Abstract
Objective
To examine the short-term effects of the nicotine patch or nasal spray on measures of nicotine exposure, withdrawal symptoms, and on maternal and fetal heart rates in pregnant smokers.
Methods
We measured nicotine/cotinine concentrations and maternal and fetal heart rates during an 8-hour monitoring session while smoking and again after 4 days of nicotine patch (15 mg/16 hours), nasal spray (recommended regimen of 24 doses per day), or placebo treatment. Nicotine withdrawal symptoms were assessed daily.
Results
Twenty-one subjects, who smoked an average of 17 cigarettes per day, completed both monitoring sessions. Nicotine concentrations decreased from baseline smoking concentrations in all groups (p = 0.002). Percent change in cotinine concentration differed across groups (reduction = 77% with placebo, 70% with nasal spray, and 48% with patch; p = 0.029). Maternal heart rate decreased in the placebo and nasal spray groups compared with the patch group (p = 0.021). The baseline fetal heart rate decreased in the placebo group throughout the second monitoring session, but increased slightly in the patch and nasal spray groups. The treatment by time interaction was marginally significant (p = 0.052). Daily cigarette craving decreased more in the patch versus the other groups (p = 0.025).
Conclusions
Nicotine patch and nasal spray reduce maternal nicotine exposure compared with smoking and may be effective for smoking cessation.
Introduction
Given the substantial health risks of smoking during pregnancy, the relative safety and potential utility of pharmacotherapy to enhance smoking cessation rates during pregnancy are important research issues [1, 2]. Nicotine replacement therapy may be particularly beneficial for heavier smokers (i.e., those who smoke at least 20 cigarettes per day), who are much less likely to quit during pregnancy than lighter smokers [2]. Moreover, the risks of smoking during pregnancy are dose proportional [3], suggesting that improving cessation rates among heavier smokers is particularly important.
The nicotine patch and nasal spray approximately double quit rates relative to placebo. A recent meta-analysis in non-pregnant smokers estimated the odds ratio (95% CI) of the nicotine nasal spray to be 2.3 (1.7–3.0) and the patch to be 1.9 (1.7–2.2) relative to placebo. Consequently, these nicotine replacement products are recommended as first-line treatment for non-pregnant smokers who smoke at least 10 cigarettes per day [4]. The patch is the slowest nicotine delivery system; releasing approximately 0.9mg nicotine/hour [5]. Treatment adherence is a major advantage of the patch since it is administered once daily. The nasal spray, the fastest delivery system, best approximates the rise in nicotine concentrations observed with smoking [6, 7]. Hence, an advantage of the nasal spray is that it relieves craving quickly, and therefore may be most useful in highly-dependent smokers [6]. On the other hand, the nasal spray is associated with a number of adverse effects (e.g., nasal irritation, sneezing) and although it relieves symptoms easily it may be difficult for patients to use, limiting its overall clinical utility.
The purpose of this study was to compare the short-term effects of the nicotine patch, nasal spray, or placebo with smoking on nicotine concentrations and withdrawal symptoms, and on maternal and fetal heart rates in pregnant smokers. These two products were chosen to compare a slow with a more rapid delivery of nicotine. Based on studies in non pregnant smokers, smoking would have the greatest effect, nicotine nasal spray would have an intermediate effect, and the patch would have the smallest effect on maternal heart rate and other measures of sympathetic activity [8,9]. To relieve withdrawal symptoms and to evaluate the effects of nicotine as delivered by two different nicotine preparations, we prescribed daily dosages of nicotine patch or nasal spray that was estimated to produce an overall nicotine exposure similar to smoking 10–15 cigarettes per day. We examined nicotine replacement therapies during the first week of use following smoking cessation, when nicotine withdrawal symptoms peak [10]. This also coincides with the occurrence of steady state concentrations of cotinine (the major metabolite of nicotine and a valid measure of overall nicotine exposure), which are expected to occur after the 3rd or 4th day of nicotine replacement therapy [5].
Methods
The Institution Review Board at the University of Connecticut Health Center approved the study. Participants were recruited from newspaper advertisements and from flyers in local physicians’ offices. Subjects were initially screened by telephone. Inclusion criteria included: 1) smoking ≥ 10 cigarettes per day; 2) ≥ 16 years of age; 3) singleton gestation; 4) good general health; 5) established prenatal care; 6) 28–36 weeks gestation; and 7) a desire to quit smoking. Exclusion criteria included: 1) an allergy to adhesive tape (as a proxy measure of sensitivity to the patch); 2) current sinus problems (which could be exacerbated by the inhaler); 3) known congenital anomaly; 4) fetal growth restriction (<5% as measured by a screening ultrasound); 5) current use of another smoking cessation treatment product; 6) current abuse or dependence on drugs other than nicotine; 7) insulin-dependent diabetes mellitus.
