Abstract
Purpose:
To evaluate and compare real world cost-effectiveness of inhaled corticosteroids (ICS) administered by metered dose inhaler (MDI), breath-actuated MDI (BAI), or dry powder inhaler (DPI) in asthma.
Patients and methods:
This retrospective database study analyzed the direct health care costs and proportion of patients (aged 5–60 years) achieving asthma control over 1 year in two population groups: those starting ICS (initiation population) and those receiving a first increase in ICS dose (step-up population). Asthma control was defined as no unplanned asthma visits, oral corticosteroids, or antibiotics for lower respiratory infection; outcomes were adjusted for confounding variables. Cost-effectiveness of BAI and DPI were compared with MDI.
Results:
For the initiation population (n = 56,347), average annual health care costs per person (adjusted results), as compared with MDIs, were £9 higher (95% CI: −1.65 to 19.71) for BAIs and £32 higher (95% CI: 19.51 to 43.66) for DPIs. The probability of BAIs being the dominant strategy (more effective and less costly than MDIs) was 5% and of BAIs being more effective and more costly than MDIs was 94%. DPIs were consistently more effective and more costly than MDIs, with an incremental cost-effectiveness ratio of £1711 (95% CI: 760 to 3,576) per additional controlled patient per year. For the step-up population (n = 9169), mean total health care costs per person, (adjusted) as compared with MDIs, were £1 higher (95% CI: −27.28 to 31.55) for BAIs and £73 higher (95% CI: 44.48 to 103.29) for DPIs. The probability of BAIs being dominant was 48% and of BAIs being more effective but more costly than MDIs was 52%; the probability of DPIs being more effective but more costly than MDIs was 96%.
Conclusion:
The real world effectiveness of ICS inhalers may vary, and inhaler device selection for patients with asthma should take into consideration not only initial device cost but also the subsequent health care resource costs.
Keywords: asthma control, breath-actuated inhaler, dry powder inhaler, metered dose inhaler, incremental cost-effectiveness ratio
Asthma affects over 300 million people worldwide and has a substantial economic impact.1 Estimated costs of asthma to the National Health Service in the United Kingdom (UK NHS) are £1 billion per year,2,3 with total associated costs in the UK of over £2.3 billion per year.4 Much of the economic burden of asthma results from poorly controlled disease and asthma exacerbations, which contribute to increased direct medical expenditures related to emergency care as well as indirect costs of lost work time and lost productivity.5–7
Asthma is often poorly controlled. In one western European asthma survey, only 5.3% of patients met all criteria for asthma control,8 and other international surveys similarly indicate that many patients do not achieve control of their asthma.9,10 Much work thus remains to improve asthma control, as it has been shown that those with poor asthma control are more likely to suffer exacerbations of asthma requiring unscheduled care.11
Inhalation therapy is the cornerstone of asthma treatment, used for delivery of ‘reliever’ bronchodilators such as salbutamol, as well as anti-inflammatory corticosteroid ‘controller’ therapies. Currently available inhaler devices include pressurized metered dose inhalers (MDIs), breath-actuated MDIs (BAIs), and dry powder inhalers (DPIs). Both BAIs and DPIs are actuated by the patient’s inhalation maneuver, while MDIs require coordination by the patient of actuation and inhalation. The clinical effectiveness of inhalation therapy derives from delivery of the drug to target sites in the lungs, and evidence is mounting that incorrect use of inhaler devices is a common problem contributing to incomplete asthma control for many patients.12,13 Indeed, decreased asthma control has been linked to the number of mistakes made when using MDIs for the delivery of inhaled corticosteroids (ICS).14 Moreover, there is evidence that patients’ abilities regarding use of the different inhaler device types are variable.13,15,16
Recent reviews of randomized controlled trials (RCTs), while recognizing the importance of inhaler technique, have concluded that inhaler devices do not differ significantly in efficacy17–19 and that the cheapest inhaler device should be used.18 However, these results, because they are based on RCTs, should be applied with caution to the broader population of patients with asthma in primary care settings, over 90% of whom do not qualify for inclusion in RCTs because of comorbidity, smoking habits, or the lack of ‘sufficient’ lung function impairment.20,21 Importantly, patients enrolled in RCTs typically receive extensive device training and must demonstrate and maintain proper inhaler technique, which can seldom be accomplished in a real world setting.
Observational studies can provide important information to supplement that from RCTs.22,23 While RCTs are designed to eliminate confounding factors and maximize internal validity, the results of well-conducted observational studies of broader populations are more applicable (ie, generalizable) to patients seen in general practice. For this reason, and because of the opportunity for long term follow up that is so important in studying a chronic disease, database studies have been recommended for health economic evaluations in asthma.24,25 Indeed, the findings of one observational study, using a large primary care database, suggest that inhaler device does impact asthma outcomes as well as health care resource use.26
Our objective in this retrospective observational study was to evaluate the ‘real world’ cost-effectiveness of three different inhaler devices – BAI or DPI as compared with the more commonly prescribed MDI – for delivering ICS in asthma, as captured in a large primary care database. Outcomes and costs, calculated from the perspective of the UK NHS, were studied for two populations of primary care patients with asthma: those initiating ICS therapy and those prescribed an increased dose of ICS.
Methods
Data for these analyses were extracted from patient records in the UK General Practice Research Database (GPRD) from January 1997 to the end of June 2007, a period when all inhaler types under study were available. The GPRD contains de-identified medical records from approximately 500 primary care practices throughout the UK, including 3.6 million active records and 13 million records overall.27 Validated and used frequently for epidemiologic research,28–30 the GPRD applies a standard set of criteria relating to registration details to define which data are acceptable for research. Specifically, data recorded after the practice ‘up-to-standard date’ are considered research quality, prospectively recorded data.
