Abstract
IL-22 is a member of the IL-10 cytokine family and signals through a heterodimeric receptor composed of the common IL-10R2 subunit and the IL-22R subunit. IL-10 and IL-22 both activate the STAT3 signaling pathway; however, in contrast to IL-10, relatively little is known about IL-22 in the host response to infection. In this study, using IL-22−/− mice, neutralizing Abs to IL-22, or both, we show that IL-22 is dispensable for the development of immunity to the opportunistic pathogens Toxoplasma gondii and Mycobacterium avium when administered via the i.p. or i.v. route, respectively. IL-22 also played little to no role in aerosol infections with Mycobacterium tuberculosis and in granuloma formation and hepatic fibrosis following chronic percutaneous infections with the helminth parasite Schistosoma mansoni. A marked pathogenic role for IL-22 was, however, identified in toxoplasmosis when infections were established by the natural oral route. Anti–IL-22 Ab-treated mice developed significantly less intestinal pathology than control Ab-treated mice even though both groups displayed similar parasite burdens. The decreased gut pathology was associated with reduced IL-17A, IL-17F, TNF-α, and IFN-γ expression. In contrast to the prior observations of IL-22 protective effects in the gut, these distinct findings with oral T. gondii infection demonstrate that IL-22 also has the potential to contribute to pathogenic inflammation in the intestine. The IL-22 pathway has emerged as a possible target for control of inflammation in certain autoimmune diseases. Our findings suggest that few if any infectious complications would be expected with the suppression of IL-22 signaling.
Developing an effective immune response to an invading pathogen is critical for host defense and survival. However, if the pathogen-specific immune response is not quickly controlled, then significant collateral tissue damage often develops. Although cytokines derived from CD4+ Th1, Th2, and Th17 cells often dictate the outcome of pathogen-specific immune responses, IL-10 has emerged as one of the most dynamic and critically important immunoregulatory cytokines in nearly all of the infectious diseases examined to date. In most cases, IL-10 functions as an immune response “rheostat” that precisely regulates the magnitude of the effector T cell response so that clearance of the invading pathogen is mediated with minimal collateral tissue damage. Five IL-10–like cytokines, a subset of type II cytokines that include IL-19, IL-20, IL-22, IL-24, and IL-26, have been recently identified and are proposed to share a six α-helical structural motif with IL-10 as well as receptor subunits derived from the cytokine receptor family 2 (1, 2). Although the role of IL-10 in infectious immunosurveillance is relatively well understood, the function of the other IL-10–like cytokines is just beginning to be explored.
Unlike IL-10, which is produced by a variety of cell types, IL-22 is made by only a subset of activated immune cells. Although originally demonstrated to be made by human CD4+ Th1 cells and conventional NK cells (3), more recent studies indicate that IL-22 is made preferentially by Th17 T cells (4). It is also produced by γδ CD3+ T cells (5, 6), noncytolytic NK cells, lymphoid tissue inducer cells (7), and skin-homing IL-13+ T cells (8, 9). The receptor for IL-22 is distinct and consists of IL-22R, which most studies indicate is restricted to the surfaces of epithelial cells, keratinocytes, and some fibroblasts, and IL-10R2, which is expressed ubiquitously (10). Hence, and in contrast to IL-10, IL-22 is not thought to have direct effects on immune cells. Instead, it is proposed that IL-22 regulates tissue responses as a result of its local expression from innate and adaptive immune cells and by direct signaling in nonhematopoietic cells (11, 12).
IL-22 participates in host defense at environmental interfaces, including mucosal surfaces of the airways and gastrointestinal tract as well as in the skin where it maintains barrier integrity and regulates wound repair (11–18). For example, Aujla et al. (13) demonstrated that T cell-derived IL-22 is critical for airway mucosal host defense against the Gram-negative bacterium Klebsiella pneumoniae. In vitro studies indicated that IL-22 maintains viability and generates transepithelial integrity after wounding of primary human bronchial epithelial cells. Zheng et al. (19) verified a role for IL-22 at mucosal surfaces and demonstrated that non-T cell-derived IL-22 induces antimicrobial proteins, such as Re-gIIIg, which are essential for early mucosal immunity in the gut to the Gram-negative bacterium Citrobacter rodentium. These studies suggest a requirement for IL-22 in immunity to airway- and gut-invading bacteria. However, IL-22 also exhibits protective activity in noninfectious models of inflammatory bowel disease. Local gene delivery of IL-22 suppressed TCRα-induced colitis, with IL-22 blockade delaying recovery following dextran sulfate sodium-induced colitis (17). Similarly, Zenewicz et al. (20) observed increased colitis when adoptively transferring il22−/− CD45RBhi cells, compared with il22+/+ CD45RBhi cells, into il22−/− Rag1−/− mice. Supporting these observations, Pickert et al. (21) illustrated a STAT3-dependent protective role for IL-22 in the colon following dextran sulfate sodium-induced colitis, with IL-22 inducing epithelial cell proliferation and wound healing. Interestingly, Weber et al. (22) determined that administration of IL-22 binding protein (IL-22BP), the proposed natural decoy receptor for IL-22, is partially protective in a peritoneal sepsis model, suggesting that IL-22 can also exacerbate immunosurveillance when infection is initiated via a shunt from the gut lumen. Thus, at mucosal surfaces both pro- and anti-inflammatory roles for IL-22 have been described, with IL-22 signaling enhancing immunity to bacteria and suppressing inflammation-associated colitis, respectively.
In the skin, IL-22 appears to be pathogenic. Zheng et al. (23) and Ma et al. (24) both demonstrated a proinflammatory role for IL-22 in the progression of psoriasis-like skin inflammation. A similar proinflammatory role for IL-22 was also described in a collagen-induced model of rheumatoid arthritis (25). In contrast, in the liver, a significant body of evidence suggests that IL-22 has protective effects in noninfectious models of hepatitis (26–28). For example, hydrodynamic delivery of IL-22 significantly attenuated Con A-induced hepatitis, with increased STAT3 activation and upregulation of several antiapoptotic proteins observed in mice overexpressing IL-22 (26).
Given the apparent pro- and anti-inflammatory properties of IL-22 exhibited in various tissues including the lung, gut, skin, and liver, in addition to its relationship with IL-10, we decided to systematically investigate the role of IL-22 in the development of immunity to a diverse group of pathogens (bacteria, protozoa, and helminth) that affect different tissues including the lung (aerosol Mycobacterium tuberculosis infections), gut (oral Toxoplasma gondii infection), peritoneal cavity (i.p. infection with T. gondii), skin (percutaneous infection with the helminth Schistosoma mansoni), and liver (systemic i.v. infections with Mycobacterium avium and hepatic immunopathology following S. mansoni infection). In marked contrast to IL-10 and TNF-α, our studies show that IL-22 plays a redundant or no role in nearly all of these infections. In particular, and in consideration of the proposed important role of IL-22 in barrier immunity, IL-22 deficiency had no impact on S. mansoni infections established through the skin or in M. tuberculosis infections established via the airway. However, and in contrast to systemic T. gondii infections in which IL-22 played no obvious role, a subset of mice orally infected with T. gondii were significantly protected from lethal infection when IL-22 was neutralized with anti–IL-22 mAb. Together, these studies for the first time illustrate both redundant and pathogenic roles for the novel IL-10 family cytokine IL-22 in models of mycobacterial, protozoan, and helminth infection.
