Abstract
Approximately 5% of people that are hospitalized for any reason develop acute kidney failure, which, in some cases, progresses to a chronic condition resulting in fibrosis of the kidney and permanent changes in the organ’s function. Two new studies suggest that cell cycle arrest of epithelial cells and epigenetic modifications have key roles in the switch to chronic disease (pages 535–543 and 544–550).
Direct damage to the kidneys caused by a critical illness or persistent irritant is the most complicated cause of renal failure and accounts for 25–40% of all cases1. When acute renal injury occurs, normal tissue repair mechanisms are usually able to restore proper kidney function, provided that the injury is quickly discovered and treated successfully. However, if these repair mechanisms are disrupted or the injury-causing irritants persist, acute renal injury can progress into a chronic disorder, characterized by marked organ remodeling and fibrosis.
Understanding the mechanisms that regulate the transition from acute kidney injury to chronic fibrotic disease is important, because once fibrogenesis is initiated it can be incredibly difficult to switch off or reverse2. New studies by Yang et al.3 and Bechtel et al.4 in this issue of Nature Medicine now reveal two distinct but complementary mechanisms of fibrosis after acute kidney injury.
Normal healthy kidneys filter toxins from the blood and preserve the balance of bodily fluids and electrolytes. They allow a person to consume a variety of foods, drugs, vitamins, minerals and excess fluids without worry that toxic by-products will build up to harmful levels. Although healthy kidneys are incredibly resilient and typically recover quickly from a variety of acute tissue-damaging insults, in some cases, acute kidney injury can contribute to a persistent inflammation of the renal tubules and surrounding tissues. This inflammation leads to incomplete kidney repair and the development of fibrosis, which is a primary determinant in the progression to end-stage renal failure5. Although the epithelial cells lining renal tubules are thought to have an active role in the development of fibrosis, the mechanisms by which they influence the progression and resolution of fibrosis remain unclear.
A key determinant of successful kidney repair is the ability of tubular epithelial cells to proliferate and replace damaged cells after injury6. Indeed, efficient epithelial cell renewal enables the kidney to recover from many acute ischemic, obstructive and toxic insults. Yang et al.3 showed that the switch from normal wound repair in the kidney to pathogenic fibrosis occurs when epithelial cells stall in the G2/M phase of the cell cycle, corresponding to the transition from interphase to mitosis7.
Using a variety of approaches to induce acute kidney damage, including an animal model of ischemia reperfusion injury, Yang et al.3 showed that tubular injury and interstitial inflammation are characterized by prominent proliferation of tubular epithelia cells, confirming that activation of epithelial cell renewal is an important feature of successful repair. However, in severe cases where acute kidney damage was accompanied by the development of fibrosis, they observed a marked increase in the number of proximal tubule epithelial cells arresting in the G2/M phase of the cell cycle3. In contrast to actively proliferating epithelial cells, the arrested cells produced high amounts of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor, suggesting that cell cycle arrest converts normal epithelial cells to a phenotype that promotes the growth and activation of fibroblasts8.
The authors observed a similar effect in vitro when they arrested human, rat and pig proximal tubular epithelial cell lines in the G2/M phase3. As would be expected with increased production of TGF-β1, conditioned media obtained from these arrested cells stimulated proliferation of fibroblasts and collagen synthesis in vitro. Furthermore, when the authors induced epithelial G2/M cell cycle arrest in vivo, either with a cyclin-dependent kinase-1 inhibitor or with taxol, a microtubule stabilizing agent, the expression of genes that induce fibroblast activation increased dramatically, as did kidney fibrosis3. Conversely, if the authors reversed epithelial G2/M arrest using ATM (mutated in ataxia-telangiectasia) and p53 inhibitors, epithelial cells produced lower amounts of profibrotic cytokines and the development of fibrosis was reduced3.
These findings confirm a direct and crucial role for cell cycle dysregulation in the progression of fibrosis after acute kidney injury.
Bechtel et al.4 also investigated the mechanisms of fibrogenesis after acute kidney injury. They, however, examined why fibrogenesis progresses even after the tissue-damaging insult is eliminated4. They hypothesized that the maintenance of fibrosis might represent a failure of activated fibroblasts to return to their resting state.
By screening the genome for changes in methylation, they identified several changes unique to fibroblasts obtained from fibrotic human kidneys as compared to nonfibrotic human kidneys. DNA methylation involves the addition of a methyl group to DNA at specific locations—for example, to the fifth carbon of the cytosine pyrimidine ring or to the nitrogen at position 6 in the adenine purine ring4. This type of epigenetic modification reduces gene expression, and it can be inherited through cell division. As a result, it is considered a stable transformation of the gene expression pattern for a particular population of cells9.
Although the authors identified 12 candidate genes in their initial methylation screen, they focused on RASAL1, a suppressor of the Ras protooncogene4. Ras hyperactivity is known to promote growth factor–independent autonomous cell proliferation, essentially turning normal cells into tumor cells10. The authors hypothesized that the epigenetic silencing through hypermethylation of RASAL1 has a key mechanistic role in the persistent activation of fibroblasts in kidneys after acute injury4.
In their first series of experiments, they showed that fibroblast activation after injury could be suppressed by demethylating agents and that fibrosis was attenuated if the hyper-methylation of the human RASAL1 was reversed4. Conversely, when RASAL1 activity was knocked down in fibroblasts, the corresponding increase in Ras activity induced spontaneous fibroblast proliferation and collagen synthesis. Finally, studies with DNA methyltransferase–deficient mice (Dnmt1−/− mice) and Ras inhibitors confirmed that epigenetic silencing of Rasal1 and the resulting increase in Ras activity promote fibroblast activation. Together, these studies provide a new molecular explanation for the sustained activation of fibroblasts that is often observed during the conversion of acute kidney injury to chronic fibrosis.
