Abstract
Objective
CXCL13 is a potent chemokine, produced by mature and recently recruited macrophages to sites of inflammation, which has anti-microbial and anti-angiogenic properties. The purpose of this study was to determine whether CXCL13 is present in maternal serum, umbilical cord blood and amniotic fluid (AF); if AF concentration changes with intra-amniotic infection/inflammation (IAI); and localize the production of CXCL13 in chorio-amniotic membranes and umbilical cord.
Study design
A cross-sectional study on maternal serum was performed including patients in the following groups: 1) non-pregnant women (n=20); 2) normal pregnant women (n=49); 3) patients at term not in labor (n=30); and 4) patients in spontaneous labor at term (n=29). Umbilical cord blood was collected from term neonates with (n=30) and without labor (n=28). Amniotic fluid was attained from patients in the following groups: 1) midtrimester (n=65); 2) term not in labor (n=22); 3) term in labor (n=47); 4) preterm labor (PTL) with intact membranes leading to term delivery (n=70); and 5) PTL leading to preterm delivery with IAI (n=79) and without IAI (n=60). CXCL13 concentrations were determined by ELISA. Chorio-amniotic membranes and umbilical cords were examined with immunohistochemistry. Non-parametric statistics were used for analysis.
Results
1) CXCL13 was present in 100% of serum and cord blood samples, and 99% of AF samples (339/343); 2) Serum CXCL13 concentration was significantly higher in pregnant women when compared to non-pregnant women [median 313.3 pg/mL (IQR: 197.2–646.9) vs. 40.5 pg/mL (IQR: 29.5–93.5), respectively; p<0.001]; 3) Serum CXCL13 concentration decreases with advancing gestational age (Spearman’s rho = −0.424; p<0.001); 4) There were no significant differences in the median serum CXCL13 concentration between women at term with and without labor [371.6 pg/mL (IQR: 194.3–614.3) vs. 235.1 pg/mL (IQR: 182.8–354.7), respectively; p=0.6]; 5) The concentration of CXCL13 in AF did not change with gestational age (p=0.11); 6) Patients with PTL and delivery with IAI had a significantly higher median concentration of CXCL13 than those without IAI [median 513.2 pg/mL (199.7–2505.5) vs 137.3 pg/mL (96.7–209.6), respectively; p<0.001] and those who delivered at term [133.7 pg/mL (97.8–174.8); p<0.001]; 7) Spontaneous labor did not result in a change in the median AF concentration of CXCL13 [labor: 86.9 pg/mL (55.6–152) vs no labor: 77.8 pg/mL (68–98); p=0.75]; 8) CXCL13 was immunolocalized to macrophages in fetal membranes and umbilical vein.
Conclusions
1) We report for the first time the presence of CXCL13 in AF; 2) AF CXCL13 concentrations are dramatically increased in intra-amniotic infection/inflammation; 3) Unlike other chemokines, AF and serum CXCL13 concentrations did not change with spontaneous parturition.
Keywords: amniotic fluid, BLC, chorioamnionitis, preterm delivery, preterm labor
INTRODUCTION
The preterm parturition syndrome can be caused by multiple pathologic processes, however, the only process that has been proven to be causal is intra-uterine infection/inflammation.1–5 The understanding of the specific roles and the identification of all the key components of the innate inflammatory defense system is still a work in progress, and preterm labor and its related adverse consequences are on the increase.6
Successful pregnancy requires tolerance to the fetal allograft as well as effective protection against infection. The mechanisms responsible for immune tolerance during pregnancy have been the subject of investigation for more than 30 years and remain controversial. On the other hand, a complex system exists to protect the conceptus and mother from infection. This system involves physical barriers to infection such as the mucous plug,7–11 placenta,12 fetal membranes,13,14 and components of the innate and adaptive immune system such as neutrophils, macrophages, natural killers cells and trophoblasts.12,15–19 Many of these cells require recruitment to the site of inflammation which is accomplished by chemokines.
Chemokines are classified into four families by the structure of the conserved cysteine-motif in the amino-terminus of the proteins.20 Less than half of recognized chemokines have been identified in amniotic fluid (AF), and several have been associated with preterm birth, intra-amniotic infection or inflammation including CCL2(MCP-1),17 CCL3(MIP-1α), CCL5(RANTES),21 CXCL1(GRO-α),22 and CXCL8(IL-8)23 among others.24–29
CXCL13, a chemokine previously named B-lymphocyte chemoattractant (BLC) in mice30 or B cell-attracting chemokine-1 (BCA-1) in humans,31 was first identified in 1998 in liver and lymph nodes. CXCL13 is a potent chemokine secreted by monocytes, lymphocytes and dendritic cells32 and is detected in serum,33 normal lymphoid tissue and in acute and chronically inflamed tissue.34 Its role in the inflammatory response is to attract B and T lymphocytes to areas of infection and inflammation.35 CXCL13 is involved in normal chemotaxis in response to routine physiological stress in the lymph nodes during development.36 In other tissues, parasitic and microbial exposure induces local production of CXCL13 leading to the formation of ectopic germinal centers,37 which serve as tertiary lymphoid organs in inflamed and infected tissues.
In pregnancy, the identification of CXCL13 has been reported only in cases of placental malaria.38 Present in both acute and chronic placental malaria, CXCL13 expression in placenta was up-regulated >130 fold in active malaria and >1,000 fold in cases of massive malarial infection. In addition, CXCL13 has been observed to form lymphoid-like aggregates similar to ectopic germinal centers in other tissues.
The purpose of this study was to determine if: 1) CXCL13 is present in maternal serum and the amniotic cavity in normal pregnancy; 2) concentrations of CXCL13 in AF and maternal serum change with increasing gestational age and in the presence of spontaneous labor at term; 3) CXCL13 concentrations in AF is affected by the presence of intra-amniotic infection/inflammation; 4) CXCL13 is present in cord blood of term neonates; and 5) if the production of CXCL13 can be localized in chorio-amniotic membranes and umbilical cord.
MATERIALS AND METHODS
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples including patients in the following groups: 1) non-pregnant women in the secretory phase of their cycle (n=20); 2) normal pregnant women (n=49); 3) patients at term not in labor (n=30); and 4) patients in spontaneous labor at term (n=29). Umbilical cord blood from term neonates was collected from mothers with (n=30) and without labor (n=28).
For the analysis of CXCL13 in AF, a cross-sectional study was conducted including patients in the following groups: 1) normal women in the mid-trimester of pregnancy (16–18 weeks) who underwent amniocentesis for genetic indications and delivered normal neonates at term (n=65); 2) normal pregnancies at term (≥37 weeks) with (n=47) and without (n=22) spontaneous labor; and 3) women with an episode of spontaneous preterm labor (PTL) and intact membranes who were further subdivided into three groups: a) PTL who delivered at term without intra-amniotic infection/inflammation (IAI) (n=70); b) PTL who delivered preterm without IAI (n=60); and c) PTL who delivered preterm with IAI (n=79).
All patients provided written informed consent prior to the collection of samples. The collection and utilization of samples for research purposes was approved by the Institutional Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICH/NIH/DHHS, Bethesda, MD) and participating institutions. These samples have been previously utilized in published studies of other inflammatory markers and antimicrobial peptides in normal and abnormal pregnancies.
Definitions
Patients were considered to have a normal pregnancy if they did not have any medical, obstetrical, or surgical complications, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age.39,40 Spontaneous preterm labor was defined by the presence of at least two regular uterine contractions every 10 minutes associated with cervical changes before 37 completed weeks of gestation, requiring admission to the hospital. Intra-amniotic infection was defined as a positive AF culture for microorganisms. Intra-amniotic inflammation was defined as AF interleukin-6 (IL-6) concentration >2.6 ng/mL.41
Amniotic fluid collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for genetic indications, evaluation of microbial status of the amniotic cavity, or assessment of fetal lung maturity in patients approaching term. The AF samples were transported to the laboratory in a sterile capped syringe. AF samples from patients with PTL were cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed after collection as previously described.41–45 The results of these tests were used for subsequent clinical management. In addition, IL-6 concentrations were determined from the AF of PTL patients the results were used only for research purposes. AF not utilized for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at −70°C until analysis.
Human CXCL13 immunoassays
Concentrations of CXCL13 in AF, maternal and umbilical cord serum were determined using specific and sensitive enzyme-linked immunosorbent assays obtained from R&D Systems, Inc. (Minneapolis, MN, USA) as per manufacturer’s instructions. AF CXCL13 assays were validated in our laboratory prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that AF matrix constituents did not interfere with antigen-antibody binding in this assay system. The calculated inter-assay coefficients of variation for serum and AF in our laboratory were 7.2% and 3.9%, respectively. The calculated intra-assay coefficients of variation for serum and AF were 4.9% and 3.0%, respectively. The sensitivity was calculated to be 3.7 pg/mL for serum and 2.8 pg/mL for AF.