To be included in the current analyses, patients must have been registered at the same practice and had up-to-standard follow up data for at least 12 months before and 12 months after the index prescription date of the start of ICS therapy (initiation population) or a first increase in ICS dose (step-up population). Patients eligible for the step-up population had to have at least one recorded prescription for ICS during the year before the index date (baseline year). Eligible patients in both the initiation and step-up populations were aged between 5 and 60 years on the index date and had persistent asthma, as evidenced by a recorded diagnosis of asthma or two or more prescriptions for ICS for asthma at more than one time point during the year after the index date (outcome year). Patients were excluded if their record contained a diagnostic code for any chronic respiratory condition other than asthma, including chronic obstructive pulmonary disease (COPD), or if they were prescribed more than one ICS or ICS device on the index date.
The GPRD Independent Scientific Advisory Committee approved the use of GPRD data for this study as part of a broader evaluation of the effectiveness of differing interventions in obstructive lung disease.
Outcomes
All outcomes and approaches to analysis were predefined before the analyses were performed according to Standard Operating Procedures published by the study group.31
Data from the baseline year were used to establish patient eligibility and identify likely confounding factors. The primary end point was the cost to achieve an additional person meeting the asthma control measure during the outcome year. The ‘asthma control measure’ was a composite proxy measure defined as: 1) no recorded hospital attendance for asthma (neither admission nor attendance at the emergency department or outpatient department, or use of afterhours services); 2) no prescription for oral corticosteroids; and 3) no consultation, hospital admission, or emergency department attendance for lower respiratory tract infection requiring antibiotics. A second composite, the ‘revised asthma control measure’, which approximates to the well controlled asthma status in the GINA guidelines,32 incorporated an additional parameter, namely: 4) average daily prescribed dose of salbutamol of ≤200 μg (or terbutaline ≤500 μg).
Health care resource use, including asthma therapy and respiratory-related consultations and hospitalizations, was drawn from the GPRD for the baseline year and the outcome period of 1 year after the index prescription date. Quantities were multiplied by unit costs derived from UK national data sources to calculate total costs from the UK NHS perspective in 2007 pounds sterling (£). Hospital-related costs were derived from the 2006–2007 NHS Reference Costs,33 and general practice costs were drawn from the Personal Social Services Research Unit at the University of Kent.34 Unit costs applied for respiratory- and nonrespiratory-related consultations and visits are summarized in Table 1.
Table 1.
Unit costs for health care resources applied in the study (2007 pounds sterling, £)
| Health care resource type | Unit cost, £ |
|---|---|
| Primary care consultation | 34.00 |
| Respiratory outpatient consultation | 127.00 |
| Respiratory inpatient admissions | 761.50 |
| Respiratory emergency room visit | 144.61 |
| Nonrespiratory inpatient admission | 1,576.57 |
| Nonrespiratory outpatient attendances | 167.95 |
| Nonrespiratory emergency room visit | 89.40 |
Unit costs for medications were sourced from the GPRD and, if not available there, from the Prescription Cost Analysis for England for 2007.35 Product names used in the GPRD were cross-referenced to the British National Formulary (BNF) drug names as closely as possible.36 The cheapest price was taken if there were any ambiguity. For preparations that were not available in 2007, unit costs were used from either 2002, 2000, or 1998 and multiplied by inflation factors based on the retail price index of 1.17, 1.21, and 1.27, respectively. The numbers of tablets and inhalers were used when possible, rather than assuming a standard pack size.
Statistical analyses
Patients prescribed ICS using BAIs or DPIs were defined as the cohorts of interest and were compared with patients prescribed MDIs as the reference cohort. Results were examined separately for the populations new to ICS and those receiving their first increased dose of ICS.
The probabilities of achieving the two asthma control measures were calculated using a binary logistic regression model (1,000 replications) with asthma control as the dependent variable and cohort, together with potential confounding factors (year of index date, age, sex, socioeconomic status, comorbidity, and treatment with medication that could affect respiratory outcomes), as explanatory variables. Confounding factors included variables that were significantly different (P ≤ 0.05) among cohorts at baseline or that were predictive of asthma control based on univariate analysis (P ≤ 0.05). Adjustments were made for the following confounding variables as noted: age, categorized as 5–12, 13–20, 21–30, 31–40, 41–50, 51–60 years; sex; socioeconomic status (log scale); comorbidities including gastroesophageal reflux disease diagnosis, cardiac disease diagnosis, rhinitis diagnosis, Charlson comorbidity index (CCI) score; and beta blockers, nonsteroidal anti-inflammatory agents, and paracetamol. Respiratory-related outcomes included as confounding factors were oral corticosteroid courses, categorized as 0, 1, 2, or ≥3; antibiotics; hospital admissions for asthma and respiratory disease; outpatient attendance; asthma prescriptions; asthma consultations; short-acting β2 agonist (SABA) dose, categorized as 0, >0–100 μg, 101–200 μg, 201–400 μg, 401–800 μg, >800 μg; ICS dose at index prescription date, categorized as 0–200 μg, 201–400 μg, 401–800 μg, >800 μg; and year of index prescription. The dose of ICS was normalized to the beclomethasone dipropionate (BDP) equivalent dose, using ratios of 1:1:2:2:2 for BDP, budesonide, fluticasone propi-onate, BDP in solution (QVAR®, Teva UK), and mometasone, respectively.
Socioeconomic status was the score assigned by the GPRD to each practice using the Index of Multiple Deprivation (IMD) as a proxy measure. The CCI score,37 a weighted index accounting for number and severity of comorbidities, was used to represent comorbidities, as calculated for each patient using ICD-9 matching algorithms produced by CliniClue software (http://www.cliniclue.com/software).
For the cost analysis, unadjusted total health care costs per person in the baseline and outcome periods were summarized using medians and compared across study groups using the Kruskal–Wallis test. Multivariate generalized linear models with a log link and gamma distribution were used to estimate total health care costs 1 year after the index date, controlling for age, sex, baseline asthma control status, and baseline total health care costs (logged). Adjusted costs were reported with 95% confidence intervals (CIs) for the difference in costs compared with MDI, which were obtained by the bootstrap method with 1000 replications.
