Abstract
The Escherichia coli NusA protein modulates pausing, termination, and antitermination by associating with the transcribing RNA polymerase core enzyme. NusA can be covalently cross-linked to nascent RNA within a transcription complex, but does not bind RNA on its own. We have found that deletion of the 79 carboxy-terminal amino acids of the 495-amino-acid NusA protein allows NusA to bind RNA in gel mobility shift assays. The carboxy-terminal domain (CTD) of the α subunit of RNA polymerase, as well as the bacteriophage λ N gene antiterminator protein, bind to carboxy-terminal regions of NusA and enable full-length NusA to bind RNA. Binding of NusA to RNA in the presence of α or N involves an amino-terminal S1 homology region that is otherwise inactive in full-length NusA. The interaction of the α-CTD with full-length NusA stimulates termination. N may prevent termination by inducing NusA to interact with N utilization (nut) site RNA rather than RNA near the 3′ end of the nascent transcript. Sequence analysis showed that the α-CTD contains a modified helix–hairpin–helix motif (HhH), which is also conserved in the carboxy-terminal regions of some eubacterial NusA proteins. These HhH motifs may mediate protein–protein interactions in NusA and the α-CTD.
Keywords: NusA, α subunit, RNA-binding, HhH motifs, termination, antitermination
The NusA protein of Escherichia coli binds to core RNA polymerase shortly after the initiation of transcription and stimulates pausing and termination at certain sites (for review, see Richardson and Greenblatt 1996). The mechanism by which NusA influences pausing during transcription is not yet clear, but RNase protection experiments suggest that NusA may bind and stabilize the stem-loop RNA structures often associated with pause sites (Landick and Yanofsky 1987). By increasing the dwell time of RNA polymerase at such a pause site, this stabilization may serve to couple transcription and translation (Zheng and Friedman 1994). In vitro studies have shown that NusA also enhances termination of transcription at intrinsic terminators (Greenblatt et al. 1981; Grayhack and Roberts 1982; Schmidt and Chamberlin 1987; Whalen et al. 1988). These terminators also contain a GC-rich stem-loop in the nascent transcript upstream of the termination site, and it is possible that NusA helps promote release of the transcript by stabilizing the RNA stem-loop and blocking its interaction with a single-stranded RNA-binding site on RNA polymerase (Artsimovich and Landick 1998). Consistent with this, the NusA in a transcription complex could be cross-linked to the nascent RNA located more than 10 nucleotides from the 3′ end of the transcript (Liu and Hanna 1995).
NusA binds directly to the α subunit of RNA polymerase (Liu et al. 1996). It can also be cross-linked to the large β and β′ subunits of RNA polymerase (J. Li and J. Greenblatt, unpubl.) and may be capable of binding directly to these subunits as well (Liu et al. 1996). The α subunit of RNA polymerase has two domains: The amino-terminal domain (NTD) is required for dimerization and for interaction with the β and β′ subunits of RNA polymerase, whereas the carboxy-terminal domain (CTD) is a contact surface for DNA-binding activator proteins (for review, see Ebright and Busby 1995) and possesses a minor dimerization interface (Blatter et al. 1994). The CTD of α also binds the UP element, a DNA element that enhances initiation of transcription at certain promoters (Ross et al. 1993; Blatter et al. 1994). Additionally, it has been suggested that a direct interaction between the α-CTD and NusA is important for NusA's ability to control pausing and termination (Liu et al. 1996).
NusA influences not only pausing and termination by RNA polymerase, but also transcriptional antitermination by the bacteriophage λ N protein (Friedman 1971). Antitermination by N requires a cis-acting RNA element, called the nut site, which consists of two functional components, boxA and boxB (Salstrom and Szybalski 1978; de Crombrugge et al. 1979; Olson et al. 1982; Das and Wolska 1984; Horwitz et al. 1987). NusA, as well as the E. coli proteins NusB, NusE (ribosomal protein S10), NusG, RNA polymerase, and the nut site on the phage RNA, take part in multiple interactions within the N-modified transcription complex (for review, see Friedman 1988; Greenblatt et al. 1993). The resulting highly stable ribonucleoprotein complex is capable of suppressing transcription termination over long distances and through multiple terminators (Mason et al. 1992; Mogridge et al. 1995). Within this complex, NusA interacts with the N protein (Greenblatt and Li 1981b), and both the amino- and carboxy-terminal regions of NusA interact with RNA polymerase (Mah et al. 1999).
The 107-amino-acid phage λ N protein has been dissected into an amino-terminal arginine-rich motif (amino acids 1–22), which binds as a bent α-helix to the boxB RNA hairpin in the nut site (Legault et al. 1998), and an activating region (amino acids 23–107) also required for transcriptional antitermination (Mogridge et al. 1998). At least two portions of the activating region are important for antitermination: Amino acids 34–47 interact with NusA, and amino acids 73–107 interact with RNA polymerase. A carboxy-terminally truncated λ N protein (amino acids 1–47), which can interact with both the nut site RNA and NusA, has partial antitermination activity: It is sufficient to reverse the enhancing effect that NusA has on the efficiency of an intrinsic terminator, but does not provide complete terminator read-through (Mogridge et al. 1998). This suggests that an interaction of N with NusA may reverse the effect of NusA on termination.
Sequence comparisons revealed two types of putative RNA-binding domains in NusA, an S1 homology region and tandemly duplicated KH homology regions (Gibson et al. 1993a,b; Bycroft et al. 1997). S1 and KH domains are both found in proteins that can associate with RNA nonspecifically (Gibson et al. 1993a,b; Bycroft et al. 1997). The S1 domain was first identified in ribosomal protein S1, which has six of them, whereas the KH domain was initially identified in the hnRNP K protein (Subramanian 1983; Siomi et al. 1993). Recent studies with other S1- and KH-domain-containing proteins and with the isolated domains themselves have suggested that these domains can be capable of sequence-specific RNA binding (Ringquist et al. 1995; Dejgaard and Leffers 1996; Dodson and Shapiro 1997).
There is evidence that NusA may interact directly with nucleotides in both the boxA and boxB components of the nut site (Olson et al. 1982; Friedman and Olson 1983; Olson et al. 1984; Mogridge et al. 1995). The effects of mutations in the S1 homology region of NusA between amino acids 136 and 240 suggest that this region is important for antitermination. The nusA1 (L183R) and nusA R199A mutations both cause temperature-sensitive λ growth because of an inability of N to function at high temperature (Friedman 1971; Friedman and Baron 1974; T. Mah, Y. Zhou, N. Yu, J. Mogridge, E. Olsen, J. Greenblatt, and D. Friedman, unpubl.). Unlike wild-type NusA, both mutant proteins are unable to supershift an N–nut-site-RNA complex in a gel mobility shift experiment, even though they bind N directly with wild-type affinity. This suggests that both mutations cause a defect in the interaction of the S1 homology region of NusA with nut-site RNA (T. Mah, Y. Zhou, N. Yu, J. Mogridge, E. Olsen, J. Greenblatt, and D. Friedman, unpubl.). Other experiments have shown that both an amino-terminal RNA polymerase-binding region in amino acids 1–137 and a portion of NusA that contains the S1 and KH homology regions are essential for NusA to enhance both termination at an intrinsic terminator and antitermination by N (Mah et al. 1999).
Despite the evidence suggesting that NusA can interact with RNA, the full-length NusA protein does not cause a mobility shift of the phage λ nut-site RNA in a gel retardation assay unless the N protein is also present (Mogridge et al. 1995). A direct interaction between NusA and N has been demonstrated (Greenblatt and Li 1981b), and it is likely that this interaction confers an RNA-binding ability on NusA. Since NusA does cross-link to RNA in a transcription complex in the absence of N (Liu and Hanna 1995), it seemed likely that an interaction of NusA with RNA polymerase might also alter the conformation of NusA so as to allow for RNA binding.
In this work, we report that the α subunit of E. coli RNA polymerase and the λ N protein bind to the carboxy-terminal regions of NusA, suggesting that α and N may act in similar ways to control the binding of NusA to RNA, and that interaction of α with NusA promotes the association of NusA with RNA. Our results suggest that an interaction of NusA with α in a transcription complex would allow NusA to bind the nascent transcript and stimulate pausing and termination by RNA polymerase. This interaction appears to be inhibited by the 79 carboxy-terminal amino acids of NusA, as judged from the ability of the truncated form of NusA to bind RNA independently. Sequence analysis shows that both the α-CTD and the NusA carboxy-terminal regulatory region contain modified versions of a helix–hairpin–helix (HhH) motif. This motif has been found in a wide variety of DNA-binding and RNA-binding proteins, including many enzymes involved in DNA replication and repair, and is thought to mediate non-sequence-specific binding of proteins to nucleic acids. Our data may be indicative of a different kind of function for the HhH motifs in the α-CTD and NusA, namely, participation in intermolecular α-CTD–NusA complex formation and intramolecular protein–protein interactions within NusA. The λ N protein may reverse the effects of NusA on pausing and termination by causing NusA to interact with nut-site RNA rather than the RNA near the 3′ end of the nascent transcript.
