This review covers historical and recent progress in understanding how respiration, fermentation and mitochondria contribute to pollen tube growth. It also summarizes what is known about the energetic requirements of this growth. Molecular mechanisms are viewed in the context of pollen tube physiology, a necessary perspective to understanding pollen tube growth.
Abstract
Background
Pollen tubes grow by transferring chemical energy from stored cellular starch and newly assimilated sugars into ATP. This drives myriad processes essential for cell elongation, directly or through the creation of ion gradients. Respiration plays a central role in generating and regulating this energy flow and thus in the success of plant reproduction. Pollen tubes are easily grown in vitro and have become an excellent model for investigating the contributions of respiration to plant cellular growth and morphogenesis at the molecular, biochemical and physiological levels.
Scope
In recent decades, pollen tube research has become increasingly focused on the molecular mechanisms involved in cellular processes. Yet, effective growth and development requires an intact, integrated set of cellular processes, all supplied with a constant flow of energy. Here we bring together information from the current and historical literature concerning respiration, fermentation and mitochondrial physiology in pollen tubes, and assess the significance of more recent molecular and genetic investigations in a physiological context.
Conclusions
The rapid growth of the pollen tube down the style has led to the evolution of high rates of pollen tube respiration. Respiration rates in lily predict a total energy turnover of 40–50 fmol ATP s−1 per pollen grain. Within this context we examine the energetic requirements of cell wall synthesis, osmoregulation, actin dynamics and cyclosis. At present, we can only estimate the amount of energy required, because data from growing pollen tubes are not available. In addition to respiration, we discuss fermentation and mitochondrial localization. We argue that the molecular pathways need to be examined within the physiological context to understand better the mechanisms that control tip growth in pollen tubes.
Introduction
One of the unique features of flowering plants is the rapidly growing tube produced by the male gametophyte that delivers the sperm cell to the ovule. Produced in the anther and then transferred to the stigmatic surface by wind, insect, bird, animal or direct contact, the pollen grain germinates and a pollen tube emerges. The pollen tube elongates rapidly through the style to find the ovules. The preponderance of pollen tubes relative to the ovules, and the fact that only one tube per ovule will be successful, creates conditions that foster vigorous competition among tubes. Through evolution, this has led to rates of cellular elongation unequalled elsewhere in the plant world. For example, in maize, pollen tubes can grow as fast as 1 cm h−1 (Mascarenhas 1993). To support this extremely rapid growth, there must be an equally powerful energy transduction mechanism.
The pollen tube presents an excellent model for the study of respiration for several reasons. First, because pollen tubes lack chloroplasts, respiration is not confounded by photosynthesis. Similarly, the commonly used fluorescent probes are not masked by chlorophyll autofluorescence. Second, the pollen tube is heterotrophic (O'Kelley 1955; Dickinson 1968; Labarca and Loewus 1973) and absorbs prodigious quantities of sugar and other ions from its external environment. Third, pollen tubes are easily grown in vitro, including in a well-aerated aqueous environment, allowing for measurement of inputs and outputs, specifically oxygen uptake and carbon dioxide efflux. The speed of growth, together with the large number of tubes that can be sampled at any time, permits short experiments and accurate measurements.
Respiration alone does not support the growth of the pollen tube in lily and tobacco. Aerobic fermentation also plays a role (Tadege and Kuhlemeier 1997; Rounds et al. 2010), although it is unclear whether this is true for all pollen tubes. In our recent report we showed that when respiration is inhibited, growth stops briefly before resuming using aerobic fermentation as the source of energy (Rounds et al. 2010). During this brief cessation of growth, processes such as cyclosis continue while others stop. These data suggest the need to account for the energy budgeting of the cell. This need has led to the present review.
The article summarizes historical work and current knowledge concerning the energy poise of the pollen tube. Recent research on pollen tube growth has focused on molecular mechanisms, particularly the signalling and control networks. Yet, these mechanisms must function in the physiological context of the cell. We strive to synthesize data from both physiological and molecular approaches to give a clearer understanding of growth regulation. We first describe what is known about respiration and energy production in the pollen tube. We then discuss why pollen tubes need so much energy by investigating the cell's ATP demands. The role of energy homeostasis and mitochondrial localization is also considered. Finally, we examine various outstanding questions that remain to be answered concerning pollen tube energetics.
Respiration
Pollen tubes are heterotrophic
Pollen of many species germinates in water alone (Johri and Vasil 1961). Nevertheless, in vitro studies of pollen generally include a growth medium with 5–10 % sugar. Many different sugars have been used in growth media, although sucrose generally shows the best germination and growth rates (Vasil 1987). Whether this added sugar acts merely as an osmoticum, or whether it is absorbed and metabolized by the pollen grains or tubes, received early research attention (Vasil 1987). Using 14C-labelled sugar, O'Kelley (1955, 1957) showed that the carbon dioxide produced by growing pollen tubes contained the labelled carbon. This work also demonstrated that pollen from Lonicera japonica, Tecoma radicans and Nicotiana tabacum grew better in sucrose than in other sugars. Subsequent work using labelled glucose injected into lily styles showed that pollen tubes absorbed the glucose and used it to construct the growing cell walls (Labarca and Loewus 1973). These data show that the growing pollen tube is heterotrophic, absorbing sugars for energy and for construction of the cell wall. This is not surprising given the extremely rapid growth, together with the large increase in cell volume and deposition of cell wall material that occurs between pollen grain germination and fertilization of the ovule.
The pollen tube has evolved the machinery for ample respiratory production
Several lines of evidence show that the pollen grain builds a substantial respiratory apparatus to support the rapid growth of the pollen tube. Even before germination, maize pollen grains have been shown to have 20 times more mitochondria than sporophytic cells (Lee and Warmke 1979). Growing tobacco pollen tubes respire at a rate ∼10 times higher than that of sporophytic tissues (Tadege and Kuhlemeier 1997). In addition, Itoh and Sekiya (1994) showed that purified lily FoF1 ATPases from pollen tubes respired at 20 times the rate of bulb ATPases and almost twice the rate of leaf ATPases.
To examine the genes necessary for pollen tube growth, a number of research groups have performed transcriptome profiling on various stages of pollen grain maturation (Twell et al. 2006; Becker and Feijó 2007). The analysis of the growing pollen tube has proved less productive. Nevertheless, a few recent studies have compared the transcriptome in pollen grains and growing pollen tubes in both Arabidopsis thaliana (arabidopsis) and Oryza sativa (Wang et al. 2008; Qin et al. 2009; Wei et al. 2010). Pollen tubes were shown not to express the alternative oxidase (AOX) (Fujii and Toriyama 2008). In most plant mitochondria, AOX allows for a shunt in the electron transport chain during biotic and abiotic stress (Sieger et al. 2005). This finding suggests that full-on energy-generating respiration is very important to the pollen tube. Of course, transcriptome profiling only gives part of the picture. It does not report protein levels or turnover and it does not describe mRNA longevity. Proteomic comparison of mature rice pollen to growing pollen tubes reveals a substantial up-regulation of carbon metabolism proteins (Dai et al. 2007), showing that the pollen tube dramatically up-regulates its respiratory machinery as growth begins.
Pollen tubes respire at extremely high rates
Pollen tubes readily germinate and grow in either a Clarke oxygen electrode or a Warburg flask, and thus are a good choice for investigating respiration. As a result, oxygen consumption by lily pollen was measured nearly 50 years ago (Dickinson 1965, 1966, 1967, 1968). These experiments revealed three phases of respiration using bulk lily pollen cultures. The first phase, that of rapid respiration, lasted ∼30 min and preceded germination (Dickinson 1965). During the second phase, respiration slowed to ∼40 % of the initial rate while the cells germinated and began growing (Dickinson 1965). The final phase continued indefinitely and was the phase of highest respiration, roughly double that of phase 2 (Dickinson 1965). In a subsequent study, Dickinson (1968) showed that sugar conversion to starch was most rapid before germination, i.e. during the initial period of high respiration. This suggests that in the pre-germination phase, the cell is preparing for the energetic demands of the high respiration observed in phase 3.
The work of Dickinson (1965, 1968) is supported by studies performed on isolated mitochondria from pollen of several species. Hoekstra (1979) isolated mitochondria from Typha latifolia, Nicotiana alata, Tradescantia paludosa and Aster tripolium pollen, and compared respiration rates in different sugars and with different inhibitors. He concluded that all of the pollen samples had mitochondria capable of very high levels of respiration but that the developmental stage of the mitochondria at pollen maturity varies and that this influences germination (Hoekstra 1979). After ultrastructural examinations, Hoekstra (1979) showed that more recent plant lineages with tri-cellular rather than bi-cellular pollen tend to have more mature mitochondria at grain maturity, with the pollen tubes from these species tending to grow more rapidly than those from bi-cellular grains (Hoekstra 1979; Hoekstra and Bruinsma 1979).
