Abstract
Objective:
To evaluate adherence to antihypertensive therapy (AHT) and the association between adherence to AHT, all-cause mortality, and cardiovascular (CV) morbidity in a large cohort of patients newly treated with antihypertensives in a clinical practice setting.
Methods:
An administrative database kept by the Local Health Unit of Florence (Italy) listing patient baseline characteristics, drug prescription, and hospital admission information was used to perform a population-based retrospective study including patients newly treated with antihypertensives, ≥18 years of age, with a first prescription between January 1, 2004 and December 31, 2006. Patients using antihypertensives for secondary prevention of CV disease, occasional spot users, and patients with early CV events, were excluded from the study cohort. Adherence to AHT was calculated and classified as poor, moderate, good, and excellent. A Cox regression model was conducted to determine the association among adherence to AHT and risk of all-cause mortality, stroke, or acute myocardial infarction.
Results:
A total of 31,306 patients, 15,031 men (48.0%), and 16,275 women (52.0%), with a mean age of 60.2 ± 14.5 years was included in the study. Adherence to AHT was poor in 8038 patients (25.7% of included patients), moderate in 4640 (14.8%), good in 5651 (18.1%), and excellent in 12,977 (41.5%). Compared with patients with poor adherence (hazard ratio [HR] = 1), the risk of all-cause death, stroke, or acute myocardial infarction was significantly lower in patients with good (HR = 0.69, P < 0.001) and excellent adherence (HR = 0.53, P < 0.001).
Conclusions:
These findings indicate that suboptimal adherence to AHT occurs in a substantial proportion of patients and is associated with poor health outcomes already in primary prevention of CV diseases. For health authorities, this preliminary evidence underlines the need for monitoring and improving medication adherence in clinical practice.
Keywords: antihypertensive drug therapy, adherence, all-cause mortality, stroke, acute myocardial infarction
Introduction
Clinical trials have shown that continuous treatment with antihypertensive drug therapy (AHT) significantly lowers blood pressure in a very high proportion of patients, thus significantly decreasing both cardiovascular (CV) morbidity and mortality1–4 and, ultimately, overall costs associated with high blood pressure.5–7
However, in clinical practice, adherence (whether a patient takes medications as prescribed) and persistence (whether a patient remains on therapy as long as needed) to AHT are suboptimal,8–11 ranging from 30% to 70%, depending on measurement methods and definitions.12,13 Poor adherence to AHT causes suboptimal blood pressure control and, ultimately, preventable CV morbidity, hospitalizations, mortality, and health care expenditure,10,14–16 all consequences that have been documented, but still remain overlooked.
The objective of this study was to evaluate adherence to AHT and the association between adherence to AHT, all-cause mortality, and hospitalizations for stroke and acute myocardial infarction (AMI) in a large cohort of patients newly treated with antihypertensives in a clinical practice setting.
Patients and methods
Data source
The study was based on administrative databases maintained by the Local Health Unit (LHU) of Florence, Italy, with a population of approximately 800,000 inhabitants. The LHU Ethics Committee approved the study. In the Medications Prescription Database, the LHU routinely measures the volume of expenditure generated by the dispensing of drugs to the enrollees. The data available in each prescription claim include the patient’s national health number, the prescribing physician’s number, the ATC (anatomical-therapeutic-chemical) code of the drug delivered, the number of packs, the number of units per pack, the dosages, the unit cost per pack, and the prescription date. Using the numeric code released to each citizen by the LHU as a unique identifier, this database was linked with the Beneficiaries’ Database, listing some patients’ demographic characteristics such date of birth, sex, place of residence, physician licence number, and registration and end of registration date, and the Hospital Discharge Database, which includes all hospitalization data with the discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision (ICD 9), and the Mortality Database, where death data are recorded. It was not possible to retrieve the cause of death from death certificates. Universal health-care coverage in Italy allows completeness and comprehensiveness of the information contained in these databases, which have been used in previous epidemiological studies.17 The Italian Ministry of Health reported that Tuscany archives are 100% complete and 95% accurate.18
In order to guarantee patient privacy, each subject was assigned an anonymous univocal alphanumeric code.
