Abstract
Introduction: Denosumab, a fully human monoclonal antibody, targets the receptor activator of nuclear factor-kappaB (RANK) ligand, a protein essential for osteoclast differentiation, activity and survival. Loss of osteoclasts from the bone surface reduces bone turnover and bone loss in malignant and benign diseases. In breast cancer, bone metastases are frequently observed; cancer treatment-induced bone loss (CTIBL) may result as a consequence of endocrine treatment or chemotherapy. Furthermore, preclinical studies suggest a direct role of the RANK/RANK-ligand pathway in breast tumorigenesis. This paper reviews preclinical and clinical data on denosumab in breast cancer.
Materials and methods: Studies were identified through the Medline database. Key search terms included: AMG-162, bisphosphonates, denosumab, RANK-ligand and zoledronic acid. Information available in abstract form only was retrieved from major oncology meetings, such as the American Society of Clinical Oncology (ASCO) annual meeting, ASCO breast meeting, European Cancer Organization, European Society of Medical Oncology and the San Antonio Breast Cancer Symposium.
Results: Denosumab was consistently well tolerated throughout clinical trials, although the observed incidence of osteonecrosis of the jaw was comparable to that with bisphosphonates. Efficacy as determined by a reduction of skeletal-related events was at least equal to zoledronic acid, and superior in one phase III study conducted in patients with metastatic breast cancer. Clinical trials investigating the role of denosumab for the prevention of CTIBL and breast cancer recurrences are currently ongoing.
Conclusion: In conclusion, denosumab appears to be an effective and safe treatment option in patients with bone metastases from breast cancer with the potential of also preventing CTIBL.
Keywords: bisphosphonates, bone metastases, cancer treatment-induced bone loss, denosumab, osteoporosis, receptor activator of nuclear factor-kappaB
Introduction
Breast cancer
Breast cancer is the most common malignant disease in women worldwide [American Cancer Society, 2009]. Bone loss and bone degradation are frequently observed in breast cancer patients and occur as a treatment side effect, that is, cancer treatment induced bone loss (CTIBL) [Amir et al. 2010] or directly due to bone metastases [Coleman, 2001]. Bone resorption requires osteoclasts, cells specialized in the degradation of bone tissue. Osteoclast activation is triggered by the receptor activator of nuclear factor-kappaB (RANK)/RANK-ligand pathway, rendering RANK-ligand an attractive target for the prevention of bone loss. Recent preclinical data suggest that this pathway may also play a role in breast tumorigenesis, opening the stage for new options of breast cancer prophylaxis and therapy [Beleut et al. 2010; Schramek et al. 2010].
Bone metastases in breast cancer
Bone metastases are common in breast cancer; indeed, up to 60% of patients with advanced breast cancer will eventually develop bone metastases during their course of disease [Coleman, 2001]. According to radiographic appearance, lesions are classified as being osteolytic, osteoblastic or mixed [Roodman, 2004], with the majority of breast cancer bone metastases belonging to the mixed or lytic types [Yin et al. 2005]. Therefore, complications such as hypercalcaemia, fractures or spinal cord compressions may occur [Mundy, 2001]. Fractures as well as spinal cord compression and the consecutive local interventions (e.g. surgery to bone, radiotherapy to bone) are summarized by the term ‘skeletal-related events’ (SREs) [Ross et al. 2003]. In most phase III clinical trials, reduction of SREs is chosen as the primary endpoint in order to measure the activity of drugs blocking bone resorption. In phase I and II studies, biomarkers of bone turnover, for example, urinary-N-telopeptide/creatinine ratio (uNTX/Cr), are often substituted as a surrogate endpoint [Brown et al. 2003].
Current treatment options for bone metastases
Apart from systemic antitumour therapy and local interventions, bisphosphonates and denosumab were developed as direct inhibitors of bone resorption. Through different mechanisms of action, these bone-specific agents block osteoclast function. Bisphosphonates have a unique property of selective uptake by their target organ. After binding strongly to hydroxyapatite bone mineral, they are internalized by osteoclasts located on the bone surface [Baron et al. 2011]. Once taken up, bisphosphonates that contain a nitrogen residue on a side chain (amino-bisphosphonates) inhibit the enzyme farnesyl-pyrophosphate synthase within osteoclasts, thereby preventing prenylation of certain signal-transduction GTPases, such as Ras, Rho and Rac, which, in turn, induces apoptosis [Luckmann et al. 1998]. Non-nitrogen-containing bisphosphonates, on the other hand, are metabolized into ATP analogues, which again eventually leads to cell degradation [Frith et al. 2001].
In contrast, denosumab acts by blocking osteoclast differentiation from osteoclast precursor cells [Bekker et al. 2004]. This mechanism and its clinical relevance will be discussed in this article.
CTIBL
Under physiological conditions, a postmenopausal woman loses 1% of bone mass per year [Kanis et al. 1997]. Tamoxifen was regarded as the standard of care for the treatment of endocrine-responsive breast cancer for nearly two decades. This selective oestrogen receptor modulator exhibits antiresorptive properties in bone tissue, thereby preventing the development of osteoporosis. Aromatase inhibitors, however, are superior to tamoxifen as adjuvant therapy for early breast cancer [Thürlimann et al. 2005]. This class of drugs acts by blocking aromatase, the main source of oestrogen production in postmenopausal women. Reduction of oestrogen blood levels, on the other hand, increases annual bone loss to 2.6% [Eastell et al. 2006; Perez et al. 2006]. This is clinically relevant, as such bone loss eventually translates into higher fracture rates (11% in patients treated with aromatase inhibitors compared with 8% on tamoxifen) [Howell et al. 2005].