The cost-effectiveness of the BAI and DPI inhalers were compared relative to the MDI in terms of cost per additional patient achieving asthma control during the outcome year. The differences in costs and proportion of patients achieving asthma control (after adjustment) for the 1,000 replications were shown graphically on a cost-effectiveness plane. Incremental cost-effectiveness ratios (ICERs) were calculated when the uncertainty in the data was restricted to one quadrant of the cost-effectiveness plane; ICERs cannot be calculated when the data cover more than one quadrant of the cost-effectiveness plane because of difficulties in interpreting and calculating CIs.38–40 The ICER is the ratio of the difference in costs to the difference in effectiveness of the two treatment arms, as depicted below:
where C1 and E1 are the cost and effectiveness of the BAI or DPI, and C2 and E2 are the cost and effectiveness of the comparator (MDI).
Baseline statistical analyses were carried out using SPSS for Windows, (version 17.0; SPSS Inc. Chicago, IL). Bootstrap analyses were carried out using STATA 9.2 for Windows (StataCorp LP, College Station, TX) and cost-effectiveness planes were derived using Microsoft Office Excel 2007 (Microsoft Corp., Redmond, WA).
Results
Patients identified in the GPRD as eligible for the study numbered 56,347 in the initiation population starting ICS and 9,169 in the step-up population receiving a first increased dose of ICS. Demographic and baseline characteristics of the two populations are summarized in Table 2.
Table 2.
Demographic and baseline characteristics of patients receiving a first prescription or an increased dose of inhaled corticosteroid, by inhaler device type
|
Patients starting ICS |
Patients receiving an increased dose of ICS |
|||||
|---|---|---|---|---|---|---|
| MDI (n = 39,746) | BAI (n = 9809) | DPI (n = 6792) | MDI (n = 6245) | BAI (n = 1388) | DPI (n = 1536) | |
| Male sex | 17,294 (43.5)b | 4,062 (41.4) | 3,013 (44.4) | 2,735 (43.8)b | 571 (41.1) | 735 (47.9) |
| Age at index date (y) | 28 (12–42)b | 30 (13–45) | 22 (11–42) | 27 (10–45) | 21 (12–44) | 20 (11–42) |
| Baseline asthma controlc | 30,129 (75.8)a | 7,328 (74.7) | 5,205 (76.6) | 3,727 (59.7)b | 927 (66.8) | 977 (63.6) |
| Total asthma/resp costs | 34.00b (0–68.00) | 35.47 (1.47–70.32) | 34.00 (0–69.59) | 103.13b (54.82–180.87) | 101.69 (58.63–173.68) | 127.42 (77.38–222.06) |
| Total asthma/resp costs excl ICS | – | – | – | 77.28b (38.98–148.47) | 78.46 (40.30–143.52) | 83.96 (45.76–159.46) |
| Total annual health care costs | 247.28b (108.30–489.01) | 261.60 (116.69–510.31) | 244.95 (108.92–470.83) | 418.39b (230.12–752.58) | 396.54 (220.61–687.39) | 428.19 (242.91–765.96) |
| Total annual health care costs excl ICS | 247.28b (108.30–489.01) | 261.60 (116.69–510.31) | 244.95 (108.92–470.83) | 390.93a (210.91–717.65) | 366.05 (198.58–649.00) | 386.32 (204.32–697.45) |
Notes: Values shown are n (%) or median (interquartile range). Costs are per person in 2007 pounds sterling (£) for the baseline year.
P ≤ 0.05,
P ≤ 0.01 for comparison among the three cohorts.
Asthma control was defined as no recorded hospital attendance for asthma, oral corticosteroid prescription, or lower respiratory tract infection requiring antibiotics.
Abbreviations: Asthma/resp, asthma and respiratory; BAI, breath-actuated inhaler; DPI, dry powder inhaler; excl, excluding; ICS, inhaled corticosteroids; MDI, metered dose inhaler.
Patients initiating inhaled corticosteroids
Of the 56,347 patients in the initiation population, 39,746 (71%) were prescribed an MDI, 9,809 (17%) a BAI, and 6,792 (12%) a DPI. During the baseline year, healthcare costs were significantly different among cohorts for several categories, including total asthma/respiratory costs and total annual healthcare costs (both highest in the BAI cohort; Table 2); these costs were included as covariates in the adjusted analyses.
During the outcome year, health care costs per person were significantly different among cohorts for most resource categories (Table 3). Total asthma-related drug costs were significantly higher in the DPI cohort, with costs for ICS double those for MDI and BAI cohorts. Total annual health care costs, both including and excluding ICS, were highest in the BAI cohort. When ICS costs were excluded, the median total annual health care resource costs were £353, £379, and £342 for MDI, BAI, and DPI cohorts, respectively (Table 3).
Table 3.
