Abstract
Melanoma is a malignancy that is highly curable in the early stages but has devastating consequences in later stages due to lack of response to traditional treatments. Improved understanding of the basic science of tumorigenesis has helped lead to novel targeted therapies which are producing beneficial results in patients with melanoma. Enhancement of the immune system by blockade of the cytotoxic T-lymphocyte associated antigen-4 by the monoclonal antibody ipilimumab is now approved by the United States Food and Drug Administration (FDA) for use in patients with unresectable melanoma. The approval of this drug was based on the first ever data in melanoma showing an improvement in overall survival. New advances in targeting components of the mitogen-activated protein kinase pathway are showing impressive responses in clinical trials in most patients harboring activating mutations in BRAF. Thus, this is a new era in the management of melanoma and we review the recent progress made in treating patients with advanced disease.
Keywords: BRAF mutation, ipilimumab, metastatic melanoma, targeted therapy, vemurafenib
Introduction
The incidence of melanoma is increasing faster than any other cancer, with about 68,000 new cases diagnosed every year in the United States [Rigel, 2010]. Melanoma is the fifth most common newly diagnosed malignancy in men and the seventh most common in women [Jemal et al. 2010]. When diagnosed early and appropriately excised, melanoma is a curable malignancy. However, mortality is high for patients with advanced disease because of lack of effective treatment options, with 1-year survival rates of about 40% [AJCC Staging Manual, seventh edition]. The only drugs approved by the FDA for treatment of advanced melanoma prior to 2011 were high-dose interleukin-2 and dacarbazine, which both have response rates of 10–20% [Agarwala 2009; Atkins et al. 1999]. Until recently, none of the agents used to treat melanoma demonstrated an improvement in overall survival, thus emphasizing the need for novel agents. Advances in the field of oncology have shown that agents which target immune system components as well as molecularly targeted therapies show great promise in malignancies which have been resistant to traditional chemotherapy.
Ipilimumab
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is a T-cell surface receptor that works as an immune system checkpoint to regulate immune responses. The antigen is expressed when the immune system is stimulated and competes with CD28 for binding on antigen presenting cells, thus leading to blockade of costimulatory signals needed for T-cell activation. Blockade of CTLA-4 releases immune system inhibition allowing for enhanced T-cell-mediated immunity and the ability to recognize cancer cells as foreign [Peggs et al. 2006].
Ipilimumab is a fully humanized monoclonal antibody directed against CTLA-4. Preclinical data showed ipilimumab to be effective in enhancing the host’s ability to generate an anticancer immune response by depletion of regulatory T cells and was used successfully in conjunction with vaccine therapies or standard chemotherapy in mouse melanoma cell lines [Sutmuller et al. 2001; Van Elsas et al. 1999].
A hallmark phase III study of ipilimumab was published by Hodi and colleagues in August 2010. In this trial, 676 patients with pretreated, unresectable stage III or stage IV melanoma were randomized to receive ipilumumab at 3 mg/kg intravenously every 3 weeks, with or without a glycoprotein-100 (gp100) vaccine versus gp100 alone. Patients treated with ipilimumab and gp100 had an overall survival of 10 months compared with 6.4 months in the gp100 control group (p < 0.001). The best overall response rate was 10.9% and disease control rate including complete response, partial response and stable disease was 28.5% in patients treated with ipilimumab. There was no difference in progression-free survival (PFS) in patients treated with ipilimumab and gp100 compared with gp100 alone (2.76 months), which highlights the latency period of about 3 months for the drug to take effect. The 1- and 2-year survival rates for patients treated with ipilimumab were 45.6% and 23.5%, respectively [Hodi et al. 2010]. In a recent, meta-analysis of phase II trials, 1-year survival rates were reported to be 25.5% and 2-year survival rates were approximately 10% [Korn et al. 2008].
Immune-related adverse events (IRAEs), including dermatitis, hepatitis, nephritis, hypophysitis and, more commonly, diarrhea and enterocolitis, are seen with ipilimumab. In the phase III trial of ipilimumab, 60% of patients experienced an IRAE, with 10–15% being grade 3 or 4. Most of these adverse events remit within 6 weeks of discontinuing ipilimumab. About 2% of patients treated with ipilimumab died of complications related to the study drug [Hodi et al. 2010]. Diarrhea and enterocolitis are two common IRAEs, occurring in about 20–30% of treated patients, although grade 4 colitis is rare. Prompt initiation of treatment (including steroids) is effective in alleviating symptoms in the vast majority of patients within 2 weeks, although a small proportion of patients require treatment with infliximab, an antibody against tumor necrosis factor alpha [Beck et al. 2006]. Severe enterocolitis leading to colonic perforation and subsequent death has been reported in the literature.