CTIBL may also occur in premenopausal women due to chemotherapy-induced ovarian failure [Hines et al. 2009] or suppression of ovarian function by gonadotropin-releasing hormone analogues [Gnant et al. 2007]. Furthermore, fracture rates are increased by androgen deprivation in male patients with prostate cancer [Smith et al. 2009].
Bisphosphonates, when given in conjunction with endocrine therapy, prevent CTIBL as evidenced by a decreased reduction of bone mineral density [Van Poznak et al. 2010; Gnant et al. 2007]. Other trials even observed an increase of bone mineral density in patients receiving bisphosphonates [Eidtmann et al. 2010; Brufsky et al. 2009]. However, this effect might not translate into a reduction in fracture rates [Valachis et al. 2010]. In this context, however, it is important to realize that the reason such a decrease in fracture rates was not observed may be related to the fact that bisphosphonates in most trials were only initiated when patients developed a T-score of less than -2.0. Furthermore, many of those studies were not adequately powered to detect such an effect. Denosumab on the other hand was found to reduce the incidence of new vertebral fractures in prostate cancer patients on androgen deprivation in a large, adequately powered phase III study [Smith et al. 2009]. Phase III clinical trials of denosumab for the prevention of CTIBL in breast cancer are currently ongoing [ClinicalTrials.gov identifier: NCT0056374; Bartsch and Steger 2009].
Bone metabolism
Osteoclast activation
As outlined, osteoclasts are key mediators of bone resorption. Osteoclasts derive from osteoclast precursors (OCPs). Those cells derive from bone marrow cells of mononuclear linage, linking bone metabolism with the immune system. Under physiological conditions, a balance of bone synthesis and degradation exists; therefore, osteoblasts were suggested to mediate osteoclast activation via a hypothetical ‘osteoclast activating factor’ [Rodan and Martin, 1981]. This assumption was eventually proven correct when the RANK-ligand was identified (reviewed by Geusens [2009]). This protein is part of a system of interacting cytokines of the tumour necrosis factor (TNF) family that regulates bone turnover. In the RANK/RANK-ligand pathway, RANK-ligand, which is secreted by osteoblasts and bone marrow stromal cells, activates RANK on the surface of OCPs, inducing differentiation of precursors into mature cells. This step is antagonized by osteoprotegerin (OPG) (reviewed by Vega et al. [2007]).
RANK/RANKL/OPG pathway in breast cancer bone metastases
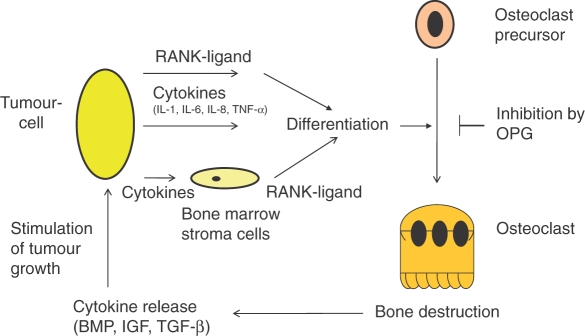
In breast cancer bone metastases, the interaction of tumour cells, bone matrix and bone cells results in a vicious cycle of bone destruction [Giuliano et al. 2004]. Tumour cells secrete cytokines and growth factors, such as parathyroid hormone-related peptide, interleukin (IL)-1, IL-6, IL-8, IL11 and TNF-α, thereby causing stroma cells and osteoblasts to secrete RANK-ligand [Kitazawa and Kitazawa, 2002; Chikatsu et al. 2000; Thomas et al. 1999]. As outlined, this leads to increased osteoclast differentiation; resulting bone resorption mobilizes growth factors, such as transforming growth factor beta (TGF-β), insulin-like growth factor, basic fibroblast growth factor and bone morphogenetic protein (BMP) from the bone matrix. Those cytokines in turn stimulate tumour proliferation and support tumour cell survival [Yin et al. 2005; Roodman, 2004; Kostenuik et al. 1992; Hauschka et al. 1986] (Figure 1).
Figure 1.
Vicious cycle of bone destruction. BMP, bone morphogenetic protein; IGF, insulin-like growth factor; IL, interleukin; OPG, osteoprotegerin; RANK-ligand, receptor activator of nuclear factor-kappaB ligand; TGF-β, transforming growth factor beta; TNF-α, tumour necrosis factor alpha.
The resulting vicious cycle is potentially inhibited by OPG, which leads to the development of compounds that inhibit the RANK/RANK-ligand pathway as therapeutic agents [Body et al. 2003].
As outlined in Figure 1, certain malignancies, such as prostate cancer and multiple myeloma, may produce RANK-ligand directly [Farrugia et al. 2003; Brown et al. 2001]. Furthermore, a RANK-ligand independent mechanism of osteoclast differentiation mediated by IL-1, IL-6, IL-8 and TNF-α exists [Bendre et al. 2005; Kudo et al. 2003], suggesting a potential mechanism of resistance to RANK-ligand inhibition.
RANK pathway in breast tumorigenesis
Recently, it was suggested that RANK-ligand may also have a direct role in breast cancer tumorigenesis. In a mouse model developed by Beleut and colleagues, adult ovarectomized mice were exposed to progesterone [Beleut et al. 2010]. In this model, progesterone drove the proliferation of mammary gland epithelial cells (MECs) in two waves. A first, smaller wave of proliferation occurred in progesterone receptor (PR)-positive cells and was found to require cyclin D1. A second, larger wave, however, relied on RANK-ligand signalling.