Cohort definition
This was a retrospective cohort study, which included only new AHT users, ≥18 years of age, with a complete history of prescriptions and clinical outcome data over the study period. Subjects were enrolled if they had at least 1 prescription of AHT (diuretics [ATC code C03], excluding loop diuretics [ATC code C03C] which are mainly used for heart failure, β-blockers [ATC code C07], calcium-channel blockers [ATC code C08], angiotensin-converting enzyme inhibitors [ATC code C09A/B], angiotensin-receptor blockers [ATC code C09C/D], or other AHT drugs [ATC code C02]) dispensed between January 1, 2004 and December 31, 2006 (enrolment period). The date of the first AHT claim was defined as the enrolment date. Subjects were defined new users if they had not been prescribed any AHT between January 1, 2002 and the enrolment date.
In order to prevent inclusion of subjects prescribed with any of the index drugs for indications other than hypertension, we excluded subjects who had been diagnosed with heart failure (ICD9 code 428.x), ischemic heart disease (ICD9 code 410 through 414.x), cerebrovascular disorders (ICD9 code 430 through 438.x), or other CV diseases (ICD9 code 390 through 400.x, 406 through 459 excluding the aforementioned diagnosis codes) before the enrolment date and those prescribed with nitrates in the year before the enrolment date.19 Furthermore, we also excluded records of subjects who died, moved to other LHU or were hospitalized with a diagnosis of stroke or AMI in the 6 months after the enrolment date, in order to ensure a minimum time interval for adherence assessment: in fact, the use of short-term time intervals to assess adherence has been shown not to reflect accurately long-term behaviors.20,21
Adherence to AHT
Adherence to AHT was estimated as the percentage of days a subject had tablets available (proportion of days covered, PDC), from the first delivery of AHT until either death, moving to another LHU, hospitalization for AMI or stroke, or July 1, 2007, whichever occurred earlier. The interval was separated into treatment episodes of continuous AHT use based on the method of Catalan and LeLorier.22 A treatment episode was measured as the time-span between the starting date of the first AHT dispensing until the last day of the final AHT supply. The latter date included allowance for a possible gap after the final dispensing within the specific episode. Prescriptions filled near the end of the interval contributed days till that date.
Prescriptions containing more than 1 drug contributed a) the sum of the days’ supply of all drugs, in case of drugs from the same AHT drug class, because a possible stockpiling of medication was considered; b) the lower days’ supply drug value, in case of drugs from different AHT drug classes, identifying them as a combined therapy.
The PDC corresponded to the total of number of days’ supply of medication dispensed within each episode, divided by the total length of the interval and multiplied by 100. Subjects were grouped into 4 adherence categories as follows: poor (PDC ≤ 40%), moderate (PDC 41% to 60%), good (PDC 61% to 80%), and excellent adherence (PDC > 80%). When PDC was ≤20%, indication for AHT treatment was considered uncertain or questionable and, therefore, the corresponding subjects were excluded,23 of whom 77.2% received only 1 and 14.6% only 2 prescriptions.
Outcomes
Study outcomes were all-cause mortality and the first hospitalization for fatal or nonfatal CV events, represented by a principal discharge diagnosis of AMI (ICD 9 code 410.x) or stroke (ICD 9 codes 430 through 438.x). Patients were followed-up from the date of enrolment until study closure (December 31, 2007) or the date of cancellation from the enrollees’ list in the LHU of Florence. Median follow-up duration was 1.9 years, with a maximum of 3.5 (first 6 months after the enrolment date excluded).
Statistical analysis
Data were summarized as mean ± standard deviation (SD) for continuous variable and as numbers (percentages) of subjects for categorical variables. Pearson’s chi-square and one-way ANOVA tests were used to evaluate differences in baseline characteristics across adherence levels.
Bivariate and multivariable Cox proportional hazard regression models (Table 2, Models 1 and 2, respectively) were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of death and CV events, as combined and separate outcomes, as a function of adherence categories. To adjust for potential confounders, in the multivariable model we included age, gender, and presence of specific treatments in the year prior to the enrolment date, taken as proxies for the diagnoses of diabetes mellitus (hypoglycemic agents, ATC code A10), dyslipidemia (hypolipidemic agents, ATC code C10), heart disease (cardiac therapy, ATC code C01, excluding nitrates, ATC code C01DA), and atherosclerotic disease (platelet aggregation inhibitors, ATC cod B01AC). Visual inspection of the survival curves confirmed that the assumption of proportional hazards was not violated.
Table 2.