Ipilimumab was the first drug to ever show an improvement in overall survival in patients with advanced melanoma. Its novel mechanism of action as an immune system activator is responsible for the latency period before anticancer responses are seen and the IRAEs observed. Prompt initiation of steroids is required upon development of IRAEs because fatal effects have been seen.
Results of a second phase III randomized trial have been presented recently at the 2011 American Society of Clinical Oncology (ASCO) conference [Wolchok et al. 2011] and then published in the New England Journal of Medicine [Robert et al. 2011]. The researchers reported that first-line treatment with a combination of ipilimumab plus dacarbazine (DTIC) improved overall survival (primary endpoint) in patients with previously untreated metastatic melanoma. This is the first study to show that combining chemotherapy and immunotherapy is safe and effective for patients with advanced melanoma.
In this study, 502 patients with treatment-naive metastatic melanoma [Eastern Cooperative Oncology Group (ECOG) performance status 0/1] were randomized 1:1 to ipilimumab (10 mg/kg) plus DTIC (850 mg/m2) or placebo and DTIC (850 mg/m2) at weeks 1, 4, 7, 10 followed by DTIC every 3 weeks through week 22 (induction). Eligible patients received ipilimumab or placebo every 12 weeks as maintenance. Overall survival rates for combination therapy versus chemotherapy alone after 1, 2 and 3 years of therapy were: 47.3% versus 36.3%, 28.5% versus 17.9% and 20.8% versus 12.2% respectively. Median overall survival was 11.2 months with ipilimumab plus DTIC versus 9.1 months for DTIC alone. Median PFS times, however, were similar: 2.8 months for combination therapy versus 2.6 months for DTIC. The lack of difference in PFS shows that the effects of immunotherapy treatment can take much longer to be seen than those from traditional chemotherapy or targeted therapies. In addition, patients' scans may sometimes even get worse before they improve; thus, overall survival is a more accurate way to gauge treatment effectiveness than PFS. Ipilimumab plus DTIC therapy had a good safety profile, with no gastrointestinal perforations and a lower rate of colitis than was expected based on prior ipilimumab monotherapy studies. However, approximately 56% of patients receiving ipilimumab plus DTIC and 27% of patients receiving DTIC alone had significant grade 3 or 4 adverse events from their therapy.
BRAF inhibition
In approximately 50% of melanomas, there is an activating mutation in BRAF, a seronine-threonine kinase that is a member of the mitogen-activated protein kinase (MAPK) pathway [Davies et al. 2002]. Over 90% of the mutations in BRAF are due to a glutamic acid for valine amino acid substitution at position 600 (V600E), which allows for unregulated signaling of the MAPK pathway and thus unregulated cell growth. Preclinical studies on human melanoma cell lines have shown that inhibition of BRAF by small inhibiting RNAs is able to restore cell turnover arrest and thus reverse the malignant features of the melanoma cell lines [Hingorani et al. 2003].
PLX4032, also known as RO5185426 (Roche) or RG7204 (Genentech) and now vemurafenib (Plexxikon; Roche/Genentech), is an agent that targets activated mutant BRAF at the V600E mutation [Tsai et al. 2008] and will be referred to as vemurafenib throughout this review. The phase I dose-escalation trial was tested on patients with various solid tumors regardless of BRAF mutational status, although there was a predominance of patients with BRAF-mutated melanoma because of the above-mentioned preclinical data. Dose-escalation phase analysis determined the maximal tolerated dose (MTD) of vemurafenib as 960 mg orally twice daily. For the expansion cohort of this trial, patient selection was limited to those with BRAF-mutated metastatic melanoma. Of the 32 patients treated in the expansion cohort, 26 (81%) showed favorable treatment response. Of these 26 patients, 24 had a partial response and two had a complete response. The duration of response is unknown at present but was more than 7 months in 50% of the patients who responded to the drug.
vemurafenib was beneficial in alleviating symptoms such as pain as soon as 1–2 weeks after drug initiation [Flaherty et al. 2010], indicating a rapid onset of treatment response. In addition, patients treated with vemurafenib showed a dramatic decrease in positron emission tomography (PET) standard uptake values within 2 weeks of initiating therapy. In 22 patients with advanced melanoma treated with at least 320 mg of vemurafenib twice daily, all showed a decrease of more than 25% in metabolic activity on PET scan in the first 2 weeks of treatment [McArthur et al. 2010].