Immunofluorescence staining
Two frozen specimens with chorioamnionits and funisitis and one control case with no evidence of inflammation were selected to determine the immuno-localization of CXCL13 in fetal membranes and umbilical cord. Immunofluorescence staining was performed on 5 μ-thick frozen sections after fixation with 4% paraformaldehyde. Following permeabilization with 0.25% TritonX-100 and blocking, sections were incubated with a rabbit polyclonal anti-CXCL13 antibody (ProteinTech Group, IL, USA). Alexa Fluor 594-labeled donkey anti-rabbit Ig (Invitrogen, CA, USA) was used as the secondary antibody. For double staining, sections were further incubated with a murine monoclonal anti-HLA-DR antibody (Dako, Glostrup, Denmark), and Alexa Fluor 488-labeled goat anti-mouse Ig (Invitrogen). The slides were mounted in ProLong Gold antifade reagent with DAPI (Invitrogen), and images were taken using a Leica TCS SP5 spectral confocal system (Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Amniotic fluid and serum CXCL13 concentrations were not normally distributed. Kruskal-Wallis with post-hoc analysis and Mann-Whitney U tests were used for the evaluation of continuous variables. Spearman’s Rho was utilized to assess correlations. Among patients with PTL and intact membranes, receiver operating characteristic (ROC) curve analysis was employed for the identification of patients who had IAI. A survival analysis with Kaplan-Meier was performed to examine the amniocentesis-to-delivery interval according to AF CXCL13 concentrations. A p-value of <0.05 was considered statistically significant. The statistical package utilized was SPSS v.12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Detection of CXCL13 in serum
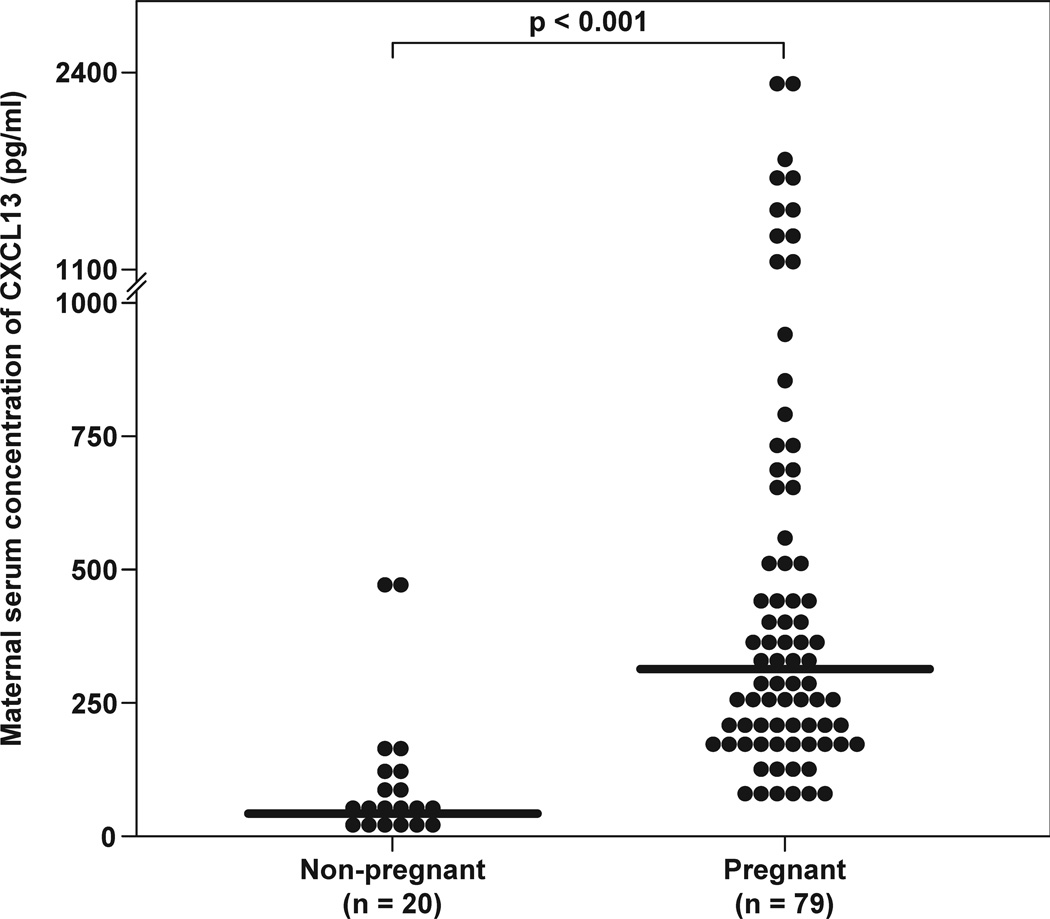
Serum CXCL13 concentrations were detected in all patients. The median serum CXCL13 concentration was significantly higher in pregnant women when compared to non-pregnant women [median 313.3 pg/mL (interquartile range [IQR]: 197.2–646.9) vs. median 40.5 pg/mL (IQR: 29.5–93.5), respectively; p<0.001] (Figure 1). In normal pregnancy, maternal serum CXCL13 concentration decreases with advancing gestational age from 8 to 40.7 weeks of gestation (Spearman’s rho = −0.424; p<0.001). There were no significant differences in the median serum CXCL13 concentration between women at term with and without labor [median 371.6 pg/mL (IQR: 194.3–614.3) vs. median 235.1 pg/mL (IQR: 182.8–354.7), respectively; p=0.6].
Figure 1.
Detection of CXCL13 in amniotic fluid
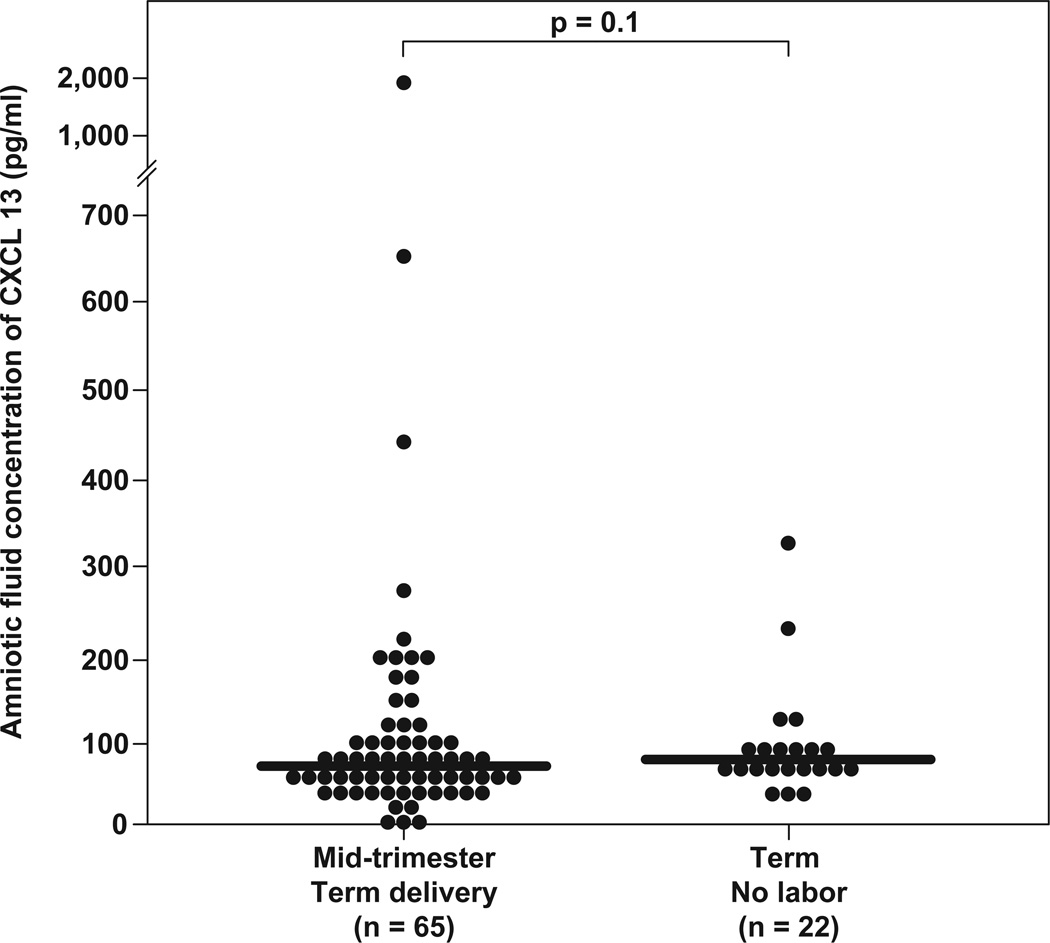
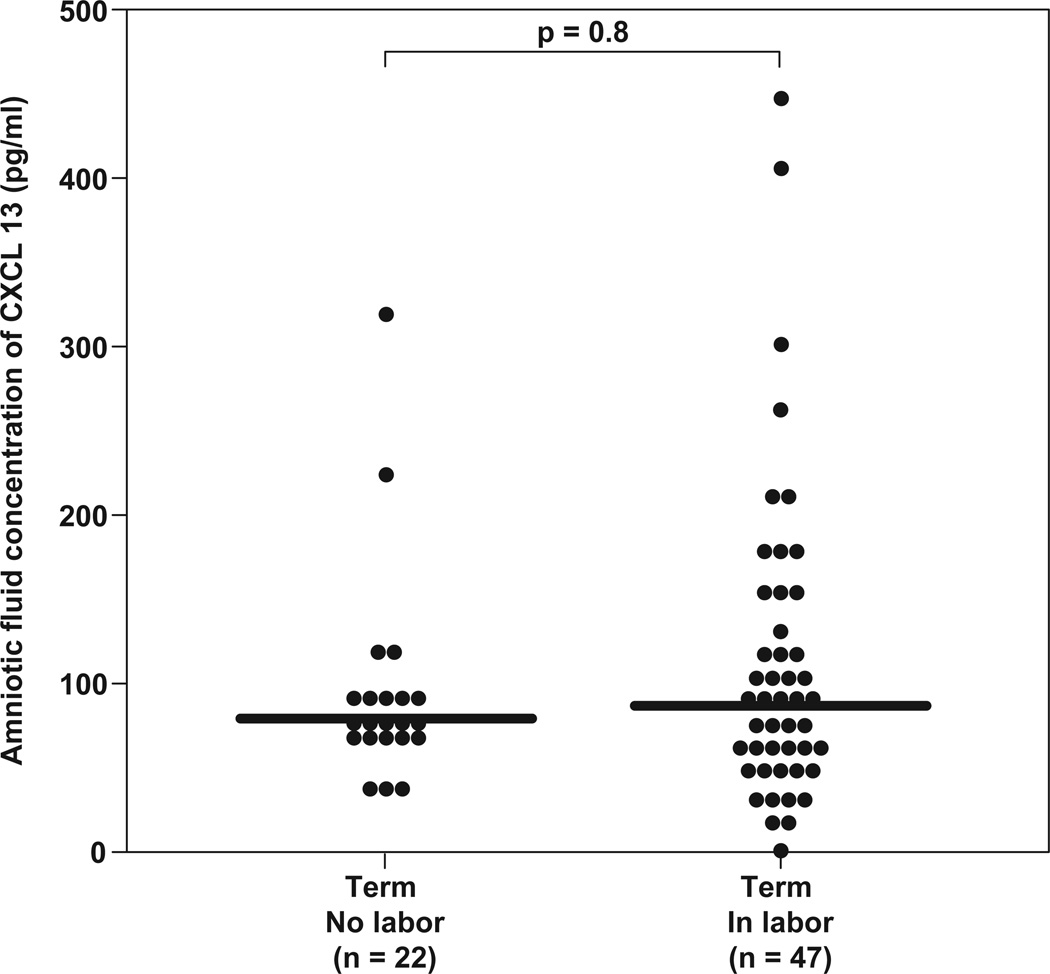
