Abstract
Background:
Whole slide imaging (WSI) makes it possible to capture images of an entire histological slide. WSI has established roles in surgical pathology, including support of off-site frozen section interpretation, primary diagnosis, educational activities, and laboratory quality assurance (QA) activities. Analyses of the cost of WSI have traditionally been based solely on direct costs and diagnostic accuracy; however, these types of analyses largely ignore workflow and cost issues that arise as a result of redundancy, the need for additional staffing, and customized software development when WSI is integrated into routine diagnostic surgical pathology. The pre-scan, scan, and post-scan costs; quality control and QA costs; and IT process costs can be significant, and consequently, pathology groups can find it difficult to perform a realistic cost–benefit analysis of adding WSI to their practice.
Materials and Methods:
In this paper, we report a “value added” approach developed to guide our decisions regarding integration of WSI into surgical pathology practice. The approach focuses on specific operational measures (cost, time, and enhanced patient care) and practice settings (clinical, education, and research) to identify routine activities in which the addition of WSI can provide improvements.
Results:
When applied to our academic pathology group practice, the value added approach resulted in expanded and improved operations, as demonstrated by outcome based measures.
Conclusion:
A value added can be used to perform a realistic cost-benefit analysis of integrating WSI into routine surgical pathology practice.
Keywords: Digital pathology, telepathology, whole slide imaging
INTRODUCTION
The development of whole slide imaging (WSI) has made it possible to capture images of an entire histological slide. While different hardware platforms support the image capture process, it is the development of user-friendly software interfaces that has enabled pathologists to begin to seriously contemplate integration of digital pathology activities into their routine clinical practice. The user-friendly interfaces make it possible to navigate to various regions of the scanned slide and change magnification for diagnosis (digital pathology), to view the image remotely by different pathologists in real time (telepathology), to capture static images of the slide for reporting or archiving, and to perform computer-aided analysis (digital image analysis). Roles for WSI in surgical pathology to support off-site frozen section interpretation;[1–4] primary diagnosis, not only as telepathology consultations,[5–7] but also to improve service to underserved areas;[8,9] educational activities;[10–14] and laboratory quality assurance (QA) activities are all well established.[15–18]
While the advantages of WSI for all of these applications are well established, the criteria for evaluating the cost of WSI-based activities have traditionally been based solely on direct costs (hardware and software) and diagnostic accuracy.[6,19–22] However, these types of analyses largely ignore a spectrum of workflow and cost issues that arise when WSI is integrated into routine diagnostic surgical pathology as a result of redundancy (due to the fact that histological sections on glass slides are an intrinsic component of surgical pathology, and the addition of WSI necessarily involves duplication of the primary diagnostic material), the need for additional staffing (so that WSI does not introduce delays in diagnosis), and customized software development (often required to address unique aspects of specific pathology practices). The pre-scan, scan, and post-scan costs; quality control and QA costs; and IT process costs that result when WSI is integrated into routine surgical pathology can be significant.[6,21,23,24]
Consequently, most pathology groups find it difficult to perform a realistic cost–benefit analysis of adding WSI to their practice, or are uncertain as to the process they should use to decide whether WSI will have utility in their spectrum of patient care activities. In this paper, we report a “value added” approach developed to guide our decisions regarding integration of WSI into our surgical pathology practice.[25–28] The approach focuses on specific operational measures (cost, time, and enhanced patient care) and the settings in which they can provide enhancement (patient care, education, and research). Application of this value added approach provided the focus to identify several routine activities in which the addition of WSI could improve our practice, and resulted in expanded and improved surgical pathology operations.