The BRIM-2 trial is a phase II study that enrolled 132 patients with pretreated, BRAF V600E mutated metastatic melanoma. The cohort was treated with 960 mg of RG7204/vemurafenib twice daily. About 60% of patients had widely metastatic visceral disease at baseline. Approximately 50% of the cohort had been treated with one prior therapy, 27% of patients had received two prior therapies and 22% had received three or more prior treatments. Prior treatments included interleukin-2 in 39% of patients and 5% had prior exposure to ipilimumab. Impressive results were seen because 52% of patients had a partial or complete response and 29.5% had stable disease. At a median follow up of 7 months, the duration of response was 6.8 months and PFS was 6.2 months. Around 69% of patients were still alive at the time of data analysis and median overall survival had not yet been reached. Of the 132 patients treated, 41 deaths were seen with 39 attributed to disease progression and one to treatment-related renal failure [Sosman et al. 2010].
Vemurafenib seems to be well tolerated with adverse events including grade 2 or 3 arthralgias, rash, nausea, pruritis, palmar-plantar dysethesia and fatigue. Cutaneous reactions including development of keratoacanthoma-type squamous cell carcinomas were seen in 31% of patients. As these are slow-growing neoplasms with little risk of significant local invasion or distant metastases, complete surgical excision is curative [Flaherty et al. 2010]. Adverse events requiring dose modification or discontinuation of vemurafenib included rash, fatigue and elevations in gamma-glutamyl transpeptidase levels.
A phase III study of vemurafenib versus dacarbazine (BRIM-3) enrolled 675 treatment-naïve patients with stage IIIc or IV BRAF V600E mutant melanoma. Results were recently reported at ASCO 2011 and then recently published in the New England Journal of Medicine [Chapman et al. 2011]. The trial compared the effectiveness (overall survival and PFS as co-primary endpoints) of treatment with PLX4032 (960 mg orally twice daily) or DTIC (1000 mg/m2 intravenously every 3 weeks).
At the planned interim analysis, patients receiving vemurafenib had a 63% reduction in risk of death [95% confidence interval (CI) 0.26 to 0.55; p < 0.0001] compared with those receiving DTIC. There was also a 74% reduction in the risk of progression (or death) with vemurafenib compared with DTIC (95% CI 0.26 to 0.55; p < 0.0001). Response rates were 48.4% with vemurafenib versus 5.5% with DTIC. Because of this significant result, it was recommended that patients receiving DTIC crossover to receive vemurafenib.
Less than 10% of patients who received vemurafenib experienced problems with high levels of toxicity (grade 3 or worse). The most common side effects were skin rashes, photosensitivity and joint pain. About 20–30% of patients developed a low-grade squamous cell carcinoma of the skin.
The researchers commented that because the study findings showed improvements in PFS and response rate along with greater overall survival, PFS may now become a validated study endpoint for future trials with similarly targeted therapies of melanoma. The next step is to test vemurafenib in combination with other agents in patients with advanced melanoma. A phase I trial has already begun with vemurafenib and ipilimumab.
GSK2118436 is a BRAF inhibitor that competes with adenosine triphosphate (ATP) for binding on mutated BRAF. Phase I/II studies of melanomas with BRAF-activating mutation as well as other solid tumors have shown dramatic responses. Of 30 patients treated with this compound, 18 had a decrease of more than 20% in the size of metastatic lesions as determined by Response Evidence Criteria in Solid Tumors (RECIST) at the time of first restaging 8 weeks after drug initiation. In this study, the MTD had not yet been reached because treatment-related toxicity was minimal [Kefford et al. 2010].