Ablation of RANK in the mammary epithelium blocked progesterone-induced morphogenesis. On the other hand, systemic administration of RANK-ligand also triggered proliferation in the absence of PR signalling, and injection of recombinant OPG as a RANK signalling inhibitor blocked progesterone-induced proliferation [Beleut et al. 2010].
In line with these data, Schramek and colleagues showed that the administration of medroxyprogesterone acetate (MPA) triggered an induction of RANK-ligand in MECs. Results showed that inactivation of the RANK pathway in MECs prevented the expansion of a stem cell-enriched population in response to MPA and, furthermore, sensitized those cells to DNA damage-induced cell death. Consequently, deletion of RANK decreased incidence and delayed onset of MPA-driven mammary cancers [Schramek et al. 2010].
This data may offer a possible hypothesis for the induction of breast cancers by hormone replacement therapy with a combination of oestrogen and progestins and opens an opportunity for new approaches in breast cancer prevention.
Development of denosumab
Early development of drugs targeting RANK/RANK-ligand started with recombinant OPG (AMGN-0007). While OPG was active and well tolerated [Body et al. 2003; Bekker et al. 2001], AMG162, later named denosumab, a fully human antibody targeting RANK-ligand, reduced levels of bone turnover markers to a greater extent [Bekker et al. 2004].
In patients with osteoporosis, the effects of denosumab on levels of bone turnover markers were sustained for up to 6 months, enabling an administration schedule similar to that for intravenous bisphosphonates [Bekker et al. 2004]. Further studies were conducted in patients with bone metastases from solid cancers and multiple myeloma. Importantly, neither antibodies against denosumab nor any drug-related serious adverse events were observed [Body et al. 2006].
Phase II clinical trials of denosumab in malignancies and CTIBL
Two randomized phase II studies of denosumab in advanced cancer patients were initiated based upon those results. One trial included 255 bisphosphonate-naïve patients with metastatic breast cancer. Patients were randomly assigned to different doses and schedules of denosumab or a control group receiving zoledronic acid. Reduction of uNTX/Cr was defined as a primary study endpoint. Overall, denosumab and zoledronic acid yielded similar results and denosumab at 120 mg administered every 4 weeks was identified as the optimal schedule for further investigations. Serious adverse events were observed less frequently in patients treated with denosumab compared with the bisphosphonate group (9% vs. 16%) [Lipton et al. 2007].
A second randomized phase II study included patients already receiving various bishosphonates (i.e. ibandronate, pamidronate and zoledronic acid) for bone metastases of solid cancers and multiple myeloma. It consisted of 111 patients with intermediate-to-high levels of uNTX at baseline randomized to denosumab or continuation of bisphosphonates. The percentage of patients reaching uNTX levels of more than 50 nmol/L at week 13 was defined as the primary study endpoint. This endpoint was met by 71% of patients in the denosumab group compared with 29% of patients who continued on bisphosphonates (p < 0.001). Adverse events were comparable between both groups [Fizazi et al. 2009]. When considering this trial, however, it is important to understand that the patients included were resistant to biphosphonates as evidenced by intermediate-to-high baseline uNTX; it is not entirely clear whether results can be transferred to a general population.
The role of denosumab for the prevention of CTIBL was evaluated in another phase II trial: 252 patients with early breast cancer and reduced bone mass who received aromatase inhibitors in the adjuvant setting were included and randomized to denosumab 60 mg or placebo every 6 months. In the denosumab group, bone mineral density increased significantly over time (5.5% and 7.6%, at 12 and 24 months, respectively; p < 0.0001 [both time points]). Again, treatment was generally well tolerated and adverse events were similar between the respective denosumab and placebo groups [Ellis et al. 2008].
Denosumab for the treatment of bone metastases: results from phase III clinical trials
Recently, results of the first phase III trial to compare directly denosumab to zoledronic acid in patients with metastatic breast cancer patients were published (Amgen 20050136 [Clinical Trials.gov identifier: NCT00321464]). A total of 2046 biphosphonate-naïve patients (except treatment with oral bisphosphonates for osteoporosis) were included and randomized to denosumab 120 mg or zoledronic acid 4 mg every 4 weeks in a randomized, double-blind, active-controlled trial. Primary study endpoint was time to first on-study SRE (noninferiority); secondary endpoints consisted of time to first on-study SRE (superiority) and time to first and subsequent on-study SREs. In the denosumab group, a significant delay in time to first on-study SRE was observed (hazard ratio [HR] 0.82; 95% confidence interval [CI] 0.71–0.95; p < 0.001 noninferiority; p = 0.01 superiority). Indeed, median time to first on-study SRE was 26.4 months in patients receiving zoledronic acid, and was not reached in the denosumab group [Stopeck et al. 2010b]. This was recently updated at the 2010 San Antonio Breast Cancer Symposium where a median time to first on-study SRE of 32.4 months was reported in the denosumab group [Stopeck et al. 2010c]. Furthermore, denosumab reduced the risk of experiencing multiple SREs (analysis of time to first and subsequent on-study SRE) significantly (HR 0.77; 9% CI 0.66–0.89; p = 0.001) [Stopeck et al. 2010b]. The results are summarized in Table 1.
Table 1.