Materials and Methods
Animals and reagents
C57BL/6, BALB/c (wild-type [WT]), and OVA-specific OT-2 (C57BL/6-Tg [TCRαTCRβ]) mice were obtained from National Institute of Allergy and Infectious Diseases animal facilities at Taconic Farms (Germantown, NY). C57BL/6/129/SvJ il22−/− and BALB/c il22−/− mice were provided by Wyeth (Cambridge, MA), the design of the deficient mice as previously described (23). All of the animals were housed under specific pathogen-free conditions at the National Institutes of Health in an American Association for the Accreditation of Laboratory Animal Care-approved facility. The National Institute of Allergy and Infectious Diseases animal care and use committee approved all of the experimental procedures. A minimum of five mice per group was used in each experiment, unless indicated. The IL-22 neutralizing Abs (clones IL-22-01 and IL-22-03) were generated at Wyeth (4) and were used at 1 mg per mouse, where indicated. Anti–TNF-α (clone XT3.11; BioXcell, West Lebanon, NH) was also used at 1 mg per mouse.
ELISA and biochemical analysis
Soluble egg Ag (SEA)-specific IgG1 and IgG2b isotype-specific Ab titers were evaluated by ELISA. Immulon 4 plates (Thermo LabSystems, Waltham, MA) were coated with 10 μg/ml SEA (100 μl per well) diluted in PBS for 24 h. Plates were blocked with 5% milk for 2 h, and serum samples were added, using serial 2-fold dilutions. Biotin-conjugated rabbit anti-Mouse IgG1 (Zymed) or HRP-conjugated rabbit anti-mouse IgG2b (Zymed; Invitrogen, Carlsbad, CA) was used at a 1:1000 dilution for 2 h, followed by peroxidase-labeled streptavidin (Kirkegaard & Perry Laboratories, Gaithersburg, MD) substrate enzyme at a 1:1000 dilution. Absorbance was read at 405 nm using a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA) after adding 100 μl ABTS peroxidase substrate (Kirkegaard & Perry Laboratories). Cytokines were measured by ELISA using suppliers’ guidelines. Capture Abs for IL-12/23p40 (4 μg/ml; R&D Systems, Minneapolis, MN) or IFN-γ (4 μg/ml; R&D Systems) were diluted in carbonate buffer and incubated at 4°C overnight. Plates were washed with 0.05% Tween 20 in PBS (PBST) and blocked with 5% milk in PBST. Mouse serum (at a 1:10 or 1:40 dilution) and assay standard diluted in PBST plus 1% BSA were added for 2 h at 37°C. The detection Abs (1 μg/ml biotinylated anti–IL-12/23p40 or 4 μg/ml biotinylated anti–IFN-γ; R&D Systems) were added for 2 h at 37°C. Peroxidase-labeled streptavidin (1:1000 dilution for 1 h at 37°C) and ABTS peroxidase substrate were used to detect biotinylated Abs. The concentration of cytokine in the sample was determined from a serial-fold–diluted standard curve. The absorbance in the wells was read at 405 nm using a Vmax Kinetic Microplate Reader. The liver transaminases glutamic oxalacetic transaminases/aspartate aminotransferase (AST) and glutamic pyruvic transaminases/alanine aminotransferase (ALT) were measured in individual serum samples using a Vista Analyzer (Siemens, New York, NY) in the clinical center at the National Institutes of Health.
RNA isolation, purification, and quantitative real-time PCR
Samples were homogenized in 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA) using a tissue polytron (Omni International, Kennesaw, GA) with total RNA extracted according to the manufacturers’ recommendations. RNA was further purified using the RNeasy Mini Kit from (Qiagen, Valencia, CA). Individual sample RNA (0.1 μg) was reverse-transcribed using SuperScript II Reverse Transcriptase (Invitrogen) and a mixture of oligo(dT) and random primers. Real-time RT-PCR was performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Relative quantities of mRNA for several genes were determined using SYBR Green PCR Master Mix (Applied Biosystems) and the comparative threshold cycle method as described by Applied Biosystems for the ABI Prism 7700/7900HT Sequence Detection Systems. In this method, mRNA levels for each sample were normalized to hypoxanthine guanine phosphoribosyl transferase mRNA levels and then expressed as a relative increase or decrease compared with levels in uninfected controls. For Arg1, tnfα, il4, il13, il10, ifnγ, and il13rα2, primers have previously been described (29, 30). The following primers were designed with Primer Express software (version 2.0; Applied Biosystems), PrimerBank (http://pga.mgh.harvard.edu/primerbank/), or ProbeFinder (version 2.3; www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp): inducible NO synthase (iNOS), sense, 5′-CTTTGCCACGGACGAGAC-3′, antisense, 5′-TCATTGTACTCTGAGGGCTGAC-3′; IL-25, sense, 5′-TGG-AGCTCTGCATCTGTGTC-3′, antisense, 5′-CGATTCAAGTCCCTGTCC-A-3′; IL-17A, sense, 5′-CCACACCCACAGCATCTC-3′, antisense, 5′-CT-GAGAGCTGCCCCTTCAC-3′; IL-22BP, sense, 5′-CCAGAAGGTCCGAT-3, antisense, 5′-CATTGGTCAGGTCA-3′.
Histopathology and hydroxyproline assay
Collagen was measured as hydroxyproline after hydrolysis of a known weight of tissue in 5 ml 6 N HCl at 110°C for 18 h. The increase in hydroxyproline was calculated based upon total tissue weight and expressed as micromoles per 10, 000 parasite eggs or per organ. For histopathological analyses, formalin (4% paraformaldehyde in PBS)-fixed tissues were processed and embedded in paraffin for sectioning (Histopath of America, Millersville, MD). Wright’s Giemsa or H&E stains were used for analysis of inflammation and pathological changes. Evaluations were scored by a blinded observer on an arbitrary 1–4+ basis, unless specified. The same individual scored all of the histological features and had no knowledge of the experimental design.
Flow cytometry
Freshly isolated cells were stimulated for 3 h with PMA (10 μg/ml), ionomycin (1 μg/ml), and brefeldin A (10 μg/ml) and stained with Abs diluted in PBS with 0.5% BSA (Sigma-Aldrich, St. Louis, MO) and 0.05% sodium azide (Sigma-Aldrich) for 20 min at 4°C. Surface molecule staining (CD4 [BD Biosciences, San Jose, CA], CD8 [eBioscience, San Diego, CA], CD44 [BioLegend, San Diego, CA], CD62L [BD Biosciences], and CD25 [BD Biosciences]) followed by fixation and permeabilization (BD Cytofix/Cytoperm) and intracellular cytokine staining (IL-17A [BD Biosciences], IFN-γ [BD Biosciences], TNF-α [BD Biosciences], IL-13 [Centocor, Horsham, PA], IL-4 [BD Biosciences], IL-5 [BD Biosciences], Foxp3 [eBioscience], or IL-22 [kindly provided by Wyeth; Ref. 24]) was carried out. The expression of surface and intracellular molecules was analyzed on a BD LSR II flow cytometer using FlowJo version 8 software (Tree Star, Ashland, OR).
Infection models
M. avium. Mice were i.v. infected with 5 × 106 CFU of M. avium (strain SmT 2151). Bacterial loads in infected mice were determined at day 30 from spleen and lung homogenates as previously described (31).