Results
NusA binds to RNA in vitro only in the presence of the full-length RNA-polymerase α subunit or a fragment of α containing its carboxy-terminal domain
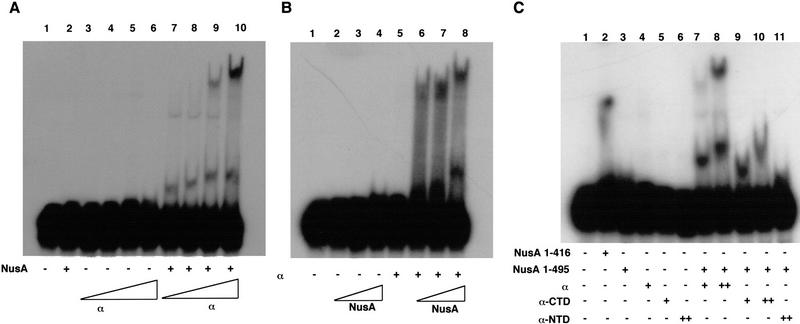
In view of the known involvement of the α subunit of RNA polymerase in NusA function (Liu et al. 1996), we tested whether α could promote RNA binding by NusA in gel mobility shift assays containing 32P-labeled RNA with a wild-type λ nut site (Fig. 1). The addition of increasing amounts of α to a constant amount of NusA resulted in the appearance of two bands, and sometimes a weak third band, with lower mobility than the free RNA (Fig. 1A, cf. lane 1 with lanes 7–10). These bands were absent in lanes containing either NusA alone (lane 2) or the full concentration range of α alone (lanes 3–6), indicating that formation of a complex on the RNA required both proteins. In the converse experiment, in which the concentration of α was held constant and that of NusA was varied (Fig. 1B), NusA alone was not able to bind nut-site-containing RNA, even at very high concentrations (lanes 2–4), but complexes of lower mobility appeared and increased in intensity as the concentration of NusA was increased in the presence of α (lanes 6–8). In view of the known ability of α to dimerize, primarily via its amino-terminal domain (Blatter et al. 1994; Kimura et al. 1994), these complexes may represent different combinations of α and NusA. The apparent Kd of the interaction of NusA and α with the RNA is at least 100 μM because only a small fraction (2%–5%) of the RNA is bound when the concentrations of NusA and α are about 10 μM. It is possible that the gel mobility shift assay is overestimating the Kd if not all the molecules of NusA and α are active. However, such weak binding may not be surprising because NusA and α are both bound to RNA polymerase and therefore are both in the vicinity of each other and the nascent RNA during transcription.
Figure 1.
Binding of NusA to RNA in the presence of the RNA polymerase α subunit. (A) Addition of increasing amounts of α to a constant amount of NusA results in an increase in complex formation. Reactions containing 32P-labeled nut-site RNA and various combinations of 14 μM NusA and 1.25, 2.5, 5, or 10 μM α (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film. (B) Addition of increasing amounts of NusA to a constant amount of α results in increased complex formation. Reactions containing 32P-labeled nut-site RNA and various combinations of 9 μM α and 3.5, 7, or 14 μM NusA (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film. (C) α-CTD stimulates RNA binding by NusA. Reactions containing 32P-labeled nut-site RNA and various combinations of 13 μM NusA or NusA (amino acids 1–416), 4.5 or 9 μM α, 4.5 or 9 μM α-CTD or 11 μM α-NTD (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film.
Since the CTD of the RNA-polymerase α subunit is known to be important for NusA activity in pausing and termination (Liu et al. 1996), we tested whether the α-CTD (amino acids 249–329) or α-NTD (amino acids 1–235) alone could promote RNA binding by NusA (Fig. 1C). We added full-length α, α-CTD, or α-NTD to gel mobility shift reactions with NusA and 32P-labeled nut-site-containing RNA. Neither the α subunit nor its isolated amino- and carboxy-terminal domains alone could retard the mobility of the RNA (lanes 3–6), unlike the carboxy-terminally truncated NusA molecule containing amino acids 1–416, which is capable of direct binding to nut-site-containing RNA (lane 2; also see below). The addition of either intact α (lanes 7 and 8) or the α-CTD (lanes 9 and 10) to intact NusA (amino acids 1–495) caused shifts in the mobility of the RNA. The complex formed with the α-CTD and NusA (lanes 9 and 10) was similar, but not identical, in mobility to the more rapidly migrating complex obtained when full-length α was incubated with NusA (lanes 7 and 8). Since the α-CTD lacks the principal dimerization domain of α, which is located in the α-NTD (Blatter et al. 1994; Kimura et al. 1994), the complex obtained with NusA and the α-CTD is likely to contain only one molecule each of the α-CTD and NusA. In fact, we have reason to believe that the weak dimerization activity of the α-CTD is abolished by the presence of NusA (see below). It is also possible, though unlikely, that NusA causes the α-CTD to bind RNA, leading to the formation of a complex that contains the α-CTD and RNA but no NusA. The additional lower-mobility complex in the reactions containing α and NusA (lanes 7 and 8) is likely to contain two molecules of, α and one or more molecules of NusA as a consequence of dimerization of the α subunits. In contrast, no distinct shift was obtained with NusA and the α-NTD (lane 11). These results suggest that the CTD of the RNA-polymerase α subunit is capable of stimulating RNA binding by NusA.
RNA binding by NusA in the presence of α is sequence-specific and sensitive to a mutation in the S1 homology region of NusA
To evaluate potential RNA sequence or structure specificity in the RNA binding observed with NusA and α, we compared the abilities of RNAs containing either a wild-type nut site or a nut site in which the sequence of the boxA element had been switched from 5′–3′ to 3′–5′ (boxA reverse; Mogridge et al. 1995) to support formation of NusA–α–nut-site complexes (Fig. 2A). Whereas N only requires the boxB RNA element for binding, NusA is unable to supershift an N–nut-site complex when boxA is reversed (Mogridge et al. 1995). This suggests that there is a direct and specific interaction between boxA and an RNA-binding domain in NusA. As shown in Figure 2A, the low-mobility complexes formed with the wild-type probe, NusA, and α were not present in reactions when the reverse nut-site probe was used (cf. lane 4 with lane 8). Thus, the NusA binding promoted by α in these gel mobility shift experiments has structure- or sequence-specificity. Moreover, the importance of the boxA element for RNA binding provided additional evidence that the RNA binding observed in experiments containing NusA and α involves NusA.
Figure 2.
RNA binding by NusA in the presence of α is sequence-specific and sensitive to a mutation in the S1 domain of NusA. (A) RNA binding by NusA in the presence of α is prevented by a mutation in the boxA portion of the nut site. Reactions containing wild-type or mutant 32P-labeled nut-site RNA (as indicated) and various combinations of 10 μM α and 14 μM NusA (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film. (B) RNA binding by NusA in the presence of α is prevented by a mutation in the S1 domain of NusA. Reactions containing 32P-labeled nut-site RNA and various combinations of 14 μM NusA, 14 μM NusA (amino acids 1–416), or 12 μM NusA R199A and 11 μM α (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film.
The nusA R199A mutation causes a defect in antitermination by N in vivo, as well as a slight defect in the ability of NusA to supershift an N–nut-site complex, even though the NusA R199A mutant protein binds with normal affinity to N (T. Mah, Y. Zhou, N. Yu, J. Mogridge, E. Olsen, J. Greenblatt, and D. Friedman, unpubl.). Therefore, this mutation in the S1 homology region of NusA appears to cause a defect in the binding of NusA to nut-site RNA. As shown in Figure 2B, the nusA R199A mutation also prevented NusA from binding the nut-site RNA in the presence of α (cf. lanes 6 and 7). This result indicates that the S1 homology region of NusA is likely to participate in nut-site binding stimulated by α and provided further evidence that RNA binding observed in the presence of α and NusA reflects direct RNA binding by NusA.