Adherence to antihypertensive medications and risk of the combined outcome of all-cause death, stroke, or acute myocardial infarction, estimated by Cox proportional hazards models
| Model 1
|
Model 2
|
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Adherencea | <0.001a | <0.001b | ||
| Poor | 1.00 | 1.00 | ||
| Moderate | 0.94 (0.80–1.11) | 0.464 | 0.87 (0.74–1.03) | 0.104 |
| Good | 0.77 (0.65–0.90) | 0.001 | 0.69 (0.58–0.81) | <0.001 |
| Excellent | 0.61 (0.53–0.70) | <0.001 | 0.53 (0.46–0.61) | <0.001 |
| Age, years | ||||
| <45 | – | – | 1.00 | |
| 45–65 | – | – | 2.67 (1.84–3.88) | <0.001 |
| >65 | – | – | 10.48 (7.31–15.03) | <0.001 |
| Gender | ||||
| Male | – | – | 1.00 | |
| Female | – | – | 0.62 (0.55–0.69) | <0.001 |
| Associated drug therapyc,d | ||||
| Hypoglycemic agents | – | – | 1.67 (1.41–1.99) | <0.001 |
| Lipid-lowering agents | – | – | 0.70 (0.54–0.91) | 0.006 |
| Cardiac therapy | – | – | 1.69 (1.29–2.23) | <0.001 |
| Platelet aggregation inhibitors | – | – | 1.66 (1.42–1.93) | <0.001 |
Notes: Bivariate and multivariable risks are shown in Models 1 and 2, respectively; a total of 1263 events in 31,306 subjects was considered in the models;
defined as in Table I;
for trend;
absence of medication as reference;
one year before the enrolment date.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Sensitivity analyses were used to assess the robustness of our analysis in the presence of potential biases.
Two-tailed P-values < 0.05 were considered statistically significant. All analyses were conducted using SPSS for Windows, version 15.0.
Results
Out of an initial selection of 63,027 records of a new prescription for AHT agents, 31,721 (50.3%) were excluded because of hospitalization for CV disease prior to enrolment (2,896, 4.6%), use of nitrates in the previous year (539, 0.9%), cancellation from the LHU list or occurrence of a study outcome in the 6 months after enrolment (1269, 2.0%), or PDC ≤ 20% (27,017, 42.8%). Thus, 31,306 patients (15,031 men, 48.0%) were included, whose mean age was 60.2 ± 14.5 years (range 18 to 101 years). Use of hypoglycemic agents, lipid-modifying agents, cardiac therapy, and platelet aggregation inhibitors is shown in Table 1.
Table 1.
Characteristics of subjects newly treated with antihypertensive medications, by level of adherence to treatment
| Adherencea | Total | |||||
|---|---|---|---|---|---|---|
| Poor | Moderate | Good | Excellent | P value | ||
| Subjects, n (%) | 8038 (25.7) | 4640 (14.8) | 5651 (18.1) | 12977 (41.5) | 31306 (100.0) | |
| Age, mean (SD), years | 57.3 (17.3) | 60.3 (15.2) | 61.6 (13.5) | 61.4 (12.5) | <0.001 | 60.2 (14.5) |
| Male (%) | 43.6 | 43.9 | 45.0 | 53.5 | <0.001 | 48.0 |
| Associated drug therapyb | ||||||
| Hypoglycemic agents (%) | 4.4 | 5.2 | 5.6 | 7.0 | <0.001 | 5.8 |
| Lipid lowering agents (%) | 4.3 | 4.6 | 4.9 | 5.3 | 0.007 | 4.9 |
| Cardiac therapy (%) | 1.3 | 1.7 | 1.3 | 1.6 | 0.190 | 1.5 |
| Platelet aggregation inhibitors (%) | 5.8 | 6.1 | 7.2 | 7.1 | <0.001 | 6.6 |
Notes:
Defined on the basis of the proportion of days covered (PDC): poor, PDC ≤ 40%; moderate, PDC 41% to 60%; good, PDC 61% to 80%; excellent, PDC > 80%.
One year before the enrolment date.
Adherence to AHT
Adherence to AHT was poor in 8038 patients subjects (25.7%), moderate in 4640 (14.8%), good in 5651 (18.1%), and excellent in 12,977 (41.5%) (Table 1). Poorly adherent patients were younger, predominantly women, and with lower prevalence of associated hypoglycemic, lipid-lowering, and platelet aggregation inhibitor agents (Table 1), whereas use of cardiac therapy was limited, uncommon, and unrelated to adherence level.