Pollen tubes grow through the style and oxygen diffusion is perforce affected by the local environment. Tobacco has a solid style whereas lily has a hollow style, which presumably allows for more rapid oxygen diffusion. Linskens and Schrauwen (1966), working with various cultivars of Hippeastrum, showed that, within the style of an unpollinated stigma, there are regions with substantially different oxygen partial pressure (pO2). Most dramatically, they showed that pO2 varies as the pollen tubes grow down the style. Indeed, the tip of the growing pollen tubes corresponds to the site of lowest pO2 (Linskens and Schrauwen 1966), indicating that the tip in particular consumed substantial amounts of oxygen as it grew through the style.
The activity of the mitochondrial electron transport chain can be assessed directly by measuring the fluorescence from NAD(P)H (Cárdenas et al. 2006; Rounds et al. 2010). Taking advantage of the endogenous fluorescence properties of this cofactor, in which the reduced form is more fluorescent than the oxidized form, Cárdenas et al. (2006) have shown that the activity of the electron transport chain oscillates in tandem with oscillatory changes in pollen tube growth rate. Statistical analyses of these data further reveal that the increase in the oxidized form (NAD(P)+) precedes the increase in growth (Cárdenas et al. 2006; Rounds et al. 2010) (Fig. 1). These observations directly link growth rate and oxidative phosphorylation.
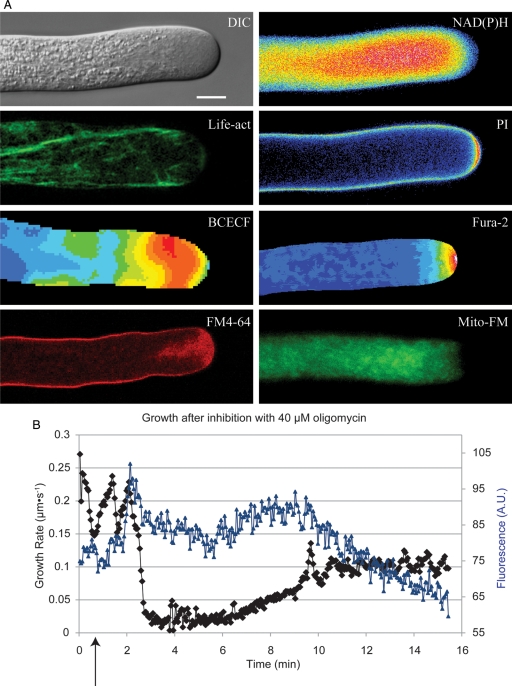
Fig. 1.
Lily pollen tubes and growth rate oscillations. (A) Lily pollen tubes imaged using different techniques. DIC is a differential image contrast micrograph of a growing lily pollen tube. NAD(P)H shows fluorescence from endogenous NAD(P)H (Cárdenas et al. 2006). Life-act shows labelling with Life-act-GFP, which labels actin (Vidali et al. 2009). PI shows propidium iodide fluorescence labelling the cell wall (McKenna et al. 2009). BCECF represents a pollen tube injected with dextranated BCECF, a ratiometric dye for pH (Feijó et al. 1999). Fura-2 is a cell injected with Fura-2 dextran, a Ca2+-sensitive ratiometric dye (Pierson et al. 1996). FM4-64 stains membranes, primarily involved with exocytosis (Parton et al. 2001). Mito-FM shows Mitrotracker-FM staining of mitochondria (Lovy-Wheeler et al. 2007). The scale bar represents 10 µm. (B) Growth rate oscillations (black) continue during growth in oligomycin. The arrow denotes addition of oligomycin. The blue trace represents NAD(P)H signal. NAD(P)H does not oscillate during growth in oligomycin. The figure was originally printed in Rounds et al. (2010).
Several studies have attempted to elucidate the role of the electron transport chain using oligomycin. For example, Gass et al. (2005) showed that, over periods of growth longer than 12 h, oligomycin, which blocks the final step in ATP synthesis, inhibits growth. Interestingly, the drug did not inhibit tobacco pollen germination (Gass et al. 2005). Other work demonstrated that oligomycin inhibits growth in lily even in short-term experiments (<2 h) (Dickinson 1966; Rounds et al. 2010). Taken together, these studies further underscore the role of respiration in driving pollen tube growth. They also suggest that in the pollen grain and shortly following imbibition, a rapidly respiring chondriome emerges to meet the pollen tube's energetic needs.
Aerobic fermentation
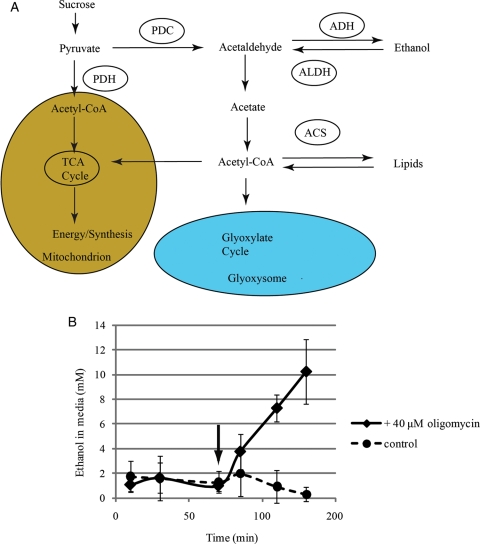
While the studies noted above highlight the massive energy needs and the role of respiration during pollen tube growth, these cells, like many other plant cells, can use fermentation during oxygen stress to produce ethanol and ATP. Surprisingly, tobacco and petunia pollen produce ethanol even in the absence of oxygen stress (Bucher et al. 1995). Using genetic and biochemical tools, Kuhlemeier and co-workers (Tadege et al. 1999; Mellema et al. 2002; Gass et al. 2005) dissected a pathway that they termed the pyruvate dehydrogenase (PDH) bypass (Fig. 2). In a series of experiments using 14C-ethanol, Mellema et al. (2002) showed that growing tobacco pollen tubes could use ethanol to support respiration through the citric acid cycle and lipid biosynthesis. Later, they showed that the presence of this bypass gave a competitive advantage to petunia pollen tubes over pollen tubes from a deletion mutant for the critical enzyme pyruvate decarboxylase (PDC) (Gass et al. 2005). The proposed pathway uses PDC to shunt pyruvate into either acetate or ethanol (Fig. 2). Acetate can then be further processed to acetyl-CoA by acetyl-CoA synthetase (Tadege et al. 1999; Gass et al. 2005). Gass et al. (2005) suggest that the massive energetic and lipid demands of the growing pollen tube led to the adaptation of this pre-existing pathway to give the pollen tube a growth advantage (Gass et al. 2005). The PDH bypass has been subsequently shown to be functional in sporophytic tissue in arabidopsis (Wei et al. 2009). Although the PDH bypass has not been demonstrated in lily pollen, pollen tubes from this species are able to grow in the absence of aerobic respiration (Rounds et al. 2010) (Fig. 1B). Indeed, during growth in oligomycin, lily pollen produces substantial quantities of ethanol, presumably to support continued ATP production (Rounds et al. 2010) (Fig. 2B). However, the characteristics of the growth are changed dramatically. The pollen tubes grow at ∼50 % of control rate and the NAD(P)H signal no longer correlates with the growth rate. Most unexpectedly, the cells still oscillate in growth rate although the oscillations have a longer wavelength when compared with controls (Rounds et al. 2010) (Fig. 1B).
Fig. 2.
(A) Pyruvate dehydrogenase bypass redrawn from Gass et al. (2005). (B) Oligomycin treatment increasing ethanol production rate. In (B) pollen tubes were grown in lily pollen growth medium for 70 min before the addition of oligomycin to a final concentration of 40 μM (arrow) to half of the cells. The black solid line represents ethanol concentration in the KCN-treated samples. The dashed line represents the control sample. The experiment was performed in quintuplicate and the error bar represents standard deviation. Figure from Rounds et al. (2010).