M. tuberculosis. Mice were exposed to aerosolized M. tuberculosis (Erdman strain) in a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, IN). Each mouse received ~100 CFU measured in the lung at 24 h after exposure. Bacterial loads in infected organs were quantified by culture on 7H11 agar as previously described (31).
S. mansoni. Mice were infected percutaneously via the tail with 30 or 100 cercariae, as indicated, of a Puerto Rican strain of S. mansoni (NMRI) obtained from Biomphalaria glabrata snails (Biomedical Research Institute). SEA was obtained from purified and homogenized S. mansoni eggs as previously described (30). Animals were perfused at sacrifice so that worm and tissue egg burdens could be determined as previously described (30).
T. gondii. Mice were infected i.p. with 20 cysts of T. gondii (ME49 strain) or orally with 20, 50, or 100 cysts of T. gondii (ME49 strain) as indicated. At day 5 or 12, serum was recovered for cytokine analysis. Brain cyst frequencies, at indicted times post infection, were evaluated in an aliquot of a 5 ml PBS/brain suspension.
Statistical analysis
Data sets were compared by Mann-Whitney U test or one-way ANOVA as specified in figure legends using GraphPad (San Diego, CA) Prism (version 5.0). Differences were considered significant at p ≤ 0.05.
Results
IL-22BP is increased in a variety of infections
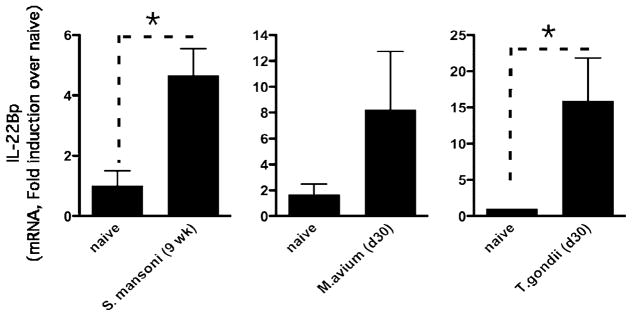
IL-22 protects the liver following chemical-, Fas ligand-, and cytokine-induced liver injury (26, 27) and during liver regeneration (32); however, the role of IL-22 in infections that affect the liver has not been previously investigated. Surprisingly, although IL-22 was detectable in vivo, we observed little to no change in IL-22 expression following infection with S. mansoni (week 8, 1.34 ± 0.23-fold induction; week 9, 1.52 ± 0.36-fold induction; week 16, 1.41 ± 0.33-fold induction, relative to uninfected). Nevertheless, a significant increase in the expression of IL-22BP, which can attenuate IL-22 activity (33), was observed in the liver (Fig. 1). Indeed, following infection with S. mansoni, expression of IL-22BP increased nearly 5-fold over background levels (Fig. 1, left panel). These data suggest that the endogenous activity of IL-22 may be tightly regulated by IL-22BP expression. Interestingly, following M. avium and T. gondii infection, both of which preferentially promote type 1 immune responses and classical macrophage activation (34, 35), significant increases in IL-22BP expression were again observed (T. gondii [15-fold, Fig. 1, right panel] and M. avium [8-fold, Fig. 1, middle panel]). Together, these observations suggest that IL-22 activity is not exclusively linked with the development of Th2- or Th1/Th17-type immune responses.
FIGURE 1.
Elevated IL-22BP in the liver following S. mansoni, M. avium, and T. gondii infection. IL-22BP gene expression in liver tissue taken from 9-wk S. mansoni-infected liver (left panel), 30-d M. avium-infected liver (middle panel), or 30-d T. gondii-infected liver (right panel). One of three experiments is shown with mean ± SEM. *p < 0.05.
IL-22 does not influence percutaneous infection, immunity, or hepatic pathology in schistosomiasis
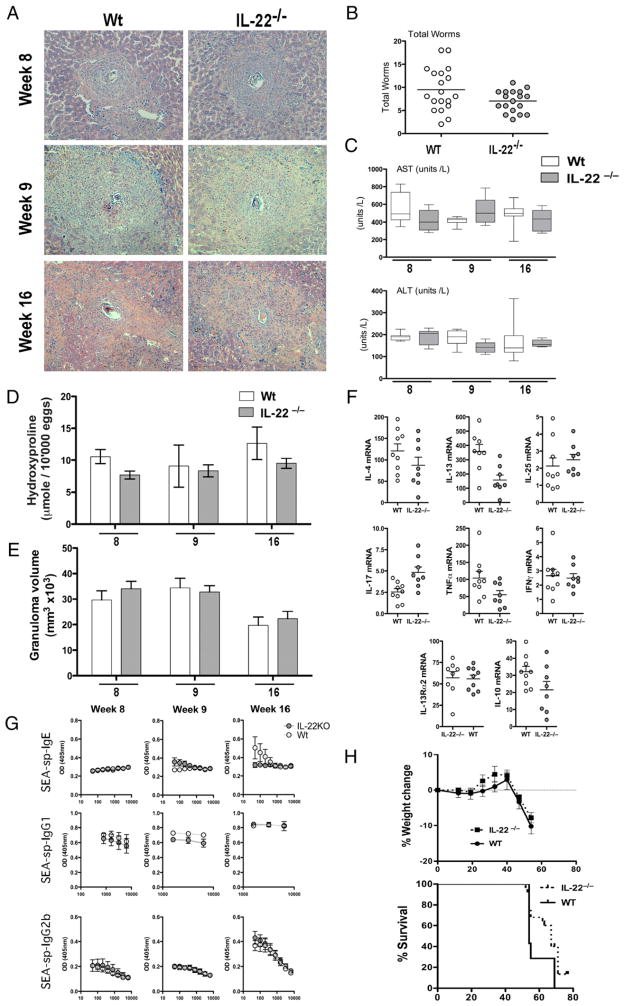
To dissect the role of IL-22 in Th2-associated dermal barrier function, we investigated the role of IL-22 following percutaneous infection with the helminth parasite S. mansoni. A patent percutaneous infection with the trematode helminth S. mansoni requires dermal penetration, larval migration via the lungs and heart, and finally adult worm maturation within the veins of the liver and mesentery. Gravid worm pairs produce hundreds of ova per day, which induce a systemic Th2 response with eosinophil-rich hepatic granulomas surrounding parasite ova (Fig. 2A). Deletion of IL-22 did not impact the establishment of infection because a similar parasite burden was observed in both groups (Fig. 2B). Serum AST and ALT levels, indicative of liver damage due to parasite ova-induced liver injury, were also similar, suggesting that there was no change in hepatotoxicity in the absence of IL-22, even when chronically infected with S. mansoni (Fig. 2C). There was a modest reduction in hepatic fibrosis in il22−/− mice at the early (week 8) and late (week 16) stages of infection (Fig. 2D); however, these did not reach statistical significance. Failure to mount a Th2 response and Th2 cytokine-driven granulomatous inflammatory reaction around parasite ova are fatal in mice (36–38). As infection proceeds beyond the acute stage (9 wk), granuloma formation is downmodulated in chronically infected mice, with less aggressive inflammation at week 16 versus that at weeks 8–9 (Fig. 2E). Peak granuloma formation also occurred normally between weeks 8 and 9 in the liver, with granuloma size successfully downmodulated by week 16 in both WT and il22−/− mice (Fig. 2A, 2E).
FIGURE 2.