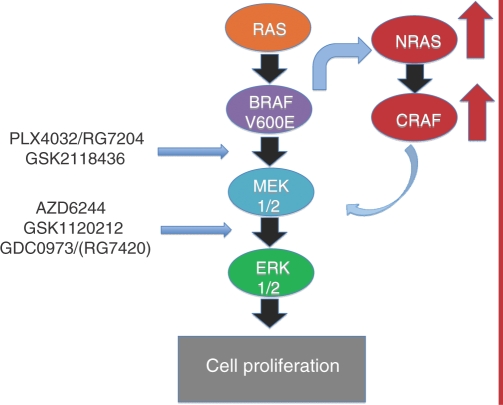
Resistance to BRAF inhibition with use of vemurafenib has been observed. A group of patients were shown to have primary resistance to the drug and another group had secondary resistance because they became resistant after initially responding to the drug [Flaherty et al. 2010]. Experiments in melanoma cell lines exposed to the BRAF inhibitor vemurafenib identified a small population of viable cells that were able to proliferate despite drug administration. Those cells were analyzed and found to maintain signaling through phospho-ERK. The combination of BRAF and MEK inhibition completely destroyed the population of resistant cells [Paraiso et al. 2010]. These data provide support for simultaneous use of BRAF and MEK inhibitors to circumvent the population of primary resistant cells. Additional potential mechanisms of resistance include activation of alternative receptor tyrosine kinase pathways or reactivating the MAPK pathway by upregulation of NRAS. Once NRAS is upregulated, CRAF is overexpressed and proliferation through the MAPK pathway is continued by bypassing BRAF (Figure 1). Secondary mutations in the BRAF gene have not yet been identified [Nazarian et al. 2010]. These data indicate that additional therapeutic agents aimed towards blockade of additional components in the MAPK pathway, like MEK, in conjunction with BRAF inhibition may prevent the development of primary and secondary resistance seen with use of BRAF inhibition alone.
Figure 1.
The mitogen-activated protein kinase (MAPK) pathway with BRAF and MEK inhibitor sites of action. One mechanism of resistance to BRAF inhibition is hypothesized to occur through upregulation of NRAS and CRAF with subsequent evasion of BRAF and thus continued signaling and cell proliferation.
MEK inhibition
Similar to inhibition of BRAF, inhibition of mitogen-activated protein kinase kinase (MEK) is also an attractive target for drug development especially since the only substrates for MEK1/2 are ERK 1/2. Since MEK is downstream of BRAF, it can be reasoned that MEK inhibition would be most effective when BRAF is mutated and constitutively activated. This hypothesis has been validated in preclinical studies indicating inhibition of MEK was more effective in BRAF-mutated melanoma cell lines as opposed to BRAF wild-type or RAS-mutated cell lines [Solit et al. 2006].
AZD6244 (ARRY-142886) is a selective, ATP-uncompetitive inhibitor of MEK1/2 with promising preclinical data in a variety of solid tumors, including inducing growth arrest and tumor regression in melanoma cell lines when combined with docetaxel [Haass et al. 2008]. Based on phase I analysis in various solid tumors, the recommended phase II dosing is 100 mg orally twice daily [Adjei et al. 2008]. A randomized phase II study used AZD6244 versus standard dosing of temozolomide in patients with stage III or IV melanoma who were chemotherapy naïve. The primary outcome was PFS and this study failed to show any difference between the two arms. However, those patients harboring a BRAF mutation had a favorable hazard ratio for death at 0.68. At the time of interim analysis, six patients treated with AZD6244 had a partial response, including five patients that had BRAF-mutated disease. AZD6244 is generally well tolerated with adverse events including diarrhea, acneiform dermatitis, nausea, fatigue and peripheral edema [Dummer et al. 2008]. Another study used AZD6244 combined with dacarbazine, docetaxel or temsirolimus. Partial response and stable disease was seen in 28% and 50% respectively, with all patients who responded to the drug harboring BRAF mutations [Patel et al. 2010].