Screening
Potentially eligible subjects were invited to an in-person screening visit, at which time they gave written informed consent, underwent a medical history and physical exam, and were given a fetal ultrasound to identify anomalies and to measure growth. Subjects who smoked more than 15 cigarettes per day were instructed to decrease their use to 10–15 cigarettes per day at least 4 days prior to the first ultrasound session. Subjects were instructed not to smoke for 8 hours prior to their first monitoring session (i.e., overnight cigarette abstinence).
Monitoring Session 1
At 8 AM on the day of the first monitoring session, all subjects received a small breakfast. The smoking monitoring session was conducted in a negative pressure room equipped with a HEPA air filter. Subjects smoked an average of 1 cigarette per hour from 10 AM to 4 PM (i.e., a total of 7 cigarettes). Subjects smoked cigarettes of their own choice and baseline levels of nicotine during usual smoking were obtained. Timing of fetal measurements was planned to correspond with trough and peak nicotine concentrations associated with smoking. A baseline fetal heart rate strip (at least a 20 minute strip) was obtained prior to the first cigarette of the day and again after the fourth cigarette of the day. Nicotine and cotinine concentrations were monitored repeatedly throughout the 8-hour monitoring session (before and 2 minutes after smoking the first cigarette of the day, 2 minutes after the 4th cigarette of the day, and before and 2 minutes after the 7th cigarette of the day) to correspond with peak and trough nicotine concentrations of smoking.
Subjects who completed this first monitoring session were randomly assigned to one of three groups in double-blind fashion: nicotine nasal spray and placebo patch, placebo nasal spray and nicotine patch, placebo nasal spray and placebo patch. The research pharmacy used an urn randomization procedure [11], which balanced the assignment to groups using gestational age (i.e., using a cutoff of ≥ 32 weeks gestation). Subjects were given their medication at the end of the first monitoring session and instructed to begin it on their quit date. The placebo nasal spray contained black oleoresin (piperine) to mimic the sensation of nicotine in the nasal passage. The women were provided with pregnancy-specific smoking cessation materials, counseled to stop smoking (focusing on the risks of smoking during pregnancy and the benefits of cessation, identification of smoking triggers and coping strategies that could be used to manage triggers and nicotine withdrawal symptoms) and were instructed on the proper use of the medication (which included viewing a video on proper nasal spray use).
Subjects were instructed to use 24 doses of the nasal spray each day (one dose is 1 spray to each nostril; each dose contains 1 mg of nicotine, of which 60% is systemically absorbed), and to use the nicotine patch daily (16 hours of use, which delivers 15 mg of nicotine) beginning on their quit date. It was estimated that 24 doses of the spray per day and 16-hour use of the patch would provide nicotine concentrations comparable to those resulting from smoking 10–15 cigarettes per day (each cigarette delivers a systemic dose of approximately 1 mg nicotine based on studies in non-pregnant smokers [5, 12]).
The study nurse collected data using standardized questionnaires beginning on the quit date to evaluate medication adherence (number of times the nasal spray was used each day; on and off times of the nicotine patch), nicotine withdrawal symptoms [13] and adverse effects. We collected the data during a clinic visit on Days 1 and 3, and by telephone on Days 2 and 4. Cigarette abstinence was assessed throughout the study by self-report and confirmed using an exhaled CO concentration of < 8 ppm at the first and third visits and at the second monitoring session.
Monitoring session 2
On medication treatment Day 5, subjects underwent a second monitoring session that was identical to the first. Subjects were instructed not to use the patch or nasal spray for at least 8 hours before the session. The patch was placed on the subject’s arm at 10 am and it remained there until the end of the monitoring session. The subject administered 11 doses of nasal spray during this session. Since the nasal spray is the nicotine product that most closely mimics the pharmacokinetic and pharmacodynamic actions of nicotine delivered via smoking [7], the second monitoring session substituted 2 doses of nasal spray (administered 5 minutes apart)for a cigarette at key monitoring times from session 1 (i.e., 1st, 4th and 7th cigarettes).