Table 1 displays the demographic and clinical characteristics of patients of the study groups. Amniotic fluid CXCL13 was detected in 98.8% (339/343) of patients. Four patients had CXCL13 concentrations below the limit of detection (3 patients in the mid-trimester group and 1 in the term in labor group). All four patients delivered at term without complication. The median AF concentration of CXCL13 was not different between women in the midtrimester of pregnancy and women at term not in labor [median 63.7 pg/mL (IQR: 42.9–94.9) vs. median 77.8 pg/mL (IQR: 68–98), respectively; p=0.11] (Figure 2a). Similarly, no differences were found in the median AF CXCL13 concentration between women not in labor at term and those with spontaneous labor [median 77.8 pg/mL (IQR: 68–98) vs. median 86.9 pg/mL (IQR: 55.6–152), respectively; p=0.8] (Figure 2b).
Table 1.
Distribution of maternal characteristics in the study categories.
| Mid-trimester (n = 65) |
Term not in labor (n = 22) |
Term in labor (n = 47) |
Preterm labor (n = 209) |
|
|---|---|---|---|---|
| Maternal age (years) | 37 (35–38) | 28.5 (21.8–32) | 23 (20–28) | 23.9 (20–27) |
| Gravity | 3 (2–3) | 3 (2–4) | 2 (1–3.3) | 3 (2–4) |
| Gestational age at amniocentesis (weeks) | 16 (16–17) | 39.3 (38.4–40) | 39 (38–40) | 28.5 (24.7–32.4) |
| Amniotic fluid sample storage time (years) | 11.7 (10.9–12.2) | 16.6 (16.5–16.6) | 16.6 (16.6–16.7) | 5.2 (4.7–6.1) |
| Gestational age at delivery (weeks) | 39 (38–40) | 39.3 (38.4–40) | 39 (38–40) | 33.1 (28.7–37.7) |
| Birth weight (grams) | 3345 (3144–3632) | 3405 (3130–3688) | 3220 (3090–3680) | 2044 (1110–2760) |
Values are expressed as median (interquartile range).
Figure 2.
AF CXCL13 in preterm labor
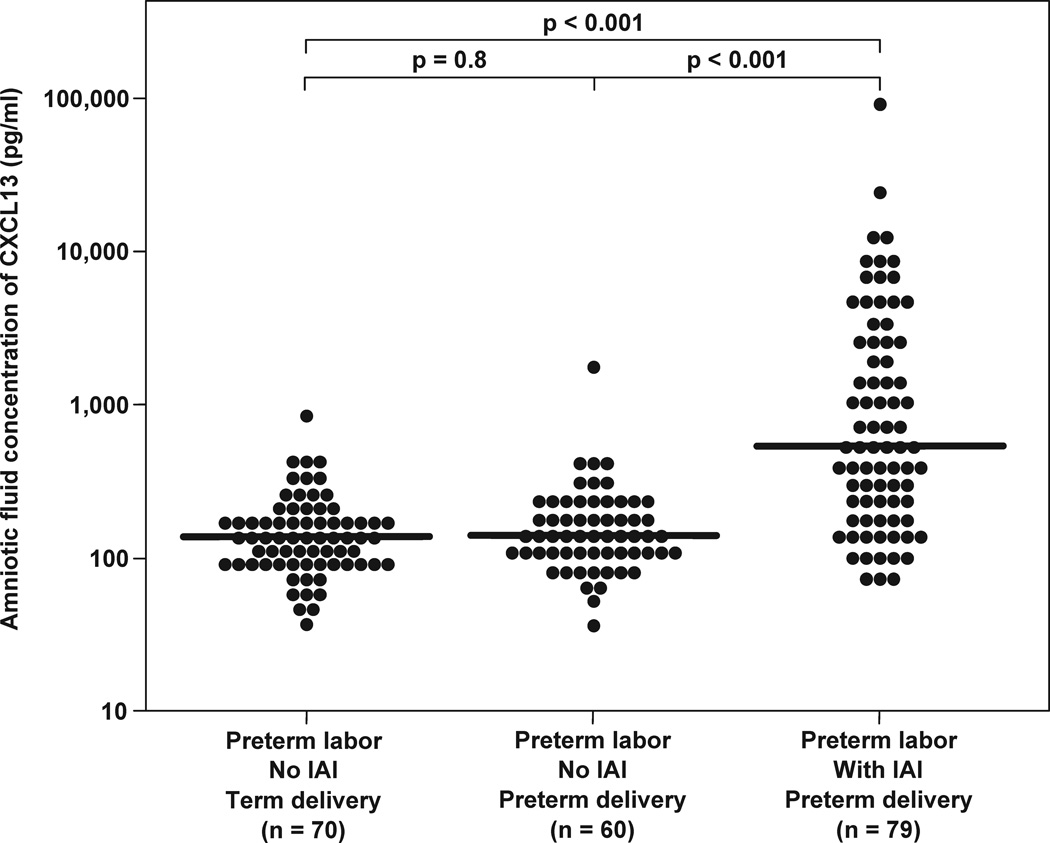
Table 2 displays the demographic and clinical characteristics of patients with spontaneous preterm labor with intact membranes. Among patients with spontaneous PTL with intact membranes, those with IAI had a significantly earlier gestational age at amniocentesis and delivery than those without IAI. In addition, patients with PTL with IAI had a significantly higher median AF concentration of CXCL13 than those without IAI who delivered preterm [median 513.2 pg/mL (IQR: 199.7–2505.5) vs. median 137.3 pg/mL (96.7–209.6), respectively; p<0.001]. Similarly, patients with PTL and IAI had significantly higher median AF concentrations of CXCL13 than those with an episode of PTL who delivered at term [median 133.7 pg/mL (IQR: 97.8–174.8), p<0.001] (Figure 3a). The median AF CXCL13 concentration in patients with PTL without evidence of IAI were not different between those who subsequently deliver preterm and at term (p=0.8).