GSK1120212 is an oral selective allosteric inhibitor of MEK1/2 currently being used in a variety of early stage clinical trials. Initially the drug was used in phase I/II studies in advanced solid tumors, including 29 patients with melanoma. In the 11 patients with BRAF-mutated disease, three patients showed partial response, five patients had stable disease and three with disease progression. Three of nine patients with BRAF wild-type mutation had stable disease, two showed partial response and four had disease progression [Infante et al. 2010]. Updated data were recently presented at the European Society for Medical Oncology meeting with a total of 202 patients enrolled including 97 patients with metastatic melanoma. Patients with melanoma had been mostly pretreated, with 37% having received one or two prior treatments and 57% having had three or more prior therapies. In addition, over 90% of patients with melanoma had widely metastatic disease in visceral organs, including 48% of patients with previously treated brain metastases. In patients harboring the BRAF mutation, a preliminary response rate of 41% was seen, with the majority of patients having partial responses. Patients with BRAF wild-type disease had a response rate of around 8%. Preliminary PFS was around 7 months in patients with BRAF-mutated disease. Stable disease was seen in two patients who had previously been treated with the BRAF inhibitor, PLX4032. Pharmacodynamic analysis showed 92% decreased expression in immunohistochemical staining of ERK1/2 and Ki67 on day 15 of treatment [Falchook et al. 2010].
The GSK1120212 MEK inhibitor is well tolerated with a MTD of 3 mg daily and the recommended phase II dosing is 2 mg daily. MTD adverse events include rash, diarrhea and central serous retinopathy [Infante et al. 2010]. At the phase II dosing, most adverse events were limited to rash, diarrhea, fatigue and decreased appetite. All adverse events were grade 1–3 with no grade 4 toxicities noted [Falchook et al. 2010].
Targeted therapies inhibiting BRAF and MEK in patients with BRAF-mutated melanoma are safe and efficacious. Estimated PFS is at least 7 months based on existing data and there is a suggestion that overall survival will also be significantly increased. It is unknown if using BRAF and MEK inhibitors in combination will be more effective than using either drug alone. Combination therapy may be able to prevent the development of resistance seen with BRAF inhibitors and studies exploring this are underway.
Conclusion
Traditionally melanoma has been resistant to treatment with conventional chemotherapy leading to poor survival rates in patients with metastatic disease. Now, however, multiple new treatment options have been studied in clinical trials that are showing encouraging response rates with improvement in PFS. In addition, ipilimumab and BRAF inhibitors have been shown to improve overall survival and there is evidence that MEK inhibitors may do the same. Studies with BRAF and MEK inhibitors have shown impressive results in the patients with BRAF-mutated disease thus providing a model for how targeted therapies should be utilized. These drugs are well tolerated and have a rapid time to initial treatment response, with sustained responses of 7 months or more. Developments in melanoma therapies have ushered in a new era for the treatment of patients with advanced disease. We anticipate that therapeutic advances made in the next few years will revolutionize the field of melanoma treatment by significantly prolonging and improving the quality of patients’ lives.
Funding
Dr Lewis received research funding from GlaxoSmithKline and Genentech. Dr Gonzalez received research funding from GSK, Genentech and Roche.
Conflict of interest statement
Dr Amaria declares no conflicts of interest in preparing this article. Dr Gonzalez is a consultant for Roche and Genentech.
References
- Adjei A.A., Cohen B., Franklin W., Morris C., Wilson D., Molina J.R., et al. (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase ½ inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26: 2139–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwala S.S. (2009) Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther 9: 587–595 [DOI] [PubMed] [Google Scholar]
- American Joint Committee on Cancer Staging Handbook Seventh Edition. Published by Springer, New York, NY 2010.
- Atkins M.B., Lotze M.T., Dutcher J.P., Fisher R.I., Weiss G., Margolin K., et al. (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17: 2105–2116 [DOI] [PubMed] [Google Scholar]
- Beck K.E., Blansfield J.A., Tran K.Q., Feldman A.L., Hughes M.S., Royal R.E. (2006) Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 24: 2283–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, P.B., Hauschild, A., Robert C., Larkin, J.M.G., Haanen, J.B.A.G., Ribas, A. et al. (2011) Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. Paper presented at the 47th Annual Meeting of the American Society of Clinical Oncology, 3–7 June, Chicago, IL abstract LBA4.