Health outcomes
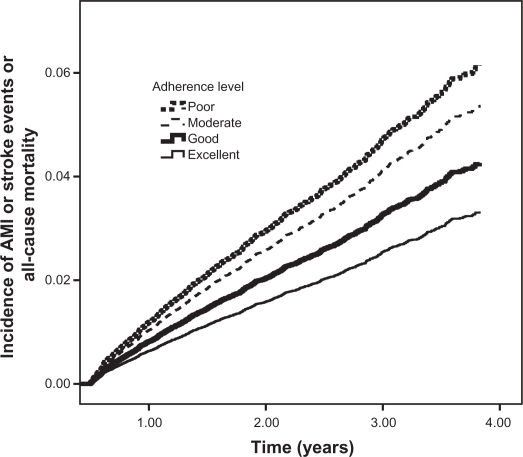
Out of 31,306 patients, a total of 1263 (4.0%) combined endpoints was seen in the follow-up, including 768 deaths, 370 strokes, and 191 AMI. Incidence of the combined endpoint decreased significantly with increasing adherence, being 29.5, 24.8, 19.6, and 16.1 per 1000 person-years in patients whose adherence was poor, moderate, good, and excellent, respectively. The corresponding bivariate HRs are shown in Table 2, Model 1. This association remained unaffected when adjusting for covariates in multivariable Model 2, Table 2 and Figure 1: the risk of the combined endpoint increased stepwise with decreasing adherence as well as with advancing age, was greater in males and in users of hypoglycemic agents or platelet aggregation inhibitors or cardiac therapy, and lower in the presence of treatment with lipid-lowering agents.
Figure 1.
Combined endpoint of all-cause death, stroke, or acute myocardial infarction (AMI) curves in 31,340 subjects newly treated with antihypertensive therapy, by levels of adherence to treatment.
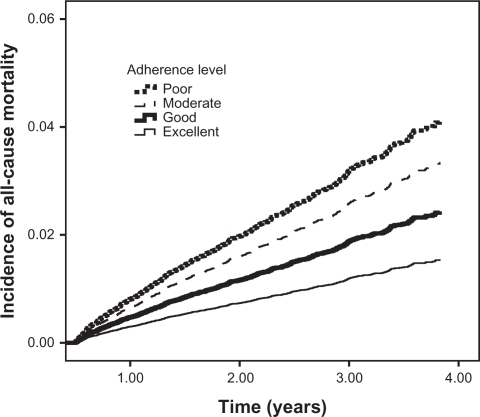
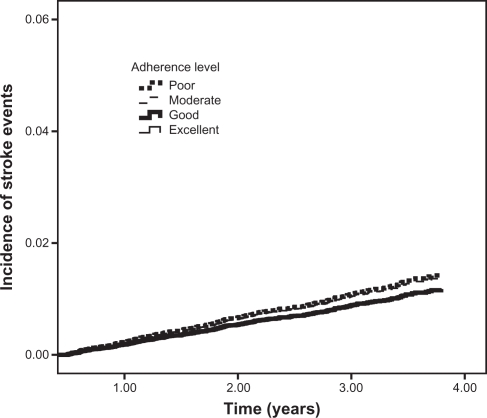
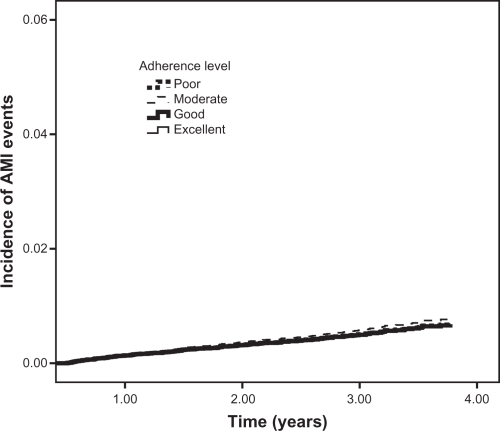
The results obtained for the individual endpoints in separate analyses were similar for the outcome of all-cause mortality, as shown by P for trend values (Table 3 and Figure 2). The analyses for the outcomes of stroke and AMI showed no statistically significant differences between individual levels of adherence and the study outcome (Table 3 and Figures 3 and 4).
Table 3.