Th2 development during acute and chronic infection with S. mansoni is unaffected in the absence of IL-22. Mice were infected with 35 cercariae, unless indicated, with worm burden, hepatic pathology, and immunological parameters assessed at week 8, 9, or 16 postinfection. One of three experiments with mean ± SEM (C–E, G, H), or individual mice per data point (B, F) are shown. A, Giemsa-stained liver section taken during acute (week 8), peak (week 9), and chronic (week 16) phases of S. mansoni infection (×10 magnification). B, Susceptibility and infection intensity expressed as worm burden. C, Serum AST and ALT levels throughout infection. D, Liver fibrosis expressed as hydroxyproline content per 10,000 eggs throughout infection. E, Granuloma volume determined from histological analysis. F, mRNA for IL-4, IL-13, IL-25, IL-17A, TNF-α, IFN-γ, IL-13Rα2, and IL-10 at week 9 of infection. G, Serum Ab isotypes specific for SEAs throughout infection. H, Weight change and percentage Kaplan–Meier graph showing survival following a high-dose (100 cercariae) infection.
Only minorchanges inTh1-, Th2-, and Th17-associated cytokines were observed in the livers of il22−/− mice. Of particular note, IL-4 and IL-13 responses were slightly but consistently reduced in the il22−/− mice (Fig. 2F), which may explain the modest reductions in fibrosis (39) and SEA-specific IgE (Fig. 2G). We next examined whether il22−/− mice were more or less susceptible to a high-dose infection with S. mansoni. As expected, WT mice challenged with 100 cercariae succumbed to infection between weeks 8 and 10 postinfection. Similar weight loss and survival were observed in il22−/− mice (Fig. 2H). Taken together these data indicate that IL-22 deficiency does not impact percutaneous infection or compromise CD4+ Th2 cell differentiation, Th2-dependent granuloma formation, or hepatic fibrosis following acute and chronic infection with S. mansoni.
Systemic control of M. avium infection and hepatic lesion formation are not compromised in the absence of IL-22
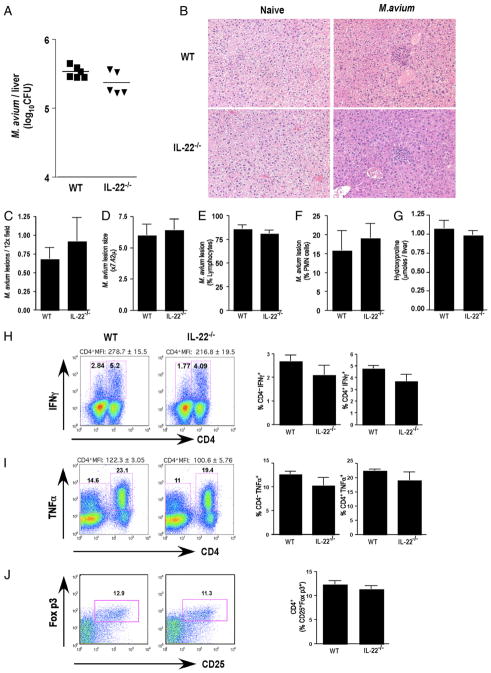
Resistance to many pathogenic bacteria, including M. avium, is dependent on the development of a competent cell-mediated Th1-type immune response (40). To test the importance of the endogenous IL-22/IL-22R signaling pathway in a systemic mycobacterial infection, the impact on liver damage, and granuloma formation, we infected il22−/− or WT littermates with M. avium by i.v. injection. Within the liver, il22−/− mice displayed similar bacterial burdens to those of M. avium-infected WT littermates (Fig. 3A), with comparable frequencies and appearances of lesions in the liver (Fig. 3B, 3C) on day 30 postinfection. Lesion size (Fig. 3D), composition (Fig. 3E, 3F), and collagen content (Fig. 3G) were also unaffected in il22−/− mice. iNOS production was also the same (data not shown). The frequency of Th1 cells and mean fluorescent intensities of IFN-γ+ (Fig. 3H) and TNF-α+ (Fig. 3I) CD4 T cells were slightly reduced in the spleens of M. avium-infected mice; however, this did not reach statistical significance. Thus, IL-22 deficiency did not compromise Th1 immunity, regulate liver damage, or alter lesion formation during infection with this important opportunistic pathogen.
FIGURE 3.
Efficient control of M. avium despite IL-22 deficiency. Mice were i.v. infected with 5 × 106 CFU of M. avium (Strain SmT 2151). Bacterial loads in infected mice were determined at day 30 postinfection. One of two experiments with five mice per group showing mean ± SEM (C–G, H–J) or individual mice per data point are shown (A). A, M. avium CFU in the liver following 30 d of infection. B, Paraffin-embedded liver sections stained with H&E (×10 magnification). C, M. avium lesion frequency in the liver following 30 d of infection. D, M. avium lesion size in the liver following 30 d of infection. E, Percentage of lymphocytes in M. avium lesions. F, Percentage of polymorphonuclear neutrophils in M. avium lesions. G, Hydroxyproline content, as an indicator of hepatic fibrosis, in M. avium-infected liver following 30 d of infection. H, Frequency of IFN-γ+ lymphocytes in spleen. I, Frequency of TNF-α+ lymphocytes in spleen. J, Frequency of CD25+Foxp3+ in CD4+ cells in spleen.
TNF-α but not IL-22 blockade compromises immunity to aerosol M. tuberculosis infection
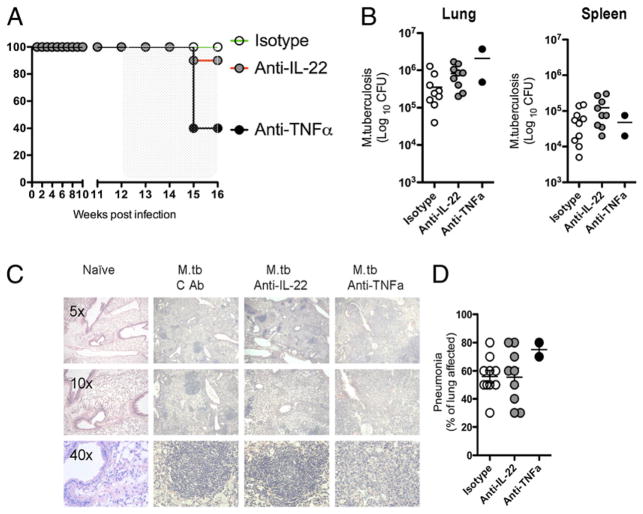
M. tuberculosis can establish latent infections in humans (41) and in susceptible immunocompetent mice (42, 43). Genetic deletion or Ab-mediated removal of TNF-α, IFN-γ, or iNOS results in reactivation of infection with granuloma disruption, weight loss, and ultimately accelerated morbidity and mortality (42, 44). We examined whether IL-22 blockade would have any impact on aerosol-mediated M. tuberculosis infection. This model also allowed us to examine whether IL-22 influenced the establishment of bacterial infections at mucosal surfaces. For these studies, chronically infected mice were given anti–TNF-α, anti–IL-22, or control mAbs at 12 wk postinfection. Four weeks later, 60% of mice receiving anti–TNF-α mAb had succumbed to the infection (Fig. 4A), as previously reported (44). However, only one tenth of mice treated with anti–IL-22 mAb died. At 16 wk of infection, bacterial burdens in the lungs and spleen were comparable between control and anti–IL-22 Ab-treated mice (Fig. 4B). In contrast to anti–TNF-α Ab-treated mice that developed highly disrupted granulomas and significant interstitial pneumonia, the lesions in the anti–IL-22 Ab-treated mice appeared relatively normal (Fig. 4C), with control and anti–IL-22 Ab-treated mice showing a similar degree of interstitial pneumonia in the lung (Fig. 4D). As observed with M. tuberculosis, TNF-α blockade exacerbated M. avium infection, whereas IL-22 inhibition had no effect (Supplemental Fig. 1A–D), further illustrating that IL-22 is not required for efficient control of mycobacterial infections delivered systemically or via the airway.