SETTING
Our group is a large surgical pathology practice (33 attending pathologists, 35 residents, and 16 subspecialty fellows from 10 different pathology subspecialty fellowship programs) at an academic tertiary care medical center and affiliated medical school. The practice has a subspecialty emphasis model with 12 subspecialty sections and multiple sign-out areas (individual pathologist's offices, common sign-out rooms, two frozen section areas that are in different buildings one city block apart); handles a large volume of high complexity cases (approximately 55,000 cases per year, including in-house cases and consultations); staffs over 40 patient care conferences per month; supports clinical, translational, and basic research activities of the department and medical school; and is involved in medical student teaching. In support of the diagnostic activities of the group, the histology laboratory processes approximately 200,000 blocks per year (approximately 800 blocks per day) and produces about 380,000 slides per year (approximately 1500 slides per day) including over 28,200 immunohistochemical stains per year, over 1600 in situ hybridization (ISH) slides per year, and over 3100 immunofluorescence stains per year.
Value added in our practice is defined in purely operational terms in three categories [Table 1], specifically cost savings, time savings, and improvements in patient care.[1–3,5–12,15,16] Although added value can be assessed on a number of different axes within each of these three categories, analysis was limited to support of patient care, educational activities, and research. Some components of the analysis are quantifiable and objective (see below), other components of the analysis are subjective. Added value can be achieved overall despite negative impacts in some categories and/or along some axes within each category. Finally, it should be emphasized that some aspects of WSI are specifically not included in a value added approach; for example, the mere capability to produce a digital image that can be used for diagnosis is, in and of itself, not value added simply because it is technically feasible or novel.
Table 1.
Operational definition of “Value added”

VALUE ADDED ANALYSIS
Table 2 presents the operational costs associated with WSI in our practice. Note that the time to scan slides in routine surgical pathology workflows is significantly higher than advertised by vendors, which reflects the operational realities of whole slide scanning as part of daily practice. The capital and personnel costs to incorporate WSI into our practice are summarized in Table 2. In the most recently completed calendar year (2010), we scanned 2.7% of the slides from our laboratory, for which we needed one scanner (Aperio ScanScope, Aperio Technologies Inc., Vista, CA, USA) at approximately 30% maximal utilization, one digital imaging technician (at approximately 0.5 FTE) and one information technology (IT) support person (at approximately 0.3 FTE). Were we to scan every slide in our practice, it would require an additional initial capital investment of approximately $2,000,000 (for hardware and software), and a yearly indirect cost of approximately $650,000 for support personnel and $10,000 for data storage.
Table 2.
Operational costs for incorporating WSI into routine surgical pathology workflows

Value added: Enhanced patient care
There are five areas in which WSI provides capabilities which are superior to current options, or for which other options are simply not available [Table 3]. Although these areas do not improve diagnostic accuracy of the specimen of origin of the scanned slides, they provide operational advantages that improve patient care in our practice in four ways. First, the use of WSI of selected slides from cases sent in consultation (the medicolegal climate in the United States dictates return of all diagnostic materials to the referring institution) provides the opportunity to enhance patient care by providing an immediately available permanent record of the slides to guide frozen section diagnosis at the time of subsequent definitive excision; for comparison at sign-out of subsequent excision or post-therapy specimen; for presentation at patient care conferences; in QA activities; and so on. Second, WSI of selected slides sent to other institutions as requested or required by their policies for patient care, or slides encumbered by medicolegal proceedings, provides us with a permanent record for use in patient care activities even though we lose control of the original glass slides. Third, WSI of slides that will be destroyed as part of ancillary testing makes it possible to retain the diagnostic content of the slides; given the demonstration that molecular tests can be performed on nucleic acids collected from diagnostic areas microdissected from glass slides,[29] the electronic record of slides produced by WSI will likely become more important. Fourth, WSI of slides for digital image analysis, for example, HER-2/neu analysis, supports emerging slide-based diagnostic paradigms.[30,31]
Table 3.
Value added in enhanced patient care

Value added: Educational programs
The use of WSI to support educational activities is well established.[10–14] At our institution, the added value was provided by the opportunity to produce virtual study sets. WSI also made it possible to view selected slides remotely from anywhere in the institution, which can be used to support medical lectures, medical student labs, and both intramural and extramural departmental educational activities.