Table 2.
Demographic and clinical characteristics of the preterm labor groups
| PTL with Term Delivery (n=70) |
PTD without IAI (n=88) |
p† | PTD with IAI (n=51) |
p* | |
|---|---|---|---|---|---|
| Maternal age (years) | 23 (19–26) |
22 (1–27) |
NS | 23 (20–28) |
NS |
| Gravity | 3 (1–4) |
3 (2–5) |
NS | 3 (2–4) |
NS |
| Race | African American: 97% Caucasian: 3% Other: 0% |
African American: 82% Caucasian: 7% Other: 12% |
NS | African American 86% Caucasian: 13% Other: 1% |
NS |
| Gestational age at amniocentesis (weeks) | 31.2 (26.4–32.9) |
30.8 (26.9–32.3) |
<0.05 | 25.9 (24–32.3) |
<0.05 |
| Amniotic Fluid Sample Storage time (years) | 4.9 (4.6–5.4) |
5.2 (4.8–6.1) |
NS | 5.7 (4.9–6.2) |
NS |
| Gestational age at delivery (weeks) | 38.3 (37.1–39.2) |
34.3 (32.3–35.3) |
<0.05 | 27.0 (24.4–32.4) |
<0.01 |
| Birthweight (grams) | 2970 (2725–3255) |
1810 (1715–2382) |
<0.01 | 1040 (634–1752) |
<0.01 |
Values are expressed as medians (inter-quartile range).
PTD: preterm delivery; IAI: intra-amniotic infection and/or inflammation; PTL: preterm labor; NS: not significant.
p†: Comparison between patients with spontaneous PTL without IAI who delivered preterm and those who subsequently delivered at term.
p*: Comparison between patients with spontaneous PTL without IAI and those with spontaneous PTL with IAI.
Figure 3.
AF CXCL13 in preterm labor with evidence of histological chorioamnionits
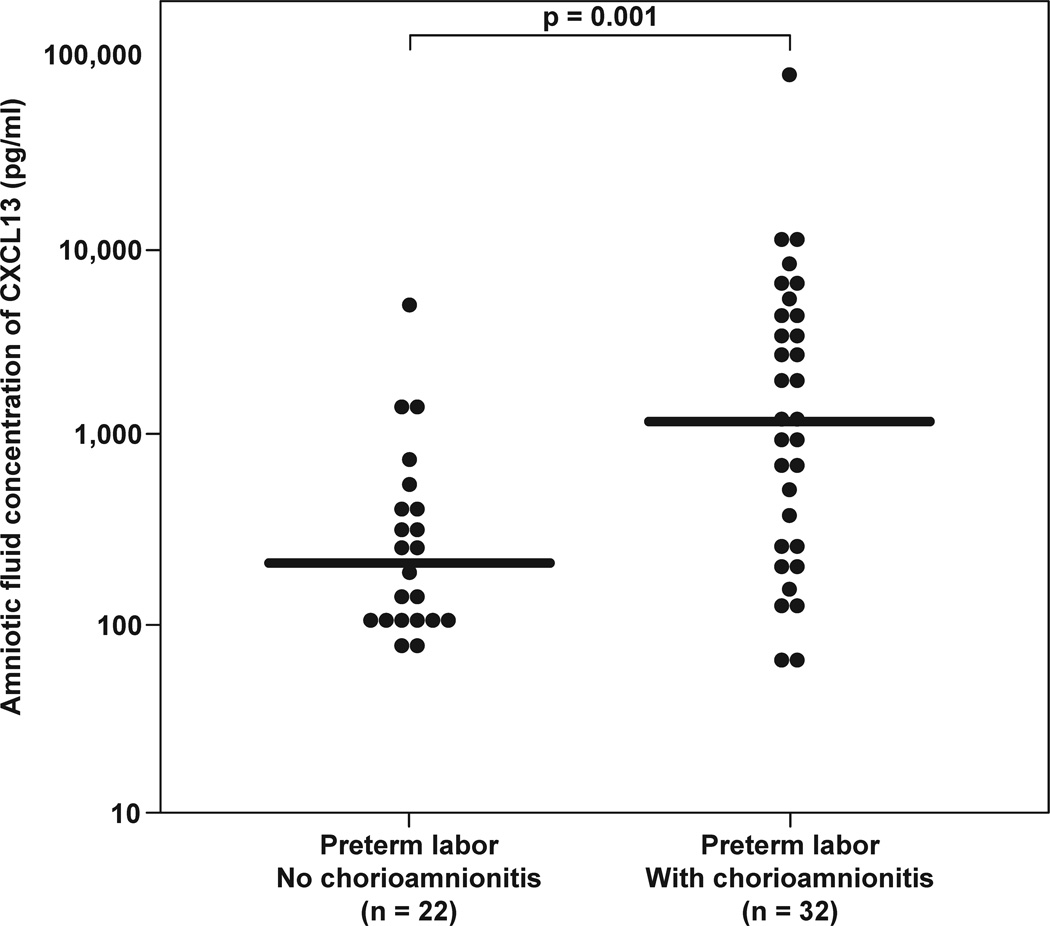
In patients with PTL who delivered within 72 hours of amniocentesis, placental pathology was available in 100% of them. Patients with histological chorioamnionitis and/or funisitis had higher median AF CXCL13 concentrations than those without [median 1201.1 pg/mL (IQR: 246.5–4643.8), vs median 216.0 pg/mL (IQR: 113.4–430.3), repectively; p=0.01] (Figure 3b).