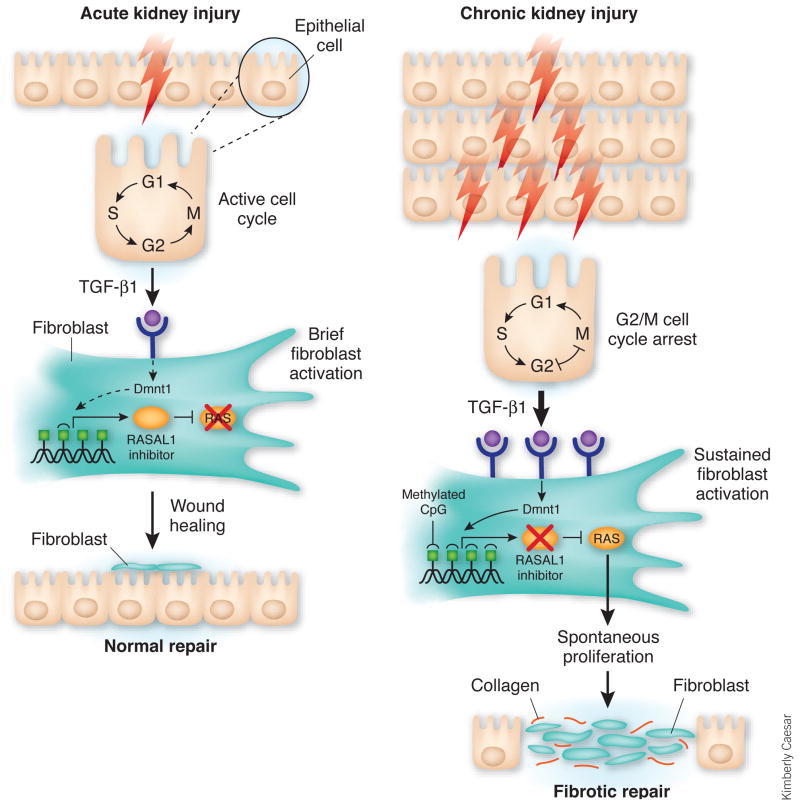
Although both groups identified previously undescribed mechanisms of kidney fibrosis, the two studies point to the profibrotic cytokine TGF-β1 as a possible common link. When epithelial cells stall in the G2/M phase of the cell cycle after injury, they produce TGF-β1 (Fig. 1). This cytokine, in turn, activates the wound-healing pathway. When the kidney-damaging insult is of short duration, the G2/M cell cycle arrest is brief, resulting in minimal TGF-β1 production by epithelial cells and rapid restoration of normal tissue architecture3. However, when the tissue-damaging insult persists, sustained production of TGF-β1 by the stalled epithelial cells induces epigenetic changes in fibroblasts and slowly transforms these cells into tumor-like myofibroblasts that proliferate and secrete collagen in a progressively growth factor–independent manner4.
Figure 1.
Epithelial cell cycle arrest in G2/M and epigenetic changes in fibroblasts coordinately promote kidney fibrosis. After acute kidney injury, damaged epithelial cells initiate a wound-healing program. When the injury is of short duration (left), epithelial cells located near the lesion secrete modest amounts of TGF-β1, which induces fibroblast proliferation and secretion of extracellular matrix components such as collagen I. Epithelial cells also activate the cell cycle and begin to proliferate. In the absence of TGF-β1 and other profibrotic mediators, fibroblasts quickly return to the resting state, undergo apoptosis or both, whereas epithelial cells expand in number and quickly repair the site of tissue damage. In cases where the kidney injury is persistent or repeated (right), increasing numbers of epithelial cells stall between the G2 and M phases of the cell cycle, which stimulates the production of considerable amounts of TGF-β1. If the cell cycle is reactivated, TGF-β1 production decreases, and normal wound repair commences once again. However, if the epithelial cell cycle is arrested for an extended length of time, TGF-β1 production persists and contributes to downstream epigenetic modifications in fibroblasts that slowly transform these cells into activated myofibroblasts. Myofibroblasts proliferate and secrete collagen in a progressively growth factor–independent manner. Although a variety of genetic modifications take place in fibroblasts at this stage, the gene encoding RASAL1 seems to become hypermethylated by the methytransferase Dnmt1. This epigenetic modification turns down RASAL1 expression leading to persistent Ras activation, perpetual fibroblast proliferation and fibrogenesis in the kidney. Epigenetic changes, such as RASAL1 methylation, are stable modifications that can be inherited through multiple cell divisions. This pathway shows how normal kidney repair mechanisms can quickly transform into pathogenic fibrotic responses.
It seems likely that the mechanisms of kidney fibrosis described in both reports are directly and sequentially linked. Indeed, it will be interesting to determine whether inducing G2/M cell cycle arrest in epithelial cells or reversing it during chronic kidney injury directly influences the downstream hypermethylation of RASAL1 in fibroblasts.
Several soluble mediators besides TGF-β1 might also participate in the activation of collagen-secreting myofibroblasts11. Therefore, it will be important to establish whether other profibrotic cytokines, chemokines and growth factors induce epigenetic changes in fibroblasts during the evolution of fibrosis.
Finally, it will be exciting to see if these findings can be exploited therapeutically and lead to the development of new drugs that prevent or possibly even reverse established kidney fibrosis.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Eddy AA. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechtel W, et al. Nat Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 6.Duffield JS, et al. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kops GJ, Weaver BA, Cleveland DW. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 8.Branton MH, Kopp JB. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 10.Kolfschoten IG, et al. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]