Value added: Research
WSI makes it possible to produce virtual study sets that can be viewed for expert panel review, eliminating the need to produce multiple recuts of the study sets, mailing the various panel numbers, and even travel to a common site for real-time synchronous viewing by panel members. WSI is also used to support various clinical trials (in which digital images can substitute for glass slides for consensus for centralized review, e.g., by the Gynecologic Oncology Group), as well as to create a permanent record of slides that will be destroyed by the ancillary testing that is part of a clinical trial.
Not value added
In our practice, WSI does not provide added value in many areas classically associated with digital imaging, although it must be emphasized that aspects of WSI that are not value added for our group may well provide a benefit in other practice settings.
Since we have a large laboratory, we already have on site the glass slides from both routine histochemical and immunohistochemical stains, and so WSI does not provide the opportunity to save money or time in primary diagnosis. Also, since our practice has a subspecialty emphasis model with experts on site in virtually every organ system (quite literally across the hallway, if not one block away), there is no value added component for WSI to save time or increase diagnostic accuracy.
Similarly, since we already have a well-developed “bricks and mortar” consultation service, there is no immediate advantage to be gained in our practice by WSI for telepathology or branding. In fact, several impediments were identified that actually impede the capture of value added components of WSI in our consultation practice. First, among our referral base, the 1-day delay introduced by overnight express shipping of slides for primary or subspecialty review is not viewed as a significant detriment to patient care (in fact, overnight shipping of slides for early morning delivery integrates nicely into the routine workflow of our group). Second, although overnight express shipping is somewhat cumbersome and introduces the risk of slide loss or damage, it is extremely cost-effective (at current rates, the cost of express overnight shipping one 18 × 12.5 × 3 inch box that can hold up to 100 slides and 100 blocks, every day for 1 year, is less than $4000; in contrast, the current cost of entry level WSI hardware and software is approximately $135,000–$160,000 for a 5–10 slide scanner). Third, significant IT resources are required to develop, implement, and support the HIPAA compliant processes necessary to transfer the images; to support automated reporting to numerous outside client lab information systems; and to bill for the patient care activities (to say nothing of navigating the quagmire of state licensure requirements).
COSTS ASSOCIATED WITH IMPLEMENTATION
It became clear early in our evaluation of WSI that the faculty and trainees at our institution varied in their experience with the software for viewing digital slide images, their willingness to incorporate WSI into their routine practice, and in the increases in time they were willing to tolerate (even among faculty who were willing to include WSI into their workflow). The faculty and trainees were vocal in their unwillingness to incorporate a WSI process that required them to move back and forth between different software packages (with concomitant multiple log-ons using different usernames and passwords, after time-outs due to inactivity of the different programs, and so on), and most were unenthusiastic about a need to have several computer monitors so that multiple software packages could be open at the same time (notably, faculty that already had two computer screens running various software packages simultaneously were reluctant to add a third computer monitor to accommodate WSI). Space constraints limited the feasibility of multiple computer screens at all work stations, and the department was reluctant to purchase additional monitors. Finally, from a purely operational perspective, we were reluctant to pursue a model incorporating a requirement for multiple monitors that might work well at the medical center, but would not support remote faculty sign-out (either at home or while traveling), remote faculty and trainee research activities, or remote faculty and trainee educational activities.
Consequently, in collaboration with several vendors, we pursued a model of “one-stop-shopping” in which there was an automatic in-house interface between our lab information system (Cerner CoPath Plus, Cerner Corporation, Kansas City, MO, USA) and the imaging software (Aperio Spectrum). Development of this new functionality took approximately 11 months overall, including 9 months to design the system architecture and write the associated software code and 2 months to implement within our existing workflow. Implementation of the automatic interface had an overall cost of approximately $70,000 [for software development for the Aperio and the CoPath HL7 interfaces, and for purchase of the underlying CoPath Advanced Bar Coding and Tracking (AB and T) module]. The “one-stop-shopping” solution is illustrated in Figure 1.