Comparison of denosumab vs. zoledronic acid in metastatic breast cancer (n = 2046).
| Endpoint | HR | 95% CI | p value |
|---|---|---|---|
| Primary endpoint | |||
| Time to first on-study SRE (noninferiority) | 0.82 | 0.71-0.95 | >0.0001 |
| Secondary endpoints | |||
| Time to first on-study SRE (superiority) | 0.82 | 0.71-0.95 | 0.01 |
| Time to first and subsequent on-study SRE | 0.77 | 0.66-0.89 | 0.001 |
| Exploratory endpoints | |||
| Overall disease progression | 1.00 | 0.89-1.11 | NS |
| Overall survival | 0.95 | 0.81-1.11 | NS |
| Skeletal morbidity rate (mean) (number of SREs per year) | D: 0.45; ZA: 0.58 | 0.004 |
CI, confidence interval; D, denosumab; HR, hazard ratio; NS, not significant; SRE, skeletal-related event (i.e. pathologic fracture, irradiation to bone, surgery to bone, spinal cord compression); ZA, zoledronic acid.
Bisphosphonates are known to have considerable activity on control and palliation of pain associated with bone metastases [Kretzschmar et al. 2007]. In a separate analysis evaluating the respective effects of zoledronic acid and denosumab on pain in all patients included in Amgen 20050136, a similar time to pain improvement was observed in both treatment arms. Patients with a baseline score of no/mild pain had a significantly longer median time to development of moderate/severe pain when treated with denosumab compared with zoledronic acid [Stopeck et al. 2010a].
Rates of severe (defined as Common Terminology Criteria of Adverse Events grade ≥3) and serious adverse events (e.g. life threatening or requiring hospitalization) once again were similar between both treatment groups. In general, those adverse events were mainly attributable to concomitant anticancer therapies. As expected, significantly more cases of pyrexia, bone pain, arthralgia and renal failure were observed in the zoledronic acid group, while hypocalcaemia and toothache, not associated with the development of osteonecrosis of the jaw (ONJ), were seen more often in patients receiving denosumab. Importantly, the overall rate of ONJs, defined as exposed necrotic bone that persists for at least 8 weeks [Cartsos et al. 2008], was similar in the respective treatment groups. Furthermore, known risk factors for ONJ, such as prior dental extractions and poor oral hygiene, were present in the vast majority of all ONJ cases [Stopeck et al. 2010b].
Another phase III trial was conducted in a mixed population of patients with different advanced solid cancers (excluding prostate and breast cancer) and multiple myloma. Similar to NCT00321464, the primary study endpoint, noninferiority to zoledronic acid, was met (HR 0.84; 95% CI 0.71–0.98; p = 0.0007), although a superiority of denosumab was not established [Henry et al. 2011].
Finally, another phase III trial of denosumab versus zoledronic acid was conducted in men with castration-resistant prostate cancer [ClinicalTrials.gov identifier: NCT00321620]. Overall, 1904 patients with bone metastases, all naïve for intravenous bisphosphonates were included. Again, time to first on-study SRE was chosen as the primary study endpoint. In patients receiving denosumab, median time to first on-study SRE was 20.7 months compared with 17.1 months in the zoledronic acid group (HR 0.82; 95% CI 0.71–0.95; p < 0.001 noninferiority; p = 0.008 superiority). In line with the aforementioned studies, the overall rate of serious adverse events was similar (63% vs. 60%), while once again a numerical increase in the rate of ONJ was observed in patients receiving denosumab (22 [2%] vs. 12 [1%]; p = 0.09) [Fizazi et al. 2011].
Trials of denosumab in CTIBL
As outlined, bisphosphonates are active in the prevention of CTIBL [Van Poznak et al. 2010; Gnant et al. 2007]. It is still not proven, however, whether this effect eventually translates into a reduction in fracture rates [Valachis et al. 2010]. Denosumab, on the other hand, was found to lower the rate of vertebral fractures significantly in patients on androgen-deprivation therapy for prostate cancer [Smith et al. 2009]. Further studies are currently ongoing: the Austrian Breast and Colorectal Cancer Study Group Study 18 (ABCSG-18) randomized postmenopausal patients treated with aromatase inhibitors as adjuvant therapy for hormone receptor-positive early breast cancer to denosumab or placebo. This study is among the first phase III trials evaluating denosumab in the prevention of CTIBL in breast cancer and also includes relevant oncological treatment goals as secondary endpoints [ClinicalTrials.gov identifier: NCT00556374].
Clinical antitumour efficacy of bisphosphonates and denosumab
Preclinical data suggested a direct antitumour effect of zoledronic acid. Among other mechanisms, synergistic activity of bisphosphonates in combination with chemotherapy, immunomodulatory properties as well as an anti-angiogenic effect were proposed [Neville-Webbe and Coleman, 2010]. While there is some clinical data in support of these assumptions, there is no clear-cut evidence yet. The ABCSG-12 study randomized premenopausal patients receiving adjuvant endocrine therapy to additive treatment with zoledronic acid or control. A significant reduction of breast cancer recurrence events was observed in the bisphosphonate group [Gnant et al. 2009].
In the postmenopausal bone protection trial (ZO-FAST [ClinicalTrials.gov identifier: NC T00171314]), early breast cancer patients on letrozole were randomly assigned to a group that received upfront zoledronic acid or a group that received bisphosphonates only when a drop in bone mineral density was observed [Eidtmann et al. 2010]. In support of ABCSG-12 results, significantly fewer recurrences occurred in the upfront group (HR 0.59; 95% CI 0.38–0.92; p = 0.0176). In the smaller ZO-FAST study, however, no significant difference between both groups was observed [Brufsky et al. 2009]. EZO-FAST even found a nonsignificantly increased recurrence risk with immediate bisphosphonate administration [Coleman et al. 2009]. A combined analysis of those study results could not be performed as the Gail-Simon test was statistically significant for a quantitative interaction between the studies (p = 0.047) [Coleman et al. 2009].