Laboratory assessments
Nicotine/cotinine concentrations were measured by gas chromatography at Hennepin County Medical Center in Minneapolis, MN. The limits of assay detection are 5ng/mL for nicotine and 10ng/mL for cotinine. An obstetrician (WC) who was blinded to the monitoring session read the fetal heart rate strips for baseline heart rate.
Samples size
In previous studies total nicotine withdrawal symptom scores increased from 4.9 (SD = 1.0) to 10.0 (SD = 1.6) after overnight cigarette abstinence. Based upon this, we estimated that, after overnight abstinence, nicotine withdrawal scores for the placebo subjects would be 10.0 and for TDN and for NNS subjects they would be 4.9–10.0. A conservative estimate for the TDN and NNS groups is a nicotine withdrawal score of 8.0, with a pooled SD of 1.6. Nine subjects per group would have 80% power (p<.05) to detect this difference.
Data analysis
Baseline measures were compared across treatment conditions (nicotine nasal spray, nicotine patch and double placebo) using one-way analysis of variance (ANOVA) for continuous variables and Fisher’s exact test for categorical variables (with a Bonferroni correction for multiple comparisons). Repeated measures ANOVA was used to determine the effect of time (linear and quadratic), treatment conditions, and interaction between time and treatment conditions on craving and withdrawal symptoms over five measurement time points (screening, quit day, day 2, day 3, and day 4). The proportion of women in each group who quit was compared using Fisher’s exact test.
Daily average cotinine concentration at monitoring session 1 was compared across treatment conditions using one-way ANOVA. The change in daily average cotinine concentrations between monitoring sessions (pre and post treatment) was measured in two ways: first, paired differences between sessions were calculated (daily average cotinine at session 2 minus daily average cotinine at session 1 for each of the 21 subjects); and second, percentage difference between sessions (100% × paired difference/daily average cotinine at session 1 for each of the 21 subjects). One-way ANOVA was also used to test the treatment effect on these measures of cotinine concentration changes at the 5% level of significance.
A mixed model was used to examine the profile of nicotine concentration over time (up to the 4th order) within each monitoring session, and to test the effect of treatment condition. A similar approach was used to model the profile of percent time of fetal breathing during 30-minute periods at monitoring session 2 with the corresponding measurement at monitoring session 1 as a covariate, and to test the effect of treatment condition.
Paired differences in daily average maternal heart rate were compared across the treatment condition using one-way ANOVA at a 5% level of significance. Repeated measures ANOVA was used to model fetal heart rate at monitoring session 2 with fetal heart rate at monitoring session 1 as a covariate and time (morning or noon), treatment condition, and their interactions as factors.
Finally Fisher’s exact test was used to determine whether adverse effects differed by treatment condition (with a Bonferroni correction for multiple comparisons).
Results
Twenty-nine subjects signed consent to participate in the study. Of this number, 6 subjects were not randomized to double-blind treatment: 1 subject served as a pilot subject, 3 subjects did not attend the first smoking monitoring session, and two subjects had pregnancy complications before randomization. Two study subjects dropped out of the study after the first day of medication treatment for reasons unrelated to the study (i.e., death in the family, family illness). Consequently, results are available for 21 women who used medication for 5 days while trying to quit smoking and who also completed both monitoring sessions (i.e., during smoking and again while on study medication).
The characteristics of the study population are shown in Table 1. The subjects were an average of 30 years old, with the majority being Caucasian and in the third trimester of pregnancy. They smoked an average of 17 cigarettes per day. Approximately 30% of subjects had prior treatment for alcohol or drug problems. The majority of subjects were either married or cohabitating with their partner. There was no statistically significant difference in any of these characteristics across the three treatment conditions (all p-values > 0.05).
Table 1.
Demographic and Baseline Clinical Characteristics*
| Characteristic | Overall (n = 21) | Placebo (n = 7) | Nicotine Patch (n = 7) | Nasal Spray (n = 7) |
|---|---|---|---|---|
| Age | 29.95 (5.75) | 29.71 (6.45) | 29.86 (6.52) | 30.29 (5.09) |
| Race (Caucasian) | 85.7% | 100.0% | 85.7% | 71.4% |
| Years of Education | 13.29 (1.95) | 13.57 (2.76) | 13.00 (0.58) | 13.29 (2.14) |
| Marital Status (Married/Cohabiting) | 76.2% | 71.4% | 71.4% | 85.7% |
| Gestational Age | 31.26 (2.61) | 30.61 (1.63) | 32.06 (2.64) | 31.70 (3.21) |
| Number of Daily Cigarettes | 17.43 (4.77) | 15.93 (4.31) | 19.64 (3.66) | 16.71 (5.90) |
| Total Fagerstrom Score [32] | 6.24 (1.45) | 5.71 (1.60) | 6.71 (1.60) | 6.29 (1.11) |
| Number cups of Daily Coffee | 0.90 (1.00) | 1.57 (0.98) | 0.71 (0.95) | 0.43 (0.79) |
| Previous Substance Abuse Treatment | 28.6% | 28.6% | 14.3% | 42.9% |
Continuous variables are reported as mean (SD) and categorical variables are reported as percentages. Fagerstrom Score is a measure of nicotine dependence with a ranges from 0–11.