Figure 1.
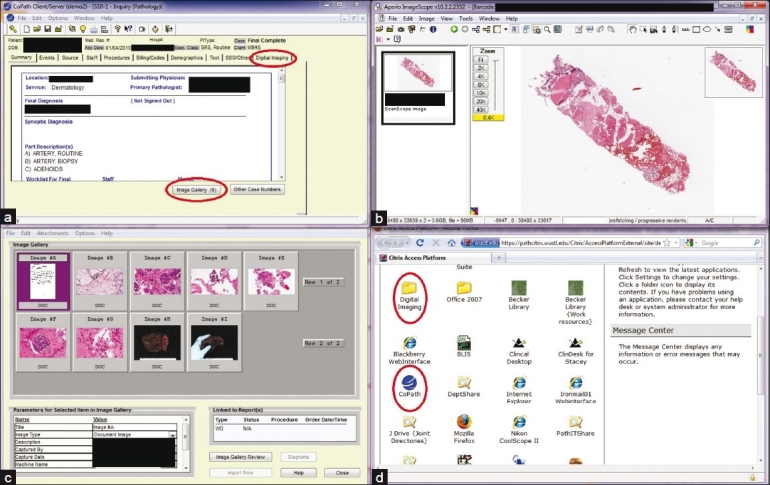
One-stop-shop solution. In CoPath, clicking on the Digital Imaging tab (upper red oval, panel a) immediately opens an Aperio Spectrum WSI window (panel b) that automatically displays the scanned slides from that case. Clicking on the Image Gallery tab (lower red oval, panel a) immediately opens a static image gallery (panel c) that includes the scanned paperwork from the case, gross images, and microscopic images. Secure remote access via the Citrix solution opens a window (panel d) with links (red ovals) to CoPath (for access to clinical WSI, as above) or directly to Spectrum (for access to research and educational WSI)
The need for the automatic interface emphasizes the types of additional costs that are often overlooked in evaluations of the utility of WSI in routine pathology workflow. Specifically, so-called off-the-shelf hardware and software packages, regardless of the vendor, have generic functionality. Integration of WSI into specific pathology practices often requires hardware and software changes to the standard products which can be costly and time consuming to develop and implement.
OUTCOMES
We also sought to evaluate whether the value added approach was successful in guiding integration of WSI into our clinical practice in ways that enhanced our patient care, research, and education activities. Our metrics in this analysis included changes in the number of scans, changes in faculty and trainee acceptance, and utilization [Table 4].
Table 4.
Outcomes of a value added approach

Number of scans
The number of cases scanned per year has shown consistent growth (at least 33% per year over the last 3 years). Of note, the average number of slides scanned per cases has decreased, which we interpret as evidence that the faculty and trainees are gaining experience with WSI and are learning which slides contribute the most to patient care. This change in the number of slides scanned per case suggests that the economic model presented above may require revision as we gain more experience with WSI.
Acceptance
Measurement of faculty and trainee acceptance is not a simple endeavor. However, we interpret faculty and trainee demands for remote access to clinical WSI activities via laptop computer, tablet personal computers, and smart phones as evidence that the faculty and trainees are integrating WSI into their routine workflows. In this regard, our pathologists are voicing an expectation in line with use by their clinical colleagues of mobile devices to access lab test results[32] and imaging studies.[33]
We have implemented the same one-stop approach to support the demands for remote access through development of a secure infrastructure in which all data are housed in an HIPAA approved environment. Access to the environment is via a Citrix application (Citrix Systems, Fort Lauderdale, FL, USA), specifically a Citrix Presentation server 4.5 installed on a Windows 2003 server-based system, which permits delivery of applications as services and thus provides on-demand secure access for users. Although this solution gives us the capability to access patient reports as well as view whole slide images in a HIPAA compliant environment, our use of Citrix's Independent Computer Architecture (ICA) protocol has clear limitations when accessing and scrolling through large amounts of graphical data; since ICA protocols were not expressly designed for this functionality, current user experience can be choppy.