Interaction of α with the carboxy-terminal region of NusA via modified helix–hairpin–helix motifs
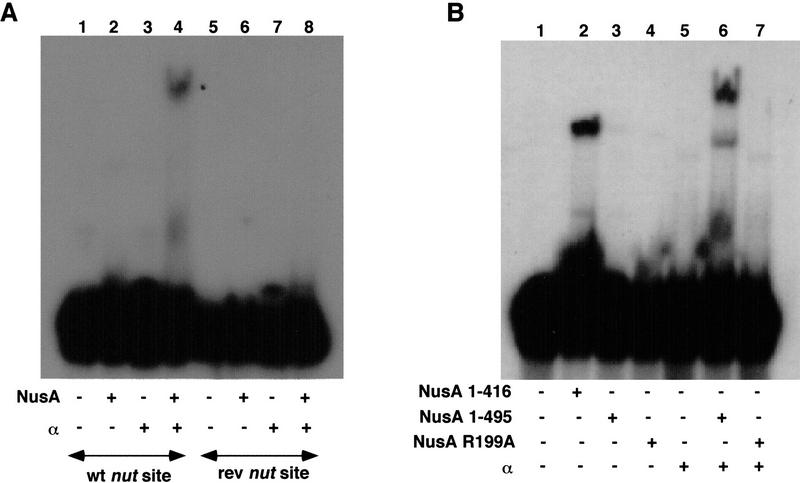
Our observation that NusA could bind nut-site RNA in the presence of α, but not in its absence, suggested that there may be a direct interaction between NusA and α. To test for such a direct interaction between α and portions of NusA, a mixture of full-length NusA and three carboxy-terminally deleted mutant proteins (Fig. 3A, lane 2) was passed over columns containing various concentrations of covalently bound α. Specific binding to α, over and above the non-specific binding to the column matrix (lane 3), was observed only for the full-length NusA protein, whose binding increased in concert with the α concentration on the column (lanes 4–6). Since NusA (amino acids 1–416) did not bind to α, the 79 carboxy-terminal amino acids of NusA are necessary for the binding of α to NusA. To further establish which regions of NusA are sufficient for interaction with α, purified α was tested for binding to various covalently immobilized portions of NusA: an amino-terminal region, NusA (amino acids 1–137), that we have shown elsewhere to bind RNA polymerase (Mah et al. 1999); a carboxy-terminal fragment, NusA (amino acids 303–495); and the full-length protein (Fig. 3B). α did not bind to the amino-terminal fragment of NusA (lane 4), although binding to the carboxy-terminal fragment of NusA was observed (lane 5), and the binding of α was best with full-length NusA (lane 6). It appears, therefore, that α interacts primarily with the carboxy-terminal region of NusA.
Figure 3.
Interaction of α with the carboxy-terminal region of NusA. (A) Carboxy-terminal truncation of NusA prevents the α–NusA interaction. A mixture of four His6-tagged NusA proteins (lane 2) was passed over columns containing affigel (lane 3) or increasing amounts of affigel-coupled α (lanes 4–6). Bound proteins were eluted with buffer containing 1M NaCl, subjected to SDS-PAGE, and stained with silver. (B) The carboxy-terminal region of NusA interacts directly with α. Buffer containing α and 0.2 mg/ml insulin (lane 2) was passed over columns containing affigel (lane 3) or affigel-coupled NusA (2 mg/ml) (lane 6) or affigel-coupled regions of NusA (lanes 4 and 5) (as indicated). The concentrations of amino- and carboxy-terminal regions of NusA on the columns were adjusted so that each had the same molar concentration as the full-length NusA. Bound protein was eluted with buffer containing 1 M NaCl, subjected to SDS-PAGE, and stained with silver. (C) α-CTD interacts with the 192 carboxy-terminal amino acids of NusA. A mixture of α, α-CTD, and α-NTD (lane 2) was passed over columns containing GST (lane 3) or increasing amounts of GST–NusA (amino acids 303–495) (lanes 4–6). As a control, buffer alone was passed over a GST–NusA (amino acids 303–495) column (lane 7). Bound proteins were eluted with buffer containing 1 M NaCl, subjected to SDS-PAGE, and stained with silver. * indicates a degradation product of α, as identified by mass spectrometry. (D) Helix–hairpin–helix motifs in the carboxy-terminal domains of bacterial RNA polymerase α subunits and NusA proteins. Identifiers in SWISSPROT and PDB databases are shown where available. Numbers indicate the distance, in amino acid residues, from the amino terminus of the protein. Roman numerals indicate the repeated motifs in the same protein. Residues conserved in many families of HhH proteins are indicated by bold type. Within the consensus line, certain categories are indicated: bulky hydrophobic residues (F, I, L, M, V, W, and Y; U in the consensus line), small side chains (A, G, and S; O in the consensus line), negatively charged residues (D and E; = in the consensus line), and positively charged residues (K and R; outlined letters in the alignment). Residues that may participate in charge–charge interactions are boxed. Underlined letters in the E. coli AlkA mismatch repair glycosylase sequence indicate the amino acids whose side chains make contacts with the phosphate residues in the DNA backbone, either directly or by coordinating a metal ion. The known elements of secondary structure are indicated by H for helix and h for hairpin.
Since the α-CTD, but not the α-NTD, allowed RNA binding by NusA (Fig. 1), we also tested which region of α was involved in the direct binding of α to NusA (Fig. 3C). Full-length α, α-NTD, and α-CTD were mixed together (lane 2) and loaded onto columns containing GST (lane 3) or various concentrations of GST–NusA (amino acids 303–495) (lanes 4–6). The bound proteins were eluted with salt. None of the α fragments were retained on the control GST column. In contrast, as the concentration of immobilized GST–NusA (amino acids 303–495) on the columns was increased, increasing amounts of full-length α and α-CTD were present in the salt eluates from this matrix. In contrast, the binding of α-NTD to the immobilized GST–NusA (amino acids 303–495) was barely detectable (lanes 4–6). Therefore, the result of this direct protein–protein-binding study was consistent with the results of the gel mobility shift experiments, which indicated that only full-length α and the α-CTD would interact with NusA. Because the α-CTD bound less tightly to NusA than did intact α, the weak binding of the α-NTD to NusA may indicate that the α-NTD makes a small contribution to the α–NusA interaction.
Sequence analysis of the regions of NusA located carboxy-terminal to the KH domains (Gibson et al. 1993a,b) revealed heterogeneity of this region among bacteria and archaea. Many bacteria and all archaea lack the carboxy-terminal domain corresponding to the last 150 residues of E. coli NusA, including the 79-residue autoinhibitory sequence identified in this study. However, this segment is conserved in representatives of several divisions of Proteobacteria, such as α-Proteobacteria (Rickettsia prowazekii), β-Proteobacteria (Neisseria meningitides), and γ-Proteobacteria (E. coli and others), as well as in two distant lineages of bacteria, namely, in Chlamydia and Treponema.
Using the PSI-BLAST program (Altschul et al. 1997), we detected sequence similarities among this carboxy-terminal region of E. coli NusA, the amino-terminal domains of the eukaryotic/archaeal recombinases Rad51/RadA, and the α-CTD sequences from all completely sequenced bacteria. For example, when the carboxy-terminal segment of NusA from Chlamydophila pneumoniae was used as a query, the first Rad51-like sequence was detected at the second iteration with the probability of a random match, p = 2 × 10−3, and the first α-CTD sequence was detected at the third iteration with p =1 × 10−4. When a homologous domain from E. coli was used to scan the database, it matched the Rad51 sequence with a p value of 2 × 10−4. If α-CTD sequences were used as queries, the NusA regions were retrieved, typically interspersed with the Rad51 amino termini and the helix–hairpin–helix (HhH) motifs from bacterial NAD+-dependent DNA ligases. Searches initiated with Rad51 protein sequences also retrieved ligases, NusA proteins, and the α-CTDs, followed by other HhH proteins. Sequence similarities among NusA, the Rad51 amino-terminal domains, and the HhH motifs in DNA ligases have been mentioned recently (Aravind et al. 1999), and matches to the α-CTD of Thermus and Synechocystis have been automatically detected using the Hidden Markov Model of aligned HhH proteins (http://smart.embl-heidelberg.de/; Schultz et al. 2000).
Multiple sequence alignment of the NusA proteins, the α-CTDs, and HhH motifs (Fig. 3D) revealed two copies of an HhH-like motif in NusA proteins from the three above-mentioned divisions of Proteobacteria and in Chlamydia. Treponema appears to have lost one of the two copies owing to sequence drift, whereas in another spirochaete, Borrelia, both copies are disrupted (Fig. 3D; data not shown). Bacterial and chloroplast α subunits have one copy of the HhH motif, and its sequence is significantly deviated in most chloroplast proteins.
Superposition of the alignment and the known three-dimensional structures of the various HhH proteins and the α-CTD of E. coli indicates that the most conserved region corresponds to the two helices and the folded hairpin loop between them, with negatively charged residues frequently found in the first helix, small side-chain residues apparently required for folding of the hairpin, and a charged patch of varying polarity always found at the beginning of the second helix (Fig. 3D). The presence of the HhH motifs in DNA-binding and RNA-binding proteins has raised the suggestion that these motifs might mediate nucleic acid–protein interactions (Doherty et al. 1996), and direct involvement of the residues in the hairpin of the DNA glycosylase AlkA in hydrogen bonding with the phosphate backbone of DNA has been demonstrated (Hollis et al. 2000). Intriguingly, neither the α-CTD nor NusA seems to utilize its HhH motif for nucleic acid binding; instead, the second HhH motif in NusA may prevent other parts of NusA from binding to RNA, and the HhH motif in the α-CTD may facilitate α-CTD–α-CTD homodimer and α-CTD–NusA heterodimer formation.