Subjects reported using the nicotine patch daily for an average of 14 hours/day. Average nasal spray doses per day by subjects assigned to the nasal spray condition were 12.8, 13.8, 13.4, and 12.3 on Days 1–4, respectively.
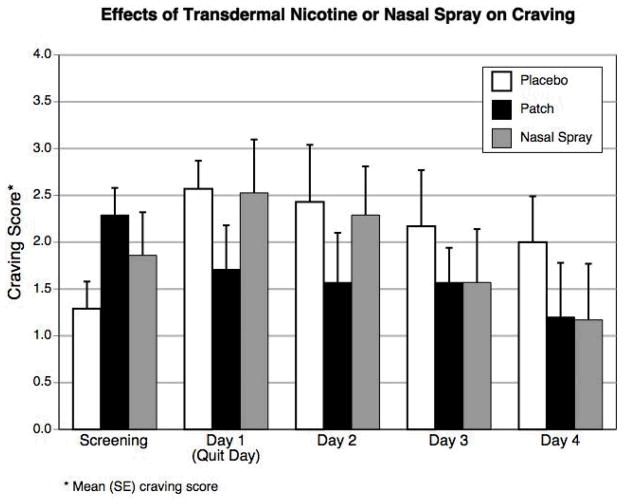
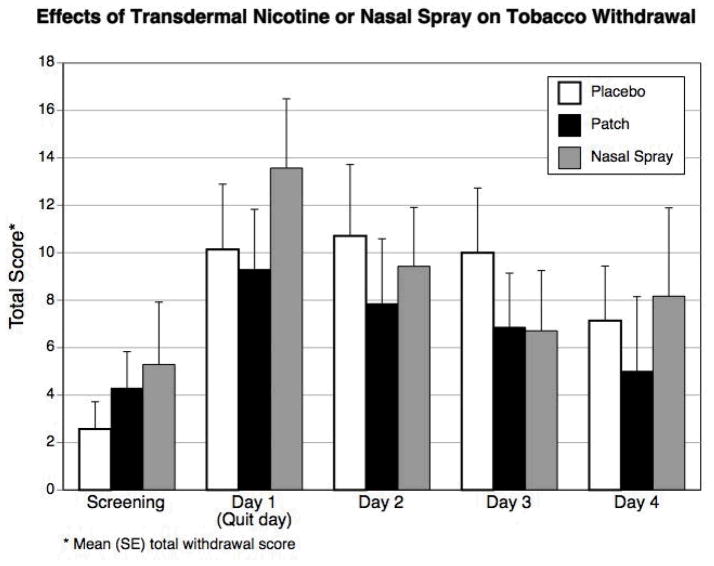
Figures 1a and 1b show the effects of the nicotine patch or nasal spray on craving and on tobacco withdrawal symptoms as measured by the Minnesota Nicotine Withdrawal Scale (which measures severity of craving, irritability/frustration/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed mood, impatience, and insomnia) across five times points: screening, quit day, and Days 2–4 following smoking cessation. Each item was scored on a 5-point scale (0 = no symptoms and 4 = severe symptoms). As recommended, craving was analyzed separately from other withdrawal symptoms, because it is does not necessarily correlate with other withdrawal items [13].
Figure 1a and b.
Mean craving scores and total withdrawal scores while smoking (screening) and while using nicotine patch, nicotine nasal spray, or placebo for smoking cessation.
Figure 1a shows a significant treatment by time interaction (p = 0.025) on craving. Accounting for baseline craving levels prior to the quit attempt, craving symptoms decreased throughout the 5-day treatment for the patch condition. In the other two conditions, craving increased initially on the quit day and decreased over the subsequent time points for the placebo and nasal spray groups, though the changes in the placebo group were much smaller. The best fit for the total withdrawal symptom score for all three groups was as a quadratic trend (p = 0.0002), peaking within the first 48 hours after cigarette abstinence. However, the change in symptoms did not differ among groups (p = 0.79) (Figure 1b). Short-term quit rates for the entire study were 6/7 (86%) with the nasal spray, 4/7 (57%) with the nicotine patch, and 2/7 (29%) with the placebo treatment (p = 0.147).