FIGURE 4.
Neutralization of TNF-α, but not IL-22, reactivates lethal M. tuberculosis. Mice were exposed to aerosolized M. tuberculosis with ~100 CFU measured in the lung at 24 h after exposure. One of two experiments, with 10 mice per group, showing individual mice per data point is shown (B, D). Groups of mice were given 1 mg/wk anti–IL-22, anti–TNF-α, or control Ab (isotype) i.p. from week 12 to week 16. A, Kaplan–Meier graph showing percent survival following Ab treatment starting at week 12 until week 16. B, Bacterial burden in the lung and spleen 16 wk postinfection. C, Giemsa-stained lung sections taken from mice at week 16 (5×, 10×, and 40× magnification as indicated). D, Percentage of lung affected by pneumonia at week 16.
Control of acute and chronic T. gondii infection is not affected by IL-22
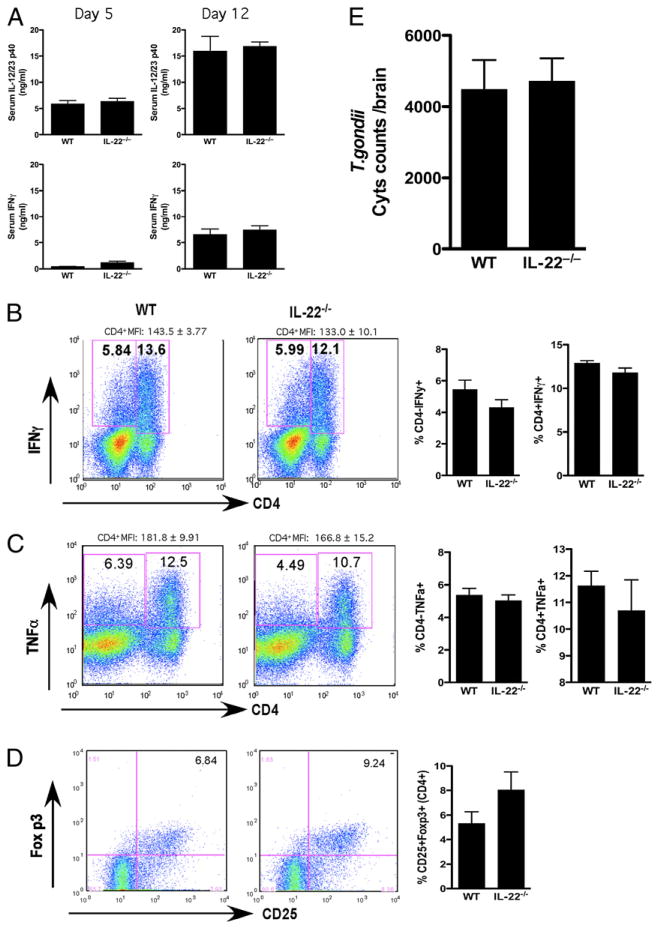
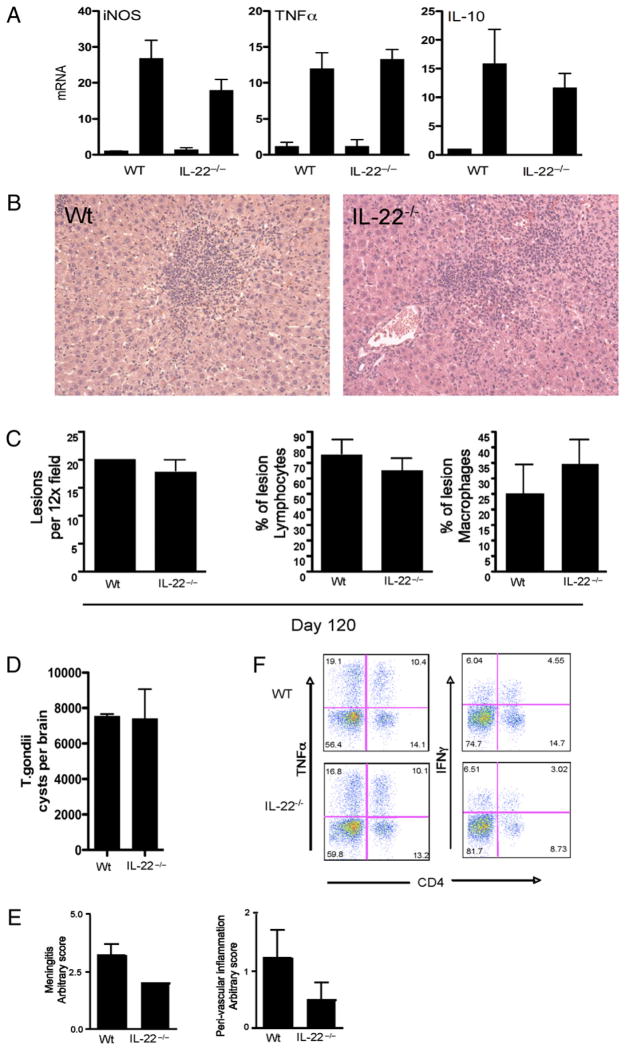
Next, we examined the role of IL-22 in systemic immunity to another important opportunistic pathogen, T. gondii. This infection also allowed us to assess the impact of IL-22 on the development of liver pathology following i.p. infection. For these studies, il22−/− mice were first infected i.p. with ME49, a type II strain of T. gondii. Between day 5 and day 12, marked increases in IL-12/23p40 and IFN-γ were detected in the serum; however, there was no significant difference noted between WT and il22−/− mice (Fig. 5A). In contrast, slight reductions in the frequencies and mean fluorescent intensities of CD4+ IFN-γ+ and TNF-α+ cells were observed in the spleen, similar to the studies with M. avium (Fig. 5B, 5C). The slight decrease in the Th1-type response was also associated with a small increase in Foxp3+ regulatory T cells (Fig. 5D). Despite these changes, IL-22 deficiency had no significant impact on the number of T. gondii cysts in the brain (Fig. 5E). The size, composition, and number of lesions in the liver were also similar in both groups (Fig. 6B, 6C), even though iNOS levels were marginally reduced in il22−/− mice (Fig. 6A). No significant differences in TNF-α or IL-10 production were observed (Fig. 6A). In separate studies, infected WT and il22−/− mice were allowed to develop chronic infections (120 d) to monitor the brain cyst burden and development of CNS pathology. Here again, the cyst burdens in both groups were nearly identical (Fig. 6D), although there was a slight reduction in meningitis and peri-vascular inflammation in il22−/− mice (Fig. 6E). The frequencies of brain-infiltrating IFN-γ+ and TNF-α+ cells were largely unaffected by the absence of IL-22 (Fig. 6F). Thus, similar to the studies with M. avium, in general a proficient Th1 response developed in the absence of IL-22, which was sufficient to control both acute and chronic infections with T. gondii. The importance of TNF-α but not IL-22 was also documented during systemic T. gondii infection, with rapid weight loss and death observed only during treatment with anti–TNF-α mAb (Supplemental Fig. 1D).