A carboxy-terminally truncated NusA also binds specifically to nut-site RNA
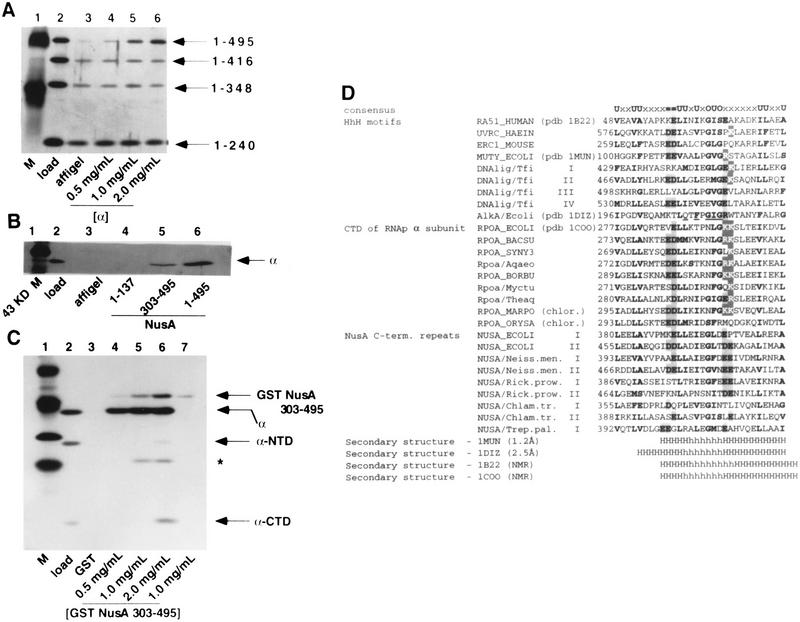
Our observations that α could provoke RNA binding by NusA and interact with NusA (amino acids 1–495), but not with NusA (amino acids 1–416), was consistent with the possibility that the 79 carboxy-terminal amino acids of NusA might inhibit the RNA-binding activity of NusA. As shown in Figure 4A, NusA (amino acids 1–416), unlike full-length NusA, could bind RNA containing a wild-type nut site in a gel mobility shift experiment (lanes 2–7), but only weakly to RNA containing a nut site with a reversed boxA sequence (Fig. 4A, lanes 12–14). This indicates that the binding of NusA to nut-site RNA is indeed inhibited by the 79 carboxy-terminal amino acids of NusA and suggests that this inhibition could be relieved by an interaction of this portion of NusA with the CTD of the RNA polymerase α subunit.
Figure 4.
A carboxy-terminally truncated NusA binds specifically to nut-site RNA. (A) RNA binding by NusA (amino acids 1–416) is prevented by a mutation in the boxA portion of the nut site. Reactions containing wild-type or mutant 32P-labeled nut-site RNA (as indicated) and 3.5, 7, or 14 μM NusA or NusA (amino acids 1–416) (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film. (B) RNA binding by NusA (amino acids 1–416) is stabilized by amino acids 1–137 and 348–415 of NusA. Reactions containing 32P-labeled nut-site RNA and 10 μM NusA (amino acids 132–416), NusA (amino acids 132–495), NusA (amino acids 1–348), NusA (amino acids 1–416) or NusA (as indicated) were electrophoresed on 7.5% nondenaturing gels, dried, and exposed to film.
In order to further characterize this α-independent RNA binding by NusA (amino acids 1–416), other deletion mutants of NusA were tested for their ability to bind RNA containing a wild-type nut site in a gel mobility shift experiment (Fig. 4B). Carboxy-terminal truncation of NusA to amino acid 348 weakened RNA binding, even though NusA (amino acids 1–348) retains the S1 and KH homology regions (lane 4). The deletion of NusA's amino-terminal RNA-polymerase-binding region (Mah et al. 1999), as in NusA (amino acids 132–416), also prevented RNA binding by NusA (lane 2). Since two disruptions of NusA (amino acids 1–416) that do not delete its S1 and KH RNA-binding domains interfere with RNA binding, the use of these deletions alone did not allow us to assign any localized RNA-binding region. Because NusA (amino acids 132–416) and NusA (amino acids 1–348) appear to be folded proteins (Mah et al. 1999), perhaps the amino-terminal RNA-polymerase-binding region in amino acids 1–137 of NusA, as well as amino acids 348–415 of NusA, may be required to stabilize the RNA-binding ability of NusA (amino acids 1–416).
The λ N protein binds the carboxy-terminal region of NusA
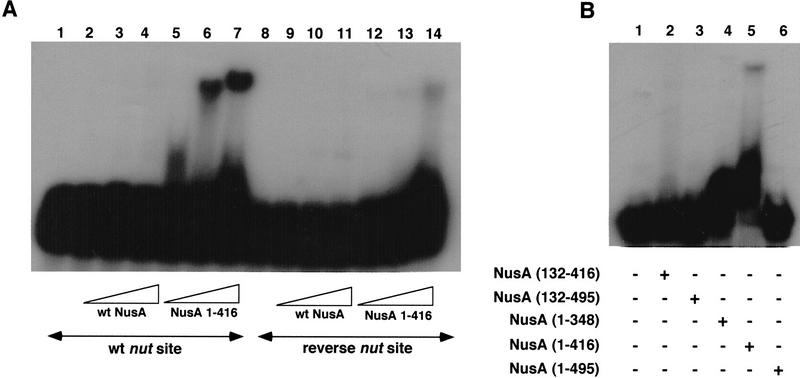
The N protein of the bacteriophage λ also binds NusA (Greenblatt and Li 1981b), and we have shown that this binding requires carboxy-terminal amino acids of NusA (Mah et al. 1999). In view of the possibility that α and N might act in similar ways to provoke RNA binding by NusA, we tested various fragments of NusA for binding to N. Full-length NusA, a carboxy-terminally truncated NusA, NusA (amino acids 1–399), and a carboxy-terminal fragment of NusA, NusA (amino acids 303–495), were mixed with E. coli extracts and passed over GST and GST–N affinity columns (Fig. 5). None of the major E. coli proteins present in the extracts applied to the columns bound to the GST or GST–N columns (lanes 2, 3, 5, 6, 8, and 9). Both NusA and NusA (303–495) bound selectively to GST–N and therefore were present in the high salt eluates from the GST–N columns, but not the GST control columns (lanes 2, 3, 8, and 9). NusA (1–399) was absent from the high salt eluates of both columns (lanes 5 and 6). Thus, N binds directly to the carboxy-terminal region of NusA. This result suggested that N might activate the RNA-binding ability of NusA by binding to the same region of NusA that we have shown binds α.
Figure 5.
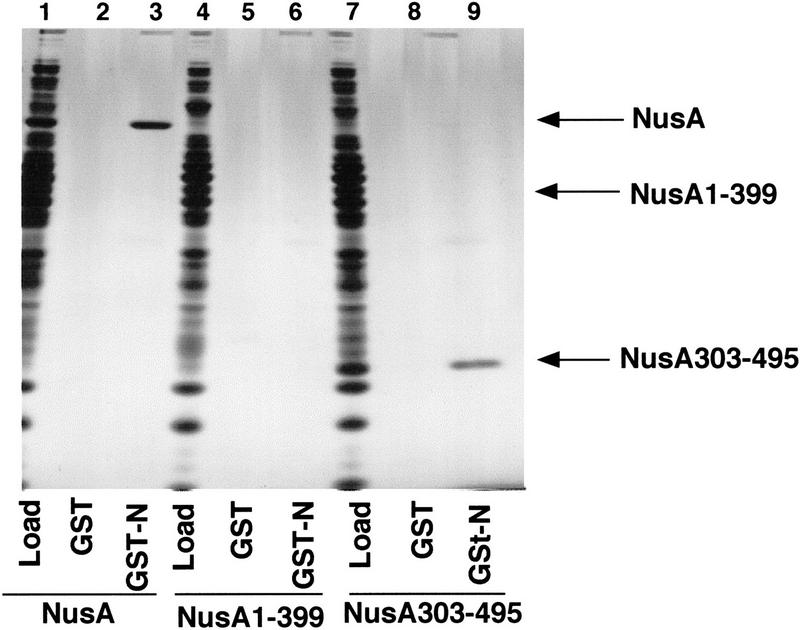
The λ N protein binds directly to the 192 carboxy-terminal amino acids of NusA. E. coli extract containing additional NusA (lane 1), NusA (amino acids 1–399) (lane 4), or NusA (amino acids 303–495) (lane 7) was passed over columns containing 2 mg/ml GST (lanes 2, 5, and 8) or 0.5 mg/ml GST–N (lanes 3, 6, and 9). Bound proteins were eluted with buffer containing 1M NaCl, subjected to SDS-PAGE, and stained with silver.