Unadjusted median health care costs (2007 pounds sterling) over 1 year after index prescription for patients receiving a first prescription of inhaled corticosteroid
|
Metered dose inhaler (n = 39,746) |
Breath-actuated inhaler (n = 9809) |
Dry powder inhaler (n = 6792) |
||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Inhaled corticosteroids | 15.74a | 7.60–34.42 | 20.98 | 10.49–41.96 | 37.00 | 18.74–74.00 |
| Oral corticosteroids | 0a | 0–0 | 0 | 0–0 | 0 | 0–0 |
| SABA inhalers | 4.41a | 1.47–8.82 | 6.30 | 2.32–13.95 | 8.39 | 2.94–20.76 |
| Total asthma-related drugs | 25.18a | 13.27–49.91 | 34.41 | 17.84–67.16 | 55.50 | 28.53–108.87 |
| Total asthma-related drugs excl ICS | 4.83a | 2.32–12.00 | 8.34 | 2.94–20.08 | 11.83 | 5.16–25.10 |
| Total drug costs | 54.66a | 26.86–118.31 | 66.63 | 34.03–141.62 | 86.03 | 46.33–179.62 |
| Total drug costs excl ICS | 29.18a | 11.34–80.85 | 35.38 | 14.10–96.00 | 33.91 | 14.25–87.43 |
| Asthma consult, primary care | 68.00a | 34.00–102.00 | 68.00 | 34.00–102.00 | 68.00 | 34.00–102.00 |
| Other GP consultation | 204.00a | 102.00–340.00 | 204.00 | 102.00–340.00 | 170.00 | 102.00–306.00 |
| Total asthma-related costs | 87.62a | 48.87–154.63 | 107.53 | 57.84–181.31 | 128.76 | 74.33–221.98 |
| Total asthma-related costs excl ICS | 70.32a | 36.32–114.96 | 75.40 | 40.02–141.41 | 76.84 | 40.92–142.92 |
| Total annual health care costs | 378.87a | 212.04–676.00 | 412.47 | 228.75–720.23 | 400.84 | 226.75–699.24 |
| Total annual health care costs excl ICS | 353.04a | 188.66–642.93 | 379.13 | 206.04–680.29 | 342.38 | 183.70–626.05 |
Notes:
P ≤ 0.01 for comparisons among cohorts. Combination inhalers, long-acting β2 agonists, leukotriene receptor antagonists, theophylline, antibiotics, and all hospital visits were infrequently prescribed/recorded; thus median (IQR) costs were 0 (0–0) for these categories.
Abbreviations: excl, excluding; GP, general practice; ICS, inhaled corticosteroids; IQR, interquartile range; SABA, short-acting β2 agonist.
Adjusted mean total annual health care costs per person were significantly higher (P ≤ 0.05) for patients prescribed a DPI by approximately £32, as compared with costs for those prescribed an MDI. Costs were on average £9 more for a BAI compared with an MDI, a nonsignificant difference (Table 4).
Table 4.
Incremental cost and incremental effectiveness analyses for the 1 year after index prescription for patients receiving an increased dose or first prescription of inhaled corticosteroid: adjusted results
|
Patients starting ICS |
Patients receiving an increased dose of ICS |
|||||
|---|---|---|---|---|---|---|
| MDI (n = 39,746) | BAI (n = 9,809) | DPI (n = 6,792) | MDI (n = 6,245) | BAI (n = 1,388) | DPI (n = 1,536) | |
| Adjusted total annual health care costs, 2007 £b: | ||||||
| Mean total costs (95% CI) | 541.13 (535.93–546.18) | 550.42 (540.67–559.60) | 573.03 (560.89–584.56) | 671.29 (656.76–686.09) | 672.34 (645.20–700.75) | 744.05 (717.99–774.86) |
| Difference from MDI (95% CI) | – | 9.30 (–1.65–19.71) | 31.90a (19.51–43.66) | – | 1.05 (–27.28–31.55) | 72.57a (44.48–103.29) |
| Effectiveness resultsc: | ||||||
| % Asthma controlledd (95% CI) | 75.49 (75.04–75.92) | 76.67 (75.82–77.50) | 77.53 (76.56–78.48) | 68.58 (67.38–69.80) | 72.24 (69.94–74.54) | 70.85 (68.75–73.14) |
| Difference from MDI (95% CI) | – | 1.18a (0.25–2.11) | 2.05a (0.97–3.11) | – | 3.66a (1.15–6.19) | 2.27 (–0.26–4.90) |
| % Asthma controlled (revised)e (95% CI) | 55.48 (55.03–55.95) | 56.80 (55.82–57.72) | 60.64 (59.56–61.85) | 37.74 (36.50–39.15) | 38.89 (36.54–41.39) | 38.36 (36.04–40.87) |
| Difference from MDI (95% CI) | – | 1.32a (0.28–2.34) | 5.16a (3.95–6.32) | – | 1.15 (–1.27–3.81) | 0.62 (–2.06–3.21) |
Notes:
P ≤ 0.05 for comparison with MDI cohort.
Total health care costs from 1,000 repetitions, adjusted for age, sex, baseline asthma control status, and baseline total health care costs including ICS.
Adjusted results based on 1000 repetitions.
Asthma control was defined as no recorded hospital attendance for asthma, oral corticosteroid prescription, or lower respiratory tract infection requiring antibiotics.
Revised asthma control measure including average daily SABA use restricted to ≤200 μg salbutamol and ≤500 μg terbutaline.
Abbreviations: BAI, breath-actuated inhaler; CI, confidence interval; DPI, dry powder inhaler; MDI, metered dose inhaler.
The asthma control measure was achieved during the outcome year by 29,961 (75%), 7,518 (77%), and 5,307 (78%) patients in MDI, BAI, and DPI cohorts, respectively (P ≤ 0.001). The revised asthma control measure incorporating SABA use was achieved by 21,956 (55%), 5,605 (57%), and 4,185 (62%) of patients in MDI, BAI, and DPI cohorts, respectively (P ≤ 0.001).
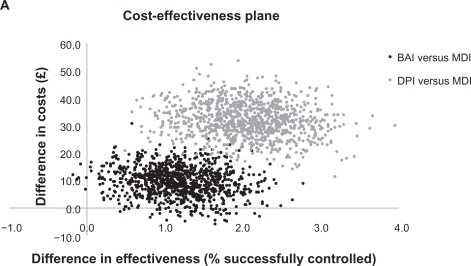
After adjustment for confounding factors, the proportions of patients achieving asthma control relative to the MDI cohort were significantly greater (by 1% −2%; P ≤ 0.05) for those patients who received a BAI or DPI (Table 4). There was a 94% probability of BAIs being more effective but also more costly than MDIs; a 5% probability of BAIs being the dominant strategy (more effective and less costly than MDIs); and a 1% probability that BAIs were less effective and more costly (Figure 1a). DPIs were consistently more effective and more costly than MDIs, representing a trade-off between more patients achieving asthma control but at greater cost than with an MDI. The ICER for DPIs was £1711 (95% CI: 760–3576) per additional patient with asthma control.