FIGURE 5.
IL-22 deletion does not impact T. gondii-induced Th1 responses in vivo. Mice were infected i.p. with 20 cysts of T. gondii (ME49 strain). One of three experiments with five mice per group is shown. Data are presented as mean ± SEM. A, Serum IL-12/23p40 and IFN-γ at day 5 and day 12. B, Frequency of IFN-γ+ lymphocytes in spleen. Representative FACS plots are shown on the left, with mean ± SEM of five mice per group shown on the right. C, Frequency of TNF-α+ lymphocytes in spleen. Representative FACS plots are shown on the left, with mean ± SEM of five mice per group shown on the right. D, Frequency of CD25+Foxp3+ in CD4+ cells in spleen. Representative FACS plots are shown on the left, with mean ± SEM of five mice per group shown on the right. E, T. gondii cyst burden in the brain of mice infected for 30 d.
FIGURE 6.
T. gondii -associated liver pathology and control of chronic infection are not altered inil22−/− mice. Micewere infected with T. gondii (ME49 strain) as in Fig. 5. One of two experiments, with five mice per group, is shown. Data are shown as mean ± SEM (A, C–E). A, Expression of iNOS, TNF-α, and IL-10 mRNA in liver of 30-d T. gondii-infected mice. B, H&E-stained liver section taken from WT and il22−/− mice infected for 30 d (×20 magnification). C, Liver lesion frequency, percentage of lymphocytes, and percentage of macrophages. D, T. gondii cyst frequency in brain tissue infected for 120 d. E, Brain pathology with meningitis and peri-vascular inflammation score. F, Brain-infiltrating lymphocytes stained for CD4, TNF-α, and IFN-γ.
IL-22 regulates intestinal pathology during oral T. gondii infection
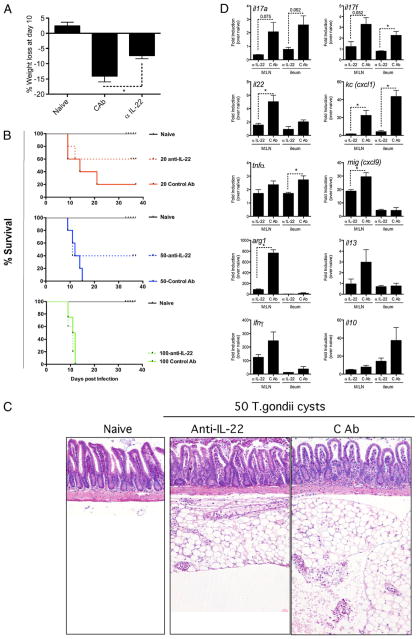
IL-22 has been proposed to have a protective role in human and mouse gut, based on its associative human expression and impact on deficiency in animal models of intestinal inflammation (13, 15, 17, 19–21, 32, 45, 46). Because oral T. gondii infections are often associated with the development of pathology in the small intestine, we performed another series of experiments using the oral, rather than i.p. (Figs. 5, 6), route of infection. In these experiments, C57BL/6 mice were infected with 20, 50, or 100 T. gondii cysts, and separate groups were treated with either anti–IL-22 or control mAb. Within 10 d of infection, WT mice infected with 50 cysts and treated with control Ab lost significant weight (Fig. 7A) and rapidly succumbed to infection in a parasite dose-dependent manner (Fig. 7B), whereas anti–IL-22 Ab-treated mice did not lose as much weight (Fig. 7A) and had reduced mortality (Fig. 7B). All of the mice administered 100 cysts were dead by day 12, whereas 20% of mice given a low-dose infection (20 cysts) survived through day 40. In contrast, mice treated with anti–IL-22 mAb succumbed at a much slower rate when infected with 20 or 50 cysts (Fig. 7B). At the highest infectious dose, the protective effect of IL-22 blockade appeared to be completely lost (Fig. 7B). Submucosal and intramuscular inflammation was observed in the ileum of the highly susceptible control Ab-treated mice. The lamina propria was also disrupted, with evidence of serositis surrounding the ileum and a marked macrophage infiltrate following oral infection of control Ig-treated mice (Fig. 7C). In contrast, mice treated with anti–IL-22 mAb developed significantly less inflammation in the ileum (control Ab histology score, 2.66 ± 0.23; anti–IL-22 Ab histology score, 1 ± 0.33; p < 0.05), with a visible reduction in serositis (Fig. 7C). The decrease in inflammation and increased survival were associated with reduced IL-17A, IL-17F, IL-22, TNF-α, and IFN-γ expression in the mesenteric lymph node and ileum (Fig. 7D). The chemokines Cxcl9 (Mig) and Cxcl1 (Kc) were also reduced in the absence of IL-22. Similarly, expression of Arg1 and IL-13 were also decreased. Together, these observations suggest that a wide array of inflammatory cytokines and chemokines were reduced when IL-22 is blocked. Furthermore, IL-10, which exhibits significant regulatory activity in the gut, was also reduced, presumably due to the reduced inflammatory response. Thus, a marked pathogenic role for IL-22 was revealed in the intestine during oral T. gondii infection.
FIGURE 7.
Neutralization of IL-22 reduces T. gondii-associated intestinal pathology and mortality. Mice were infected orally with 20, 50, or 100 cysts of T. gondii (ME49 strain) as indicated. One of two experiments, with five mice per group, is shown. Data are shown as mean ± SEM (A, D) with representative images (B). Groups of mice were given 1 mg/wk anti–IL-22 or control Ab (isotype) i.p. from day 1 until day 35. *p < 0.05. A, Weight loss at day 10 post-infection with 50 T. gondii cysts. B, Kaplan–Meier graph showing percentage survival following oral T. gondii infection with mice treated with anti–IL-22 from day 1 to day 30. C, H&E-stained section of the small intestine at day 8 after oral infection with 50 T. gondii cysts (original magnification ×100). D, Expression of il17a, il17f, il22, tnfα, ifnγ, mig (cxcl9), kc (cxcl1), il10, arg1, and il13 mRNA in the ileum of infected mice at day 9 of mice infected with 50 cysts.
Discussion
IL-10, the first identified member of the IL-10 cytokine family, exhibits critical immunoregulatory activity in many infectious diseases (47). In T. gondii infections, IL-10 prevents host protective Th1-type responses from going out of control and killing the infected host (48). IL-10 plays a similar role in M. avium and M. tuberculosis infections, where IL-10 has been shown to both reduce immunity and reactivate chronic pulmonary tuberculosis by regulating IFN-γ production (49–53). IL-10 also plays a key regulatory role in many Th2-associated helminth infections by slowing the development of morbidity and mortality (36, 54). IL-22, an IL-10–like cytokine, signals through the common IL-10R2 subunit and the IL-22R subunit and activates the STAT3 signaling pathway (55, 56). In consideration of the possible therapeutic blockade of the IL-22 pathway for the amelioration of autoimmune pathology (23–25), a better understanding of the contribution of IL-22 to infectious immunity could be beneficial. The results of this comprehensive study suggest that, unlike IL-10, IL-22 is redundant in the control of chronic percutaneous infections with S. mansoni, i.v. infections with M. avium, airway mucosal infection with M. tuberculosis, and acute and chronic i.p. infections with T. gondii. However, a pathogenic role for IL-22 was identified in oral T. gondii infections, because anti–IL-22 Ab-treated mice were significantly protected from intestinal immunopathology, thus supporting the hypothesis that IL-22 functions as a modulator of gut mucosal inflammation (57). Although prior studies have shown that IL-22 is protective in the context of gut infectious and noninfectious inflammation (13, 17, 19, 20), we show that IL-22 also has the capability to contribute to a pathogenic response in the intestines.