At baseline, while smoking, the cotinine concentrations were higher in the patch group 138 (SD = 55) ng/mL and the spray group 130 (SD = 57) ng/mL than the placebo group 77 (SD = 29) ng/mL, though the difference among groups did not reach statistical significance (F2,18 = 3.23, p = 0.063). The paired-difference in cotinine concentrations between the two monitoring sessions (i.e., while smoking and after 5 days of treatment) was similar across groups [placebo: −62 (SD = 34) ng/mL, patch: −63 (SD = 33) ng/mL, spray: −91 (SD = 38) ng/mL] (F2,18 = 1.45, p = 0.26). However, the percentage change in cotinine differed significantly by group (F2,18 = 5.16, p = 0.017), with the placebo group showing a 77% reduction, the spray group a 70% reduction, and the patch group a 48% reduction. A post hoc comparison found significant differences between placebo and patch groups (95% CI = −53, −5; p=0.017, but no statistical difference between placebo and spray groups (95% CI = −31, 17; p= 0.75) or between patch and spray groups (95% CI = 2, 46; p= 0.076).
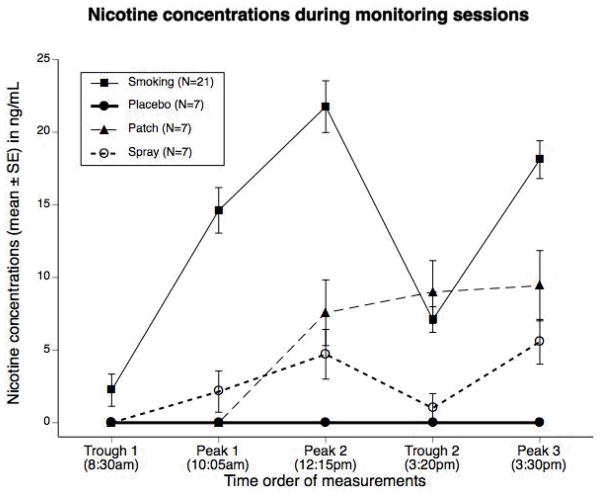
Nicotine concentrations while smoking (N = 21) and during treatment (N = 7 for each group) are shown in Figure 2. As would be expected, smoking during the initial monitoring session produced significantly greater increase in nicotine concentrations, with time effects significant time up to the 4th order (p < 0.0001). Although the study was designed to deliver systemic doses of nicotine comparable to those of smoking, the nicotine concentrations for the active treatments were markedly lower than during smoking. There were significant differences between nicotine concentrations in the first and second monitoring sessions (F1,184 = 10.41, p = 0.002), over time within sessions (F2,184 = 19.48, p < 0.0001), and across treatment groups (F2,184 = 3.24, p = 0.041).
Figure 2.
Nicotine concentrations while smoking (N=21) and after four days of nicotine patch, or nasal spray or placebo use. While smoking, nicotine concentrations were obtained after overnight abstinence (Trough 1), 2 minutes after completion of the first and fourth cigarettes of the day (Peak 1 and 2) and immediately before and 2 minutes after the 7th cigarette of the day (Trough 2 and Peak 3). The patch was placed at 10 am and nicotine levels were obtained at the abovenamed times. Eleven doses of nasal spray were administered during the session (at 9:55 am, 10 am, 10:40 am, 11am, 12:05 and 12:10 am, 1:30pm. 2:00pm, 3:20 and 3:25, 4:20).
The effects of smoking or medication treatment on maternal and fetal heart rate are presented in Table 2. During smoking, maternal heart rate increased throughout the day (from approximately 79 bpm in the morning to 88 bpm in the afternoon). Controlling for maternal heart rate during session 1, maternal heart rate during treatment (i.e., at the second monitoring session) differed by treatment group. Specifically, paired differences on the mean heart rate aggregated across the whole day differed significantly by group, with the placebo and nasal spray groups having a greater reduction in heart rate than the patch group (F2,18 = 4.80, p = 0.021).
Table 2.