Energy transduction
Rapid pollen tube growth requires high rates of energy flow from starch and sugars into cellular metabolism and development. In this section we will review what is known and make suggestions about the energetic demands of the growing pollen tube. The respiratory load of a given organism comes from the sum of growth, ion uptake and maintenance (Bouma 2005). As a conceptual model, this is usually stated as r = rG + rU + rM, where r is total respiration, rG is the respiration necessary for the growth rate, rU is the respiration for nutrient uptake and rM is the respiration for maintenance. From this simple conceptual model, it is clear that due to their rapid growth rate, pollen cells need to transduce much more energy per unit of dry weight than most other types of plant cell. Pollen cells must also maintain a constant influx of ions and sugars in order to maintain the membrane potential and turgor given the ever-increasing volume (Bouma 2005). Before we investigate where ATP is required in the cell, a rough estimate of how much ATP is available at any given time would be helpful. ATP production by a growing pollen tube can be calculated from oxygen consumption data. Using an oxygen consumption rate (Dickinson 1965; Rounds et al. 2010) of 4 nmol mg−1 min−1, a P/O ratio of 3 (Dickinson 1965) and an estimate of 4490 grains of pollen mg−1 in lily, the pollen tube synthesizes ∼4.4 × 10−14 mol of ATP grain−1 s−1 (Table 1).
Table 1.
Summary of ATP turnover in growing pollen tubes. This table lists several significant processes that require ATP turnover in the growing pollen tube. The Evidence column describes the type of data used for making the calculations contained in the text.
| Process | Absolute rate (1 × 10−14 mol–s) | Relative rate | Evidence | Reference |
|---|---|---|---|---|
| Respiration | 44 × 10−14 | 100 % | O2 consumption by electrode | Dickinson (1965) and Rounds et al. (2010) |
| Membrane potential/turgor | 0.1 × 10−14 | (2–3 %) | Growth, [K+], H+ flux | Messerli et al. (1999) and Raven (1982) |
| Cyclosis | n.a. | (0.2 %) | Chara coralina myosin activity | Ito et al. (2003) |
| Wall synthesis | 0.41 × 10−14 | (10 %) | Growth, polyhexose synthesis | Raven (1982) and Holdaway-Clarke et al. (1997) |
| Actin | n.a. | (50 %) | Chicken neuron inhibition studies | Bernstein and Bamburg (2003) |
| Protein synthesis | n.a. | Trace | Inhibition studies of pollen | Hoekstra and Bruinsma (1979), Hoekstra (1979) and Roy et al. (2011) |
| Unaccounted for | 35 % |
Several processes are likely to be energy intensive. For example, once the tube begins expanding, it grows by balancing turgor pressure and the extensibility of the apical cell wall (Winship et al. 2010). Maintenance of turgor pressure requires constant transport of ions. The cell wall must be synthesized and a great deal of vesicle trafficking occurs to effect the rapid additions to the cell wall (Fig. 1A: PI) (McKenna et al. 2009; Y. Zhang et al. 2010). In turn, this vesicle trafficking relies on actin dynamics and the coordination of many small GTPases (Vidali and Hepler 2001; Vidali et al. 2001; Gu et al. 2005; Šamaj et al. 2006; Chen et al. 2007; Cárdenas et al. 2008; Lee et al. 2008; Kroeger et al. 2009). We will review the data concerning ion fluxes, osmoregulation, synthesis of pectin and proteins, vesicle trafficking, actin dynamics and cyclosis. All these processes require ATP hydrolysis either directly or indirectly through GTP.
Ion fluxes
Ion fluxes and gradients within the pollen tube tip have received considerable research attention. Inward gradients of Ca2+ (Rathore et al. 1991; Miller et al. 1992; Pierson et al. 1996) and H+ (Messerli and Robinson 1998; Feijó et al. 1999; Lovy-Wheeler et al. 2006) have been shown by several groups. In addition, external influx of Ca2+ (Pierson et al. 1996), K+ (Messerli et al. 1999) and H+ (Messerli et al. 1999; Certal et al. 2008) and efflux of Cl− (Zonia et al. 2002) have been measured. The ion gradient that has received the most attention is that of Ca2+ (Fig. 1, Fura). While no individual channel or pump mechanism has emerged, it has been proposed that the gradient is regulated by stretch-activated channels (Dutta and Robinson 2004), reactive oxygen species (ROS), Rho of plants (Yan et al. 2009), d-serine (Michard et al. 2011), outward-directed Ca2+ ATPases (Wu et al. 2010) and cyclic nucleotide-gated channels (Frietsch et al. 2007; Wu et al. 2011). It is probable that several of these play a role, although definitive evidence is lacking.
In recent years, the H+ gradient has emerged as a potentially important feature (Lovy-Wheeler et al. 2006). The first investigations suggested that pollen tubes lacked a consistent pH gradient (Fricker et al. 1997; Messerli and Robinson 1998). But by lowering the concentration of the dye to avoid a buffering effect, more recent work shows not only a consistent gradient, but also a subapical alkaline band (Feijó et al. 1999; Lovy-Wheeler et al. 2006). These features match the distribution of H+ ATPases in tobacco pollen tubes, suggesting that the creation of the gradient and the band may rely on these pumps (Certal et al. 2008).
Ion fluxes have received somewhat less attention. The magnitudes of Cl− efflux and H+ influx are in dispute (Robinson and Messerli 2002). Along with the K+ influx, the influx of H+ at the tip is thought to be related to volume changes during growth but to be relatively passive (Messerli et al. 1999; Messerli and Robinson 2003). The dramatic Cl− effluxes that are in phase with growth may also be connected to volume changes at the tip but are not thought to involve ATPases (Zonia et al. 2002). Ca2+ influx has been better characterized. Particularly striking is the finding that the external efflux does not occur at the same time as increases in the internal gradient (Holdaway-Clarke et al. 1997), suggesting different mechanisms. All these ion movements should follow their electrochemical gradients and thus not require ATP hydrolysis directly. One probable and potentially very large sink for ATP is the plasma membrane H+ ATPases. These localize along the shank but are excluded from the tip in tobacco pollen tubes (Certal et al. 2008). H+ efflux in the subapical zone of lily and tobacco has been observed, although the presence of this efflux zone is in dispute (Fig. 1) (Feijó et al. 1999; Robinson and Messerli 2002; Messerli and Robinson 2003). The presence of the efflux zone could explain the alkaline band observed in lily (Fig. 1A) (Feijó et al. 1999; Lovy-Wheeler et al. 2006). Much remains unclear concerning ion fluxes and gradients into and within the pollen tube. Some of the ion fluxes have particular bearing on osmoregulation (see below).
Osmoregulation
Pollen tubes and grains depend on the activity of plasma membrane H+ ATPases to sustain an inwardly negative electrical membrane potential, which drives the passive accumulation of solutes and maintains turgor pressure (Robertson et al. 2004; Lefebvre et al. 2005; Certal et al. 2008; Michard et al. 2009; Pertl et al. 2010). While turgor pressure in a population of pollen tubes may vary from cell to cell, the pressure in individual pollen tubes does not vary despite massive changes in volume over hours of rapid growth (Benkert et al. 1997; Winship et al. 2010). The pollen tube's rapid growth means a continuous, ongoing volume change. This, in turn, requires a continuous uptake of solutes and adjustment of the plasma membrane potential to maintain turgor. In guard cells, dramatic hyperpolarizations occur to draw K+ into the cell (Vavasseur and Raghavendra 2005; Kim et al. 2010). Similar changes in plasma membrane potential have not been seen in pollen tubes (Messerli et al. 1999; Messerli and Robinson 2003). It has been proposed that these influxes are part of maintaining the osmolarity of the added volume of the growing pollen tube (Messerli et al. 1999; Messerli and Robinson 2003). Because the external concentration of K+ is typically ∼100× lower than the internal concentration (external 1–3 mM, internal 125 mM), K+ uptake must be driven by an electrochemical energy gradient, generated by the pumping of H+ across the plasma membrane.