Extracellular helminth infections are largely controlled by Th2-type responses, whereas most intracellular bacterial and protozoan pathogens are generally controlled by Th1-type responses (58). A new class of Th cell designated Th17 has received considerable attention in recent years because of its involvement in a variety of inflammatory, autoimmune, and infectious diseases (59). IL-22, along with IL-21 and IL-17A, is among a suite of cytokines derived from αβ+CD4+ Th17 cells (4). However, IL-22 and IL-17A are clearly differentially regulated because either IL-23 or IL-6 alone is sufficient to induce IL-22 but not IL-17A production (23). These and related studies have suggested that IL-22 is not exclusively associated with Th17 cells. Th17 cells have been identified in the acute response to the extracellular helminth S. mansoni (60), in Mycobacterium infections (61, 62), and during acute (63) and chronic (64) infections with the intracellular protozoan parasite T. gondii. In the mouse model of schistosomiasis, acutely infected CBA mice and preimmunized C57BL/6 mice develop large hepatic granulomas that are characterized by a mixed IFN-γ and IL-17A response (60). Studies conducted with neutralizing IL-17 mAbs identified an important role for IL-17 in the S. mansoni egg-induced granulomatous responses (65). Nevertheless, the specific contribution of the Th17-associated cytokine IL-22 in schistosomiasis and other helminth infections was not previously investigated. In our studies, IL-22 deficiency had no significant effect on the establishment or maintenance of dermal infections because both WT and il22−/− mice displayed similar worm and tissue egg burdens. Infective S. mansoni cercariae penetrate through the dermis and migrate to the vasculature within hours of infection (66), a rate that may be too fast for immunological control. We did however observe a modest decrease in IL-13 production, SEA-specific IgE, and hepatic fibrosis in the infected il22−/− mice. Mortality was also delayed slightly during a high-dose infection; however, none of these parameters consistently reached significance, suggesting that IL-22 plays a relatively minor role in this infection. Finally, the size and cellular composition of hepatic and intestinal granulomas were nearly identical during both acute and chronic infection. Similar observations were generated with both il22−/− mice and in WT mice treated with anti–IL-22 mAb (data not shown). When viewed together, these data clearly demonstrate that IL-22 plays little to no role in the pathogenesis of schistosomiasis.
Although immunity to mycobacteria is mediated primarily by Th1-type responses, bacterial infections also induce IL-17A+ CD4+ T cells that can enhance antibacterial defenses (61, 62). IL-23 has also been shown to enhance purified protein derivative-specific IL-17A and IFN-γ responses and increase resistance to M. tuberculosis infection (67). Furthermore, mice vaccinated with an I-Ab–restricted peptide ESAT-61–20 exhibited an early antibacterial IL-17A–dependent response that accelerated the recruitment of IFN-γ–producing cells upon challenge, resulting in reduced M. tuberculosis infection (68). Although these studies failed to directly implicate IL-22, increased levels of IL-22 have been reported in nonhuman primates suffering from severe tuberculosis (69). Similarly, humans infected with M. tuberculosis have also been found to have elevated frequencies of purified protein derivative-specific IL-22+ cells in PBMCs and increased IL-22 in bronchoalveolar lavage fluid (70). Despite the many correlative observations in both human and nonhuman primates as well as the involvement of IL-17A+ T cells in murine studies, we found that IL-22 was redundant in our infection studies with systemic M. avium and aerosol M. tuberculosis infections. Thus, unlike previous reports that demonstrated a significant role for IL-22 in the control of respiratory Gram-negative bacterial infections (13), IL-22 appears to be dispensable for the control of Gram-positive mycobacterial infections.
Immunity to T. gondii requires the establishment of a tightly controlled Th1-type immune response. In the absence of IL-10, although infected mice develop stronger T. gondii-specific IFN-γ responses and reduced parasite burdens (71), animals quickly succumb to infection due to the development of an uncontrolled proinflammatory Th1-type response (48). Previous studies have linked IL-17A production with the development of pathology in chronic toxoplasmosis (64). Stumhofer et al. (64) showed that in the absence of the IL-27 receptor, mice chronically infected with T. gondii display severe neuroinflammation and accelerated mortality, which is associated with the development of a marked IL-17A response. They also found that IL-27 triggers the production of the anti-inflammatory cytokine IL-10. Thus, like IFN-γ and IL-10 (48), the outcome of T. gondii infection appears to be controlled by the IL-17A/IL-10 ratio (72). The influence of the highly related cytokine IL-22, however, has not been previously investigated in toxoplasmosis. Because of the reported involvement of IL-17A in the pathogenesis of toxoplasmosis and the fact that Th17 cells produce IL-22 (4), a series of studies were performed to dissect the role of IL-22 in acute and chronic T. gondii infection. Surprisingly, although iNOS production was decreased in the absence of IL-22, immunity was not significantly altered in il22−/− mice following either i.p. or oral infections with T. gondii. Indeed, WT and il22−/− both developed robust IL-12/23p40, IFN-γ, IL-10, and TNF-α responses following i.p. infection. The number and cellular composition of hepatic lesions were also similar as well as the degree of hepatic damage. Interestingly, however, anti–IL-22–treated mice infected orally developed significantly less intestinal pathology, even though their parasite burdens were similar to those of WT mice. Natural transmission of T. gondii is via the oral route, and acute toxoplasmosis is often characterized by the development of significant small intestine immunopathology and ileitis (73).
Orally infected mice treated with anti–IL-22 mAb were significantly protected from T. gondii-induced immunopathology, suggesting that IL-22 was either directly contributing to the development of intestinal pathology or indirectly regulating downstream targets that trigger intestinal inflammation. IL-22 can stimulate TNF-α and IL-8 release from epithelial cells (74) and colonic fibroblasts (75). Interactions between CD4 T cells and epithelial cells during T. gondii-mediated intestinal pathologies have previously been described (76), suggesting that IL-22–producing lymphocytes acting upon T. gondii-infected epithelial cells may be a significant interaction. Recently, studies have identified a CCR2-dependent Th1/Th17-mediated inflammatory response in the ileum following peroral infection with T. gondii. However, in these studies, IL-22 was not reported. CCR2-expressing lymphocytes are also recruited to the ileum during Crohn’s disease (77), and unlike TNF-α and IL-6, IL-22 correlates with Crohn’s disease activity (75, 78, 79), suggesting that IL-22 may be central to intestinal inflammatory pathologies. CCR2 (80) and IL-22 (7) have also been associated with activated NK cells, an essential cell type required for the control of T. gondii (81). NK cell-derived IL-22 may also be responsible for the immunopathology, as previously reported in a C. rodentium model (15). Interestingly, Sanos et al. (45) found that intestinal microflora were required for production of IL-22 by NK cells. Because oral T. gondii infections can disrupt gut barrier function, intestinal microflora could also be contributing to the T. gondii-induced IL-22 response, and consequently to the development of intestinal inflammation. The relative contribution of intestinal microflora to T. gondii-induced immunopathology requires further dissection.