Adherence to antihypertensive medications and risk of all-cause death, stroke, and acute myocardial infarction, estimated in separate multivariable Cox proportional hazards models
| All-cause death
|
Stroke
|
Acute myocardial infarction
|
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Adherencea | <0.001b | 0.381b | 0.877b | |||
| Poor | 1.00 | 1.00 | 1.00 | |||
| Moderate | 0.81 (0.66–0.99) | 0.038 | 0.96 (0.69–1.33) | 0.799 | 1.14 (0.71–1.83) | 0.592 |
| Good | 0.59 (0.48–0.72) | <0.001 | 0.81 (0.59–1.11) | 0.197 | 0.98 (0.62–1.55) | 0.939 |
| Excellent | 0.37 (0.31–0.45) | <0.001 | 0.82 (0.63–1.07) | 0.138 | 0.96 (0.65–1.40) | 0.825 |
Notes: Totals of 768 deaths in 30,668 subjects and 370 strokes and 191 acute myocardial infarctions in 31,340 subjects were considered in the models; HRs with 95% CIs adjusted for age, gender, and use of hypoglycemic agents, lipid-lowering agents, cardiac therapy, and platelet aggregation inhibitors before the enrolment date;
defined as in Table I;
for trend.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 2.
Incidence of all-cause mortality among new antihypertensive therapy users.
Figure 3.
Incidence of stroke events among new antihypertensive therapy users.
Figure 4.
Incidence of acute myocardial infarction (AMI) events among new antihypertensive therapy users.
Sensitivity analysis
To examine the robustness of our results, we did several additional analyses.
First, to ensure that our method did not introduce a survival bias, we re-estimated the association between adherence-study outcomes among subjects surviving and free of AMI or stroke for at least 3 months and 1 year after the enrolment date.
Second, the primary categorization of adherence based on PDC level: poor (PDC ≤ 40%), moderate (PDC, 41% to 60%), good (PDC, 61% to 80%), excellent (PDC > 80%) was arbitrary. However, we examined the effect of medication adherence on outcomes using different definitions of PDC cutoffs: quartiles, ≤25%, 26% to 50%, 51% to 75%, >75%.
Third, as previous studies described the relationship between CV disease severity factors, level of adherence to AHTs and, consequently, the development of adverse outcome events,19,23,24 to minimize the bias, the adherence-study outcomes association was also evaluated across different risk subgroups: older patients (>65 years), patients with at least 1 medication evaluated in the year before the enrolment date.
The results of these additional analyses were consistent with our primary findings. Data are shown in Tables 4 and 5.
Table 4.
One-way sensitivity analysis for the risk of the combined outcome of all-cause death, stroke, or acute myocardial infarction
| Adherencea |
||||||
|---|---|---|---|---|---|---|
| Moderate
|
Good
|
Excellent
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Absence of study outcomes for at least 3 months | 0.94 (0.81–1.08) | 0.374 | 0.70 (0.60–0.81) | <0.001 | 0.55 (0.50–0.64) | <0.001 |
| Absence of study outcomes for at least 1 year | 0.87 (0.71–1.07) | 0.196 | 0.72 (0.59–0.88) | 0.001 | 0.56 (0.47–0.67) | <0.001 |
| Adherence categories: quartilesb | 0.88 (0.72–1.07) | 0.152 | 0.73 (0.64–0.83) | <0.001 | 0.51 (0.43–0.59) | <0.001 |
| Adherence categories: ≤25, 26–50, 51–75, >75%c | 0.83 (0.68–1.01) | 0.058 | 0.72 (0.59–0.88) | 0.002 | 0.50 (0.40–0.59) | <0.001 |
| Age > 65 | 0.85 (0.71–1.03) | 0.100 | 0.67 (0.55–0.79) | <0.001 | 0.52 (0.44–0.61) | <0.001 |
| At least 1 comorbidity | 0.96 (0.84–1.13) | 0.417 | 0.70 (0.55–1.02) | 0.059 | 0.54 (0.49–0.70) | 0.006 |
Notes:
Cases of proportion of days covered (PDC) ≤ 20% were excluded;
the first quartile corresponds to the poor category of adherence, the second quartile to the moderate category, the third quartile to the good category, the fourth quartile to the excellent category;
PDC ≤ 25% corresponds to the poor category, PDC 26% to 50% to moderate, PDC 51% to 75% to good, PDC > 75% to excellent.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 5.