The interaction between NusA and α is not necessary for NusA to stimulate termination if NusA lacks its carboxy-terminal autoinhibitory domain
The interaction between NusA and α may be important for NusA function in termination (Liu et al. 1996) because it allows NusA to interact with RNA. We have previously shown that a NusA fragment lacking the carboxy-terminal inhibitory domain retains wild-type function in termination and antitermination assays in vitro (Mah et al. 1999). A similarly truncated form of NusA supports the growth of E. coli and transcriptional antitermination by N in vivo (Tsugawa et al. 1988). If the interaction between NusA and α is necessary for full-length NusA to interact with RNA and stimulate termination, then a carboxy-terminally truncated NusA protein would still be functional in transcription termination assays performed with a mutant RNA polymerase lacking the α-CTD, even though full-length NusA would not be functional in these conditions. To test this idea, in vitro transcription assays were performed on a template containing the T7 A1 promoter and the λ tR2 terminator (Fig. 6). In reactions with wild-type RNA polymerase, both the wild-type NusA (1–495) and the carboxy-terminally truncated NusA (1–348) decreased the terminator read-through of the tR2 terminator from 39% to 11% and 15%, respectively (lanes 5–7). In contrast, whereas reactions with RNA polymerase lacking the α-CTD yielded a lower level of terminator read-through (29%) compared to the level that was obtained with wild-type RNA polymerase (39%), only NusA (1–348) but not wild-type NusA decreased the amount of terminator read-through in this case (lanes 2–4). These results are consistent with the idea that the interaction of the α-CTD with NusA is necessary for full-length NusA to stimulate termination because the interaction allows full-length NusA to bind the nascent RNA.
Figure 6.
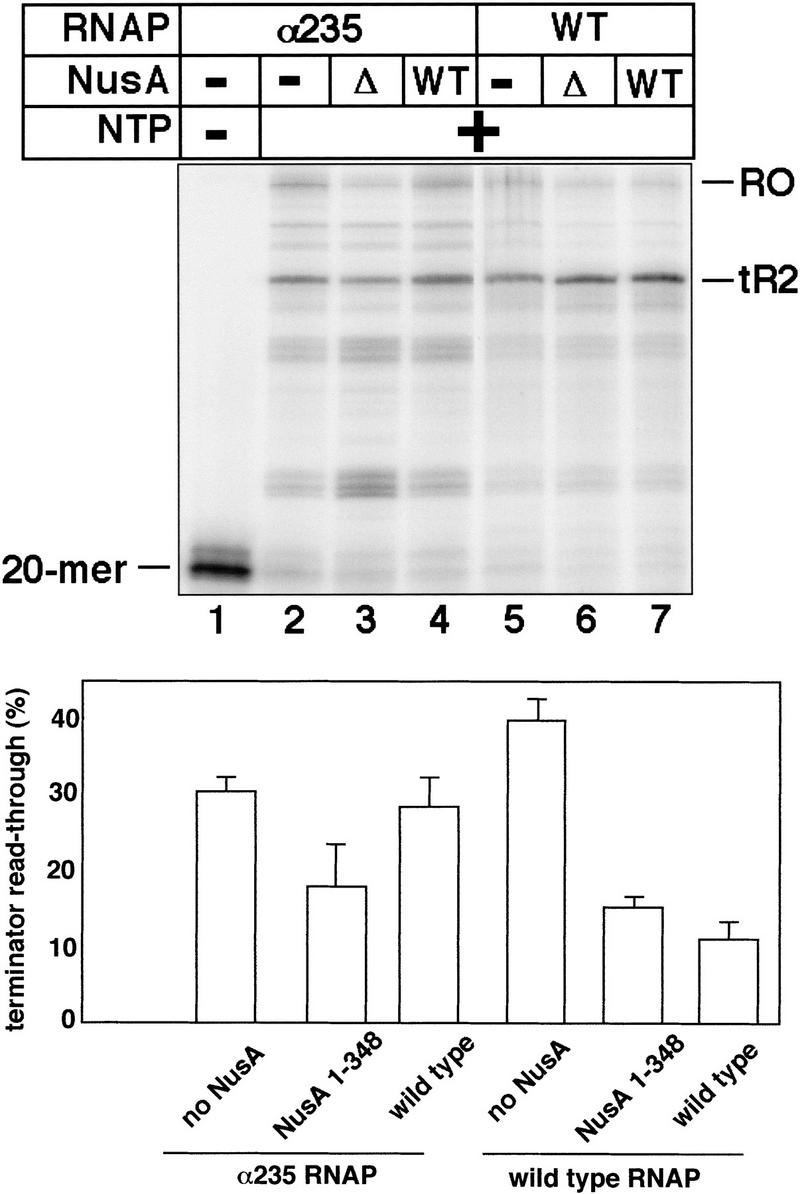
The carboxy-terminal region of NusA is not necessary for enhancement of termination by NusA if a mutant RNA polymerase lacking the α-CTD is used. In vitro transcription reactions with wild-type RNA polymerase or a mutant RNA polymerase lacking the carboxy-terminal domain of the α subunit were incubated with either no NusA, NusA 1–348 (Δ), or NusA 1–495 (WT). The bar graph is based on the average values from 4 different experiments, only one of which is shown here.
Discussion
An autoinhibition domain may inhibit RNA binding by NusA
Whereas full-length NusA does not bind RNA, we have shown here that a carboxy-terminal deletion mutant, NusA (1–416), which retains the S1 and KH homology regions of NusA but only one of its two HhH motifs, can bind RNA in the absence of α or N. This suggests that one or more of the RNA-binding domains of NusA may be occluded by the second HhH motif or other determinants within the 79 carboxy-terminal amino acids of NusA, as diagramed in the model shown in Figure 7A. Since neither NusA (1–348) nor NusA (132–416) binds RNA as strongly as NusA (1–416), amino acids 1–131 and 349–416 must also alter the folding, alignment, or accessibility of the RNA-binding domains (Fig. 7A), and it is possible that the first HhH motif in amino acids 381–410 of NusA contributes to RNA binding. Interaction of carboxy-terminally truncated NusA (1–416) with RNA is sensitive to alteration of the boxA portion of the nut site (Fig. 4A), as is also the case when full-length NusA binds the nut-site RNA in the presence of N (Mogridge et al. 1995) or α (Fig. 2A). RNA binding by NusA (1–416) is also inhibited when alterations in the loop of boxB prevent boxB from forming a GNRA tetralooplike structure (Legault et al. 1998; T. Mah, and J. Greenblatt, unpubl.). The inability of full-length NusA to bind RNA resembles the inability of the intact initiation subunit ς70 of RNA polymerase to bind DNA unless it is part of the RNA polymerase holoenzyme (Dombroski et al. 1992, 1993). Just as deletion of the carboxy-terminal HhH motif of NusA enables NusA to bind RNA in the absence of RNA polymerase, deletion of the 130 amino-terminal amino acids of ς70 enables ς70 to interact specifically and nonspecifically with DNA in the absence of the other RNA polymerase subunits (Dombroski et al. 1992). It is postulated that this autoinhibition of DNA binding by ς70 is relieved when ς70 interacts with the core polymerase subunits and undergoes a conformational change that uncovers or reorients its DNA-binding domains (Dombroski et al. 1992, 1993; Malhotra et al. 1996). It is intriguing that two prokaryotic proteins that compete for binding to the RNA polymerase core enzyme (Greenblatt and Li 1981a) may use similar mechanisms to control their nucleic acid-binding ability.
Figure 7.