To estimate K+ ion flux into a growing pollen tube, we used the following equations and assumptions:
| (1) |
| (2) |
| (3) |
| (4) |
Combining terms,
| (5) |
where r is the mean radius of the pollen tube cell (8.0 × 10−6 m), dx/dt is the rate of increase in length (2.0 × 10−7 m s−1), dv/dt is the rate of volume increase (4.0 × 10−17 m3 s−1), [Ki+] is the internal concentration of K+ ions (1.25 × 102 mol m−3), 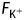 is the total flux of K+ into the cell (5.0 × 10−15 mol s−1), Atip is the area of the apical dome through which K+ flows (4.0 × 10−10 m−2) and
is the total flux of K+ into the cell (5.0 × 10−15 mol s−1), Atip is the area of the apical dome through which K+ flows (4.0 × 10−10 m−2) and 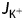 is the flux density of potassium at the tube tip (1.25 × 10−5 mol m−2 s−1). Our estimated K+ flux is several times greater than the maximum rates measured by Messerli et al. (1999), perhaps due to the difficulty of the vibrating electrode method. The minimum theoretical energy cost of K+ influx can be estimated as the work done by the moving ions, as current times voltage, across the plasmalemma,
is the flux density of potassium at the tube tip (1.25 × 10−5 mol m−2 s−1). Our estimated K+ flux is several times greater than the maximum rates measured by Messerli et al. (1999), perhaps due to the difficulty of the vibrating electrode method. The minimum theoretical energy cost of K+ influx can be estimated as the work done by the moving ions, as current times voltage, across the plasmalemma,
| (6) |
where 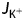 is flux density of monovalent charged ions (5.0 × 10−15 mol s−1), ΔE is the membrane electrical potential (0.10 V), N is Avogadro's number (6.023 × 1023 atoms mol−1) and F is Faraday's constant (6.241 × 1018 electrons per coulomb) so that the ion flux represents an energy cost (
is flux density of monovalent charged ions (5.0 × 10−15 mol s−1), ΔE is the membrane electrical potential (0.10 V), N is Avogadro's number (6.023 × 1023 atoms mol−1) and F is Faraday's constant (6.241 × 1018 electrons per coulomb) so that the ion flux represents an energy cost (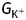 ) of 5.3 × 10−11 J s−1. With ATP hydrolysis able to supply 55 kJ mol−1 (Raven 1982), this flux would require 1 × 10−15 mol of ATP per second, or ∼2–3 % of the total ATP supply of a growing pollen tube (Table 1). The proton motive force also supplies the free energy for uptake of sugars, so this estimate is almost certainly a minimum. Messerli et al. (1999) report that H+ influx accompanies K+ influx at the pollen tube tip. If K+ influx requires a one-to-one influx of H+ (Palmgren 2001), then the electrogenic plasmalemma H+ ATPase would have to consume one ATP per H+ pumped to maintain the electrochemical gradient, at a rate of 5 fmol s−1 for a total energy cost of 10–15 % of cellular ATP synthesis (Slayman and Sanders 1985; Briskin and Reynolds-Niesman 1991; Palmgren 2001). Interestingly, when a pollen tube stops respiring, growth stops, but plasmolysis does not become apparent (Rounds et al. 2010). Possibly the pollen tube stops growing because of a drop in turgor pressure that is due to insufficient ATP to maintain the membrane potential and keep K+ flux high (Winship et al. 2010). In tobacco, it has been shown that H+ ATPases are excluded from the tip but occur all along the shank where a low but constant H+ efflux of ∼0.4 pmol cm−2 s−1 has been detected (Certal et al. 2008). If we assume that an efflux occurs along the entire shank of ∼0.4 pmol cm−2 s−1, then the ATP cost would be roughly 0.2 fmol of ATP grain−1 s−1, or roughly 0.5 % of the ATP budget of the cell. However, this measured efflux does not match the theoretical amount of pumping described above. In addition, Messerli et al. (1999) found no H+ efflux from the shank of lily. It is difficult to reconcile the conflicting data with the absolute need to balance and support the rapid influx of ions at the tip.
) of 5.3 × 10−11 J s−1. With ATP hydrolysis able to supply 55 kJ mol−1 (Raven 1982), this flux would require 1 × 10−15 mol of ATP per second, or ∼2–3 % of the total ATP supply of a growing pollen tube (Table 1). The proton motive force also supplies the free energy for uptake of sugars, so this estimate is almost certainly a minimum. Messerli et al. (1999) report that H+ influx accompanies K+ influx at the pollen tube tip. If K+ influx requires a one-to-one influx of H+ (Palmgren 2001), then the electrogenic plasmalemma H+ ATPase would have to consume one ATP per H+ pumped to maintain the electrochemical gradient, at a rate of 5 fmol s−1 for a total energy cost of 10–15 % of cellular ATP synthesis (Slayman and Sanders 1985; Briskin and Reynolds-Niesman 1991; Palmgren 2001). Interestingly, when a pollen tube stops respiring, growth stops, but plasmolysis does not become apparent (Rounds et al. 2010). Possibly the pollen tube stops growing because of a drop in turgor pressure that is due to insufficient ATP to maintain the membrane potential and keep K+ flux high (Winship et al. 2010). In tobacco, it has been shown that H+ ATPases are excluded from the tip but occur all along the shank where a low but constant H+ efflux of ∼0.4 pmol cm−2 s−1 has been detected (Certal et al. 2008). If we assume that an efflux occurs along the entire shank of ∼0.4 pmol cm−2 s−1, then the ATP cost would be roughly 0.2 fmol of ATP grain−1 s−1, or roughly 0.5 % of the ATP budget of the cell. However, this measured efflux does not match the theoretical amount of pumping described above. In addition, Messerli et al. (1999) found no H+ efflux from the shank of lily. It is difficult to reconcile the conflicting data with the absolute need to balance and support the rapid influx of ions at the tip.
Protein and starch synthesis
Unimbibed pollen is relatively quiescent in terms of metabolism (Dickinson 1965). Shortly after imbibition, lily pollen begins rapid starch synthesis from sugars (Dickinson 1968). Presumably, these stores will supply the growing pollen tube with energy as it moves down the style. Synthesis of proteins and cell wall materials will consume even more ATP (Mascarenhas and Bell 1969). Holdaway-Clarke et al. (1997) estimated that a growing lily pollen tube would need to add 1.6 µm3 s−1 of pectin to the tip to maintain average growth rates (Fig. 1, PI). The cost of cell wall polyhexose synthesis has been calculated at 55 kJ mol−1 of monomer (Raven 1982). Given a pollen tube of 18 µm diameter, a wall thickness of 0.2 µm and a growth rate of 0.2 µm s−1, the pollen tube would have to export 804 fg s−1 of cell wall material (Holdaway-Clarke et al. 1997). This would cost the cell 4.15 × 10−15 mol of ATP grain−1 s−1, or ∼10 % of the cell ATP budget.
Proteins must also be synthesized for pollen tube growth as new proteins will be needed for the ever-expanding plasma membrane and cell wall, as well as for replacing any turnover. It is unlikely, however, that this is a major draw on energy for several reasons. In experiments using cycloheximide and [3H]leucine, Hoekstra and Bruinsma (1979) show that in rapidly respiring pollen very little leucine is incorporated. Most surprisingly, in rapidly respiring tri-cellular Compositae species, cycloheximide does not inhibit growth or respiration, although in the low-respiring bi-cellular species tested it prevents germination (Hoekstra and Bruinsma 1979). As already noted, tri-cellular species have more mature mitochondria (Hoekstra 1979) and therefore need not produce protein for respiration. These data suggest that the amount of protein synthesis, and therefore the energy necessary, would be species dependent. In more recent work, Roy et al. (2011) examined the ability of pollen carrying mutant alleles of components of eIF3 to germinate. Both eIF3 subunits are represented by single genes in arabidopsis and are ultimately necessary for complete fertility. However, pollen lacking functional copies of these two genes can still germinate and grow, though at a reduced rate. This supports the idea that a large amount of protein translation is unnecessary for growth, suggesting that it probably is not a large energetic drain (Table 1) (Hoekstra and Bruinsma 1979).
Vesicle transport
We have described the potential energy drain of maintaining turgor. The other side of maintaining the growth balance is regulating extensibility of the cell wall. This is modulated, at least in part, by vesicle transport (Fig. 1, FM4-64 and PI), exocytosis and the constant synthesis of new wall material. Inspection of the ultrastructure of the apical clear zone of the pollen tube shows a dense population of vesicles (Lancelle et al. 1987). As the cell grows, new pectin and wall enzymes must be constantly secreted (McKenna et al. 2009; Winship et al. 2010). It is entirely unclear how much energy it takes to maintain the secretory pathway in pollen. In other systems, it requires surprisingly little. In Escherichia coli, ATP production can be reduced to as little as 10 % of the normal without disrupting exocytosis. Carbonyl cyanide m-chloro phenyl hydrazone (CCCP), an H+ ionophore, knocked out all exocytosis (Possot et al. 1997). The pollen tube must add a great deal of cell wall and many of the steps in exocytosis use GTP hydrolysis. It seems quite likely that more ATP is necessary for exocytosis in the pollen tube than in a bacterium. However, similar studies are lacking in plants.