One-way sensitivity analysis for the risk of all-cause death
| Adherencea |
||||||
|---|---|---|---|---|---|---|
| Moderate
|
Good
|
Excellent
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Absence of study outcomes for at least 3 months | 0.86 (0.72–1.03) | 0.095 | 0.60 (0.50–0.72) | <0.001 | 0.39 (0.35–0.48) | <0.001 |
| Absence of study outcomes for at least 1 year | 0.81 (0.63–1.04) | 0.098 | 0.62 (0.48–0.79) | <0.001 | 0.39 (0.33–0.51) | <0.001 |
| Adherence categories: quartilesb | 0.79 (0.53–1.07) | 0.103 | 0.61 (0.54–0.75) | <0.001 | 0.36 (0.30–0.42) | <0.001 |
| Adherence categories: ≤25, 26–50, 51–75, >75%c | 0.84 (0.66–1.06) | 0.140 | 0.60 (0.51–0.78) | 0.001 | 0.35 (0.28–0.45) | <0.001 |
| Age > 65 years | 0.83 (0.64–1.07) | 0.148 | 0.57 (0.41–0.73) | <0.001 | 0.36 (0.31–0.45) | <0.001 |
| At least 1 comorbidity | 0.75 (0.50–1.12) | 0.162 | 0.59 (0.47–0.71) | 0.001 | 0.40 (0.29–0.68) | <0.001 |
Notes:
cases of proportion of days covered (PDC) ≤20% were excluded;
the first quartile corresponds to the poor category of adherence, the second quartile to the moderate category, the third quartile to the good category, the fourth quartile to the excellent category;
PDC ≤ 25% corresponds to the poor category, PDC 26% to 50% to moderate; PDC 51% to 75% to good, PDC > 75% to excellent.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
Despite the considerable magnitude and the remarkable consequences of suboptimal adherence to AHT shown in randomized controlled trials as well as in observational studies,1–3 in clinical practice, evidence of the association between adherence to AHT, all-cause mortality, and CV morbidity is still limited. Existing publications focus on newly diagnosed hypertensive patients, diabetics, and patients with previous CV events – that is, mostly on secondary prevention. Studied outcomes include CV hospitalizations (mainly in primary prevention studies19,23,24), and all-cause hospitalizations or all-cause mortality (mainly in secondary prevention studies20,25–27). The present study analyzed all-cause mortality, and the first occurrence of stroke and AMI among hypertensive patients newly treated with AHT, in primary prevention.
The risk of all-cause mortality, stroke, and AMI decreased progressively as adherence to AHT increased. Compared with poor adherence, the risk of experiencing a major health outcome decreased stepwise from good (31% lower risk) to excellent (47% lower risk) (Table 2). The risk gradient did not attain statistical significance in subjects with moderate adherence, thus possibly suggesting some treatment effectiveness threshold. This is an original finding of the present study, due to the large number of records available, which enabled the definition of 4 levels of adherence, whereas most of the previous studies referred to just 2 levels of adherence.19,23 As this is an observational study, the allocation of patients into different adherence groups was not randomized and it might have produced study groups not similar in their baseline CV risk profile. So this bias might have altered the observed reduction of the occurrence of events associated with increased adherence. However, the results obtained after controlling for baseline differences in the regression model and stratifyng the patient population into different risk subgroups in the sensitivity analisys confirmed the robustness of our findings.
In separate analyses of the individual endpoints, the risk associated with poor adherence was significant for all-cause mortality and directionally similar for both stroke and AMI, though with lower, nonsignificant gradients. Thus, the present study shows that adherence to AHT is associated with a substantial reduction in mortality risk also in primary prevention, whereas previous findings were restricted to secondary prevention.20,25,26 In the analysis of the occurrence of each single health outcome, the reduction in the risk of stroke and AMI was lower than that reported by Breekveldt-Postma et al.19 However, in our study, the duration of the follow-up was much shorter (median of 1.9 years in our study versus up to a maximum of 10 years) and the observed number of strokes (370 versus 1293) and AMI (191 versus 826) was lower. Another study, recently published by Mazzaglia et al, reported greater risk reduction with increasing adherence. Compared with the present study, the endpoint of the study by Mazzaglia et al was a combination of a wider range of CV events, not only stroke and AMI, and the follow-up available was longer and extended up to 4.6 years.24 Previous studies with almost the same follow-up duration (2.4 years) as ours reported higher risk reduction only in highest-risk patients (eg, patients with a previous episode of AMI20). Nevertheless, the favorable trend in reducing CV events is more evident for nonfatal stroke than nonfatal AMI, thus confirming that for cause-specific events AHT is associated with a major reduction in the risk of fatal or nonfatal stroke, but coronary events are reduced as well, though to a lesser degree.6
Limitations
Several potential limitations of this study should be considered.