Diagnostic cutoff and amniocentesis-to-delivery interval of an elevated AF CXCL13
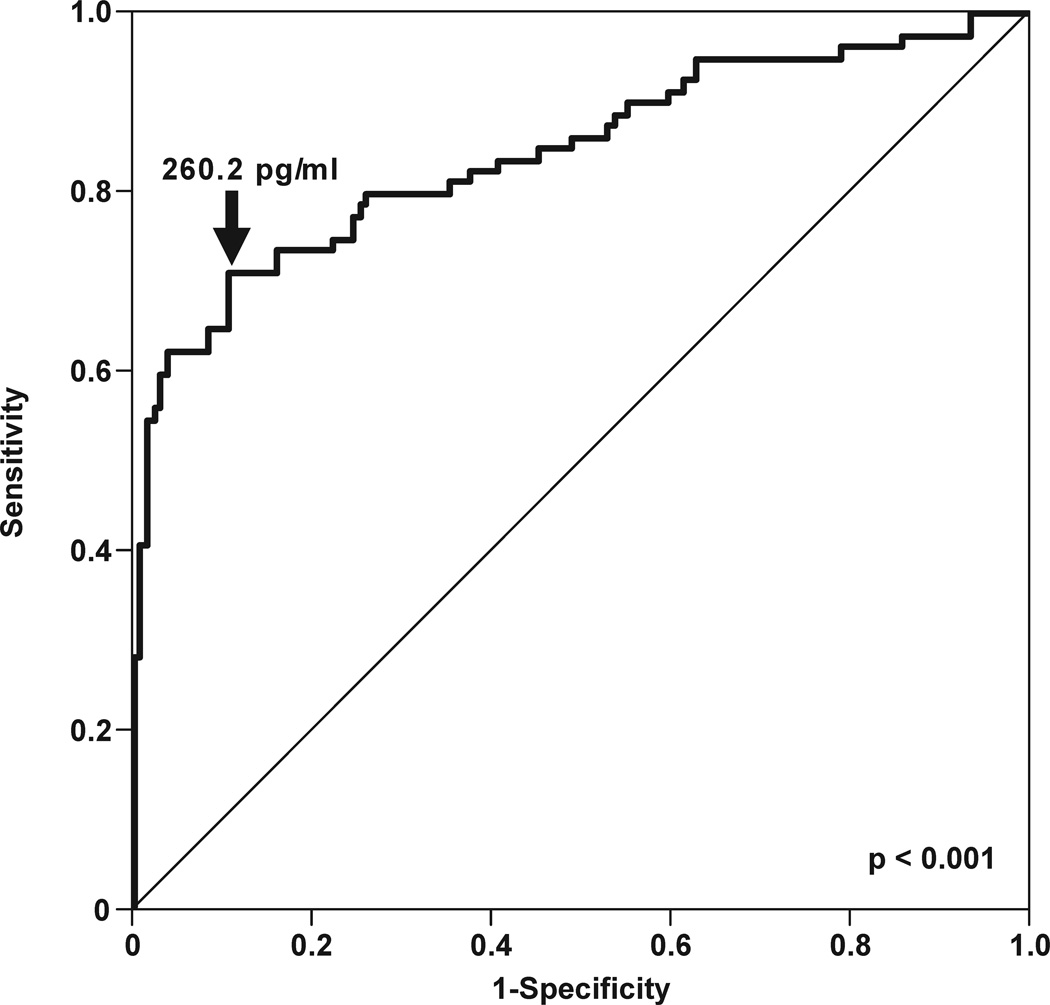
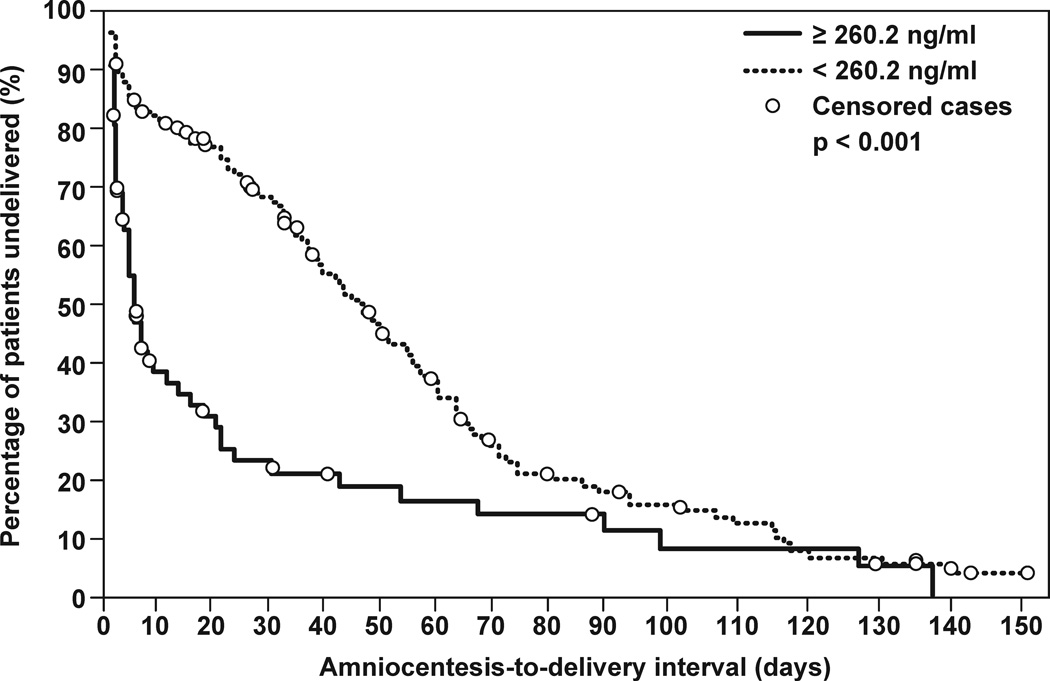
The diagnostic efficacy of CXCL13 concentration in AF was assessed among patients with PTL and intact membranes. A cutoff of 260.2 pg/mL was derived from the ROC curve analysis for the identification of patients who had IAI (sensitivity 70.9%, specificity 89.2%; area under ROC curve 84%; p<0.001) (Figure 4a). Survival analysis was employed to assess the relationship between IAI defined by CXCL13 concentration and the duration of the amniocentesis-to-delivery interval. Spontaneous labor and delivery was entered in the model as the event of interest and patients delivered for fetal or maternal indications were censored. The median amniocentesis-to-delivery interval was significantly shorter in patients whose AF CXCL13 concentrations were above 260.2 pg/mL (median amniocentesis-to-delivery interval: 4 days vs. 41 days; p<0.001) (Figure 4b).
Figure 4.
Immunohistochemistry of fetal membranes and umbilical cord
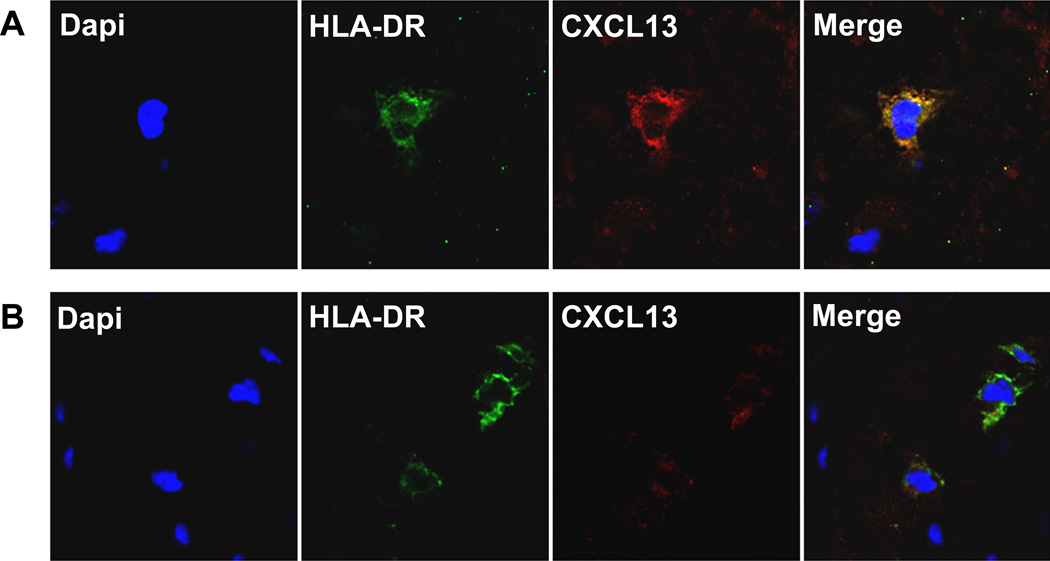
Immunohistochemical analysis of chorioamniotic membranes with histological inflammation demonstrated that CXCL13 was present only in HLA-DR positive macrophages in both chorion and decidua of fetal membranes (Figure 5). In cases of funisitis, fetal macrophages in the umbilical vein were also immunoreactive to CXCL13 (Figure 6a–d). Control sections of umbilical vein without funisitis also demonstrated evidence of CXCL13 immunoreactivity (Figure 6e–h), but was qualitatively diminished in comparison to cases with funisitis. In all cases, CXCL13 was localized mainly in the cytoplasm of the macrophages.
Figure 5.
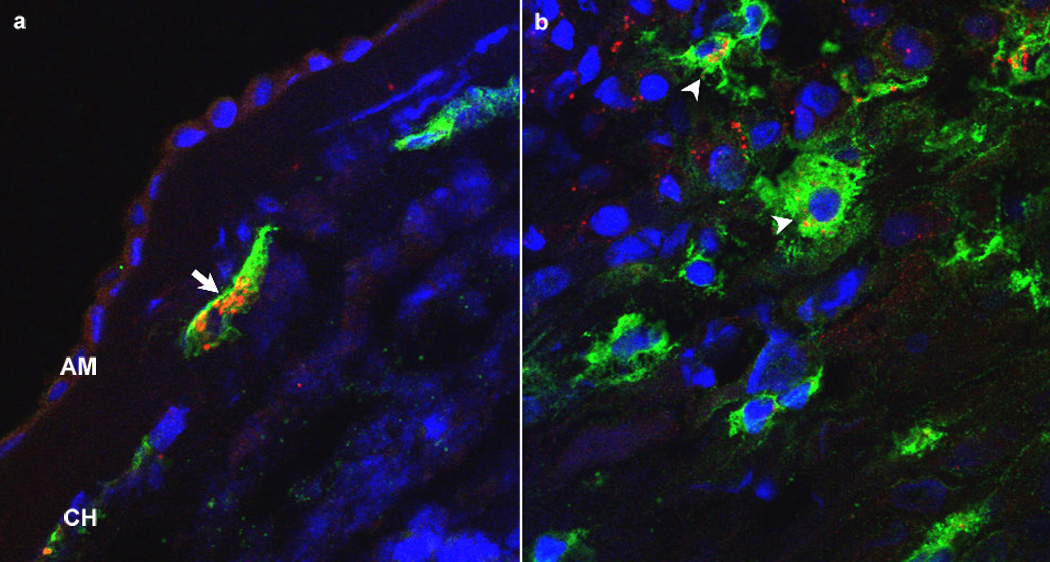
Figure 6.