The AZURE trial (BIG 01/04; chemotherapy and/or hormone therapy with or without zoledronate in women with stage II or stage III breast cancer [ClinicalTrials.gov identifier: NCT00072020]) was the most recent of adjuvant bisphosphonates studies to report results [Coleman et al. 2010]. In contrast to ABCSG-12, addition of zoledronic acid caused no reduction in recurrence-free survival events in 3360 patients randomized to receive (neo) adjuvant chemotherapy and/or endocrine therapy with or without zoledronic acid (377 disease-free survival events with zoledronic acid, 375 in the control group; HR 0.98; 95% CI 0.85–1.13; p = 0.79). Therefore, the exact role of bisphosphonates in the prevention of breast cancer recurrences awaits further clarification. Table 2 outlines the different results in terms of recurrence risk in the respective trials.
Table 2.
Randomized trials of zoledronic acid in early breast cancer.
| Study | Number Design | HR | Comment | |
|---|---|---|---|---|
| ABCSG-12 | 1803 | Premenopausal | HR 0.64; 95% CI 0.46–0.91; p = 0.01 | Significantly fewer recurrences in ET +/- ZA patients receiving ZA |
| Z-FAST | 602 | Postmenopausal | HR 0.80; 95% CI 0.45–1.41; NS | Trend towards fewer recurrences in ET + early vs. delayed ZA patients treated with ZA |
| ZO-FAST | 1065 | Postmenopausal | HR 0.59; 95% CI 0.38–0.92; p = 0.0176 | Significantly fewer recurrences in ET + early vs. delayed ZA patients receiving ZA |
| E-ZO-FAST | 527 | Postmenopausal | HR 1.76; 95% CI 0.83–3.69; NS | Trend towards more recurrences in ET + early vs. delayed ZA patients treated with ZA |
| AZURE | 3360 | Pre/postmenopausal | HR 0.98; 95% CI 0.85–1.13; NS | No effect of ZA in addition to standard CT and/or ET +/- ZA therapy |
CI, confidence interval; CT, chemotherapy; ET, endocrine therapy; HR, hazard ratio; NS, not significant; ZA, zoledronic acid. See text for explanation of study name acronyms.
As for denosumab, there are currently no clinical data concerning a direct antitumour effect, although preclinical studies implicate a considerable role for the RANK/RANK-ligand pathway in breast cancer tumorigenesis [Beleut et al. 2010; Schramek et al. 2010]. As mentioned, ABCSG-18 evaluates the role of denosumab in the prevention of CTIBL [ClinicalTrials.gov identifier: NCT00556374]. Here, recurrence-free survival is assessed as a secondary endpoint. Another ongoing phase III trial, D-CARE (study of denosumab as adjuvant treatment for women with high-risk early breast cancer receiving neo-adjuvant or adjuvant therapy [ClinicalTrials.gov identifier: NCT01077154]) even defined bone metastasis-free survival as the primary study endpoint. Results are eagerly awaited, as these trials will yield important information concerning a potential role of denosumab in the prevention of breast cancer recurrences.
Side effects
Denosumab was generally well tolerated in several clinical trials conducted in advanced cancer patients. As RANK-ligand was identified as a costimulatory cytokine for T-cell activation, a higher risk for infectious diseases was anticipated [Wong et al. 1997]. Preclinical studies, however, revealed no increased risk of bacterial infections [Stolina et al. 2003], or altered virus clearance in response to influenza infections in vivo [Miller et al. 2007]. Importantly, in the aforementioned phase III study comparing denosumab to zoledronic acid in metastatic breast cancer, there was no increase in the number of infectious adverse events (48.8% zoledronic acid vs. 46.4% denosumab) or infectious serious adverse events (8.2% zoledronic acid vs. 7.0% denosumab). In contrast, a meta-analysis of nine randomized controlled trials involving 10,329 participants with postmenopausal osteoporosis, early breast cancer and rheumatoid arthritis, identified a significant increase in the risk of serious infection in patients receiving denosumab (odds ratio 4.45; 95% CI 1.15–17.14; p = 0.03) [Anastasilakis et al. 2009].
The FREEDOM study [ClinicalTrials.gov identifier: NCT00089791; Cummings et al. 2009], a large randomized study including more than 7000 patients with osteoporosis, however, yielded different results: no increase in the risk of infections was observed in the denosumab group. Furthermore, the risk for developing cancer was not increased. This is of considerable importance, as denosumab might bind to a TNF-related apoptosis-inducing ligand, thereby increasing tumour cell survival. Therefore, at the moment, there is no clear signal that the risk for serious infections is increased with denosumab treatment.
In studies of denosumab in osteoporosis, no cases of ONJ were observed. Therefore, a lower incidence of ONJ was anticipated also in metastatic cancer patients. Results from three phase III clinical trials including 5677 patients with bone metastases, however, clearly indicated a risk of ONJ associated with denosumab treatment similar to that with bisphosphonates: 37 (1.3%) cases were recorded in patients treated with zoledronic acid compared with 52 (1.8%) cases in patients receiving denosumab [Brown et al. 2010]. Recently, Van den Wyngaert and colleagues reported pooled ONJ safety data from all three randomized phase III trials. A total of 89 ONJ cases were reported with 52 (1.83%; 95% CI 1.37–2.39) occurring in the denosumab group and 37 (1.30%; 95% CI 0.92–1.79) in the zoledronic acid group, respectively. Overall, there was no significant difference in the pooled risk ratio (RR) for ONJ (RR 1.40; 95% CI 0.92–2.13; p = 0.11). It is necessary to remember, however, that neither separately nor pooled, those trials had adequate statistical power (>80%) to detect an excess relative risk of ONJ [Van den Wyngaert et al. 2011]. Therefore, postmarketing risk-benefit studies focusing on incidence of ONJ appear warranted.