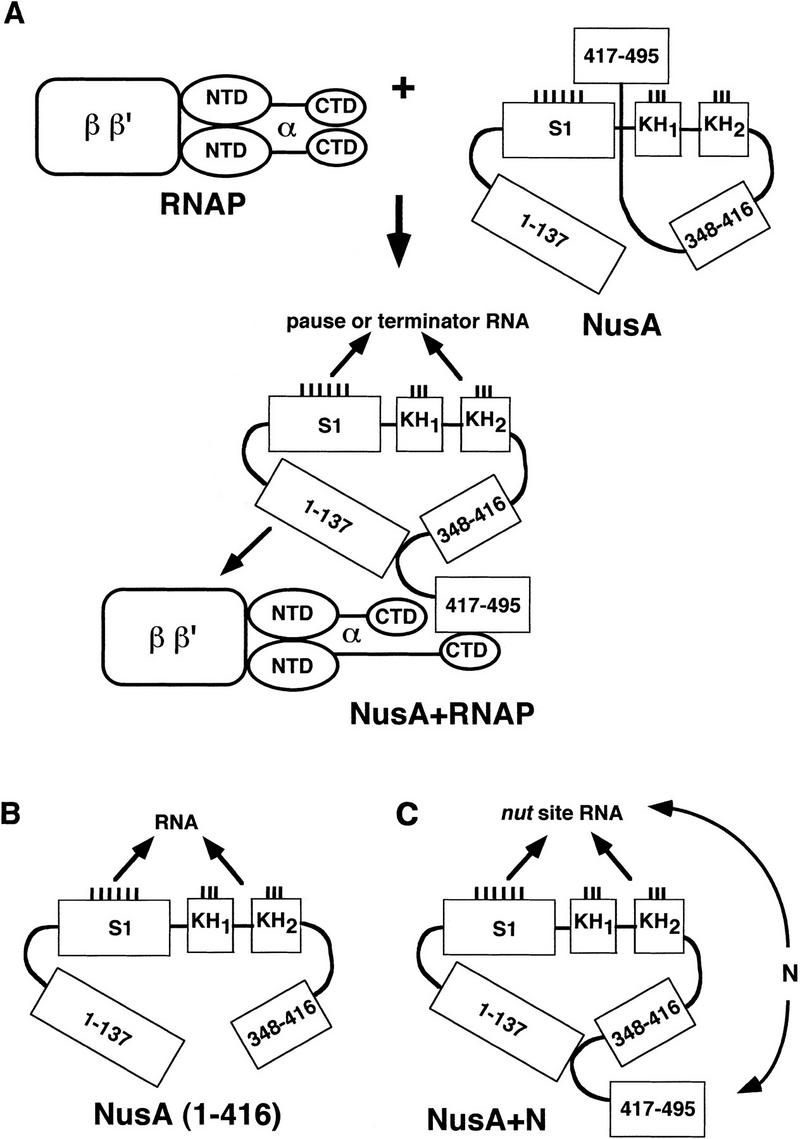
Model for NusA function in elongation, termination, and antitermination. See text for details.
Interaction of NusA with α: relationship to RNA binding and transcription termination
Our observation that the α subunit of RNA polymerase stimulates RNA binding by NusA is consistent with previous studies showing that NusA can be cross-linked to the nascent transcript in transcription complexes (Liu and Hanna 1995). We propose that this effect of α on RNA binding by NusA is the consequence of a direct interaction between the two proteins, as documented in our affinity chromatography experiments and illustrated in Figure 7A. The binding of NusA to α and stimulation of RNA binding by α appear to be mediated mainly by the carboxy-terminal domain of α (Figs. 1 and 3C). The α-CTD was implicated previously in the ability of NusA to stimulate pausing and termination by RNA polymerase (Liu et al. 1996). The interaction between NusA and α is probably mediated by a region of homology between these two proteins, consisting of one or two copies of the helix–hairpin–helix motif per protein. This region is most likely also involved in α-CTD dimer formation, and we propose that the homologous region in NusA interacts with one of the α monomers to disrupt α-dimer formation (Fig. 7A). In the NAD+-dependent DNA ligase from Thermus filiformis, four copies of the HhH motif form a compact cluster with all hairpins pointing in one direction; this cluster is apparently stabilized through the interactions between side chains from different helices (Lee et al. 2000). Given that the interaction between NusA and the α-CTD is likely to involve their HhH-like motifs, one can speculate that similar helix bundling may occur when these motifs are found on separate molecules. A preponderance of positively charged residues at the amino termini of the longer helices in the α-CTD and frequent occurrence of negative charges in the equivalent positions of the NusA HhH motifs provoke the speculation that these amino acids might be required for the interaction between NusA and the α-CTD.
RNA binding either by truncated NusA or by full-length NusA in the presence of α is weak. Nevertheless, the dependence of RNA binding by truncated or full-length NusA in the presence of α on the boxA sequence in the nut site is similar to the effect of boxA on RNA binding by NusA in the presence of N (Mogridge et al. 1995). Our ability to abrogate RNA binding by deleting the α-CTD or portions of NusA also suggests that RNA binding genuinely involves both α and NusA.
From the data presented here, we hypothesize that full-length NusA is prevented from interacting with RNA by an autoinhibition mediated by its 79 carboxy-terminal amino acids (cf. Fig. 7A with Fig. 7B). We suggest that during elongation, NusA uses its RNA-polymerase-binding region in amino acids 1–137 (Mah et al. 1999) to interact with RNA polymerase subunits β and β′, and its carboxy-terminal region to interact with α (Fig. 7A). The interaction with α may then cause a conformational change in NusA such that its RNA-binding domains either fold or become exposed and competent to bind the nascent RNA. Thus, as part of the transcription complex, NusA would be in a position to bind and stabilize pause and termination motifs in the nascent RNA, leading to enhancement of pausing and termination at certain sites. We suggest that the interaction of the α-CTD with NusA is essential for NusA to stimulate termination only if the inhibitory carboxy-terminal region of NusA is present and not if it is deleted. In other experiments we have shown that NusA (1–416) is able to stimulate termination (Mah et al. 1999). This fragment of NusA cannot interact with α, but it lacks the inhibitory region and can bind RNA on its own. In many bacteria (e.g., the Gram-positive, blue-green, and ɛ-Proteobacteria), the NusA proteins lack the carboxy-terminal HhH region. In these organisms, NusA may be able to bind RNA and stimulate pausing and termination in the absence of any direct interaction with α. E. coli strains that lack the carboxy-terminal domains of NusA are temperature sensitive (Tsugawa et al. 1988).
The interaction between the amino-terminal RNA polymerase-binding region of NusA (amino acids 1–137) and RNA polymerase is essential for function because the loss of the amino-terminal RNA polymerase-binding region results in the inability of NusA to participate in termination and antitermination (Mah et al. 1999). Since we have shown that α alone does not bind the amino-terminal region of NusA, and since there is cross-linking data (J. Li and J. Greenblatt, unpubl.) and other evidence (Liu et al. 1996) to suggest that β and β′ interact directly with NusA, it is likely that one or both of the two large RNA polymerase subunits contact the amino-terminal RNA-polymerase-binding region of NusA (Fig. 7A). Nevertheless, a weak but important interaction between α and this region of NusA cannot be ruled out.
Transcriptional antitermination by N
We also showed that N interacts with this same carboxy-terminal autoinhibitory region of NusA. Therefore, we propose that N activates the RNA-binding activity of NusA in a manner similar to that of α, as modeled in Figure 7C. In this scheme, the close proximity of NusA to both the nut site and N could result in exclusive binding of NusA to the nut-site RNA. Such an interaction might then serve two purposes: first, the interaction of NusA with nut-site RNA would prevent pause and termination sequences in the nascent RNA from binding to NusA, and this would prevent NusA from enhancing pausing and termination; second, the NusA–nut-site interaction, together with interactions involving other Nus factors, N, RNA polymerase, and the nut-site RNA, would increase the overall stability of the antitermination complex containing N.
Ordinary elongation complexes assembled in vitro contain one molecule of NusA, whereas there is evidence that elongation complexes containing the λ N protein contain two molecules of NusA (Horwitz et al. 1987). Therefore N-modified elongation complexes may have one molecule of NusA interacting with N and the other with α, or, alternatively, each of the two NusA molecules may interact with one of the two α molecules in RNA polymerase.
Friedman and colleagues have shown that a point mutation in the α-CTD, as well as deletion of the entire α-CTD, enhances antitermination by N in vivo (Schauer et al. 1996). Furthermore, even though loss of the α-CTD prevents NusA from stimulating termination in vitro, it has no effect on the ability of NusA to stimulate antitermination mediated by the N protein in vitro (Liu et al. 1996). These data suggest that there may be a competition between α and N for binding to the carboxy-terminal region of NusA. Interaction of α with NusA may direct NusA to the nascent transcript near its 3′ end and facilitate pausing and termination, whereas interaction of N with NusA would block the interaction between NusA and α, direct NusA to the nut-site RNA, and prevent NusA from stimulating pausing and termination. If this model is correct, it would resolve the long-standing paradox that the same molecule, NusA, can participate in both termination (Greenblatt et al. 1981; Grayhack and Roberts 1982; Schmidt and Chamberlin 1987; Whalen et al. 1988) and antitermination (Friedman 1971; Das and Wolska 1984; Horwitz et al. 1987) of transcription.