Maternal and Fetal Heart rate*
| Morning | Noon | Afternoon | Whole Day | ||
|---|---|---|---|---|---|
| Average | Paired Difference | ||||
| Maternal Heart Rate | |||||
| Smoking (n = 21) | 78.81 (9.99) | 85.67 (11.14) | 88.44 (7.50) | 83.70 (7.46) | |
| Placebo (n = 7) | 81.43 (6.70) | 78.00 (7.02) | 82.00 (5.29) | 80.48 (4.85) | −5.81 (4.54) |
| Nicotine Patch (n = 7) | 81.14 (6.72) | 78.43 (11.49) | 80.29 (7.87) | 79.95 (7.74) | 0.33 (4.94) |
| Nasal Spray (n = 7) | 78.29 (7.61) | 86.00 (7.39) | 84.29 (8.36) | 82.86 (7.03) | −2.33 (2.74) |
| Fetal Heart Rate | |||||
| Smoking (n = 21) | 133.81 (9.73) | 135.95 (9.44) | 134.88 (9.10) | ||
| Placebo (n = 7) | 137.86 (7.56) | 132.86 (6.36) | 135.36 (5.09) | −0.71 (5.54) | |
| Nicotine Patch (n = 7) | 129.29 (7.87) | 136.43(14.92) | 132.86 (10.84) | 0.36 (5.29) | |
| Nasal Spray (n = 7) | 132.86 (9.06) | 136.43 (5.56) | 134.64 (6.36) | −1.43 (5.56) | |
All measurements are reported as beats per minute mean (SD)
The effects of smoking on fetal heart rate characteristics from a subset of this sample has been reported previously [14]. Consequently, we include these measures here only as a covariate in the model examining treatment and time effects on fetal heart rate, during medication treatment. The baseline fetal heart rate decreased in the placebo group throughout the second monitoring session, but increased slightly in the patch and nasal spray groups (Table 2). The treatment by time interaction was marginally significant (p = 0.052).
There were no serious adverse events during the study. Treatment emergent adverse events that were moderate or severe are listed in Table 3. As anticipated, skin erythema and irritation near the patch site were more common in the nicotine patch group and nasal symptoms were more common in the active spray group, though the difference among groups did not reach statistical significance (all p-values > 0.05).
Table 3.
Treatment Emergent Adverse Effects
| Adverse Effects | Placebo (n = 7) | Nicotine Patch (n = 7) | Nasal Spray (n = 7) |
|---|---|---|---|
| Nasal irritation, runny nose, sneezing, and eyes watering | 14.3% | 28.5% | 42.9% |
| Throat irritation, coughing, and dry mouth | 0.00% | 14.39% | 14.3% |
| Skin redness, and warmth near patch | 0.00% | 42.9% | 0% |
| Diarrhea, stomach upset, nausea, and heartburn | 14.3% | 42.9% | 0% |
| Muscle aches, sweating, and light-headedness | 42.9% | 42.9% | 0% |
Discussion
Our findings indicate that use of the nicotine patch (15 mg/16 hours) and nasal spray (12–13 doses/day) reduced overall maternal nicotine exposure (as measured by nicotine and cotinine concentrations) in pregnant women who smoke approximately 17 cigarettes per day. The nicotine patch reduces cigarette craving, compared with nicotine nasal spray or placebo treatment, particularly during the first two days of abstinence. The overall picture of the effects of the various treatments (compared with smoking) on hemodynamic measurements suggests that the greatest reduction in sympathetic activity occurs with placebo. In contrast, the effects of nicotine administered via either patch or nasal spray were similar to those of smoking.
These data suggest that nicotine concentrations and overall maternal nicotine exposure (as measured by % change in cotinine) can be reduced with use of the nicotine patch or nasal spray. Data have been published on nicotine concentrations resulting from use of the patch during pregnancy [15, 16], though we are not aware of any studies evaluating nicotine concentrations associated with use of the nasal spray during pregnancy. The dosage recommended is a minimum of 8 doses and a maximum of 40 doses of nasal spray per day [17]. Although the frequency of nasal spray use in this study was less than instructed, the number of doses per day was similar to the frequency of ad libitum use in smoking cessation trials in non-pregnant smokers [6, 7]. The nasal spray resulted in nicotine replacement (as measured by cotinine) of about 30% of that seen with smoking, which is similar to findings from studies in non-pregnant individuals [7]. In contrast, the patch provided approximately 50% replacement. This is less than expected given that the patch usually delivers 15 mg of nicotine/16 hours, and we instructed subjects to decrease smoking to 10–15 cigarettes per day for four days prior to initiating use of the patch.