Expanded utilization
Our initial value added approach identified WSI of slides seen in consultation as an enhancement to patient care. We interpret faculty interest in extending WSI to include select in-house cases as evidence of increasing recognition of a role for WSI in patient care activities. We likewise interpret faculty and trainee interest in extending WSI into a broader range of educational and research roles as evidence that our value added approach was successful in demonstrating key areas in which WSI could provide utility.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2011/2/1/39/84232
REFERENCES
- 1.Weinstein RS, Graham AR, Richter LC, Barker GP, Krupinski EA, Lopez AM, et al. Overview of telepathology, virtual microscopy, and whole slide imaging: Prospects for the future. Hum Pathol. 2009;40:1057–69. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Wilbur DC, Madi K, Colvin RB, Duncan LM, Faquin WC, Ferry JA, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: A pilot study using paired subspecialist correlations. Arch Pathol Lab Med. 2009;133:1949–53. doi: 10.1043/1543-2165-133.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 4.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network Experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: A validation study. BMC Clin Pathol. 2006;6:4. doi: 10.1186/1472-6890-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juki DM, Drogowski LM, Martina J, Parwani AV. Clinical examination and validation of primary diagnosis in anatomic pathology using whole slide digital images. Arch Pathol Lab Med. 2011;135:372–8. doi: 10.5858/2009-0678-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Fine JL, Grzybicki DM, Silowash R, Ho J, Gilbertson JR, Anthony L, et al. Evaluation of whole slide image immunohistochemistry interpretation in challenging prostate needle biopsies. Hum Pathol. 2008;39:564–72. doi: 10.1016/j.humpath.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Desai S, Ghosh TK, Chinoy R, Mohan A, Dinshaw KA. Telepathology at Tata Memorial Hospital, Mumbai and Barshi, a rural centre in Maharashtra. Natl Med J India. 2002;15:363–4. [PubMed] [Google Scholar]
- 9.Schneider J. Telepathology at Tikur Anbessa Hospital: how telemedicine works. Ethiop Med J. 2005;43:51–3. [PubMed] [Google Scholar]
- 10.Ho J, Parwani AV, Jukic DM, Yagi Y, Anthony L, Gilbertson JR. Use of whole slide imaging in surgical pathology quality assurance: Design and pilot validation studies. Hum Pathol. 2006;37:322–31. doi: 10.1016/j.humpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Downing SW. A multimedia-based histology laboratory course: Elimination of the traditional microscope laboratory. Medinfo. 1995;8(Pt 2):1695. [PubMed] [Google Scholar]
- 12.Mars M, McLean M. Students’ perceptions of a multimedia computer-aided instruction resource in histology. S Afr Med J. 1996;86:1098–102. [PubMed] [Google Scholar]
- 13.Kumar RK, Freeman B, Velan GM, De Permentier PJ. Integrating histology and histopathology teaching in practical classes using virtual slides. Anat Rec B New Anat. 2006;289:128–33. doi: 10.1002/ar.b.20105. [DOI] [PubMed] [Google Scholar]
- 14.Bruch LA, De Young BR, Kreiter CD, Haugen TH, Leaven TC, Dee FR. Competency assessment of residents in surgical pathology using virtual microscopy. Hum Pathol. 2009;40:1122–8. doi: 10.1016/j.humpath.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Foster K. Medical education in the digital age: Digital whole slide imaging as an e-learning tool. J Pathol Inform. 2010;1:14. doi: 10.4103/2153-3539.68331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnon M, Inhorn S, Hancock J, Keller B, Carpenter D, Merlin T, et al. Comparison of cytology proficiency testing: glass slides vs. virtual slides. Acta Cytol. 2004;48:788–94. doi: 10.1159/000326447. [DOI] [PubMed] [Google Scholar]