Figure 1A.
Cost-effectiveness plane for patients receiving a first prescription of ICS.
Note: Asthma control was defined as no recorded hospital attendance for asthma, oral corticosteroid prescription, or lower respiratory tract infection requiring antibiotics.
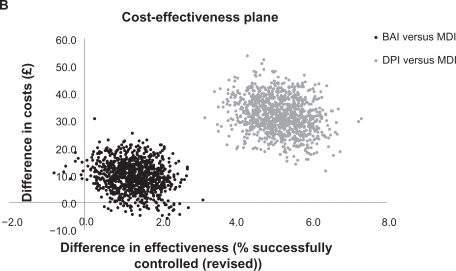
In terms of the revised asthma control measure that included SABA use, after adjustment the proportion of patients with asthma control remained significantly greater by 1% for the BAI and 5% for the DPI cohorts as compared with the MDI cohort (Table 4). As for the primary asthma control measure, there was a 94% probability of greater effectiveness but greater costs for BAIs compared with MDIs; a 5% probability of BAIs being the dominant strategy; and a 1% probability of BAIs being less effective and more costly (Figure 1b). DPIs remained consistently more effective but also more costly compared with MDIs; the ICER was £631 (95% CI: 349–983).
Figure 1B.
Cost-effectiveness plane for patients receiving a first prescription of ICS (revised asthma control).
Note: Asthma control (revised) was defined as asthma control plus average daily short-acting β2 agonist use restricted to ≤200 μg salbutamol and ≤500 μg terbutaline.
Patients receiving an increased dose of inhaled corticosteroids
Of the 9,169 patients in the step-up population, 6,245 (68%) were prescribed an MDI, 1,388 (15%) a BAI, and 1,536 (17%) a DPI. During the baseline year, health care costs were signifi-cantly different across cohorts for several resource categories, including total asthma/respiratory costs and total annual health care costs (both highest in the DPI cohort; Table 2).
Asthma-related and total health care costs during the outcome year are summarized in Table 5. Median costs were significantly different among the three cohorts and were highest in the DPI cohort for most health care resource categories. Large cohort differences were evident in the costs of ICS and SABA inhalers, with costs for the DPI cohort being twice as high as those for the MDI cohort. When ICS costs were excluded, the median total annual health care resource costs were £429, £398, and £440 for MDI, BAI, and DPI cohorts, respectively (Table 5).
Table 5.
Unadjusted median health care costs (2007 pounds sterling) over 1 year after index prescription for patients receiving an increased dose of inhaled corticosteroid, by inhaler device type
|
Metered dose inhaler (n = 6245) |
Breath-actuated inhaler (n = 1388) |
Dry powder inhaler (n = 1536) |
||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Inhaled corticosteroids | 41.94b | 20.97–79.23 | 42.99 | 20.98–81.92 | 85.04 | 40.86–166.50 |
| Oral corticosteroids | 0b | 0–0 | 0 | 0–0 | 0 | 0–0 |
| SABA inhalers | 7.35b | 2.94–16.27 | 12.42 | 5.88–25.20 | 13.98 | 6.92–34.60 |
| Total asthma-related drugs | 66.14b | 32.32–149.53 | 73.44 | 38.34–147.16 | 131.79 | 65.69–265.78 |
| Total asthma-related drugs excl ICS | 13.30b | 4.65–55.79 | 18.61 | 6.30–54.09 | 27.34 | 9.95–79.81 |
| Total drug costs | 114.37b | 54.13–262.15 | 115.74 | 60.19–242.07 | 183.96 | 92.25–369.53 |
| Total drug costs excl ICS | 56.89b | 20.48–179.74 | 58.35 | 23.47–157.47 | 66.35 | 25.66–195.03 |
| Asthma consult, primary care | 68.00 | 34.00–102.00 | 68.00 | 34.00–102.00 | 68.00 | 34.00–136.00 |
| Other GP consultation | 204.00a | 102.00–374.00 | 204.00 | 102.00–374.00 | 204.00 | 102.00–340.00 |
| Total asthma-related costs | 146.53b | 80.87–280.43 | 149.88 | 86.45–258.51 | 221.13 | 123.50–398.89 |
| Total asthma-related costs excl ICS | 88.88b | 41.44–198.04 | 93.20 | 42.82–182.60 | 109.52 | 51.64–227.10 |
| Total annual health care costs | 484.68b | 275.15–878.44 | 462.34 | 257.81–829.67 | 549.69 | 318.02–967.20 |
| Total annual health care costs excl ICS | 428.98 | 231.59–787.60 | 398.20 | 214.19–764.30 | 439.82 | 225.71–800.34 |
Notes:
P < 0.05.
P < 0.01 for comparison among cohorts. Combination inhalers, long-acting β2 agonists, leukotriene receptor antagonists, theophylline, antibiotics, and all hospital visits were infrequently prescribed/recorded; thus median (IQR) costs were 0 (0–0) for these categories.
Abbreviations: excl, excluding; GP, general practice; ICS, inhaled corticosteroids; IQR, interquartile range; SABA, short-acting β2 agonist.
After adjusting for confounding factors, mean total annual health care costs per person were significantly higher (by £73; P ≤ 0.05) for patients prescribed a DPI as compared with those prescribed an MDI (Table 4). The £1 difference between MDI and BAI cohorts was not statistically significant.