Consistent with studies in non-pregnant individuals, we observed that the patch reduced the rise in craving usually observed after smoking cessation [18]. The nasal spray group reduced craving only during days 3 and 4 of therapy. Also, there was not a reliable effect of either nicotine treatment on total withdrawal score. It has been suggested that because of a faster clearance of nicotine during pregnancy, pregnant smokers may actually need higher doses of nicotine replacement than non-pregnant persons to reduce withdrawal symptoms and to quit smoking [19]. Moreover, pregnant smokers have elevated levels of nicotine withdrawal symptoms prior to smoking cessation [20]. It is noteworthy that some of the DSM-IV [21] symptoms of withdrawal (e.g., irritability, insomnia) also commonly occur with pregnancy, which would not necessarily be affected by nicotine replacement therapy and could influence the success of pharmacotherapy for smoking during pregnancy.
Consistent with studies in non-pregnant smokers [22], we observed a reduction in maternal heart rate with smoking cessation in the absence of nicotine replacement therapy. The magnitude of the decrease in maternal heart rate is consistent with symptoms of nicotine withdrawal observed in other studies in non-pregnant individuals [8, 22]. The reduction in maternal heart rate is generally considered permanent since it does not rebound with continued abstinence [22].
In this study, we measured fetal heart rate after overnight abstinence and again approximately 2.5 hours later after continued smoking or use of nicotine replacement therapy when significant effects of smoking or nicotine would be expected to occur [23–26]. We did not find a significant reduction in overall daily fetal heart rate among groups as we did with maternal heart rate. However, we did observe a treatment by time interaction, such that fetal heart rate increased throughout the day with smoking or nicotine replacement, but it decreased with placebo use. The clinical significance of this finding is not known, and given the small sample size, it needs to be validated in future studies.
The primary limitation of this study is the small sample size. We had originally planned to examine the effects of the different nicotine delivery systems on fetal biophysical parameters and on maternal urinary catecholamine concentrations, but the small sample size limited the potential for these analyses. The small sample size was a reflection of the difficulty we encountered in recruiting subjects to participate in the two 8-hour monitoring visits at the end of pregnancy, which is also an important consideration in the design of future studies.
The nicotine patch or nasal spray may be a useful adjunct to smoking cessation treatment in pregnant women; however, more data are needed to evaluate their safety and efficacy in this patient population. Nicotine at any concentration may produce some risk to the fetus [1]. Most clinical studies have examined the nicotine patch rather than an intermittent form of nicotine replacement therapy for cessation. Studies of the nicotine patch in pregnancy have yielded conflicting findings on whether it is effective during pregnancy [27, 28, 29]. The only published, placebo-controlled study of the use of the nicotine patch in pregnancy suggests that it does not improve cessation [31]. An intermittent therapy such as the nasal spray has the potential advantage of limiting nicotine exposure while providing a tool to deal with urges to smoke. This short-term study suggests that both the nasal spray and the patch may reduce overall nicotine exposure and may be beneficial for cessation. More studies are needed to examine the efficacy of nicotine replacement in pregnancy and to examine the risk/benefit ratio in relation to long-term smoking cessation rates and on infant outcomes.
Acknowledgments
Supported by NIH grants R01 DA15167, K24 AA13736, and M01 RR06192 (University of Connecticut General Clinical Research Center). Glaxo-SmithKline Pharmaceuticals donated nicotine and placebo patches and nasal spray.
Footnotes
This study was conducted at the University of Connecticut Health Center
Disclosure Statement: Dr. Oncken has received consulting fees and honoraria from Pfizer (New York, NY). She has received at no cost nicotine and/or placebo products from Glaxo-SmithKline (Philadelphia, PA) for smoking cessation studies (i.e., for pregnant women, postmenopausal women). She has received grant funding from Pfizer and from Nabi Biopharmaceuticals (Boca Raton, FL). Dr. Kranzler has received research support from Ortho-McNeil Pharmaceuticals and Bristol-Myers Squibb Co. He has received consulting fees from Ortho-McNeil Pharmaceuticals, H. Lundbeck A/S, Forest Pharmaceuticals, elbion NV, Sanofi-Aventis, Solvay Pharmaceuticals, and Alkermes, Inc. He has received honoraria from Forest Pharmaceuticals and Alkermes, Inc.