IL-22 is a potential target for the treatment of autoimmune and inflammatory diseases (25, 82). The results of our study suggest that few if any infectious complications would be expected with an immunotherapeutic that blocks this cytokine. This is particularly relevant for M. avium, M. tuberculosis, and T. gondii infections, which are typically found as latent opportunistic infections that pose a constant risk of reactivating during prolonged immunosuppressive therapy (13, 19). Indeed, this complication has emerged as an important problem with drugs that target the TNF-α pathway (83). In our studies, IL-22 blockade had little to no effect on the establishment or progression of infection with M. avium, M. tuberculosis, or S. mansoni. In addition, in the case of oral T. gondii infections, IL-22 blockade had the added benefit of reducing the development of intestinal inflammation. When viewed together, our studies suggest therapeutics targeting the IL-22 pathway might provide an important alternative or complimentary strategy to treat chronic inflammatory diseases.
Acknowledgments
We thank Roger Askew for the initial coordination of the IL-22–deficient mice development and Khetemenee Lam for purification of the IL-22 and control Abs. The authors also acknowledge the meticulous care of animals used in this study by Lauren Donato, Joy McFarlane, and colleagues (SoBran, Fairfax, VA). We thank Thirumalai Ramalingam, John Pesce, Margaret Mentink-Kane, Satish Madala, and Luke Barron for helpful and thought-provoking discussions. We thank Sandy White and Rob Thompson for technical assistance and Dr. Fred Lewis and colleagues (Biomedical Research Institute) for SEA and schistosome material. We also appreciate the efforts of Dr. Sundar Natarajan for preparation of material.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Abbreviations used in this paper
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- IL-22BP
IL-22 binding protein
- iNOS
inducible NO synthase
- PBST
0.05% Tween 20 in PBS
- SEA
soluble egg Ag
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
M.C. and L.F. are both current employees of Wyeth Research in Cambridge, MA.
References
- 1.Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–676. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- 2.Wu PW, Li J, Kodangattil SR, Luxenberg DP, Bennett F, Martino M, Collins M, Dunussi-Joannopoulos K, Gill DS, Wolfman NM, Fouser LA. IL-22R, IL-10R2, and IL-22BP binding sites are topologically juxtaposed on adjacent and overlapping surfaces of IL-22. J Mol Biol. 2008;382:1168–1183. doi: 10.1016/j.jmb.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 4.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 9.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 10.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 13.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 15.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 20.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber GF, Schlautkötter S, Kaiser-Moore S, Altmayr F, Holzmann B, Weighardt H. Inhibition of interleukin-22 attenuates bacterial load and organ failure during acute polymicrobial sepsis. Infect Immun. 2007;75:1690–1697. doi: 10.1128/IAI.01564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 24.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 26.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetra-chloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- 27.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 28.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson MS, Elnekave E, Mentink-Kane MM, Hodges MG, Pesce JT, Ramalingam TR, Thompson RW, Kamanaka M, Flavell RA, Keane-Myers A, et al. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest. 2007;117:2941–2951. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng CG, Kullberg MC, Jankovic D, Cheever AW, Caspar P, Coffman RL, Sher A. Transgenic mice expressing human interleukin-10 in the antigen-presenting cell compartment show increased susceptibility to infection with Mycobacterium avium associated with decreased macrophage effector function and apoptosis. Infect Immun. 2002;70:6672–6679. doi: 10.1128/IAI.70.12.6672-6679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, Prüfer T, Olszak T, Steib CJ, Storr M, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–G1028. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, Dillon SR, Gao Z, Gilbert T, Madden K, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci USA. 2001;98:9511–9516. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu T, Tomioka H, Matsuoka M, Sano C. Profiles of the mRNA expression by macrophages infected with Mycobacterium leprae and Mycobacterium avium complex. Int J Lepr Other Mycobact Dis. 2002;70:250–259. [PubMed] [Google Scholar]
- 35.Schlüter D, Deckert-Schlüter M, Lorenz E, Meyer T, Röllinghoff M, Bogdan C. Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol. 1999;162:3512–3518. [PubMed] [Google Scholar]
- 36.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 37.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 38.Brunet LR, Kopf MA, Pearce EJ. Schistosoma mansoni: IL-4 is necessary for concomitant immunity in mice. J Parasitol. 1999;85:734–736. [PubMed] [Google Scholar]
- 39.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riska PF, Carleton S. Latent tuberculosis: models, mechanisms, and novel prospects for eradication. Semin Pediatr Infect Dis. 2002;13:263–272. doi: 10.1053/spid.2002.127198. [DOI] [PubMed] [Google Scholar]
- 42.Botha T, Ryffel B. Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology. 2002;107:350–357. doi: 10.1046/j.1365-2567.2002.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61(Suppl 2):ii54–ii58. doi: 10.1136/ard.61.suppl_2.ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171:3110–3118. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- 45.Sanos SL, V, Bui L, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 47.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 48.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 49.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 51.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 52.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, Cooper AM. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 53.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 55.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 56.Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang W, Valdez P. IL-22 in mucosal immunity. Mucosal Immunol. 2008;1:335–338. doi: 10.1038/mi.2008.26. [DOI] [PubMed] [Google Scholar]
- 58.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 59.Wynn TA. TH-17: a giant step from TH1 and TH2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 60.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 61.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 62.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 63.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 65.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 66.Wilson RA. The saga of schistosome migration and attrition. Parasitology. 2009;136:1581–1592. doi: 10.1017/S0031182009005708. [DOI] [PubMed] [Google Scholar]
- 67.Happel KI, Lockhart EA, Mason CM, Porretta E, Keoshkerian E, Odden AR, Nelson S, Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 69.Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, Hunt R, Estep J, Haynes BF, Jacobs WR, Jr, et al. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis. 2008;198:1514–1519. doi: 10.1086/592448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 72.Stumhofer JS, Silver J, Hunter CA. Negative regulation of Th17 responses. Semin Immunol. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection withToxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 75.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 76.Mennechet FJ, Kasper LH, Rachinel N, Li W, Vandewalle A, Buzoni-Gatel D. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol. 2002;168:2988–2996. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- 77.Connor SJ, Paraskevopoulos N, Newman R, Cuan N, Hampartzoumian T, Lloyd AR, Grimm MC. CCR2 expressing CD4+ T lymphocytes are preferentially recruited to the ileum in Crohn’s disease. Gut. 2004;53:1287–1294. doi: 10.1136/gut.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, Lohse P, Göke B, Brand S. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 79.te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:325–330. doi: 10.1002/ibd.20079. [DOI] [PubMed] [Google Scholar]
- 80.Polentarutti N, Allavena P, Bianchi G, Giardina G, Basile A, Sozzani S, Mantovani A, Introna M. IL-2-regulated expression of the monocyte chemotactic protein-1 receptor (CCR2) in human NK cells: characterization of a predominant 3.4-kilobase transcript containing CCR2B and CCR2A sequences. J Immunol. 1997;158:2689–2694. [PubMed] [Google Scholar]
- 81.Goldszmid RS, Bafica A, Jankovic D, Feng CG, Caspar P, Winkler-Pickett R, Trinchieri G, Sher A. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med. 2007;204:2591–2602. doi: 10.1084/jem.20070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 83.Lalvani A, Millington KA. Screening for tuberculosis infection prior to initiation of anti-TNF therapy. Autoimmun Rev. 2008;8:147–152. doi: 10.1016/j.autrev.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]