First, there was a lack of clinical data. The present study was conducted by cross-linking Medications Prescriptions Database with Health-assisted Subjects’ Database, Hospital Discharge Database, and Mortality Database. These ad hoc databases, comparable to the healthcare claim databases that have been utilized for years in United States and Canada for outcome research,28,29 were originally constructed to serve a billing role, the reimbursement for services provided. Thus, information on clinical data was not available and, as a consequence, the adjustment for blood pressure levels was not permitted.
However, the confounding effect of baseline blood pressure on the association between adherence to AHT agents and CV events is probably limited, as suggested by a recent study in a general practice population, where baseline systolic and diastolic blood pressure values were comparable between low-, intermediate-, and high-adherence patients.24 Moreover, a recent meta-analysis of 147 trials30 showed a benefit in lowering blood pressure by antihypertensive drugs whatever the patients’ blood pressure was, thus avoiding the need to measure blood pressure routinely.
Second, a formal diagnosis of arterial hypertension was not available. In the present study, indeed, the diagnosis was surrogated by prescription of AHT, thus leaving room for potential misclassification, as only some of the subjects treated with AHT drugs may have been truly hypertensive. However, the exclusion of subjects with only 1 or 2 prescriptions and of those with previous hospitalizations or therapies for CV diseases should have minimized this misclassification.
Third, the measurement of drug use was based on dispensed medications and actual drug-taking behaviors remain unknown. Empirical evidence suggests, however, a strong correlation between pharmacy claims and drug exposure.31,32
Fourth, information bias may also have been possible. As hospital discharge information was obtained from the Florence Hospital Discharge Database, we could not track hospitalizations collected in other Italian LHUs’ databases.
Finally, the data source utilized did not allow for lifestyle adjustments, such as physical activity, smoking, and other determinants of CV morbidity and mortality. The potential confounding effect of these variables on the association between adherence and CV events should be analyzed in future studies.
Conclusions
The findings from the present study indicate that suboptimal adherence to AHT is associated with an avoidable number of all-cause deaths and hospitalizations for CV disease in primary prevention. In a public health perspective, this evidence underlines the need for monitoring and improving medications adherence in clinical practice. Studies based on administrative databases, like the present one, offer several advantages, including a prompt, easily updated, and representative picture of the monitored cohorts for a prolonged duration of observation, with highly generalizable results.
Consequences of poor adherence with AHT extend beyond health prevention and involve costs for CV prevention and economic sustainability of national health services. Although the therapeutic benefits of AHT are well understood, the potential economic advantages are often overlooked in the public debate, which is dominated by concerns about escalating expenditure for prescription drugs. Increased drug utilization can provide a net economic return when it is driven by improved adherence to guidelines-based therapy.16 Although drug costs are a relatively small percentage of total healthcare costs,33,34 they have high leverage – a small increase in drug costs (associated with improved adherence) can produce a much larger reduction in the general economic burden for health.
Acknowledgments
This study was funded by Takeda Italia Farmaceutici. The cooperation of Dr Alessandra Fionda is gratefully acknowledged.
Footnotes
Disclosure
None of the authors declare any conflict of interest and all persons who contributed to the work meet the criteria for authorship.
References
- 1.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 2.Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood pressure lowering regimens on major cardiovascular events: second cycle of prospectively designed overviews. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers J. Comparison of various blood pressure lowering treatments on the primary prevention of cardiovascular outcomes in recent randomised clinical trials. Clin Exp Hypertens. 2004;26:709–719. doi: 10.1081/ceh-200032093. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. BMJ. 2008;336:1121–1123. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Heart Association High Blood Pressure. 2009. http://www.americanheart.org/presenter.jhtml. Accessed April 24, 2009.