Renal toxicity is another side effect associated with bisphosphonates. In AMG20050136, renal toxicity (defined as increased blood creatinine and blood urea, oliguria, renal impairment, proteinuria, decreased creatinine clearance, acute renal failure and chronic renal failure) was more frequently observed with zoledronic acid, especially in patients with baseline clearance of ≤60 ml/min. Therefore, denosumab represents a valid therapeutic option for patients with bone metastases suffering from chronic renal failure [Stopeck et al. 2010b].
Major differences between zoledronic acid and denosumab are summarized in Table 3.
Table 3.
Major differences between denosumab and zoledronic acid.
| Denosumab | Zoledronic acid | |
|---|---|---|
| Type of substance | Monoclonal antibody | Chemical agent |
| Mode of action | Inhibition of osteoclast differentiation | Induction of osteoclast apoptosis |
| Application | Subcutaneous | Intravenous |
| Direct antitumour effect | Possible, not yet observed | Suggested from preclinical and inconsistent clinical data |
| Inhibition of T-cell function | Suggested from in vitro data; increased infection rate not fully excluded | Not observed |
| Osteonecrosis of the jaw | Relevant side effect in patients receiving denosumab for bone metastases. Not observed in patients treated for osteoporosis | Relevant side effect in patients receiving zoledronic acide for bone metastases and osteoporosis |
| Renal toxicity | Not observed | Relevant side effects |
Conclusion
Bisphosphonates are currently the standard of care for the treatment of bone metastases in patients with advanced breast cancer, as these drugs were shown to reduce effectively the number of SREs. However, SREs might occur despite therapy, highlighting the need for alternative treatment approaches.
Derangement of the balance in the RANK/RANK-ligand/OPG pathway is a major driving force in the development of malignant bone lesions. Denosumab is a fully human antibody blocking RANK-ligand, thereby interfering with the vicious cycle of bone destruction. Clinical studies suggest at least similar efficacy as zoledronic acid, with one large prospectively randomized phase III trial also showing superiority of denosumab versus zoledronic acid in terms of delaying SREs in advanced breast cancer. Overall, denosumab was well tolerated, with generally mild side effects observed. However, an increased risk for infectious disease cannot fully be excluded, the risk for developing ONJs was similar to bisphosphonates; therefore, the same safety recommendations apply for this condition. Moreover, preclinical studies also suggest an important role for the RANK/RANK-ligand pathway in breast tumorigenesis. Thus, ongoing clinical studies are evaluating the effect of denosumab on CTIBL and also breast cancer recurrences in the adjuvant setting.
In conclusion, data from clinical trials of denosumab in bone metastases from solid tumours in general and from breast cancer in particular seriously challenge the current standard of care for these conditions, which is the use of bisphosphonates. Based on the published results of phase III clinical trials aiming at the delay of SREs in patients with bone metastases from solid tumours, denosumab has already been licensed by the US Food and Drug Administration for the prevention of SREs in patients with bone metastases from solid tumours including breast cancer (XGEVAR) in the USA. In Europe, the data have been filed with the European Medicines Agency and the decision is expected in the third quarter of 2011.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
G.Steger: fees for lectures and advisory boards from Amgen and Novartis. R.Bartsch: lecture fees from Novartis.
References
- American Cancer Society. (2009) Facts and figures. [ http://www.cancer.org/docroot/STT/STT_0.asp; last accessed 6 February 2011]
- Amir E., Ocaña A., Seruga B., Josse R., Clemons M. (2010) Medical oncology: Zoledronic acid for breast cancer therapy-induced bone loss. Nat Rev Clin Oncol 7: 187–188 [DOI] [PubMed] [Google Scholar]
- Anastasilakis A.D., Toulis K.A., Goulis D.G., Polyzos S.A., Delaroudis S., Giomisi A., et al. (2009) Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: A systematic review and a meta-analysis. Horm Metab Res 41: 721–729 [DOI] [PubMed] [Google Scholar]
- Baron R., Ferrari S., Russell C. (2011) Denosumab and bisphosphonates: Different mechanisms of action and effect. Bone 48: 677–692 [DOI] [PubMed] [Google Scholar]
- Bartsch R., Steger G.G. (2009) Role of denosumab in breast cancer. Expert Opin Biol Ther 9: 1225–1233 [DOI] [PubMed] [Google Scholar]
- Bekker P.J., Holloway D., Nakanishi A., Arrighi M., Leese P.T., Dunstan C.R. (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16: 348–360 [DOI] [PubMed] [Google Scholar]
- Bekker P.J., Holloway D.L., Rasmussen A.S., Murphy R., Martin S.W., Leese P.T., et al. (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059–1966 [DOI] [PubMed] [Google Scholar]
- Beleut M., Rajaram R.D., Caikovski M., Ayyanan A., Germano D., Choi Y., et al. (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A 107: 2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendre M.S., Margulies A.G., Walser B., Akel N.S., Bhattacharrya S., Skinner R.A., et al. (2005) Tumour derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappa B ligand pathway. Cancer Res 65: 11001–11009 [DOI] [PubMed] [Google Scholar]
- Body J.J., Facon T., Coleman R.E., Lipton A., Geurs F., Fan M., et al. (2006) A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 12: 1221–1228 [DOI] [PubMed] [Google Scholar]
- Body J.J., Greipp P., Coleman R.E., Facon T., Geurs F., Fermand J.P., et al. (2003) A phase I study of AMGN-0007 a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 97: 887–892 [DOI] [PubMed] [Google Scholar]
- Brown, J.E., Barrios, C.H., Diel, I.J., Facon, T., Fizazi, K., Ibrahim, T. et al. (2010) Incidence and outcomes of osteonecrosis of the jaw from an integrated analysis of three pivotal randomized double-blind, double-dummy phase 3 trials comparing denosumab and zoledronic acid for treatment of bone metastases in advanced cancer patients or myeloma. In: 10th International Conference on Cancer-induced Bone Disease, OC-15, Sheffield 22-25 September 2010.