Detection of CXCL13 in umbilical cord blood
In term neonates, CXCL13 was detected in all umbilical cord samples. The median concentration of CXCL13 in neonates born to mothers with and without labor was not significantly different [median 79.0 pg/mL (IQR: 62.5–103.8), vs. median 66.7 pg/mL (IQR: 58.5–88.0), respectively; p= 0.2].
DISCUSSION
Principal findings of this study
1) serum CXCL13 is increased with pregnancy and decreases as a function of gestational age, but did not change with spontaneous term labor; 2) CXCL13 was detectable in 98.8% of amniotic fluid samples; 3) CXCL13 concentration in AF was significantly increased in the presence of intra-amniotic infection and/or inflammation; 4) no changes were observed in AF CXCL13 concentration with advancing gestation or in the presence of spontaneous labor at term; 5) CXCL13 is present in chorioamniotic, decidual and umbilical cord macrophages, and the intracellular immunoreactivity of macrophages in the umbilical cord is increased in cases of histologic funisitis compared to those without evidence of histologic funisitis; and 6) CXCL13 was present in the umbilical cord sera of neonates at term, and its concentration does not change with labor.
What is CXCL13?
Chemokines are a subgroup of chemo-attracting cytokines that include approximately 50 ligands involved in normal homeostasis in many organs and in the pathophysiology of infectious and inflammatory disease.46 Chemokines are classified into four structural families according to the structure of the cysteine residues in the amino terminus (C, CC, CXC, CX3C). CXCL13 belongs to the CXC family of chemokines, all of whose genes are located on chromosome 4.47 The only known receptor for CXCL13 is CXCR5, which exclusively binds CXCL1348 and is expressed by mature B cells,49 a subset of CD4+ and CD8+ T cells, in secondary lymphoid tissue follicles50 and immature dendritic cells.51
Originally thought to be involved only in B cell chemotaxis, CXCL13 was previously known as BLC and BCA-1. CXCL13 assists in B cell trafficking to high endothelial venules (HEVs) in mesenteric lymph nodes, Peyer’s patches,52 inducible bronchus-associated lymphoid tissues (iBALT),53 and various musoca-associated lymphoid tissues (MALT).54 CXCL13 knockout mice have been used in various investigations involving lymph node development, and appear to have absent or reduced numbers of lymph nodes,55 absence of B1 cells in the peritoneal and pleural cavities,56 structurally abnormal nasal-associated lymphoid tissue that lack follicular dentritic cells57 and altered B cell adhesion in lymph nodes and Peyer’s patches.54 Lymph nodes that are present in these animals, albeit mostly abnormal, are capable of mounting an immune response to microbial challenge although B cell migration into dendritic-rich zones of lymph nodes is critically impaired. In addition, CXCL13 does not appear to be essential for survival or reproduction since the CXCL13−/−mice used in these studies were able to mate and produce litters of pups without any reproductive complications although the litter sizes or percentage of resorbed embryos were not reported. The presence of CXCL13 in both endothelium and epithelium-associated lymphoid tissues suggests an important role of CXCL13 on B cell function and the primary human defense response.
Recent studies have reported that CXCL13 also participates in T cell homing to germinal centers of lymphoid tissue,58 induction of migration of immature dendritic cells51 and maintenance of epithelial cell angiostatic activity.59 In a study using human umbilical vein endothelial cells, CXCL13 interfered with angiogenesis through competitive binding to fibroblast growth factor (FGF)-2 receptors on endothelium and through the formation of CXCL13-FGF-2 heterodimers.60
Role of CXCL13 in infection and inflammation
CXCL13 is implicated in chronic infectious diseases and is found in both the serum and tissues of infected individuals. CXCL13 expressing dendritic cells are found in high concentrations in granulomas in Cat Scratch Disease (Bartonella henselae infections).61 In neuroborreliosis, CXCL13 production is significantly correlated with spirochete load62 and measurement of serum CXCL13 has been proposed as a putative diagnostic or therapeutic marker for infection.63 In relapsing fever from Borrelia species, serum concentrations of CXCL13 have also been considered as a diagnostic marker for the disease.64 Serum concentrations of CXCL13 are elevated with human immunodeficiency virus (HIV) infection, and strongly correlate with inducible protein-10 (IP-10) and moderately correlate with viral loads.33 In addition, there is a down-regulation of CXCR5 on naïve B-cells in patients infected with HIV, which impairs the host’s immune system to mount a proper response to the virus despite the elevated concentrations of CXCL13.65
In several chronic infectious and inflammatory diseases, lymphoid neogenesis occurs in ectopic germinal centers in non-lymphoid tissue mediated by CXCL13.66 These organized centers of local interaction of B cells, T cells and dendritic cells serve to produce a local supply of immunoglobulin and chemokines. Ectopic germinal centers are located in bowel mucosa in inflammatory bowel disease,34 synovial membranes in rheumatoid arthritis,67,68 neural tissues in Lyme disease neuroborreliosis,62 vascular endothelium of the CNS in lymphoma,69 kidney in lupus nephritis,70 salivary glands of patients with Sjogren’s disease,71,72 and lung in tuberculosis.73
While it is advantageous for lymphoid neogenesis to occur near the infection site in order to assist the host immune response in antigen presentation and antibody production, the resultant inflammation may exacerbate symptoms of the disease. For instance, in Lyme disease, the hallmark of chronic infection is marked tissue inflammation with resultant tissue damage. Patients frequently suffer from myositis74,75 and tissue biopsies of diseased skeletal muscles demonstrate increased expression of CXCL13.76
There is paucity of literature about the role of CXCL13 in pregnancy. Muehlenbachs et al38 investigated placentas from women with active Plasmodium falciparum infection and identified aggregates of B cells resembling ectopic germinal centers. The maternal macrophages in the intervillous space of malaria-positive placentas were positive for CXCL13, suggesting that up-regulation of CXCL13 was from the maternal inflammatory response. This local concentration of CXCL13 attracts naïve B cells, plasma cells and facilitates antibody production that results in extensive inflammatory intervillious infiltration that can occlude the maternal circulation. In these placentas, the pathologic finding of massive chronic intervillositis is hypothesized to cause adverse fetal outcomes.77
CXCL13 in normal pregnancy
We report for the first time, the presence of CXCL13 in the AF of normal pregnancy. In addition, serum CXCL13 concentration is increased in normal pregnancy when compared to the non-pregnant state, and decreases as a function of gestational age. CXCL13 is also detected in umbilical cord blood of term neonates. In all compartments, CXCL13 is detected in the majority of samples, and at term, the concentration does not change with spontaneous labor.
The precise biological action exerted by CXCL13 in the amniotic cavity is not clear because this is not a site of lymphoneogenesis. However, the finding of dramatic up-regulation of CXCL13 in IAI does not preclude its importance in the role of maternal or fetal immunity or in maternal tolerance of the fetal allograft. Regulatory T (Treg) cells have been implicated in autoimmune disease,78 allergies,79 transplant rejection80 and pregnancy.81–83 Regulatory T cells express CXCR5, the receptor for CXCL13. Blockade of CXCL13 with an antibody prevents migration of regulatory T cells into transplanted organs and may favor rejection.84 Under normal circumstances, there is up-regulation of CXCL13 in the organ shortly after transplantation. This suggests that anti-rejection T cells are homed to the allograft in the initial host response. In addition, the evaluation of renal transplant biopsies as a function of time reveal that CXCL13 expression is 27-fold higher in biopsies (with well formed aggregates of B cells) from acutely rejected transplants and below the limit of detection in non-rejected transplants.85 CXCL13 is also highly expressed in severe transplant vasculopathy.86 These findings suggest that there is a sensitive balance of CXCL13 expression and regulatory T cell chemotaxis in the maintenance of transplanted organs. The interaction between CXCL13 in the transplant and CXCR5 on the T cells plays an important role in allogeneic organ acceptance.