Materials and methods
Plasmids, strains, and reagents
RNA polymerase, NusA, GST–NusA proteins, GST–N, His6-tagged NusA proteins, α, and α235 were purified as previously described (Burgess and Jendrisak 1975; Greenblatt and Li 1981b; Mogridge et al. 1998; Mah et al. 1999). Purified α-CTD was provided by G. Zhang and S. Darst. NusA R199A was provided by Ying Zhou and David Friedman. Purification for this protein is described elsewhere (T. Mah, Y. Zhou, N. Yu, J. Mogridge, E. Olsen, J. Greenblatt, and D. Friedman, unpubl.).
The oligonucleotides used for cloning were purchased from ACGT Corp. (Toronto). RNAguard was bought from Pharmacia Biotech. Restriction enzymes and DNA ligase were purchased from New England Biolabs. T7 RNA polymerase was obtained from Life Technologies.
Construction of GST–NusA proteins
PCR primers were designed to amplify fragments of NusA from the plasmid J1150. Forward and reverse primers contained BamHI and EcoRI restriction sites, respectively, for subsequent cloning into the vector pGEX-2T (Pharmacia).
Purification of GST–NusA proteins
The E. coli strain DH5α (Life Technologies) containing the GST–NusA fusion plasmid was grown in 1 liter of LB medium to an A600 of 0.5 and induced for 3 h with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside. Cells were harvested by centrifugation, resuspended, and sonicated in 10 ml of 1 M NaCl Buffer A (20 mM Tris-HCl, pH 7.8, 0.2 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and then centrifuged for 20 min at 12,000 rpm in a Sorval SS34 rotor. Glutathione-sepharose 4B beads (1 ml; Pharmacia) were added to the supernatant, and this slurry was rotated for 1 h at 4°C. The beads were washed and resuspended in 500 μl thrombin cleavage buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 2.5 mM CaCl2). Thrombin was added to the slurry and incubated at room temperature for 1 h. After centrifugation at 3000 rpm in a tabletop Eppendorf microfuge, the supernatant was collected and dialyzed into 0.1 M NaCl ACB (10 mM HEPES pH 7.0, 10% glycerol, 0.1 mM EDTA, 1 mM DTT).
Affinity chromatography
Experiments with GST, GST–N, and GST–NusA (amino acids 303–495) were performed as previously described (Mah et al. 1999), except that the GST and GST–N columns were loaded with E. coli extract containing either NusA or NusA fragments cleaved from GST with thrombin, and the GST and GST–NusA (amino acids 303–495) columns were loaded with a mixture of α, α-NTD, and α-CTD, buffer, and 0.2 mg/ml insulin.
Experiments with the α subunit of RNA polymerase were done in two ways. In the first case, α was coupled to affigel 10 matrix (BioRad) at three different concentrations. Twenty microliters of beads were added to 200-μl pipette tips that contained 10 μl of 212–300-micron glass beads (Sigma). The column bed was washed with 10 column volumes of 1 M NaCl ACB (10 mM HEPES at pH 7.0, 10% glycerol, 0.1 mM EDTA, 1 mM DTT) and then washed with 10 column volumes of 100 mM NaCl ACB. Columns were loaded with a mixture of his-tagged NusA deletion proteins as well as full-length his-tagged NusA, buffer, and 0.2 mg/ml insulin. In the second case, his-tagged NusA (amino acids 1–495), GST–NusA (amino acids 1–137), and GST–NusA (amino acids 303–495) were coupled to affigel 10 at equimolar concentrations. Twenty microliters of beads were added to 200-μl pipette tips. The columns were treated as described above except that these columns were loaded with buffer containing α and 0.2 mg/ml insulin.
Gel mobility shift experiments
Gel mobility shift assays were performed as previously described (Mogridge et al. 1995) except that the proteins were added together and incubated on ice for 20 min. Radiolabeled probe (20–50 pM) was then added, and incubation on ice continued for an additional 10 min. Concentrations of proteins are indicated in the figure legends.
Sequence analysis
The PSI-BLAST program (Altschul et al. 1997) was used for the iterative searches of sequence databases. In the first-pass search, sequences with a probability of random matching of 0.001 or lower were included in the profile, whereas at the further iterations the cutoff was set at 0.01. Multiple sequence alignments were constructed using the combination of segment pair overlap and Gibbs sampling options of the MACAW program (Schuler et al. 1991).
In vitro transcription
RNA polymerases containing histidine-tagged mutant or wild-type α were prepared by our standard in vitro reconstitution procedure (Tang et al. 1995). About 0.5 μg of RNA polymerase was used to prepare elongation complexes stalled at position 20 on a T7 A1 promoter-containing fragment fused to the tR2 terminator (template 1 from Nudler et al. 1995). Stalled, immobilized transcription complexes were washed, NusA was added to the final concentration of 100 nM, and elongation was resumed by the addition of nucleoside triphosphates to the final concentration of 100 μM. The reaction was allowed to proceed at 37°C for 5 min and terminated by the addition of urea-containing loading buffer. Reaction products were resolved by 6% denaturing PAGE, visualized by autoradiography and quantified by phosphoimagery.
Acknowledgments
The authors thank Joyce Li for constructing the strains of his-tagged deletion mutants of NusA. This work was supported by the Medical Research Council of Canada (MRC). J.G. is an International Research Scholar of the Howard Hughes Medical Institute and an MRC Distinguished Scientist. A.M. is supported in part by NIH grant GM58331. K.S. is supported in part by NIH RO1 grant GM59295.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jack.greenblatt@utoronto.ca; FAX (416) 978 8528.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.822900.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Walker DR, Koonin EV. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acid Res. 1999;27:1223–1242. doi: 10.1093/nar/27.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovich I, Landick R. Interaction of a nascent RNA structure with RNA polymerase is required for hairpin-dependent transcriptional pausing but not for transcript release. Genes & Dev. 1998;12:3110–3122. doi: 10.1101/gad.12.19.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter EE, Ross W, Tang H, Gourse RL, Ebright RH. Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- Burgess RR, Jendrisak JJ. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Bycroft M, Hubbard TJ, Proctor M, Freund SM, Murzin AG. The solution structure of the S1 RNA binding domain: A member of an ancient nucleic acid-binding fold. Cell. 1997;88:235–242. doi: 10.1016/s0092-8674(00)81844-9. [DOI] [PubMed] [Google Scholar]
- Das A, Wolska K. Transcription antitermination in vitro by λ N gene product: Requirement for a phage nut site and the products of host nusA, nusB, and nusE genes. Cell. 1984;38:165–173. doi: 10.1016/0092-8674(84)90537-3. [DOI] [PubMed] [Google Scholar]
- de Crombrugge B, Mudryj M, DiLauro R, Gottesman M. Specificity of the bacteriophage λ N gene product (pN): nut sequences are necessary and sufficient for antitermination by pN. Cell. 1979;18:1145–1151. doi: 10.1016/0092-8674(79)90227-7. [DOI] [PubMed] [Google Scholar]
- Dejgaard K, Leffers H. Characterisation of the nucleic-acid-binding activity of KH domains. Different properties of different domains. Eur J Biochem. 1996;241:425–431. doi: 10.1111/j.1432-1033.1996.00425.x. [DOI] [PubMed] [Google Scholar]
- Dodson RE, Shapiro DJ. Vigilin, a ubiquitous protein with 14 K homology domains, is the estrogen-inducible vitellogenin mRNA 3′-untranslated region-binding protein. J Biol Chem. 1997;272:12249–12252. doi: 10.1074/jbc.272.19.12249. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Serpell LC, Ponting CP. The helix–hairpin–helix DNA-binding motif: A structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski AJ, Walter WA, Record MT, Jr, Siegele DA, Gross CA. Polypeptides containing highly conserved regions of transcription initiation factor ς70 exhibit specificity of binding to promoter DNA. Cell. 1992;70:501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- Dombroski AJ, Walter WA, Gross CA. Amino-terminal amino acids modulate ς-factor DNA-binding activity. Genes & Dev. 1993;7:2446–2455. doi: 10.1101/gad.7.12a.2446. [DOI] [PubMed] [Google Scholar]
- Ebright RH, Busby S. The Escherichia coli RNA polymerase α subunit: Structure and function. Curr Opin Genet Dev. 1995;5:197–203. doi: 10.1016/0959-437x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Friedman DI. A bacterial mutant affecting λ development. In: Hershey AD, editor. The bacteriophage lambda. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1971. pp. 733–738. [Google Scholar]
- Friedman DI. Regulation of phage gene expression by termination and antitermination of transcription. In: Calendar R, editor. The bacteriophages. Vol. 2. New York: Plenum; 1988. pp. 263–319. [Google Scholar]