Actin
The actin cytoskeleton of pollen tubes has received a great deal of attention (Vidali and Hepler 2001; Cheung and Wu 2008; Chen et al. 2009; Vidali et al. 2009; Fu 2010). The shank of the pollen tube contains many delicate filaments and bundles that are constantly remodelled as the cell grows. At the tip, there is a prominent, dynamic cortical structure variously described as a fringe or a mesh (Fig. 1) (Lovy-Wheeler et al. 2005; Cheung et al. 2008; Vidali et al. 2009). Actin dynamics require prodigious amounts of energy in the form of ATP. Each G-actin monomer has a bound ATP, which facilitates assembly into F-actin. While no studies have addressed the net energy utilization in pollen tubes, using embryonic chicken neurons Bernstein and Bamburg (2003) showed that roughly 50 % of ATP utilization in these cells was from actin-ATP hydrolysis (Table 1). Moreover, in the neuron, the bulk of organelle motility is from interactions with microtubules. In pollen tubes, organelle movement and vesicle transport occur through interactions with actin (Lovy-Wheeler et al. 2007; Zheng et al. 2010). This suggests that an even larger portion of the energy budget may come from actin dynamics. As the actin cytoskeleton has been shown to be critical for tip growth, it is unclear what happens to the actin cytoskeleton in the absence of respiration, although growth can occur in the presence of respiratory inhibitors and in anoxic conditions (Tadege et al. 1998; Rounds et al. 2010) (Fig. 1B).
Cyclosis
Most plant cells exhibit cyclosis but, in the pollen tube, streaming takes on a particularly dramatic ‘reverse fountain’ form (Cárdenas et al. 2005). The mechanism leading to cytoplasmic streaming involves the interaction between myosin XI tethered organelles and F-actin (Kohno and Shimmen 1988; Verchot-Lubicz and Goldstein 2010). Actin-disrupting drugs stop cyclosis in Nitella pseudoflabellata (Foissner and Wasteneys 2007). Because both myosin and actin consume ATP, and because myosin XI has very high ATPase activity in Chara coralina (Ito et al. 2003), it was thought that cyclosis would require large amounts of ATP. Surprisingly, Yamamoto et al. (2006) found that only a small pool of myosin is active at any given time and therefore ATP utilization to maintain cyclosis would be quite low: they estimate 0.2 % of the dark respiration (Table 1). This study did not actually measure or inhibit respiration but, instead, relied on measurements of active myosin. Therefore, they may have underestimated energy utilization. When lily pollen respiration is inhibited by oligomycin, cyclosis continues despite a cessation of growth, suggesting that cyclosis does not consume a large proportion of the energy before inhibition (Rounds et al. 2010). From these data we conclude that maintenance of cyclosis accounts for a very small percentage of the energy budget of pollen tubes.
We have discussed several prominent examples of energy-utilizing processes in the pollen tube. If we return to the formulation, r = rG + rU + rM, we have now partially addressed the first two terms, growth and membrane transport. However, the maintenance function has only been touched on. In addition, ROS have been shown to be important for growth (Potocký et al. 2007; Lee and Yang 2008). What is the energetic cost to their production? Unfortunately, the energetic considerations are beyond the purview of most studies of pollen tube growth. Yet, to understand the in vivo system, we must understand the energy that the pollen tube devotes to a particular process. It seems likely that actin dynamics and the maintenance of turgor require a large percentage of the pollen tube's energy budget, whereas vesicle trafficking and streaming require significantly less.
Energy homeostasis
Overall plant growth can vary from day to day and from month to month, depending on the amount of light and nutrients available. But the pollen tube's mandate does not depend on weather and climate; the pollen tube must get to the ovule. Accomplishing this requires balancing the uptake of sugars, starch synthesis, starch utilization and energy production. In yeast and mammals, the master regulator of energy homeostasis is sugar non-fermenting 1 (SNF1) and AMP-activated protein kinase, respectively (J. Zhang et al. 2010). In yeast, the protein mediates the shift from fermentative to oxidative metabolism in part by sensing the AMP/ATP ratio (J. Zhang et al. 2010). It also regulates the sources of carbon—whether to use fatty acids, sugars or non-fermentable carbon sources (J. Zhang et al. 2010). The plant orthologue of SNF1, SnRK1, is not as well characterized, although significant progress has been made in recent years (Baena-González et al. 2007; Polge and Thomas 2007; Hey et al. 2010). As in yeast and mammals, SnRK1 has been implicated in many different pathways; for example, it is involved in stress signalling, abscisic acid signalling, starch synthesis and starch breakdown (Thelander et al. 2004; Hey et al. 2010). Essentially, it is the central regulator of the carbon status of the cell (Baena-González et al. 2007). In the model moss Physcomitrella patens, a deletion mutant for SnRK1 fails to accumulate starch and grows poorly with malformed chloronema and caulonema (Thelander et al. 2004). In potato tubers, overexpression of SnRK1 increases starch accumulation and decreases glucose concentration (McKibbin et al. 2006).
As already noted, pollen tube metabolism involves a phase of starch accumulation followed by starch utilization (Dickinson 1968). Furthermore, as has been shown in several studies, pollen is capable of both fermentation and respiration (Dickinson 1965; Bucher et al. 1995; Tadege and Kuhlemeier 1997; Rounds et al. 2010). A role for SnRK1 in pollen tube metabolic regulation seems probable. In Hordeum vulgare transformed with an antisense SnRK1 construct, pollen is abnormal and the plant is male sterile (Zhang et al. 2001). In arabidopsis, the beta subunit, which is thought to confer target specificity, has been shown to be expressed in most tissues, including growing pollen tubes (Polge et al. 2008).
The role of SnRK1 in pollen tube energy homeostasis remains to be explored. In lily pollen, when respiration is curtailed (Rounds et al. 2010), the cell pauses briefly, ∼5 min, before beginning to grow again, now using fermentation. Although it is possible that the pollen tube begins transcribing and translating the machinery for fermentation, it seems likely that the fermentative pathways are either already active, or ready to be activated potentially by SnRK1. This hypothesis is in keeping with the results from the Kuhlemeier lab (Bucher et al. 1995; Tadege and Kuhlemeier 1997; Tadege et al. 1998), which show that anaerobic fermentation operates constitutively in Solanaceae pollen.
Mitochondria in pollen tube growth
Mitochondrial localization: acto-myosin interactions and Ca2+
Mitochondria play a prominent role in maintaining the high rate of growth and energy transduction in the pollen tube. The specific localization of mitochondria to places of high energy demand is an emerging paradigm in both plants and animals. For example, significant attention has been given to neurons (Hollenbeck and Saxton 2005; Ly and Verstreken 2006; Lee et al. 2008; Cai et al. 2011). Although clearly neurons and pollen tubes are evolutionarily divergent cells, they are both highly polarized with large energy needs. Recent research suggests that mitochondrial positioning in neurons relies on Ca2+ concentration as well as the ratio of ADP to ATP. These molecules directly interact with the KIF2-Milton-Miro complex that regulates mitochondrial movement on microtubules (Cai et al. 2011). These interactions result in positioning mitochondria at locations in the cell where there is a large need for energy. In pollen tubes, similar positioning has been noted by several authors (Cárdenas et al. 2006; Lovy-Wheeler et al. 2007; Rounds et al. 2010; Zheng et al. 2010). Mitochondria accumulate in large numbers ∼20 µm behind the apex of the cell (Fig. 1, Mito-FM). These mitochondria also appear to have a very high membrane potential, in keeping with the ATP needs of tip growth (Cárdenas et al. 2006; Rounds et al. 2010). However, in contrast to neurons, mitochondria in pollen tubes, as in plants generally, rely on actin instead of microtubules for localization (Lovy-Wheeler et al. 2007; Peremyslov et al. 2008; Prokhnevsky et al. 2008; Zheng et al. 2010). In pollen tubes, the evidence largely comes from inhibitor studies in which the anti-actin reagents, latrunculin B and jasplakinolide, interfere with mitochondrial positioning (Lovy-Wheeler et al. 2007) and dynamics (Zheng et al. 2010). Additional evidence comes from work with arabidopsis that shows that myosin XI is involved directly in mitochondrial movement in leaves and root hairs (Prokhnevsky et al. 2008). In myosin XI-k and myosin XI-2 deletion mutants, root hairs fail to grow; however, the plants show no apparent male gametophyte defect possibly because a different myosin is involved (Prokhnevsky et al. 2008). Interestingly, when aerobic respiration is inhibited and lily pollen tubes are growing using strictly anaerobic fermentation, mitochondria no longer accumulate directly behind the apex despite the continued cyclosis (Rounds et al. 2010). It is unclear what regulates the apical accumulation of mitochondria in pollen tubes. However, it is tempting to speculate that the organelles must produce enough ATP for the myosin molecules that carry them along actin.