- Brown J.E., Thomson C.S., Ellis S.P., Gutcher S.A., Purohit O.P., Coleman R.E. (2003) Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 89: 2031–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M., Corey E., Lee Z.D., True L.D., Yun T.J., Tondravi M., et al. (2001) Osteoprotegerin and RANK ligand expression in prostate cancer. Urology 57: 611–616 [DOI] [PubMed] [Google Scholar]
- Brufsky A.M., Bossermann L.D., Caradonna R.R., Haley B.B., Jones C.M., Moore H.C., et al. (2009) Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36 months follow-up results. Clin Breast Cancer 9: 77–85 [DOI] [PubMed] [Google Scholar]
- Cartsos V.M., Zhu S., Zavras A.I. (2008) Bisphosphonate use and the risk of adverse jaw outcomes. J Am Dent Assoc 139: 23–30 [DOI] [PubMed] [Google Scholar]
- Chikatsu N., Takeuchi Y., Tamura Y., Fukumoto S., Yano K., Tsuda E., et al. (2000) Interactions between cancer and bone marrow cells induce osteoclast differentiation factor expression and osteoclast-like cell formation. in vitro. Biochem Biophys Res Comm 267: 632–637 [DOI] [PubMed] [Google Scholar]
- Coleman R., Bundred N., De Boer R., Llombarto A., Campbell I., Neven P., et al. (2009) Impact of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST, ZO-FAST, and E-ZO-FAST. Cancer Res 69: 733s–733s [Google Scholar]
- Coleman R.E. (2001) Metastatic bone disease: Clinical features, pathophysiology, and treatment strategies. Cancer Treat Rev 27: 165–176 [DOI] [PubMed] [Google Scholar]
- Coleman R.E., Thorpe H.C., Cameron D., Dodwell D., Burkinshaw R., Keane M., et al. (2010) Adjuvant treatment with zoledronic acid in stage II/III breast cancer. The AZURE Trial (BIG 01/04). In: 33rd Annual San Antonio Breast Cancer Symposium (SABCS) Abstract S4–S5 [Google Scholar]
- Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765 [DOI] [PubMed] [Google Scholar]
- Eastell R., Hannon R.A., Cuzick J., Dowsett M., Clack G., Adams J.E. (2006) Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 21: 1215–1223 [DOI] [PubMed] [Google Scholar]
- Eidtmann H., de Boer R., Bundred N., Llombart-Cussac A., Davidson N., Neven P., et al. (2010) Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol doi: 10.1093/annonc/mdq217 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ellis G.K., Bone H.G., Chlebowski R., Paul D., Spadafora S., Fan M., et al. (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 26: 4875–4882 [DOI] [PubMed] [Google Scholar]
- Farrugia A.N., Atkins G.J., To L.B., Pan B., Horvath N., Kostakis P., et al. (2003) Receptor activator of nuclear factor-κB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res 63: 5438–5445 [PubMed] [Google Scholar]
- Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L., et al. (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 377: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., Lipton A., Mariette X., Body J.J., Rahim Y., Gralow J.R., et al. (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27: 1564–1571 [DOI] [PubMed] [Google Scholar]
- Frith J.C., Mönkkönen J., Auriola S., Mönkkönen H., Rogers M.J. (2001) The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: Evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum 44: 2201–2210 [DOI] [PubMed] [Google Scholar]
- Geusens P. (2009) Emerging treatments for postmenopausal osteoporosis – focus on denosumab. Clin Interv Aging 4: 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano N., Colla S., Rizzoli V. (2004) Update on the pathogenesis of osteolysis in multiple myeloma patients. Acta Biomed 75: 143–152 [PubMed] [Google Scholar]
- Gnant M.F., Mlineritsch B., Luschin-Ebengreuth G., Grampp S., Kaessmann H., Schmid M., et al. (2007) Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: A report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 25: 820–828 [DOI] [PubMed] [Google Scholar]
- Gnant M., Mlineritsch B., Schippinger W., Luschin-Ebengreuth G., Pöstlberger S., Menzel C., et al. (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360: 679–691 [DOI] [PubMed] [Google Scholar]
- Hauschka P.V., Mavrakos A.E., Iafrati M.D., Doleman S.E., Klagsbrun M. (1986) Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-sepharose. J Biol Chem 261: 12665–12674 [PubMed] [Google Scholar]
- Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., et al. (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29: 1125–1132 [DOI] [PubMed] [Google Scholar]
- Hines S.L., Mincey B.A., Sloan J.A., Thomas S.P., Chottiner E., Loprinzi C.L., et al. (2009) Phase III randomized, placebo-controlled, double-blind trial of risedronate for the prevention of bone loss in premenopausal women undergoing chemotherapy for primary breast cancer. J Clin Oncol 27: 1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A., Cuzick J., Baum M., Buzdar A., Dowsett M., Forbes J.F., et al. (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 yearś adjuvant treatment for breast cancer. Lancet 365: 60–62 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Delmas P., Burckhardt P., Cooper C., Torgerson D. (2007) Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int 7: 390–406 [DOI] [PubMed] [Google Scholar]
- Kitazawa S., Kitazawa R. (2002) RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol 198: 228–236 [DOI] [PubMed] [Google Scholar]