The rise in maternal serum CXCL13 concentration with pregnancy and the subsequent decrease throughout gestation parallels the chemokine changes that occur with successful transplanted allografts.80,84,85 The presence of CXCL13 in AF early in gestation and its stable concentration throughout pregnancy suggests that CXCL13 may also contribute to the homeostatic balance that it may be important in the maintenance of a normal gestation.
CXCL13 in preterm labor
CXCL13 is expressed in biological fluids such as cerebrospinal fluid (CSF),64 plasma64 and serum,33 and tissues in infection and inflammation.38,61,62,73,74,76,87,88 The findings of our study reported herein indicate that this can also occur in the amniotic cavity in cases of intra-amniotic infection. While microbial invasion of the amniotic cavity is an important cause of preterm labor, inflammation has also been implicated in the etiology of preterm labor and is associated with an equal rate of adverse fetal outcomes when compared with microbial invasion of the amniotic cavity.41 Numerous inflammatory peptides have been identified and proposed to be used as diagnostic markers27,89 of increased risk of IAI and PTL. Of note, epithelial cell-derived neutrophil-activating peptide (ENA)-78,25 IL-6,90 and macrophage inhibitory factor91 are all pro-inflammatory proteins found to be elevated in IAI. Strong support of the involvement of CXCL13 in the inflammatory response is the stimulation of its production by TNF-α in the murine model.92
Lipopolysaccharide (LPS) can induce CXCL13 production by dendritic cells.93 This microbial product has been previously found in the amniotic cavity and decidua, which contain dendritic cells.94 It is possible that extra-amniotic infection stimulates the production of CXCL13 in the decidua. However, the precise mechanism responsible for the increased CXCL13 in amniotic fluid in cases of intra-amniotic infection is unknown. We have demonstrated the presence of CXCL13 in the cytoplasm of macrophages in the fetal membranes and the umbilical cord. It is possible that microbial products or bacteria stimulate the production of CXCL13 by resident macrophages in the amniotic cavity, in the fetal membranes, or in the fetus. Indeed, there is evidence that LPS can stimulate monocytes and macrophages to produce CXCL13.34 The same study indicated that macrophages secrete more CXCL13 than recently recruited monocytes or dendritic cells. It is possible that CXCL13 may be an important link between innate and adaptive immune responses because this chemokine would participate in the recruitment of B cells, which could produce antibodies.
What is unique about CXCL13 is that unlike other pro-inflammatory chemokines, such as IL-823 and CCL5/RANTES,21 its concentration in AF does not change with normal term parturition and does not decrease with advancing gestation. Increased CXCL13 concentration in AF is specific to PTL with evidence of IAI. Indeed, the increase in AF CXCL13 is not only a marker for a disease process, but may also initiate a pro-inflammatory response to IAI that leads to the activation of preterm parturition pathways.
Origin of CXCL13 in the amniotic fluid
In placental malaria, maternal macrophages in the intervillous space were hypothesized to be the source of CXCL13 production.38 However, the identification of CXCL13 in the maternal compartment does not explain its presence in AF. Evaluation of cord blood demonstrates that the fetus mounts a response to infection by activation of monocytes and neutrophils.95 Specifically, elevations in fetal umbilical cord blood cytokines have been identified in IAI,96 periventricular leukomalacia,97,98 and a fetal systemic inflammatory response syndrome.99 Indeed, the leading hypothesis for the source of inflammatory cytokines in AF has been of fetal origin, from the fetal intravascular compartment100 or from fetal membranes.101–103 It is known that CXCL13 is present in normal umbilical endothelium104 and CXCL13 expression has been identified in fetal spleen and vascular smooth muscle as early as 14 weeks of gestation.105 Therefore, the source of the increase in CXCL13 concentrations in the AF with IAI may be a fetal response to IAI.
Conclusion
The chemokine CXCL13 is a physiologic constituent of AF. Unlike other known chemokines, CXCL13 concentration does not increase with advancing gestation or with spontaneous labor at term. Concentrations of this protein are significantly increased in the presence of intra-amniotic infection/inflammation, indicating an acute response to microbial products in the amniotic cavity. Our findings suggest that CXCL13 may contribute to the innate immune response against intra-amniotic infection and/or inflammatory response. Further investigations are needed to elucidate the origin and biological actions of CXCL13 in normal and abnormal pregnancy.
Supplementary Material
Acknowledgements
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988 Sep;31(3):553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989 Sep;161(3):817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994 Sep;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 4.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006 Dec;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDorman MF, Hoyert DL, Martin JA, Munson ML, Hamilton BE. Fetal and perinatal mortality, United States, 2003. Natl Vital Stat Rep. 2007 Feb;55(6):1–17. [PubMed] [Google Scholar]
- 7.Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. 1993;168:A57. [Google Scholar]
- 8.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997 Feb;176(2):470–475. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 9.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000 Apr;15(4):778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 10.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002 Jul;187(1):137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 11.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001 Sep;185(3):586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 12.Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997 Oct;38(4):252–255. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 13.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991 May;12(3):285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 14.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001 Feb;94(2):224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 15.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000 May;6(5):589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003 Jan;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 17.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005 Jun;17(6):365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Yamamoto T, Kojima K, Tanemura M, Tateyama H, Suzumori K. Evaluation levels of cytokines in amniotic fluid of women with intrauterine infection in the early second trimester. Fetal Diagn Ther. 2006;21(1):45–50. doi: 10.1159/000089047. [DOI] [PubMed] [Google Scholar]
- 19.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007 Jan;20(1):15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000 Feb;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 21.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon BH. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999 Oct;181(4):989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, Goncalves LF, Gomez R. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996 Jan;35(1):23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 24.Shimoya K, Zhang Q, Tenma K, Ota Y, Hashimoto K, Shizusawa Y, Kimura T, Koyama M, Murata Y. Fractalkine (FRK) levels in amniotic fluid and its production during pregnancy. Mol Hum Reprod. 2003 Feb;9(2):97–101. doi: 10.1093/molehr/gag009. [DOI] [PubMed] [Google Scholar]
- 25.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, Mitchell MD. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod. 2004 Jan;70(1):253–259. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsson B, Holst RM, Andersson B, Hagberg H. Monocyte chemotactic protein-2 and -3 in amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation and preterm delivery. Acta Obstet Gynecol Scand. 2005 Jun;84(6):566–571. doi: 10.1111/j.0001-6349.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 27.Malamitsi-Puchner A, Vrachnis N, Samoli E, Baka S, Iliodromiti Z, Puchner KP, Malligianis P, Hassiakos D. Possible early prediction of preterm birth by determination of novel proinflammatory factors in midtrimester amniotic fluid. Ann N Y Acad Sci. 2006 Dec;1092:440–449. doi: 10.1196/annals.1365.043. 440-9. [DOI] [PubMed] [Google Scholar]
- 28.Mittal P, Romero R, Kusanovic JP, Gotsch F, Mazaki-Tovi S, Espinoza J, Erez O, Nhan-Chang CL, Than NG, Vaisbuch E, Edwin S, Hassan S. Granulocyte chemotactic protein-2 (CXCL6): A novel chemokine involved in the innate immune response of the amniotic cavity. Am.J.Obstet.Gynecol. 2007;197(6):S68. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, Than NG, Mittal P, Espinoza J, Friel L, Vaisbuch E, Mazaki-Tovi S, Hassan S. Exodus 1 (CCL20): Evidence for the participation of this chemokine in spontaneous labor at term, preterm labor and intrauterine infection. J.Perinat.Med. 2008 doi: 10.1515/JPM.2008.034. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998 Feb;391(6669):799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 31.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998 Feb;187(4):655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vissers JL, Hartgers FC, Lindhout E, Figdor CG, Adema GJ. BLC (CXCL13) is expressed by different dendritic cell subsets in vitro and in vivo. Eur J Immunol. 2001 May;31(5):1544–1549. doi: 10.1002/1521-4141(200105)31:5<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, Zack JA, Detels R, Martinez-Maza O. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005 Nov;25(11):702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 34.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004 Nov;104(10):3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 35.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000 Dec;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000 Aug;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 37.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24(1):39–65. doi: 10.1615/critrevimmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 38.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol. 2007 Jul;179(1):557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 39.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996 Feb;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004 Oct;132(10):1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001 Nov;185(5):1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988 Jul;159(1):114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990 Sep;163(3):968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993 Oct;169(4):805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 46.Kunkel SL. Through the looking glass: the diverse in vivo activities of chemokines. J Clin Invest. 1999 Nov;104(10):1333–1334. doi: 10.1172/JCI8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- 48.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003 Oct;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. 117-35. [DOI] [PubMed] [Google Scholar]
