–3870. doi: 10.1210/jcem.86.8.7749. [DOI] [PubMed] [Google Scholar]
- 7.Constant EL, Adam S, Seron X, Bruyer R, Seghers A, Daumerie C. Anxiety and depression, attention, and executive functions in hypothyroidism. J Int Neuropsychol. 2005;11(5):535–544. doi: 10.1017/S1355617705050642. [DOI] [PubMed] [Google Scholar]
- 8.Costa A, Arisio R, Benedetto C, Bertino E, Fabris C, Giraudi G. Thyroid hormones in tissues from human embryos and fetuses. J Endocrinol Invest. 1991;14:559–568. doi: 10.1007/BF03346869. [DOI] [PubMed] [Google Scholar]
- 9.Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- 10.Denicoff KD, Joffe RT, Lakshmanan MC, Robbins J, Rubinow DR. Neuropsychiatric manifestations of altered thyroid state. Am J Psychiatry. 1990;147(1):94–99. doi: 10.1176/ajp.147.1.94. [DOI] [PubMed] [Google Scholar]
- 11.Despuza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29(3):414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Dratmen MB, Futaesaku Y, Crutchfield FL, Berman N, Payne B, Sar M, et al. Iodine-125 labeled triiodothyronine in rat brain: evidence for localization in discrete neural systems. Science. 1982;215:309–312. doi: 10.1126/science.7053582. [DOI] [PubMed] [Google Scholar]
- 13.Dratman MB, Crutchfield FL, Gordon JT, Jennings AS. Iodothyronine homeostasis in rat brain during hypo- and hyperthyroidism. Am J Physiol. 1983;245:E185–193. doi: 10.1152/ajpendo.1983.245.2.E185. [DOI] [PubMed] [Google Scholar]
- 14.Gyulai L, Bauer M, Bauer MS, Garcia-Espana F, Cnaan A, Whybrow P. Thyroid hypofunction in patients with rapid cycling bipolar disorder after lithium challenge. Biol Psychiatry. 2003;53(10):899–905. doi: 10.1016/s0006-3223(02)01573-1. [DOI] [PubMed] [Google Scholar]
- 15.Hennessey JV, Jackson IM. The interface between thyroid hormones and psychiatry. Endocrinologist. 1996;6:214–23. [Google Scholar]
- 16.Jain VK. A psychiatric study of hypothyroidism. Psychiatr Clin. 1972;5:121–130. doi: 10.1159/000283197. [DOI] [PubMed] [Google Scholar]
- 17.Joffe R, Nobrega J, Kish S, Calvo R, Dixon L, Wilson J, et al. Regional thyroid hormone levels in rat brain. Psychoneuroendocrinology. 1994;19:773–777. doi: 10.1016/0306-4530(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 18.Marangell LB, Ketter TA, George MS, Pazzaglia PJ, Callahan AM, Parekh P, et al. Inverse relationship of peripheral thyrotropin-stimulating hormone levels to brain activity in mood disorders. Am J Psychiatry. 1997;154:224–230. doi: 10.1176/ajp.154.2.224. [DOI] [PubMed] [Google Scholar]
- 19.Meier C, Trittibach P, Guglielmetti M, Staub JJ, Müller B. Serum thyroid stimulating hormone in assessment of severity of tissue hypothyroidism in patients with overt primary thyroid failure: cross sectional survey. BMJ. 2003;326:311–312. doi: 10.1136/bmj.326.7384.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KJ, Parsons TD, Whybrow PC, Van Herle K, Rasgon N, Van Herle A, et al. Verbal memory retrieval deficits associated with untreated hypothyroidism. J Neuropsychiatry Clin Neurosci. 2007;19(2):132–136. doi: 10.1176/jnp.2007.19.2.132. [DOI] [PubMed] [Google Scholar]
- 21.Nagamachi S, Jinnouchi S, Nishii R, Ishida Y, Fujita S, Futami S, et al. Cerebral blood flow abnormalities induced by transient hypothyroidism after thyroidectomy- analysis by Tc-99m-HMPAO and SPM96. Ann Nucl Med. 2004;18:469–477. doi: 10.1007/BF02984562. [DOI] [PubMed] [Google Scholar]
- 22.Osterweil D, Syndulko K, Cohen SN, Pettler-Jennings PD, Hershman JM, Cummings JL, et al. Cognitive function in non-demented older adults with hypothyroidism. J Am Geriatr Soc. 1992;40:325–335. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 23.Reber AS, Allen R, Reber PJ. Implicit versus Explicit Learning. In: Sternberg RJ, editor. The Nature of Cognition. Cambridge, Massachusetts: The MIT press; 1999. pp. 475–513. [Google Scholar]
- 24.Regalbuto C, Alagona C, Maiorana R, Di Paola R, Cianci M, Alagona G, et al. Acute changes in clinical parameters and thyroid function peripheral markers following L-T4 withdrawal in patients totally thyroidectomized for thyroid cancer. J Endocrinol Invest. 2006;29(1):32–40. doi: 10.1007/BF03349174. [DOI] [PubMed] [Google Scholar]
- 25.Ridgeway EC, Cooper DS, Walker H, Daniels GH, Chin WW, Myers G, et al. Therapy of primary hypothyroidism with L-triiodothyronine: discordant cardiac and pituitary responses. Clin Endocrinol (Oxf) 1980;13(5):479–488. doi: 10.1111/j.1365-2265.1980.tb03414.x. [DOI] [PubMed] [Google Scholar]
- 26.Robbins J, Lakshmanan M. The movement of thyroid hormones in the central nervous system. Acta Med Austriaca. 1992;19:21–25. [PubMed] [Google Scholar]
- 27.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlated with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab. 2006;91:3389–3393. doi: 10.1210/jc.2006-0414. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz HL, Oppenheimer JH. Nuclear triiodothyronine receptor sites in brain: probable identity with hepatic receptors and regional distribution. Endocrinology. 1978;103:267–273. doi: 10.1210/endo-103-1-267. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD. Description and applications of the STAI: Manual for the State-Trait Anxiety Inventory. 2. Consulting Psychologists Press, Inc; Palo Alto, California: 1983. [Google Scholar]
- 30.Strauss E, Sherman EMS, Spreen O. Memory. A Compendium of Neuropsychological Tests. Administration, Norms, and Commentary. 3. Vol. 11. New York, New York: Oxford University Press; 2006. p. 678. [Google Scholar]
- 31.Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry. 1969;20:48–63. doi: 10.1001/archpsyc.1969.01740130050004. [DOI] [PubMed] [Google Scholar]