In plants and animals, mitochondria have also been shown to buffer Ca2+ (Wang et al. 2010; Cai et al. 2011). Although many studies have been conducted concerning the Ca2+ gradient at the tip of pollen tubes, the potential impact of mitochondrial Ca2+ buffering has not received as much attention. Arabidopsis root hairs have a Ca2+ gradient similar to the one in pollen tubes (Monshausen et al. 2008), and a recent study investigated the potential role of mitochondria in this gradient. Using the mitochondria-specific Ca2+ dye rhod-2, Wang et al. (2010) showed a high Ca2+ gradient in the tips of root hairs. Intriguingly, treatment with latrunculin B and jasplakinolide caused a loss of mitochondrial Ca2+ through the irreversible opening of the permeability transition pore (Wang et al. 2010). It seems quite possible that mitochondria buffer Ca2+ and may even play a role in forming the tip-localized Ca2+ gradient (Cárdenas et al. 2005).
Prospects
Much of the research on pollen tube growth over the past decade has focused on molecular mechanisms that contribute to growth. These studies have elucidated critical molecular pathways and have led to a much greater understanding of tip growth in plants. It is important to emphasize that tip growth does not occur only through a series of protein–protein interactions. While these interactions are clearly necessary, they take place within a physiological context, which to some extent is dominated by movement of ions, metabolites and water. It is the unification of these two ways of studying growth that will be most informative. In the current review, we focus on the energetic requirements and adaptations of pollen tube growth, an area that has received much less attention in recent years.
Many questions concerning pollen tube energetics remain to be explored. One of the most interesting recent findings concerns aerobic fermentation. Gass et al. (2005) showed that the PDH bypass operates in petunia, giving a competitive advantage to pollen tubes. Experiments in which respiration or the PDC pathway is inhibited could lead to a greater understanding of the energy demands of different processes in the growing pollen tube (Rounds et al. 2010). Can membrane trafficking continue in the absence of respiration? Are actin dynamics inhibited by a drop in respiration? Production of ROS has been shown to be critical to tip growth and generally occurs in the mitochondria. Can ROS play an important role in a pollen tube in which respiration has been inhibited? Chondriome dynamics must be regulated by both energy needs and protein interactions. It has already been shown that myosin XI is necessary for mitochondrial actin interactions, but what other proteins are involved? It is also unclear whether mitochondria play a role in Ca2+ buffering in the pollen tube or whether Ca2+ itself regulates mitochondrial positioning. The compelling findings concerning SnRK1 suggest that it may play a role in the energy homeostasis of pollen tubes, yet its mechanistic roles are uncertain. The pollen tube represents an excellent system to unravel these questions.
Sources of funding
This work was supported by a grant from the National Science Foundation USA (MCB-0847876).
Contributions by the authors
All authors contributed to a similar extent overall.
Conflict of interest statement
None declared.
Acknowledgements
We thank Professor C. Kuhlemeier for permission to redraw the proposed PDH bypass. We thank Prof. M. Bezanilla for her comments on this manuscript.
References
- Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signaling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. doi:10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- Becker JD, Feijó JA. How many genes are needed to make a pollen tube? Lessons from transcriptomics. Annals of Botany. 2007;100:1117–1123. doi: 10.1093/aob/mcm208. doi:10.1093/aob/mcm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert R, Obermeyer G, Bentrup F. The turgor pressure of growing lily pollen tubes. Protoplasma. 1997;198:1–8. doi:10.1007/BF01282125. [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. The Journal of Neuroscience. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma T. Understanding plant respiration: separating respiratory components versus a process-based approach. In: Lambers H, Ribas-Carbo M, editors. Plant respiration. Berlin/Heidelberg: Springer; 2005. pp. 177–194. [Google Scholar]
- Briskin DP, Reynolds-Niesman I. Determination of H+/ATP stoichiometry for the plasma membrane H+-ATPase from red beet (Beta vulgaris L.) storage tissue. Plant Physiology. 1991;95:242–250. doi: 10.1104/pp.95.1.242. doi:10.1104/pp.95.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Brander KA, Sbicego S, Mandel T, Kuhlemeier C. Aerobic fermentation in tobacco pollen. Plant Molecular Biology. 1995;28:739–750. doi: 10.1007/BF00021197. doi:10.1007/BF00021197. [DOI] [PubMed] [Google Scholar]
- Cai Q, Davis ML, Sheng Z. Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neuroscience Research. 2011;70:9–15. doi: 10.1016/j.neures.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motility and the Cytoskeleton. 2005;61:112–127. doi: 10.1002/cm.20068. doi:10.1002/cm.20068. [DOI] [PubMed] [Google Scholar]
- Cárdenas L, McKenna ST, Kunkel JG, Hepler PK. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiology. 2006;142:1460–1468. doi: 10.1104/pp.106.087882. doi:10.1104/pp.106.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiology. 2008;146:1611–1621. doi: 10.1104/pp.107.113035. doi:10.1104/pp.107.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguéz-Léon J, Wu H, Cheung AY, Feijó JA. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. The Plant Cell. 2008;20:614–634. doi: 10.1105/tpc.106.047423. doi:10.1105/tpc.106.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Qu X, Wu Y, Huang S. Regulation of actin dynamics in pollen tubes: control of actin polymer level. Journal of Integrative Plant Biology. 2009;51:740–750. doi: 10.1111/j.1744-7909.2009.00850.x. doi:10.1111/j.1744-7909.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- Chen T, Teng N, Wu X, Wang Y, Tang W, Šamaj J, Baluška F, Lin J. Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant and Cell Physiology. 2007;48:19–30. doi: 10.1093/pcp/pcl036. doi:10.1093/pcp/pcl036. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu H. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. doi:10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Duan Q, Costa SS, de Graaf BHJ, Di Stilio VS, Feijó J, Wu H. The dynamic pollen tube cytoskeleton: live cell studies using actin-binding and microtubule-binding reporter proteins. Molecular Plant. 2008;1:686–702. doi: 10.1093/mp/ssn026. doi:10.1093/mp/ssn026. [DOI] [PubMed] [Google Scholar]
- Dai S, Chen T, Chong K, Xue Y, Liu S, Wang T. Proteomics identification of differentially expressed proteins associated with pollen germination and tube growth reveals characteristics of germinated Oryza sativa pollen. Molecular & Cellular Proteomics. 2007;6:207–230. doi: 10.1074/mcp.M600146-MCP200. doi:10.1074/mcp.M600146-MCP200. [DOI] [PubMed] [Google Scholar]
- Dickinson DB. Germination of lily pollen: respiration and tube growth. Science. 1965;150:1818–1819. doi: 10.1126/science.150.3705.1818. doi:10.1126/science.150.3705.1818. [DOI] [PubMed] [Google Scholar]
- Dickinson DB. Inhibition of pollen respiration by oligomycin. Nature. 1966;210:1362–1363. doi:10.1038/2101362a0. [Google Scholar]
- Dickinson DB. Permeability and respiratory properties of germinating pollen. Physiologia Plantarum. 1967;20:118–127. doi:10.1111/j.1399-3054.1967.tb07149.x. [Google Scholar]
- Dickinson DB. Rapid starch synthesis associated with increased respiration in germinating lily pollen. Plant Physiology. 1968;43:1–8. doi: 10.1104/pp.43.1.1. doi:10.1104/pp.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Robinson KR. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiology. 2004;135:1398–1406. doi: 10.1104/pp.104.041483. doi:10.1104/pp.104.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. The Journal of Cell Biology. 1999;144:483–496. doi: 10.1083/jcb.144.3.483. doi:10.1083/jcb.144.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wasteneys GO. Wide-ranging effects of eight cytochalasins and latrunculin A and B on intracellular motility and actin filament reorganization in characean internodal cells. Plant and Cell Physiology. 2007;48:585–597. doi: 10.1093/pcp/pcm030. doi:10.1093/pcp/pcm030. [DOI] [PubMed] [Google Scholar]
- Fricker MD, White NS, Obermeyer G. pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. Journal of Cell Science. 1997;110:1729–1740. doi: 10.1242/jcs.110.15.1729. [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowdky SM, Schroeder JI, Harper JF. A cyclic nucleotide gated channel is essential for polarized tip growth of pollen. Proceedings of the National Academy of Sciences of the USA. 2007;104:14531–14536. doi: 10.1073/pnas.0701781104. doi:10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. The actin cytoskeleton and signaling network during pollen tube tip growth. Journal of Integrative Plant Biology. 2010;52:131–137. doi: 10.1111/j.1744-7909.2010.00922.x. doi:10.1111/j.1744-7909.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Toriyama K. DCW11, Down-Regulated Gene 11 in CW-type cytoplasmic male sterile rice, encoding mitochondrial protein phosphatase 2C is related to cytoplasmic male sterility. Plant and Cell Physiology. 2008;49:633–640. doi: 10.1093/pcp/pcn036. doi:10.1093/pcp/pcn036. [DOI] [PubMed] [Google Scholar]