During the outcome year, 4,237 (68%), 1,032 (74%), and 1,103 (72%) patients in the MDI, BAI, and DPI cohorts, respectively, achieved the asthma control measure, with the differences among cohorts being significant (P ≤ 0.001). The revised asthma control measure incorporating SABA use was achieved by 2,289 (37%), 584 (42%), and 610 (40%) of patients in the MDI, BAI, and DPI cohorts, respectively (P ≤ 0.001).
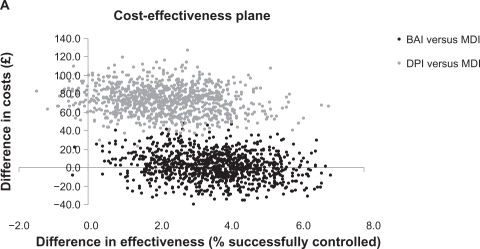
After adjustment for confounding factors, the proportion of patients achieving asthma control relative to the MDI cohort was significantly greater in the BAI cohort, by 3.7%. On average, an additional 2.3% of DPI patients achieved asthma control but this was not significantly different from the MDI cohort (Table 4). As shown in Figure 2a, there was a 48% probability of BAIs being the dominant strategy and a 52% probability that BAIs were more effective and more costly than MDIs. The probability that DPIs were more effective and more costly than MDIs was 96%, representing a trade-off between more patients achieving asthma control but at greater cost than with an MDI. There was a 4% probability of DPIs being less effective and more costly than MDIs.
Figure 2A.
Cost-effectiveness plane for patients receiving an increased dose of ICS.
Note: Asthma control was defined as no recorded hospital attendance for asthma, oral corticosteroid prescription, or lower respiratory tract infection requiring antibiotics.
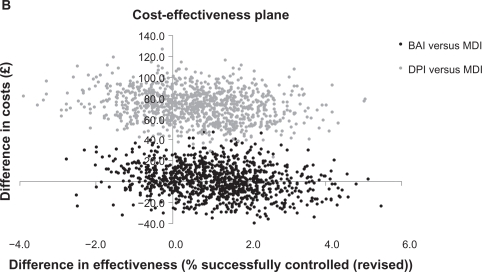
For the revised asthma control measure that included SABA use, there was no statistically significant difference in effectiveness between the BAI or DPI cohorts compared with the MDI cohort after adjustment for baseline confounding variables. The probability of BAIs being the dominant strategy was 41%; of BAIs being more effective and more costly than MDIs was 40%; of BAIs being less effective and less costly was 7%; and of BAIs being less effective and more costly was 12% (Figure 2b). For DPIs, the probability of being more effective but more costly was 67%, and of being less effective and more costly was 33%.
Figure 2B.
Cost-effectiveness plane for patients receiving an increased dose of ICS (revised asthma control).
Note: Asthma control (revised) was defined as asthma control plus average daily short-acting β2 agonist use restricted to ≤200 μg salbutamol and ≤500 μg terbutaline.
Discussion
The purpose of this study was to evaluate the cost-effectiveness of three different inhaler devices for delivering ICS to patients with asthma in a real world setting. The study results indicate that, during the first year after the initiation of ICS, both BAI and DPI devices were more effective than MDI devices, as significantly more patients achieved asthma control, by both study definitions, with a BAI or DPI than with an MDI. During the outcome year, DPIs, but not BAIs, were associated with significantly higher mean health care costs than MDIs. Overall, BAIs were more effective, by either asthma control definition, but had a 94% probability of also being more costly; DPIs were consistently more effective and more costly, with ICERs of £1711 (95% CI: 760–3576) and £631 (95% CI: 349–983) per additional patient achieving asthma control, depending on definition.
For patients receiving an increased dose of ICS, those prescribed a BAI were, on average, significantly more likely to achieve asthma control than those prescribed an MDI, with similar total annual health care resource costs. Patients prescribed a DPI were as likely to achieve asthma control as those prescribed an MDI, but with significantly higher associated costs. Overall, in the cost-effectiveness analyses, BAIs were consistently more effective than MDIs and almost evenly split with regard to costs as compared with MDIs, namely, the dominant strategy (more effective, less costly) about half the time and more effective but more costly about half the time, whereas DPIs had a high probability (96%) of being more effective but more costly than MDIs. When the revised definition of asthma control was applied, the probabilities of BAIs and DPIs being more effective but more costly were 47% and 67%, respectively; and the probability of BAIs being the dominant strategy was 41%.
The costs reported in this study are the direct medical costs from the perspective of the UK NHS (2007 pounds sterling) over 1 year after initiation or an increase in dose of ICS therapy. The total health care costs used to calculate the additional cost for one patient to achieve asthma control, adjusted for confounding factors, included the actual cost of the ICS devices, as well as all other health care expenditures captured from data recorded in the GPRD. Because there is no recognized threshold for the additional expenditure that is justified to provide one additional patient with asthma control, and because indirect costs were not measured, it is beyond the scope of this study to conclude whether the additional cost of a BAI or DPI is ‘worth it,’ as this will depend on the decision maker’s willingness to pay. However, for patients receiving an increased dose of ICS, one could argue that a BAI should be prescribed in preference over an MDI as BAIs were more effective than MDIs in this study. Moreover, BAIs appear to be the most cost-effective option as there was almost a 50% chance that a BAI would be less costly as well as more effective than an MDI for patients receiving an increased dose of ICS. In the remainder of cases there was a trade-off between greater costs and greater effectiveness.
For patients initiating ICS, the situation is less clear, although, on average, both BAIs and DPIs were more effective than MDIs, and BAIs were not significantly more costly than MDIs. The chance of BAIs being dominant (both less costly and more effective than MDIs) was 5%, and when there was a trade-off between greater costs and greater effectiveness, the trade-off was lower than for DPIs.