References
- 1.Crawford JT, Tolosa JE, Goldenberg RL. Smoking in pregnancy: Why, How, and What next…. Clin Obstet Gynecol. 2008;51:419–35. doi: 10.1097/GRF.0b013e31816fe9e9. [DOI] [PubMed] [Google Scholar]
- 2.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80(5):541–4. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Dept of Health and Human Services, . Women and Smoking: A Report of the Surgeon General. Public Health Service, Center for Disease Control and Prevention; Washington, DC: 2001. [Google Scholar]
- 4.Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence 2008 Update. Washington (DC): U.S. Department of Health and Human Services. Public Health Service; 2008. [Accessed July 21, 2008]. http://www.surgeongeneral.gov/tobacco/ [Google Scholar]
- 5.Henningfield JE. Nicotine medications for smoking cessation. N Engl J Med. 1995;333(18):1196–203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- 6.Schneider NG, et al. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction. 1995;90(12):1671–82. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland G, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet. 1992;340(8815):324–9. doi: 10.1016/0140-6736(92)91403-u. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Hansson, Jacob P. Cardiovascular effects of nasal and transdermal nicotine and smoking. Hypertension. 2002;39:1107–1112. doi: 10.1161/01.hyp.0000018825.76673.ea. [DOI] [PubMed] [Google Scholar]
- 9.Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and smoking. J Am Coll Cardiol. 1993;22:1159–67. doi: 10.1016/0735-1097(93)90431-y. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 11.Stout RL, et al. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 12.Balfour DJ, Fagerstrom KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996;72(1):51–81. doi: 10.1016/s0163-7258(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 13.Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7(1):92–3. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oncken C, et al. The effect of cigarette smoking on fetal heart rate characteristics. Obstet Gynecol. 2002;99(5 Pt 1):751–5. doi: 10.1016/s0029-7844(02)01948-8. [DOI] [PubMed] [Google Scholar]
- 15.Oncken CA, et al. Effects of transdermal nicotine or smoking on nicotine concentrations and maternal-fetal hemodynamics. Obstet Gynecol. 1997;90(4 Pt 1):569–74. doi: 10.1016/s0029-7844(97)00309-8. [DOI] [PubMed] [Google Scholar]
- 16.Ogburn PL, Jr, et al. Nicotine patch use in pregnant smokers: nicotine and cotinine levels and fetal effects. Am J Obstet Gynecol. 1999;181(3):736–43. doi: 10.1016/s0002-9378(99)70521-1. [DOI] [PubMed] [Google Scholar]
- 17.Physicians' Desk Reference. Thompson PDR; Montvale, NJ: 2006. pp. 2596–7. [Google Scholar]
- 18.Rose J, et al. Transdermal nicotine reduces cigarette craving and nicotine preference. Clin Pharmacol Ther. 1985;38:450–56. doi: 10.1038/clpt.1985.203. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–8. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 20.Heil SH, et al. Characterizing nicotine withdrawal in pregnant cigarette smokers. Exp Clin Psychopharmacol. 2006;14(2):165–70. doi: 10.1037/1064-1297.14.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 22.Oncken CA, et al. Impact of smoking cessation on ambulatory blood pressure and heart rate in postmenopausal women. Am J Hypertens. 2001;14(9 Pt 1):942–9. doi: 10.1016/s0895-7061(01)02147-1. [DOI] [PubMed] [Google Scholar]
- 23.Barrett JM, Vanhooydonk JE, Boehm FH. Acute effect of cigarette smoking on the fetal heart nonstress test. Obstet Gynecol. 1981;57(4):422–5. [PubMed] [Google Scholar]
- 24.Graca LM, et al. Acute effects of maternal cigarette smoking on fetal heart rate and fetal body movements felt by the mother. J Perinat Med. 1991;19(5):385–90. doi: 10.1515/jpme.1991.19.5.385. [DOI] [PubMed] [Google Scholar]
- 25.Lindblad A, Marsal K, Andersson KE. Effect of nicotine on human fetal blood flow. Obstet Gynecol. 1988;72(3 Pt 1):371–82. [PubMed] [Google Scholar]
- 26.Oncken CA, et al. Effects of short-term use of nicotine gum in pregnant smokers. Clin Pharmacol Ther. 1996;59(6):654–61. doi: 10.1016/S0009-9236(96)90005-3. [DOI] [PubMed] [Google Scholar]
- 27.Pollak KI, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33(4):297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotham ED, Gilbert AL, Atkinson ER. A randomised-controlled pilot study using nicotine patches with pregnant women. Addict Behav. 2006;31(4):641–8. doi: 10.1016/j.addbeh.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Wisborg K, et al. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol. 2000;96(6):967–71. doi: 10.1016/s0029-7844(00)01071-1. [DOI] [PubMed] [Google Scholar]
- 30.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–82. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]