- Chapman, P.B., Hauschild, A., Robert, C., Haanen, J.B., Ascierto, P., Larkin, J. et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Eng J Med June 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Dummer R., Robert C., Chapman P.B., Sosman J.A., Middleton M., Bastholt L., et al. (2008) AZD6244 (ARRY-142886) vs temozolamide (TMZ) in patients (pts) with advanced melanoma: An open-label, randomized, multicenter, phase II study. In ASCO 2008, Chicago, IL. J Clin Oncol 26(Suppl): 15S–15S abstract 9003 [Google Scholar]
- Falchook G., Infante J.R., Fecher L.A., Gordon M.S., Vogelzang N.J., DeMarini D.J., et al. (2010) The oral MEK1/2 inhibitor GSK1120212 demonstrates early efficacy signals. Paper presented at European Society of Medical Oncology October 8–12 2010 Milan, Italy [Google Scholar]
- Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Eng J Med 363: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass N.K., Sproessor K., Nguyen T.K., Contractor R., Medina C.R., Nathanson K.L., et al. (2008) The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res 14: 230–239 [DOI] [PubMed] [Google Scholar]
- Hingorani S.R., Jacobetz M.A., Robertson G.P., Herlyn M., Tuveson D.A. (2003) Suppression of BRAF (V599E) in human melanoma abrogates transformation. Cancer Res 63: 437–450 [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J.R., Fecher L.A., Nallapareddy S., Gordon M.S., Flaherty K.T., Cox D.S., et al. (2010) Safety and efficacy results from the first-in-human study of the oral MEK ½ inhibitor GSK1120212. In ASCO 2010, Chicago, IL. J Clin Oncol 28(Suppl): 15S–15S abstract 2503 [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Kefford R., Arkenau H., Brown M.P., Millward M., Infante J.R., Long G.V., et al. (2010) Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. In ASCO 2010, Chicago, IL. J Clin Oncol 28(Suppl): 15S–15S abstract 8503 [Google Scholar]
- Korn E.L., Liu P.Y., Lee S.J., Chapman J.A., Niedzwiecki D., Susman V.J., et al. (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26: 527–534 [DOI] [PubMed] [Google Scholar]
- McArthur G.A., Puzanov I., Ribas A., Chapman P.B., Kim K.B., Sosman R.J., et al. (2010) Early FDG-PET responses to PLX4032 in BRAF-mutant advanced melanoma. In ASCO 2010, Chicago, IL. J Clin Oncol 28(Suppl): 15S–15S abstract 8529 [Google Scholar]
- Nazarian R., Shi H., Wang Q., Kong X., Koya R.C., Lee H., et al. (2010) Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 468: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso K.H.T., Fedorenko I.V., Cantini L.P., Munko A.C., Hall M., Sondak V.K., et al. (2010) Recovery of phosphor-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer 102: 1724–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.P., Lazar A.J., Mahoney S., Vaughn C., Gonzalez N., Papadopoulos P., et al. (2010) Clinical responses to AZD6244 (ARRY-142886)-based combination therapy stratified by gene mutations in patients with metastatic melanoma. In ASCO 2010, Chicago, IL. J Clin Oncol 28(Suppl): 15S–15S abstract 8501 [Google Scholar]
- Peggs K.S., Quezada S.A., Korman A.J., Allison J.P. (2006) Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opion Immunol 18: 206–213 [DOI] [PubMed] [Google Scholar]
- Rigel D.S. (2010) Trends in dermatology: melanoma incidence. Arch Dermatol 146: 318–318 [DOI] [PubMed] [Google Scholar]
- Robert, C., Thomas, L., Bondarenko, I., O'Day, S., Weber, J., Garbe, K., et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Eng J Med Jun 5 [Epub ahead of print] [DOI] [PubMed]
- Solit D.B., Garraway L.A., Pratilas C.A., Sawai A., Getz G., Basso A., Ye Q. (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman J., Kim K., Schuchter L., Gonzalez R., Pavlick A., Weber J., et al. (2010) An open-label, multicenter phase II study of continuous oral dosing of RG7204 (PLX4032) in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. Paper presented at Society for Melanoma Research Nov 4-7th 2010 Sydney, Australia [Google Scholar]
- Sutmuller R.P., van Duivenvoorde L.M., van Elsas A., Schumacher T.N., Wildenberg M.E., Allison J.P., et al. (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 194: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J., Lee J.T., Zhang J., Cho H., Mamo S., Bremer R., et al. (2008) Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A 105: 3041–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elsas A., Hurwitz A.A., Allison J.P. (1999) Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok, J.D., Thomas, L., Bondarenko, I.N., O'Day, S., Weber, J.S., Garbe, C., et al. (2011) Phase 3 randomized study of ipilimumab (IPI) plus dacarbazine (DTIC) vs DTIC alone as first line treatment in patients with unresectable stage III or IV melanoma. In ASCO June 3-7 2011, Chicago, IL, abstract LBA5.