In general, the cost-effectiveness of ICS therapy for patients with persistent asthma is widely accepted. As much of the costs of asthma derive from poorly controlled disease, it would follow that improved control can lower asthma-related costs.5 Indeed, results of a health economic analysis alongside a long term randomized trial indicated that improvement in asthma control is associated with favorable cost per quality-adjusted life-year.41 While most prior health economic studies of asthma therapy have compared controller medications,24,42 cost-effectiveness comparisons of inhaler devices are few and different in scope to the present study.42–44 The findings of this study are supported, however, by assessments of inhaler technique, which indicate that patients tend to make fewer mistakes with BAIs and DPIs.15,16
For the initiation and step-up populations in this study, the ICS device prescribed was an MDI for 71% and 68%, respectively, a BAI for 17% and 15%, respectively, and a DPI for 12% and 17%, respectively, ratios approximating the wider market shares of these devices in the UK. Of the BAI devices for ICS prescribed in the UK over the course of the study, approximately 73% were Easi-Breathe® (Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel) and 27%, the Autohaler™ (Teva Pharmaceutical Industries Ltd., and 3M, St. Paul, MN, USA).45
Asthma control, simply defined, is the control of clinical manifestations of asthma, including day and nighttime symptoms, limitations in the activities of daily living, and exacerbations. While parameters such as lung function are important measures of successful treatment, at the end of the day, asthma control is of practical importance to patients. The composite measure of asthma control used in this study was designed to capture outcomes recorded in the GPRD indicating that an asthma exacerbation had occurred, including: unplanned medical care or hospitalization for asthma, a prescription for oral corticosteroids, and antibiotic prescribing for lower respiratory tract infection, as acute asthma may be confused in practice for respiratory infection. These outcomes are in line with recent recommendations by the American Thoracic Society/European Respiratory Society task force for assessing asthma control in clinical trials,46 as well as those of the authors of a recent review of health economic studies in asthma.24
The limitations of this study are inherent to all observational studies, including nonrandom allocation of treatments and the possibility of unrecognized confounding factors. While the GPRD is regarded as a high quality database,29,30 errors and omissions in medical record reporting are possible, and the database contains limited information on hospitalizations. Nonetheless, the large size of the GPRD, representing over 5% of the UK population,27 makes it a valuable source for study of primary care practice, where most asthma is managed in the UK. Moreover, outcomes were studied over the course of 1 year, a period sufficient to capture seasonal variations in asthma and some of the fluctuations in symptoms characteristic of this chronic respiratory condition.
Conclusion
In clinical practice, there can be considerable pressure to use the least expensive, most effective inhaled therapies and the most appropriate inhaler devices available to minimize the burden of asthma treatment costs for the UK NHS. Health economic assessments are important to aid decision makers in determining the optimal allocation of resources. The results of this retrospective observational study indicate that inhaler device selection does indeed matter for real world patients prescribed ICS. Specifically, the results indicate that for patients initiating ICS, BAIs were more effective than MDIs most of the time, and, while the probability of BAIs being more costly than MDIs was 94%, mean health care costs with BAIs were not significantly greater than with MDIs. Instead, DPIs were consistently more effective and expensive than MDIs, with an incremental cost-effectiveness ratio of £631–1,711 per additional patient achieving asthma control, depending on control definition. For those patients receiving an increased dose of ICS, more patients can achieve asthma control with little or no additional cost relative to an MDI if prescribed a BAI. DPIs were usually more effective than MDIs but also more costly for this patient population. These findings suggest that the real world effectiveness of ICS inhalers may vary and that the selection of inhaler device for patients with asthma should take into consideration not only initial cost of the device itself but also the subsequent health care resource costs.
Acknowledgments
Access to data from the General Practice Research Database was funded by Merck and Co., Inc., and the analysis was funded by Teva Pharmaceuticals Ltd.
Footnotes
Disclosures
Linda Kemp has no conflict of interest to declare. John Haughney has received some or all of the following: reimbursements for attending symposia; fees for speaking and organizing educational events; funds for research; fees for consulting; from the following pharmaceutical companies:
AstraZeneca, Boehringer-Ingelheim, Glaxo Smith Kline, Merck, Sharp and Dohme, Mundipharma, Novartis, Nycomed, Sanofi Aventis and Teva. He holds no stocks or shares. He has no relationship with the tobacco industry.
Professor Neil Barnes has lectured for and has received consulting fees from AstraZeneca, GlaxoSmithKline, Chiesi, Cipla, Nycomed, Novartis, NAPP.
Professor Barnes has had research grants from Astra-Zeneca, GlaxoSmithKline and Novartis which have gone into departmental funds. Erika Sims has worked on projects funded by Schering Plough, Merck & Co., Inc., and Teva, and has received funding to attend conferences. Julie von Ziegenweidt has no conflict of interest to declare.
Elizabeth V. Hillyer has done freelance writing work for Merck & Co., Inc., Aerocrine, and Teva Santé. Amanda J. Lee receives payment for statistical consultancy through the independent research company, Thorpe Respiratory Research. Alison Chisholm has no conflict of interest to declare David Price has consultant arrangements with Aerocrine, Boehringer Ingelheim, Dey Pharmaceuticals, GlaxoSmithKline, Merck, Sharpe and Dohme, Novartis, Schering-Plough, and Teva. He or his team have received grants and research support for research in respiratory disease from the following organizations: UK National Health Service, Aerocrine, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Sharpe and Dohme, Novartis, Pfizer, Schering Plough, and Teva. He has spoken for: Boehringer Ingelheim, GlaxoSmithKline, Merck, Sharpe and Dohme, Pfizer, and Teva.
References
- 1.Global Initiative for Asthma. Global Burden of Asthma May 2004. Available from: http://www.ginasthma.com/GuidelinesResources.asp?l1=2&l2=0. Accessed Mar 2, 2010.
- 2.Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clin Exp Allergy. 2004;34:520–526. doi: 10.1111/j.1365-2222.2004.1935.x. [DOI] [PubMed] [Google Scholar]
- 3.Asthma UK. New data reveals high cost of asthma. 2009. Available from: http://www.asthma.org.uk/news_media/news/new_data_reveals_hig.html. Accessed February 25, 2010.