- 17.Rocha R, Vassallo J, Soares F, Miller K, Gobbi H. Digital slides: present status of a tool for consultation, teaching, and quality control in pathology. Pathology Res Pract. 2009;205:735–41. doi: 10.1016/j.prp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez NC, Barr TJ, Billiter DM. Utilizing virtual microscopy for quality control review. Dis Markers. 2007;23:459–66. doi: 10.1155/2007/959376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein RS, Descour MR, Liang C, Bhattacharyya AK, Graham AR, Davis JR, et al. Telepathology overview: From concept to implementation. Hum Pathol. 2001;32:1283–99. doi: 10.1053/hupa.2001.29643. [DOI] [PubMed] [Google Scholar]
- 20.Glatz-Krieger K, Glatz D, Mihatsch MJ. Virtual slides: High-quality demand, physical limitations, and affordability. Hum Pathol. 2003;34:968–74. doi: 10.1053/s0046-8177(03)00348-4. [DOI] [PubMed] [Google Scholar]
- 21.Huisman A, Looijen A, van den Brink SM, van Diest PJ. Creation of a fully digital pathology slide archive by high-volume tissue slide scanning. Hum Pathol. 2010;41:751–7. doi: 10.1016/j.humpath.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Rojo Mg, Castro AM, Gonçalves L. COST Action “EuroTelepath”: digital pathology integration in electronic health record, including primary care centres. Diagn Pathol. 2011;6(Suppl 1):S6. doi: 10.1186/1746-1596-6-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson ES, Rayo M, Gill C, Gurcan MN. Barriers and facilitators to adoption of soft copy interpretation from the user perspective: Lessons learned from filmless radiology for slideless pathology. J Pathol Inform. 2011;2:1. doi: 10.4103/2153-3539.74940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jara-Lazaro AR, Thamboo TP, Teh M, Tan PH. Digital pathology: Exploring its applications in diagnostic surgical pathology practice. Pathology. 2010;42:512–8. doi: 10.3109/00313025.2010.508787. [DOI] [PubMed] [Google Scholar]
- 25.Taylor R. Value-Added processes in the Information Life Cycle. J American Soc Info Science. 1982;33:341–346. [Google Scholar]
- 26.Wynants M. In Sickness and in Health: The Future of Medicine: Added Value and Global Access. London: ASP-VUB Press; 2009. [Google Scholar]
- 27.Baker J. Activity-Based Costing and Activity-Based Management for Health Care. New York: Aspen Publishers; 1998. [Google Scholar]
- 28. [Last accessed on 2011 Apr 17]. Available from: http://it.toolbox.com/blogs/enterprise-solutions/determiningthe-valueadded-activities-of-a-process-20700 .
- 29.Pfeifer JD, Zehnbauer B, Payton J. The changing spectrum of DNA-based specimen provenance testing in surgical pathology. Am J Clin Pathol. 2011;135:132–8. doi: 10.1309/AJCPLNO4PFVZVA4P. [DOI] [PubMed] [Google Scholar]
- 30.Ellis CM, Dyson MJ, Stephenson TJ, Maltby EL. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol. 2005;58:710–4. doi: 10.1136/jcp.2004.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson L, Conway C, Hanley A, Johnson A, Costello S, O’Grady A, et al. Image analysis as an adjunct to manual HER-2 immunohistochemical review: A diagnostic tool to standardize interpretation. Histopathology. 2010;57:27–38. doi: 10.1111/j.1365-2559.2010.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branz K. Pathology Groups and Clinical Labs Prepare to Deliver Test Results to Smart Phones. [Last accessed on 2010 Jul 2]. Available from: http://www.darkdaily.com/pathology-groupsand-clinical-labs-prepare-to-deliever-test-results-to-smart-phones-070210 .
- 33.iPad in practice: Appyling the apps. [Last accessed on 2010 Dec 14]. Available from: http://www.healthimaging.com/index.php?option=com_articlesandview=articleandid=25573 .