- Friedman DI, Baron LS. Genetic characterization of a bacterial locus involved in the activity of the N function of phage λ. Virology. 1974;58:141–148. doi: 10.1016/0042-6822(74)90149-4. [DOI] [PubMed] [Google Scholar]
- Friedman DI, Olson ER. Evidence that a nucleotide sequence, “boxA,” is involved in the action of the NusA protein. Cell. 1983;34:143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Gibson TJ, Rice PM, Thompson JD, Heringa J. KH domains within the FMR1 sequence suggest that fragile X syndrome stems from a defect in RNA metabolism. Trends Biochem Sci. 1993a;18:331–333. doi: 10.1016/0968-0004(93)90068-x. [DOI] [PubMed] [Google Scholar]
- Gibson TJ, Thompson JD, Heringa J. The KH domain occurs in a diverse set of RNA-binding proteins that include the antiterminator NusA and is probably involved in binding to nucleic acid. FEBS Lett. 1993b;324:361–366. doi: 10.1016/0014-5793(93)80152-k. [DOI] [PubMed] [Google Scholar]
- Grayhack EJ, Roberts JW. The phage λ Q gene product: Activity of a transcription antiterminator in vitro. Cell. 1982;30:637–648. doi: 10.1016/0092-8674(82)90260-4. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, Li J. The nusA gene protein of Escherichia coli. Its identification and a demonstration that it interacts with the gene N transcription anti-termination protein of bacteriophage λ. J Mol Biol. 1981a;147:11–23. doi: 10.1016/0022-2836(81)90076-0. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, Li J. Interaction of the ς factor and the nusA gene protein of E. coli with RNA polymerase in the initiation–termination cycle of transcription. Cell. 1981b;24:421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, McLimont M, Hanly S. Termination of transcription by nusA gene protein of Escherichia coli. Nature. 1981;292:215–220. doi: 10.1038/292215a0. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, Nodwell JR, Mason SW. Transcriptional antitermination. Nature. 1993;364:401–406. doi: 10.1038/364401a0. [DOI] [PubMed] [Google Scholar]
- Hollis T, Ichikawa Y, Ellenberger T. DNA bending and a flip-out mechanism for base excision by the helix–hairpin–helix DNA glycosylase, Escherichia coli AlkA. EMBO J. 2000;19:758–766. doi: 10.1093/emboj/19.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz RJ, Li J, Greenblatt J. An elongation control particle containing the N gene transcriptional antitermination protein of bacteriophage λ. Cell. 1987;51:631–641. doi: 10.1016/0092-8674(87)90132-2. [DOI] [PubMed] [Google Scholar]
- Kimura M, Fujita N, Ishihama A. Functional map of the α subunit of Escherichia coli RNA polymerase. Deletion analysis of the amino-terminal assembly domain. J Mol Biol. 1994;242:107–115. doi: 10.1006/jmbi.1994.1562. [DOI] [PubMed] [Google Scholar]
- Landick R, Yanofsky C. Isolation and structural analysis of the Escherichia coli trp leader paused transcription complex. J Mol Biol. 1987;196:363–377. doi: 10.1016/0022-2836(87)90697-8. [DOI] [PubMed] [Google Scholar]
- Lee JY, Chang C, Song HK, Moon J, Yang JK, Kim HK, Kwon ST, Suh SW. Crystal structure of NAD(+)-dependent DNA ligase: Modular architecture and functional implications. EMBO J. 2000;19:1119–1129. doi: 10.1093/emboj/19.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault P, Li J, Mogridge J, Kay LE, Greenblatt J. NMR structure of the bacteriophage λ N peptide/boxB RNA complex: Recognition of a GNRA fold by an arginine-rich motif. Cell. 1998;93:289–299. doi: 10.1016/s0092-8674(00)81579-2. [DOI] [PubMed] [Google Scholar]
- Liu K, Hanna MM. NusA contacts nascent RNA in Escherichia coli transcription complexes. J Mol Biol. 1995;247:547–558. doi: 10.1006/jmbi.1994.0161. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang Y, Severinov K, Das A, Hanna MM. Role of Escherichia coli RNA polymerase α subunit in modulation of pausing, termination and anti-termination by the transcription elongation factor NusA. EMBO J. 1996;15:150–161. [PMC free article] [PubMed] [Google Scholar]
- Mah TF, Li J, Davidson AR, Greenblatt J. Functional importance of regions of Escherichia coli elongation factor NusA that interact with RNA polymerase, the bacteriophage λ N protein and RNA. Mol Micro. 1999;34:523–537. doi: 10.1046/j.1365-2958.1999.01618.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Severinova E, Darst SA. Crystal structure of a ς70 subunit fragment from E. coli RNA polymerase. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- Mason SW, Li J, Greenblatt J. Host factor requirements for processive antitermination of transcription and suppression of pausing by the N protein of bacteriophage λ. J Biol Chem. 1992;267:19418–19426. [PubMed] [Google Scholar]
- Mogridge J, Mah TF, Greenblatt J. A protein–RNA interaction network facilitates the template-independent cooperative assembly on RNA polymerase of a stable antitermination complex containing the λ N protein. Genes & Dev. 1995;9:2831–2845. doi: 10.1101/gad.9.22.2831. [DOI] [PubMed] [Google Scholar]
- Mogridge J, Legault P, Li J, van Oene MD, Kay LE, Greenblatt J. Independent ligand-induced folding of the RNA-binding domain and two functionally distinct antitermination regions in the phage λ N protein. Mol Cell. 1998;1:265–275. doi: 10.1016/s1097-2765(00)80027-1. [DOI] [PubMed] [Google Scholar]
- Nudler E, Kashlev M, Nikiforov V, Goldfarb A. Coupling between transcription termination and RNA polymerase inchworming. Cell. 1995;81:351–357. doi: 10.1016/0092-8674(95)90388-7. [DOI] [PubMed] [Google Scholar]
- Olson ER, Flamm EL, Friedman DI. Analysis of nutR: A region of phage λ required for antitermination of transcription. Cell. 1982;31:61–70. doi: 10.1016/0092-8674(82)90405-6. [DOI] [PubMed] [Google Scholar]
- Olson ER, Tomich CS, Friedman DI. The nusA recognition site. Alteration in its sequence or position relative to upstream translation interferes with the action of the N antitermination function of phage λ. J Mol Biol. 1984;180:1053–1063. doi: 10.1016/0022-2836(84)90270-5. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Greenblatt J. Control of RNA chain elongation and termination. In: Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium. Washington, D.C.: ASM Press; 1996. pp. 822–848. [Google Scholar]
- Ringquist S, Jones T, Snyder EE, Gibson T, Boni I, Gold L. High-affinity RNA ligands to Escherichia coli ribosomes and ribosomal protein S1: Comparison of natural and unnatural binding sites. Biochemistry. 1995;34:3640–3648. doi: 10.1021/bi00011a019. [DOI] [PubMed] [Google Scholar]
- Ross W, Gosink KK, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse RL. A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- Salstrom JS, Szybalski W. Coliphage λ nutL−: A unique class of mutants defective in the site of gene N product utilization for antitermination of leftward transcription. J Mol Biol. 1978;124:195–221. doi: 10.1016/0022-2836(78)90156-0. [DOI] [PubMed] [Google Scholar]
- Schauer AT, Cheng SW, Zheng C, St. Pierre L, Alessi D, Hidayetoglu DL, Costantino N, Court DL, Friedman DI. The α subunit of RNA polymerase and transcription termination. Mol Micro. 1996;21:839–851. doi: 10.1046/j.1365-2958.1996.451409.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Chamberlin MJ. NusA protein of Escherichia coli is an efficient transcription termination factor for certain terminator sites. J Mol Biol. 1987;195:809–818. doi: 10.1016/0022-2836(87)90486-4. [DOI] [PubMed] [Google Scholar]
- Schuler GD, Altschul SF, Lipman DJ. A workbench for multiple alignment construction and analysis. Proteins. 1991;9:180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: A web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Matunis MJ, Michael WM, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian AR. Structure and functions of ribosomal protein S1. Prog Nucleic Acid Res Mol Biol. 1983;28:101–142. doi: 10.1016/s0079-6603(08)60085-9. [DOI] [PubMed] [Google Scholar]
- Tang H, Severinov K, Goldfarb A, Ebright RH. Rapid RNA polymerase genetics: One-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc Natl Acad Sci. 1995;92:4902–4906. doi: 10.1073/pnas.92.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa A, Saito M, Court DL, Nakamura Y. nusA amber mutation that causes temperature-sensitive growth of Escherichia coli. J Bact. 1988;170:908–915. doi: 10.1128/jb.170.2.908-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W, Ghosh B, Das A. NusA protein is necessary and sufficient in vitro for phage λ N gene product to suppress a ρ-independent terminator placed downstream of nutL. Proc Natl Acad Sci. 1988;85:2494–2498. doi: 10.1073/pnas.85.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Friedman DI. Reduced ρ-dependent transcription termination permits NusA-independent growth of Escherichia coli. Proc Natl Acad Sci. 1994;91:7543–7547. doi: 10.1073/pnas.91.16.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]