- 6.Hansson L, Lloyd A, Anderson P, et al. Excess morbidity and cost of failure to achieve targets for blood pressure control in Europe. Blood Press. 2002;11:35–45. doi: 10.1080/080370502753543945. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano TA, Bitton A, Anand S, et al. The global cost of non optimal blood pressure. J Hypertens. 2009;27:1472–1477. doi: 10.1097/HJH.0b013e32832a9ba3. [DOI] [PubMed] [Google Scholar]
- 8.Degli Esposti L, Valpiani G. Pharmacoeconomic burden of undertreating hypertension. Pharmacoeconomics. 2004;22:907–928. doi: 10.2165/00019053-200422140-00002. [DOI] [PubMed] [Google Scholar]
- 9.Costa FV, D’Ausilio A, Bianchi C, et al. Adherence to anti-hypertensive medications: a review and update. High Blood Pressure and Cardiovascular Prevention. 2009;16:101–110. [Google Scholar]
- 10.Fitz-Simon N, Bennett K, Feely A. A review of studies of adherence with antihypertensive drugs using prescription databases. Ther Clin Risk Manag. 2005;1:93–106. doi: 10.2147/tcrm.1.2.93.62915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Safety. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 13.ISPOR Medication Compliance Special Interest Group http://www.ispor.org/sigs/medication.asp. Accessed 23 June, 2005.
- 14.Elliott WJ. Improving outcomes in hypertensive patients: focus on adherence and persistence with antihypertensive therapy. J Clin Hypertens. 2009;11:376–382. doi: 10.1111/j.1751-7176.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muszbek N, Brixner D, Benedict A, et al. The economic consequences of non-compliance in cardiovascular disease and related conditions: a literature review. Int J Clin Pract. 2008;62:338–351. doi: 10.1111/j.1742-1241.2007.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 17.Di Bari M, Balzi D, Roberts AT, et al. Prognostic stratification o folder persons based on simple administrative data: development and validation of the “Silver Code”, to be used in emergency department triage. J Gerontol A Biol Sci Med. 2010;65:159–164. doi: 10.1093/gerona/glp043. [DOI] [PubMed] [Google Scholar]
- 18.Ministero del Lavoro, della Salute e delle Politiche Sociali Rapporto annuale sulla attività di ricovero ospedaliero. Anno. 2005. http://www.ministerosalute.it/programmazione/sdo/sezDocumenti.jsp?id=148&label=osp. Accessed April 24, 2009.
- 19.Breekveldt-Postma NS, Penning-van Beest FJ, Siiskonen SJ, et al. The effect of discontinuation of antihypertensives on the risk of acute myocardial infarction and stroke. Curr Med Res Opin. 2008;24:121–127. doi: 10.1185/030079908x253843. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Safety. 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 22.Catalan VS, LeLorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value Health. 2000;3:417–426. doi: 10.1046/j.1524-4733.2000.36006.x. [DOI] [PubMed] [Google Scholar]
- 23.Kettani FZ, Dragomir A, Cŏté R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40:213–220. doi: 10.1161/STROKEAHA.108.522193. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 25.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonad-herence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 27.Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation. 2007;116:737–744. doi: 10.1161/CIRCULATIONAHA.106.669101. [DOI] [PubMed] [Google Scholar]
- 28.Motheral BR, Fairman KA. The use of claims databases for antihy-pertensive drugs and associated hospitalization. Clin Ther. 1997;19:346–366. doi: 10.1016/s0149-2918(97)80122-1. [DOI] [PubMed] [Google Scholar]
- 29.Birnbaum HG, Cremieux PY, Greenberg PE, et al. Using health care expenditures for healthcare claims data for outcome research and pharmaco-economic analyses. Pharmacoeconomics. 1999;16:1–8. doi: 10.2165/00019053-199916010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. J Am Board Fam Med. 2007;20:72–80. doi: 10.3122/jabfm.2007.01.060094. [DOI] [PubMed] [Google Scholar]
- 32.Elliott WJ. Compliance strategies. Curr Opin Nephrol Hypertens. 1994;3:271–278. [PubMed] [Google Scholar]
- 33.Leal J, Luengo-Fernandez R, Gray A. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J. 2006;27:1610–1619. doi: 10.1093/eurheartj/ehi733. [DOI] [PubMed] [Google Scholar]
- 34.Tarride JE, Lim M, DesMeules M, et al. A review of the cost of cardiovascular disease. Can J Cardiol. 2009;25:e195–e202. doi: 10.1016/s0828-282x(09)70098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]