- 4.Asthma UK. Where do we stand? Asthma in the UK today. 2004. Available from: http://www.asthma.org.uk/health_professionals/ordering_materials/where_do_we_stand.html. Accessed Mar 2, 2010.
- 5.Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9:636–642. doi: 10.1183/09031936.96.09040636. [DOI] [PubMed] [Google Scholar]
- 6.Cisternas MG, Blanc PD, Yen IH, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol. 2003;111:1212–1218. doi: 10.1067/mai.2003.1449. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007;62:126–133. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 8.Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–807. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 9.Boulet LP, Phillips R, O’Byrne P, Becker A. Evaluation of asthma control by physicians and patients: comparison with current guidelines. Can Respir J. 2002;9:417–423. doi: 10.1155/2002/731804. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Adachi M, Lai CK, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102:1681–1693. doi: 10.1016/j.rmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med. 2006;100:1479–1494. doi: 10.1016/j.rmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Chrystyn H, Price D. Not all asthma inhalers are the same: factors to consider when prescribing an inhaler. Prim Care Respir J. 2009;18:243–249. doi: 10.4104/pcrj.2009.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002 Feb;19:246–251. doi: 10.1183/09031936.02.00218402. [DOI] [PubMed] [Google Scholar]
- 15.Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. Respir Med. 2000;94:496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- 16.Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 17.Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5:1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- 18.Brocklebank D, Wright J, Cates C. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering corticosteroids in asthma. BMJ. 2001;323(7318):896–900. doi: 10.1136/bmj.323.7318.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 20.Herland K, Akselsen JP, Skjonsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99:11–19. doi: 10.1016/j.rmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219–223. doi: 10.1136/thx.2006.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Concato J, Horwitz RI. Beyond randomised versus observational studies. Lancet. 2004;363(9422):1660–1661. doi: 10.1016/S0140-6736(04)16285-5. [DOI] [PubMed] [Google Scholar]
- 23.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell JD, Spackman DE, Sullivan SD. Health economics of asthma: assessing the value of asthma interventions. Allergy. 2008;63:1581–1592. doi: 10.1111/j.1398-9995.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M, Cleland J, Price D. Database studies in asthma pharmacoeconomics: uses, limitations and quality markers. Expert Opin Pharmacother. 2003;4:351–358. doi: 10.1517/14656566.4.3.351. [DOI] [PubMed] [Google Scholar]
- 26.Price D, Thomas M, Mitchell G, Niziol C, Featherstone R. Improvement of asthma control with a breath-actuated pressurised metred dose inhaler (BAI): a prescribing claims study of 5556 patients using a traditional pressurised metred dose inhaler (MDI) or a breath-actuated device. Respir Med. 2003;97:12–19. doi: 10.1053/rmed.2002.1426. [DOI] [PubMed] [Google Scholar]
- 27.General Practice Research Database. 2010. Available from: http://www.gprd.com/. Accessed Mar 2, 2010.
- 28.Hansell A, Hollowell J, Nichols T, McNiece R, Strachan D. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4) Thorax. 1999;54:413–419. doi: 10.1136/thx.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollowell J. The General Practice Research Database: quality of morbidity data. Popul Trends. 1997 Spring;(87):36–40. [PubMed] [Google Scholar]
- 30.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the General Practice Research Database. Pharmacotherapy. 2003;23:686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 31.Research in Real Life: Standard Operating Procedures. Available from: http://www.optimumpatientcare.org/downloads/documents/SOP%20observational%20database%20studies.pdf. Accessed Mar 2, 2010.
- 32.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention, updated 2009. Available from: http://www.ginasthma.org. Accessed February 25, 2010.
- 33.Department of Health NHS reference costs 2007–08. 2009. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945. Accessed Mar 2, 2010.
- 34.Curtis L. Unit Costs of Health and Social Care 2007. Personal Social Services Research Unit. University of Kent; 2007. Available from: http://www.pssru.ac.uk/pdf/uc/uc2007/uc2007.pdf. Accessed Mar 2, 2010.
- 35.The NHS Information Centre Prescribing Support Unit. Prescription Cost Analysis for England 2007. Data by individual preparation; 2008. Available from: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-2007. Accessed Mar 2, 2010.
- 36.British National Formulary. V 54 ed. Londen, Uk: British Medical Association and the Royal Pharma ceutical society of Great Britain; 2007. [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Heitjan DF, Moskowitz AJ, Whang W. Problems with interval estimates of the incremental cost-effectiveness ratio. Med Decis Making. 1999;19:9–15. doi: 10.1177/0272989X9901900102. [DOI] [PubMed] [Google Scholar]
- 39.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11:415–430. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res. 2002;11:455–468. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- 41.Briggs AH, Bousquet J, Wallace MV, et al. Cost-effectiveness of asthma control: an economic appraisal of the GOAL study. Allergy. 2006;61:531–536. doi: 10.1111/j.1398-9995.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 42.Sculpher MJ, Price M. Measuring costs and consequences in economic evaluation in asthma. Respir Med. 2003;97:508–520. doi: 10.1053/rmed.2002.1474. [DOI] [PubMed] [Google Scholar]
- 43.Liljas B, Stadhl E, Pauwels RA. Cost-effectiveness analysis of a dry powder inhaler (Turbuhaler) versus a pressurised metered dose inhaler in patients with asthma. Pharmacoeconomics. 1997;12(2 Pt 2):267–277. doi: 10.2165/00019053-199712020-00017. [DOI] [PubMed] [Google Scholar]
- 44.Kelloway JS, Wyatt R. A cost-effectiveness analysis of breath-actuated metered-dose inhalers. Manag Care Interface. 1997;10:99–107. [PubMed] [Google Scholar]
- 45.IMS Health Available from: http://www.imshealth.com. Accessed January 28, 2010.
- 46.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]