- Gass N, Glagotskaia T, Mellema S, Stuurman J, Barone M, Mandel T, Roessner-Tunali U, Kuhlemeier C. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. The Plant Cell. 2005;17:2355–2368. doi: 10.1105/tpc.105.033290. doi:10.1105/tpc.105.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. The Journal of Cell Biology. 2005;169:127–138. doi: 10.1083/jcb.200409140. doi:10.1083/jcb.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey SJ, Byrne E, Halford NG. The interface between metabolic and stress signaling. Annals of Botany. 2010;105:197–203. doi: 10.1093/aob/mcp285. doi:10.1093/aob/mcp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA. Mitochondrial development and activity of binucleate and trinucleate pollen during germination in vitro. Planta. 1979;145:25–36. doi: 10.1007/BF00379924. doi:10.1007/BF00379924. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Bruinsma J. Protein synthesis of binucleate and trinucleate pollen and its relationship to tube emergence and growth. Planta. 1979;146:559–566. doi: 10.1007/BF00388832. doi:10.1007/BF00388832. [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. The Plant Cell. 1997;11:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. Journal of Cell Science. 2005;118:5411–5419. doi: 10.1242/jcs.02745. doi:10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kashiyama T, Shimada K, Yamaguchi A, Awata JY, Hachikubo Y, Manstein DJ, Yamamoto K. Recombinant motor domain constructs of Chara corallina myosin display fast motility and high ATPase activity. Biochemical and Biophysical Research Communications. 2003;312:958–964. doi: 10.1016/j.bbrc.2003.10.202. doi:10.1016/j.bbrc.2003.10.202. [DOI] [PubMed] [Google Scholar]
- Itoh A, Sekiya J. Tissue specificity of mitochondrial F0F1-ATPase activity of Lilium longiflorum plant. FEBS Letters. 1994;356:229–232. doi: 10.1016/0014-5793(94)01269-5. doi:10.1016/0014-5793(94)01269-5. [DOI] [PubMed] [Google Scholar]
- Johri BM, Vasil IK. Physiology of pollen. The Botanical Review. 1961;27:325–381. doi:10.1007/BF02860810. [Google Scholar]
- Kim T, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. doi:10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Shimmen T. Accelerated sliding of pollen tube organelles along Characeae actin bundles regulated by Ca2+ The Journal of Cell Biology. 1988;106:1539–1543. doi: 10.1083/jcb.106.5.1539. doi:10.1083/jcb.106.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger JH, Daher FB, Grant M, Geitmann A. Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophysical Journal. 2009;97:1822–1831. doi: 10.1016/j.bpj.2009.07.038. doi:10.1016/j.bpj.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Loewus F. The nutritional role of pistil exudate in pollen tube wall formation in Lilium longiflorum. Plant Physioogy. 1973;52:87–92. doi: 10.1104/pp.52.2.87. doi:10.1104/pp.52.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK. Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma. 1987;140:141–150. doi:10.1007/BF01273723. [Google Scholar]
- Lee SJ, Warmke HE. Organelle size and number in fertile and T-cytoplasmic male-sterile corn. American Journal of Botany. 1979;66:141–148. doi:10.2307/2442516. [Google Scholar]
- Lee YJ, Yang Z. Tip growth: signaling in the apical dome. Current Opinion in Plant Biology. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. doi:10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. The Journal of Cell Biology. 2008;181:1155–1168. doi: 10.1083/jcb.200801086. doi:10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B, Arango M, Oufattole M, Crouzet J, Purnelle B, Boutry M. Identification of a Nicotiana plumbaginifolia plasma membrane H+-ATPase gene expressed in the pollen tube. Plant Molecular Biology. 2005;58:775–787. doi: 10.1007/s11103-005-7875-3. doi:10.1007/s11103-005-7875-3. [DOI] [PubMed] [Google Scholar]
- Linskens HF, Schrauwen J. Measurement of oxygen tension changes in the style during pollen tube growth. Planta. 1966;71:98–106. doi: 10.1007/BF00384646. doi:10.1007/BF00384646. [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK. Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005;221:95–104. doi: 10.1007/s00425-004-1423-2. doi:10.1007/s00425-004-1423-2. [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK. Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. The Plant Cell. 2006;18:2182–2193. doi: 10.1105/tpc.106.044867. doi:10.1105/tpc.106.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Cárdenas L, Kunkel JG, Hepler PK. Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motility and the Cytoskeleton. 2007;64:217–232. doi: 10.1002/cm.20181. doi:10.1002/cm.20181. [DOI] [PubMed] [Google Scholar]
- Ly CV, Verstreken P. Mitochondria at the synapse. The Neuroscientist. 2006;12:291–299. doi: 10.1177/1073858406287661. doi:10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. Molecular mechanisms of pollen tube growth and differentiation. The Plant Cell. 1993;5:1303–1314. doi: 10.1105/tpc.5.10.1303. doi:10.2307/3869783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP, Bell E. Protein synthesis during germination of pollen: studies on polyribosome formation. Biochimica et Biophysica Acta. 1969;179:199–203. doi: 10.1016/0005-2787(69)90136-1. [DOI] [PubMed] [Google Scholar]
- McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. The Plant Cell. 2009;21:3026–3040. doi: 10.1105/tpc.109.069260. doi:10.1105/tpc.109.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin RS, Muttucumaru N, Paul MJ, Powers SJ, Burrell MM, Coates S, Purcell PC, Tiessen A, Geigenberger P, Halford NG. Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnology Journal. 2006;4:409–418. doi: 10.1111/j.1467-7652.2006.00190.x. doi:10.1111/j.1467-7652.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- Mellema S, Eichenberger W, Rawyler A, Suter M, Tadege M, Kuhlemeier C. The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. The Plant Journal. 2002;30:329–336. doi: 10.1046/j.1365-313x.2002.01293.x. doi:10.1046/j.1365-313X.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- Messerli MA, Robinson KR. Cytoplasmic acidification and current influx follow growth pulses of Lilium longiflorum pollen tubes. The Plant Journal. 1998;16:87–91. doi:10.1046/j.1365-313x.1998.00266.x. [Google Scholar]
- Messerli MA, Robinson KR. Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta. 2003;217:147–157. doi: 10.1007/s00425-003-0972-0. [DOI] [PubMed] [Google Scholar]
- Messerli MA, Danuser G, Robinson KR. Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. Journal of Cell Science. 1999;112:1497–1509. doi: 10.1242/jcs.112.10.1497. [DOI] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijó JA. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. The International Journal of Developmental Biology. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. doi:10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu L, Obermeyer G, Feijó JA. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil d-serine. Science. 2011;332:434–437. doi: 10.1126/science.1201101. doi:10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]
- Miller DD, Callaham DA, Gross DJ, Hepler PK. Free Ca2+ gradient in growing pollen tubes of Lillium. Journal of Cell Science. 1992;101:7–12. [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiology. 2008;147:1690–1698. doi: 10.1104/pp.108.123638. doi:10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kelley JC. External carbohydrates in growth and respiration of pollen tubes in vitro. American Journal of Botany. 1955;42:322–327. doi:10.2307/2438569. [Google Scholar]
- O'Kelley JC. Boron effects on growth, oxygen uptake and sugar absorption by germinating pollen. American Journal of Botany. 1957;44:239–244. doi:10.2307/2438805. [Google Scholar]