- 50.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000 Dec;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard OM, Dong HF, Su SB, Caspi RR, Chen X, Plotz P, Oppenheim JJ. Autoantigens signal through chemokine receptors: uveitis antigens induce C. Blood. 2005 Jun;105(11):4207–4214. doi: 10.1182/blood-2004-07-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, Akira S, Miyasaka M. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003 Aug;171(4):1642–1646. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 53.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A. 2007 Jun;104(25):10577–10582. doi: 10.1073/pnas.0700591104. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanemitsu N, Ebisuno Y, Tanaka T, Otani K, Hayasaka H, Kaisho T, Akira S, Katagiri K, Kinashi T, Fujita N, Tsuruo T, Miyasaka M. CXCL13 is an arrest chemokine for B cells in high endothelial venules. Blood. 2005 Oct;106(8):2613–2618. doi: 10.1182/blood-2005-01-0133. [DOI] [PubMed] [Google Scholar]
- 55.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000 Jul;406(6793):309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 56.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002 Jan;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 57.Rangel-Moreno J, Moyron-Quiroz J, Kusser K, Hartson L, Nakano H, Randall TD. Role of CXC chemokine ligand 13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and function of nasal-associated lymphoid tissue. J Immunol. 2005 Oct;175(8):4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 58.Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007 Jan;37(1):100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 59.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004 Apr;25(4):201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Spinetti G, Camarda G, Bernardini G, Romano DP, Capogrossi MC, Napolitano M. The chemokine CXCL13 (BCA-1) inhibits FGF-2 effects on endothelial cells. Biochem Biophys Res Commun. 2001 Nov;289(1):19–24. doi: 10.1006/bbrc.2001.5924. [DOI] [PubMed] [Google Scholar]
- 61.Vermi W, Facchetti F, Riboldi E, Heine H, Scutera S, Stornello S, Ravarino D, Cappello P, Giovarelli M, Badolato R, Zucca M, Gentili F, Chilosi M, Doglioni C, Ponzi AN, Sozzani S, Musso T. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood. 2006 Jan;107(2):454–462. doi: 10.1182/blood-2005-04-1342. [DOI] [PubMed] [Google Scholar]
- 62.Narayan K, Dail D, Li L, Cadavid D, Amrute S, Fitzgerald-Bocarsly P, Pachner AR. The nervous system as ectopic germinal center: CXCL13 and IgG in lyme neuroborreliosis. Ann Neurol. 2005 Jun;57(6):813–823. doi: 10.1002/ana.20486. [DOI] [PubMed] [Google Scholar]
- 63.Rupprecht TA, Pfister HW, Angele B, Kastenbauer S, Wilske B, Koedel U. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology. 2005 Aug;65(3):448–450. doi: 10.1212/01.wnl.0000171349.06645.79. [DOI] [PubMed] [Google Scholar]
- 64.Gelderblom H, Londono D, Bai Y, Cabral ES, Quandt J, Hornung R, Martin R, Marques A, Cadavid D. High production of CXCL13 in blood and brain during persistent infection with the relapsing fever spirochete Borrelia turicatae. J Neuropathol Exp Neurol. 2007 Mar;66(3):208–217. doi: 10.1097/01.jnen.0000248556.30209.6d. [DOI] [PubMed] [Google Scholar]
- 65.Chong Y, Nabeshima S, Furusyo N, Murata M, Yamaji K, Hayashi J. Downregulation of CXCR5 in. J Med Virol. 2004 Jul;73(3):362–367. doi: 10.1002/jmv.20099. [DOI] [PubMed] [Google Scholar]
- 66.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000 May;12(5):471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 67.Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, Yoshikawa H, Ochi T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001 Jan;166(1):650–655. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- 68.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003 Apr;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 69.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003 Feb;101(3):815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, Lipp M, Matsushima K. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001 Jun;193(12):1393–1402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren's syndrome. Scand J Immunol. 2002 Apr;55(4):336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 72.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 2003 Nov;48(11):3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 73.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007 Jun;178(11):7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 74.Atlas E, Novak SN, Duray PH, Steere AC. Lyme myositis: muscle invasion by Borrelia burgdorferi. Ann Intern Med. 1988 Aug;109(3):245–246. doi: 10.7326/0003-4819-109-3-245. [DOI] [PubMed] [Google Scholar]
- 75.Museteanu C, Schaible UE, Stehle T, Kramer MD, Simon MM. Myositis in mice inoculated with Borrelia burgdorferi. Am J Pathol. 1991 Dec;139(6):1267–1271. [PMC free article] [PubMed] [Google Scholar]
- 76.Pachner AR, Dail D, Narayan K, Dutta K, Cadavid D. Increased expression of B-lymphocyte chemoattractant, but not pro-inflammatory cytokines, in muscle tissue in rhesus chronic Lyme borreliosis. Cytokine. 2002 Sep;19(6):297–307. doi: 10.1006/cyto.2002.1973. [DOI] [PubMed] [Google Scholar]
- 77.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998 Aug;22(8):1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004 Aug;200(3):273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004 Nov;114(10):1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003 Feb;3(2):147–158. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 81.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004 May;136(2):373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saito S, Sasaki Y, Sakai M. CD4(+)CD25high regulatory T cells in human pregnancy. J Reprod Immunol. 2005 Apr;65(2):111–120. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon BA, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J, Volk HD. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006 Jan;36(1):82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 84.Lee BP, Chen W, Shi H, Der SD, Forster R, Zhang L. CXCR5/CXCL13 interaction is important for double-negative regulatory T cell homing to cardiac allografts. J Immunol. 2006 May;176(9):5276–5283. doi: 10.4049/jimmunol.176.9.5276. [DOI] [PubMed] [Google Scholar]
- 85.Steinmetz OM, Panzer U, Kneissler U, Harendza S, Lipp M, Helmchen U, Stahl RA. BCA-1/CXCL13 expression is associated with CXCR5-positive B-cell cluster formation in acute renal transplant rejection. Kidney Int. 2005 Apr;67(4):1616–1621. doi: 10.1111/j.1523-1755.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 86.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., III Antibody and complement in transplant vasculopathy. Circ Res. 2007 Feb;100(2):191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 87.Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999 Nov;104(10):R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou J, Law HK, Cheung CY, Ng IH, Peiris JS, Lau YL. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J Infect Dis. 2006 Jul;194(1):61–70. doi: 10.1086/504690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki Y, Yamamoto T, Kojima K, Tanemura M, Tateyama H, Suzumori K. Evaluation levels of cytokines in amniotic fluid of women with intrauterine infection in the early second trimester. Fetal Diagn Ther. 2006;21(1):45–50. doi: 10.1159/000089047. [DOI] [PubMed] [Google Scholar]
- 90.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, Edwin SS. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000 Jan;182(1 Pt 1):135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 91.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005 Dec;18(6):405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishikawa S, Nagai S, Sato T, Akadegawa K, Yoneyama H, Zhang YY, Onai N, Matsushima K. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur J Immunol. 2002 Jul;32(7):1881–1887. doi: 10.1002/1521-4141(200207)32:7<1881::AID-IMMU1881>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 93.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004 Jun;172(11):7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 94.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003 Oct;69(4):1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 95.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, Bianchi DW. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995 Oct;173(4):1315–1320. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 96.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000 Nov;183(5):1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 97.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996 May;174(5):1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 98.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997 Jul;177(1):19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 99.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999 Oct;181(4):773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 100.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, Gomez R. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002 Jun;186(6):1178–1182. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 101.Laham N, Brennecke SP, Bendtzen K, Rice GE. Differential release of interleukin-6 from human gestational tissues in association with labour and in vitro endotoxin treatment. J Endocrinol. 1996 Jun;149(3):431–439. doi: 10.1677/joe.0.1490431. [DOI] [PubMed] [Google Scholar]
- 102.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol Reprod. 1997 Dec;57(6):1438–1444. doi: 10.1095/biolreprod57.6.1438. [DOI] [PubMed] [Google Scholar]
- 103.Keelan JA, Wang K, Chaiworapongsa T, Romero R, Mitchell MD, Sato TA, Brown DA, Fairlie WD, Breit SN. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Mol Hum Reprod. 2003 Sep;9(9):535–540. doi: 10.1093/molehr/gag068. [DOI] [PubMed] [Google Scholar]
- 104.Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003 Dec;134(3):431–441. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steiniger B, Ulfig N, Risse M, Barth PJ. Fetal and early post-natal development of the human spleen: from primordial arterial B cell lobules to a non-segmented organ. Histochem Cell Biol. 2007 Sep;128(3):205–215. doi: 10.1007/s00418-007-0296-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.