- Kostenuik P.J., Singh G., Suyama K.L., Orr F.W. (1992) A quantitative model for spontaneous bone metastasis: Evidence for a mitogenic effect of bone on Walker 256 cancer cells. Clin Exp Metastasis 10: 403–410 [DOI] [PubMed] [Google Scholar]
- Kretzschmar A., Wiege T., Al-Batran S.E., Hinrichs H.F., Kindler M., Steck T., et al. (2007) Rapid and sustained influence of intravenous zoledronic acid on course of pain and analgesics consumption in patients with cancer with bone metastases: A multicenter open-label study over 1 year. Support Cancer Ther 4: 203–210 [DOI] [PubMed] [Google Scholar]
- Kudo O., Sabokbar A., Pockok A., Itonaga I., Fujikawa Y., Athanasou N.A. (2003) Interleukin-6 and Interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32: 1–7 [DOI] [PubMed] [Google Scholar]
- Lipton A., Steger G.G., Figueroa J., Alvarado C., Solal-Celigny P., Body J.J., et al. (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 25: 4431–4437 [DOI] [PubMed] [Google Scholar]
- Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13: 581–589 [DOI] [PubMed] [Google Scholar]
- Miller R.E., Branstetter D., Armstrong A., Kennedy B., Jones J., Cowan L., et al. (2007) Receptor activator of NF-κB ligand inhibition suppresses bone resorption and hypercalcemia but does not affect host immune response to influenza infection. J Immunol 179: 266–274 [DOI] [PubMed] [Google Scholar]
- Mundy G.R. (2001) Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593 [DOI] [PubMed] [Google Scholar]
- Neville-Webbe H.L., Coleman R.E. (2010) Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur J Cancer 46: 1211–1222 [DOI] [PubMed] [Google Scholar]
- Perez E.A., Josse R.G., Pritchard K.I., Ingle J.N., Martino S., Findlay B.P., et al. (2006) Effect of letrozol versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A comparison study to NCIC CTC MA.17. J Clin Oncol 24: 3629–3635 [DOI] [PubMed] [Google Scholar]
- Rodan G.A., Martin T.J. (1981) Role of osteoblasts in hormonal control of bone resorption: A hypothesis. Calcif Tissue Int 33: 349–351 [DOI] [PubMed] [Google Scholar]
- Roodman G.D. (2004) Mechanisms of bone metastases. N Engl J Med 350: 1655–1664 [DOI] [PubMed] [Google Scholar]
- Ross J.R., Saunders Y., Edmonds P.M., Patel S., Broadley K.E., Johnston S.R. (2003) Systematic review of the role of bisphosphonates on skeletal morbidity in metastatic breast cancer. BMJ 327: 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek D., Leibbrandt A., Sigl V., Kenner L., Pospisilik J.A., Lee H.J., et al. (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468: 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Egerdie B., Hernández Toriz N., Feldman R., Tammela T.L., Saad F., et al. (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361: 7445–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolina M., Guo J., Faggioni R., Brown H., Senaldi G. (2003) Regulatory effects of osteoprotegerin on cellular and humoral immune responses. Clin Immunol 109: 347–354 [DOI] [PubMed] [Google Scholar]
- Stopeck A., Fallowfield L., Patrick D., Cleeland C.S., De Boer R.H., Steger G.G., et al. (2010a) Effect of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with metastatic breast cancer: Results from a phase III clinical trial. J Clin Oncol 28(15): Abstract 1024 [Google Scholar]
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., De Boer R.H., et al. (2010b) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol 28: 5132–5139 [DOI] [PubMed] [Google Scholar]
- Stopeck A., Martin M., Ritchie D., Body J.J., Paterson A., Viniegra M., et al. (2010c) Effect of denosumab versus zoledronic acid treatment in patients with breast cancer and bone metastases: Results from the extended blinded treatment phase. Cancer Res 2010: P6-14-01–P6-14-01 [Google Scholar]
- Thomas R.J., Guise T.A., Yin J.J., Elliott J., Horwood N.J., Martin T.J., et al. (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140: 4451–4458 [DOI] [PubMed] [Google Scholar]
- Thürlimann B., Keshaviah A., Coates A.S., Mouridsen H., Mauriac L., Forbes J.F., et al. (2005) Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353: 2727–2757 [DOI] [PubMed] [Google Scholar]
- Valachis A., Polyzos N.P., Gergoulias V., Mavroudis D., Mauri D. (2010) Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: A meta-analysis. Gynecol Oncol 117: 139–145 [DOI] [PubMed] [Google Scholar]
- Van den Wyngaert T., Wouters K., Huizing M.T., Vermorken J.B. (2011) RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): Non bis in idem? Support Care Cancer doi: 10.1007/s00520-010-1061-0 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Van Poznak C., Hannon R.A., Mackey J.R., Campone M., Apffelstaedt J.P., Clack G., et al. (2010) Prevention of aromatase inhibitor-induced bone loss using risedronate: The SABRE trial. J Clin Oncol 28: 967–975 [DOI] [PubMed] [Google Scholar]
- Vega D., Maalouf N.M., Sakhaee K. (2007) The role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: Clinical implications. J Clin Endocrinol Metab 92: 4514–4521 [DOI] [PubMed] [Google Scholar]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272: 25190–25194. [DOI] [PubMed]
- Yin J.J., Pollock C.B., Kelly K. (2005) Mechanisms of cancer metastasis to the bone. Cell Res 15: 57–62 [DOI] [PubMed] [Google Scholar]