- Palmgren MG. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. doi:10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. Journal of Cell Science. 2001;114:2685–2695. doi: 10.1242/jcs.114.14.2685. [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV. Two class XI myosins function in organelle trafficking and root hair development in arabidopsis. Plant Physiology. 2008;146:1109–1116. doi: 10.1104/pp.107.113654. doi:10.1104/pp.107.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl H, Pöckl M, Blaschke C, Obermeyer G. Osmoregulation in Lilium pollen grains occurs via modulation of the plasma membrane H+ ATPase activity by 14-3-3 proteins. Plant Physiology. 2010;154:1921–1928. doi: 10.1104/pp.110.165696. doi:10.1104/pp.110.165696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK. Tip-localized calcium entry fluctuates during pollen tube growth. Developmental Biology. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. doi:10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- Polge C, Thomas M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends in Plant Science. 2007;12:20–28. doi: 10.1016/j.tplants.2006.11.005. doi:10.1016/j.tplants.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Polge C, Jossier M, Crozet P, Gissot L, Thomas M. β-subunits of the SnRK1 complexes share a common ancestral function together with expression and function specificities; physical interaction with nitrate reductase specifically occurs via AKINβ1-subunit. Plant Physiology. 2008;148:1570–1582. doi: 10.1104/pp.108.123026. doi:10.1104/pp.108.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possot OM, Letellier L, Pugsley AP. Energy requirement for pullulanase secretion by the main terminal branch of the general secretory pathway. Molecular Microbiology. 1997;24:457–464. doi: 10.1046/j.1365-2958.1997.3451726.x. doi:10.1046/j.1365-2958.1997.3451726.x. [DOI] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. The New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. doi:10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV. Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proceedings of the National Academy of Sciences of the USA. 2008;105:19744–19749. doi: 10.1073/pnas.0810730105. doi:10.1073/pnas.0810730105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes identifying genes critical for growth in a pistil. PLoS Genetics. 2009;5:e1000621. doi: 10.1371/journal.pgen.1000621. doi:10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR. A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Developmental Biology. 1991;148:612–619. doi: 10.1016/0012-1606(91)90278-b. doi:10.1016/0012-1606(91)90278-B. [DOI] [PubMed] [Google Scholar]
- Raven JA. The energetics of freshwater algae; energy requirements for biosyntheiss and volume regulation. New Phytologist. 1982;92:1–20. doi:10.1111/j.1469-8137.1982.tb03358.x. [Google Scholar]
- Robertson WR, Clark K, Young JC, Sussman MR. An Arabidopsis thaliana plasma membrane proton pump is essential for pollen development. Genetics. 2004;168:1677–1687. doi: 10.1534/genetics.104.032326. doi:10.1534/genetics.104.032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Pulsating ion fluxes and growth at the pollen tube tip. Science's STKE: Signal Transduction Knowledge Environment. 2002;2002:pe51. doi: 10.1126/stke.2002.162.pe51. [DOI] [PubMed] [Google Scholar]
- Rounds CM, Hepler PK, Fuller SJ, Winship LJ. Oscillatory growth in lily pollen tubes does not require aerobic energy metabolism. Plant Physiology. 2010;152:736–746. doi: 10.1104/pp.109.150896. doi:10.1104/pp.109.150896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Copenhaver GP, von Arnim AG. Fluorescence-tagged transgenic lines reveal genetic defects in pollen growth—Application to the Eif3 complex. PLoS ONE. 2011;6:e17640. doi: 10.1371/journal.pone.0017640. doi:10.1371/journal.pone.0017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamaj J, Müller J, Beck M, Böhm N, Menzel D. Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends in Plant Science. 2006;11:594–600. doi: 10.1016/j.tplants.2006.10.002. doi:10.1016/j.tplants.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Sieger SM, Kristensen BK, Robson CA, Amirsadeghi S, Eng EWY, Abdel-Mesih A, Møller IM, Vanlerberghe GC. The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. Journal of Experimental Botany. 2005;56:1499–1515. doi: 10.1093/jxb/eri146. doi:10.1093/jxb/eri146. [DOI] [PubMed] [Google Scholar]
- Slayman CL, Sanders D. Steady-state kinetic analysis of an electroenzyme. Biochemical Society Symposium. 1985;50:11–29. [PubMed] [Google Scholar]
- Tadege M, Kuhlemeier C. Aerobic fermentation during tobacco pollen development. Plant Molecular Biology. 1997;35:343–354. doi: 10.1023/a:1005837112653. doi:10.1023/A:1005837112653. [DOI] [PubMed] [Google Scholar]
- Tadege M, Brandle R, Kuhlemeier C. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. The Plant Journal. 1998;14:327–335. doi:10.1046/j.1365-313X.1998.00130.x. [Google Scholar]
- Tadege M, Dupuis R, Kuhlemeier C. Ethanolic fermentation: new functions for an old pathway. Trends in Plant Science. 1999;4:320–325. doi: 10.1016/s1360-1385(99)01450-8. doi:10.1016/S1360-1385(99)01450-8. [DOI] [PubMed] [Google Scholar]
- Thelander M, Olsson T, Ronne H. Snf1-related protein kinase 1 is needed for growth in a normal day-night light cycle. The EMBO Journal. 2004;23:1900–1910. doi: 10.1038/sj.emboj.7600182. doi:10.1038/sj.emboj.7600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Oh S, Honys D. Pollen development, a genetic and transcriptomic view. In: Malhó R, editor. Berlin: Springer; 2006. pp. 15–45. The pollen tube: a cellular and molecular perspective. Plant Cell Monographs Vol. 3. [Google Scholar]
- Vasil I. Physiology and culture of pollen. In: Giles KL, Prakash J, editors. Pollen: cytology and development. San Diego: Academic Press; 1987. pp. 127–174. [Google Scholar]
- Vavasseur A, Raghavendra AS. Guard cell metabolism and CO2 sensing. New Phytologist. 2005;165:665–682. doi: 10.1111/j.1469-8137.2004.01276.x. doi:10.1111/j.1469-8137.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Goldstein RE. Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma. 2010;240:99–107. doi: 10.1007/s00709-009-0088-x. doi:10.1007/s00709-009-0088-x. [DOI] [PubMed] [Google Scholar]
- Vidali L, Hepler PK. Actin and pollen tube growth. Protoplasma. 2001;215:64–76. doi: 10.1007/BF01280304. doi:10.1007/BF01280304. [DOI] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Molecular Biology of the Cell. 2001;12:2534–2545. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Rounds CM, Hepler PK, Bezanilla M. Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PloS One. 2009;4:e5744. doi: 10.1371/journal.pone.0005744. doi:10.1371/journal.pone.0005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Song L, Zou J, Su Z, Wu W. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in arabidopsis. Plant Physiology. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. doi:10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu Y, Ling Y, Zhang H, Liu P, Baluška F, Šamaj J, Lin J, Wang Q. Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs. BMC Plant Biology. 2010;10:53. doi: 10.1186/1471-2229-10-53. doi:10.1186/1471-2229-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LQ, Xu WY, Deng ZY, Su Z, Xue Y, Wang T. Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genomics. 2010;11:338. doi: 10.1186/1471-2164-11-338. doi:10.1186/1471-2164-11-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Lin M, Oliver D, Schnable P. The roles of aldehyde dehydrogenases (ALDHs) in the PDH bypass of Arabidopsis. BMC Biochemistry. 2009;10:7. doi: 10.1186/1471-2091-10-7. doi:10.1186/1471-2091-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship LJ, Obermeyer G, Geitmann A, Hepler PK. Under pressure, cell walls set the pace. Trends in Plant Science. 2010;15:363–369. doi: 10.1016/j.tplants.2010.04.005. doi:10.1016/j.tplants.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Shang Z, Wu J, Jiang X, Moschou PN, Sun W, Roubelakis-Angelakis KA, Zhang S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. The Plant Journal. 2010;63:1042–1053. doi: 10.1111/j.1365-313X.2010.04301.x. doi:10.1111/j.1365-313X.2010.04301.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Qu H, Jin C, Shang Z, Wu J, Xu G, Gao Y, Zhang S. cAMP activates hyperpolarization-activated Ca2+ channels in the pollen of Pyrus pyrifolia. Plant Cell Reports. 2011;7:1193–1200. doi: 10.1007/s00299-011-1027-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Shimada K, Ito K, Hamada S, Ishijima A, Tsuchiya T, Tazawa M. Chara myosin and the energy of cytoplasmic streaming. Plant and Cell Physiology. 2006;47:1427–1431. doi: 10.1093/pcp/pcl006. doi:10.1093/pcp/pcl006. [DOI] [PubMed] [Google Scholar]
- Yan A, Xu G, Yang Z. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proceedings of the National Academy of Sciences of the USA. 2009;106:22002–22007. doi: 10.1073/pnas.0910811106. doi:10.1073/pnas.0910811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Vemuri G, Nielsen J. Systems biology of energy homeostasis in yeast. Current Opinion in Microbiology. 2010;13:382–388. doi: 10.1016/j.mib.2010.04.004. doi:10.1016/j.mib.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shewry PR, Jones H, Barcelo P, Lazzeri PA, Halford NG. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. The Plant Journal. 2001;28:431–441. doi: 10.1046/j.1365-313x.2001.01167.x. doi:10.1046/j.1365-313X.2001.01167.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, He J, Lee D, McCormick S. Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiology. 2010;152:2200–2210. doi: 10.1104/pp.109.142349. doi:10.1104/pp.109.142349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Wang Q, Teng Y, Wang X, Wang F, Chen T, Šamaj J, Lin J, Logan DC. The speed of mitochondrial movement is regulated by the cytoskeleton and myosin in Picea wilsonii pollen tubes. Planta. 2010;231:779–791. doi: 10.1007/s00425-009-1086-0. doi:10.1007/s00425-009-1086-0. [DOI] [PubMed] [Google Scholar]
- Zonia L, Cordeiro S, Tupý J, Feijó JA. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. The Plant Cell. 2002;14:2233–2249. doi:10.1105/tpc.003830. [PubMed] [Google Scholar]