Abstract
The Bloom's syndrome gene (BLM) plays a pivotal role in the maintenance of genomic stability in somatic cells. It encodes a DNA helicase (BLM) of the RecQ family, but the exact function of BLM remains elusive. To study this question, we have cloned the BLM homolog of the frog Xenopus laevis (xBLM) and have raised antibodies to it. Immunodepletion of xBLM from a Xenopus egg extract severely inhibits the replication of DNA in reconstituted nuclei. Moreover, the inhibition can be rescued by the addition of the recombinant xBLM protein. These results provide the first direct evidence that BLM plays an important role in DNA replication, suggesting that Bloom's syndrome may be the consequence of defective DNA replication.
Keywords: Bloom's syndrome, DNA replication, Xenopus laevis, RecQ, Werner's syndrome, FFA-1
Bloom's syndrome (BS) is associated with cancer, immunodeficiency, proportional dwarfism, and sun-sensitive facial erythema (German and Ellis 1998). Despite its rarity, BS is of great significance in biomedical research because it displays dramatically the consequences of excessive genomic instability. In BS cells chromosome gaps, breakages, and rearrangements are easily observed. Interchanges between homologous chromosomes and, in particular, between sister chromatids are greatly increased (Ray and German 1983). Surprisingly, BS cells are not defective in the known DNA repair pathways (Friedberg et al. 1979). Instead, they show a retarded rate of nascent DNA chain elongation (Hand and German 1975) and accumulate abnormal replication intermediates (Lonn et al. 1990). The gene mutated in BS encodes a DNA helicase (BLM) of the RecQ family (Ellis et al. 1995), and the helicase activity has been confirmed in vitro with the recombinant protein (Karow et al. 1997). BLM is localized in the nucleus at discrete foci (identical to promyelocytic leukemia protein bodies), diffused patches, nucleoli in S phase cells, and telomeres in telomerase-negative tumor cells (Ishov et al. 1999; Neff et al. 1999; Zhong et al. 1999; Yankiwski et al. 2000). It has been reported to interact with topoisomerase III (Johnson et al. 2000; Wu et al. 2000) and Brca1 (Wang et al. 2000). Despite this progress, the function of BLM remains elusive.
The phenotypes of BS cells imply that BLM may be involved in DNA replication, but this interpretation is complicated by the very nature of genomic instability in BS cells. The replication defect may be an indirect effect of defects in other processes such as DNA repair, recombination, or even transcription, or it may result from secondary mutations in genes other than BLM. To definitively examine the role of BLM in DNA replication, we have used the interphase extract of Xenopus eggs as the model system. In this extract, a DNA substrate, such as demembranated sperm chromatin or purified lambda DNA, induces nuclear formation around itself, and the DNA within the nuclei is replicated once. It is the only cell-free system that can replicate genomic DNA in a semiconservative manner (Lohka and Masui 1983; Newport 1987). By immunodepleting a protein from the extract, one can conclusively determine whether it is important for DNA replication. We have cloned and expressed the Xenopus homolog of BLM and have raised specific antibodies to it. We have found that immunodepletion of xBLM from the egg extract severely inhibits the replication of DNA in the reconstituted nuclei. Moreover, the inhibition can be restored by the addition of the recombinant xBLM protein. These results provide the first direct biochemical evidence that BLM is involved in DNA replication. They also suggest that the clinical and cellular phenotypes of BS may be associated with a defect in DNA replication.
Results
We first cloned the helicase domain of the Xenopus BLM from an oocyte cDNA library by PCR with degenerate primers corresponding to two stretches of amino acids conserved among RecQ helicase family members. The two ends were subsequently cloned by 5′ and 3′ random amplification of cDNA ends (RACE) reactions. The complete sequence encodes an open reading frame (ORF) of 1367 amino acids (aa), slightly smaller than that of the human BLM (1417 aa) and mouse BLM (1416 aa). The predicted molecular weight is 153 kD and the pI value is 8.68. xBLM and hBLM share extensive homology throughout the ORF, especially in the second half (Fig. 1). The overall amino acid identity is 50%, and the total similarity is 64%.
Figure 1.

Sequence alignment of Xenopus and human BLM open reading frames. ClustalW in MacVector 6.5 was used for the alignment. (Asterisks) Identical amino acids; (periods) similar amino acids. The helicase motifs are underlined.
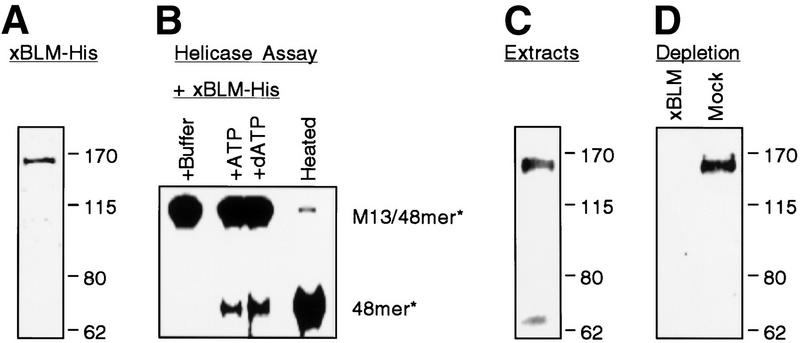
The xBLM protein was then expressed in Escherichia coli with a His tag at the carboxyl terminus and purified on a nickel affinity column. The recombinant protein ran at 160 kD on SDS-PAGE (Fig. 2A), slightly larger than the predicted molecular weight of 154 kD (including the His tag). It showed DNA helicase activity, and either ATP or dATP could drive the unwinding reaction (Fig. 2B). To study the function of xBLM, we prepared polyclonal antibodies against a glutathione S transferase (GST) fusion protein containing the amino-terminal 173 amino acids of xBLM, a part of the protein not conserved among RecQ family members. Western analysis indicated that these antibodies consistently recognized a protein of about 160 kD in the egg extracts (Fig. 2C). We believe this protein is the endogenous xBLM based on two observations: (1) Its size is similar to that of the recombinant xBLM-His protein; (2) both proteins are eluted from cation exchange columns at high salt concentrations (500–600 mM NaCl), a property consistent with the high pI value of xBLM (data not shown).
Figure 2.
Characterization of xBLM protein. (A) Coomassie blue staining of the purified His-tagged recombinant xBLM protein. (B) Helicase activity of the recombinant xBLM. The substrate is a 5′-32P-labeled 48mer oligonucleotide hybridized to m13mp18 single-stranded DNA. The purified xBLM protein was incubated with the substrate in the presence of either buffer alone or nucleotides (ATP or dATP). The right lane contains the heat-denatured substrate. The dissociated oligonucleotide was separated from m13 on 12% polyacrylamide gels. (C) Western blot of interphase egg extracts (2 μL) probed with the affinity-purified rabbit anti-xBLM antibody and detected by ECL (Amersham). The 160-kD band is xBLM. The small band is not always detected, suggesting that it is a degradation product. (D) Immunodepletion of xBLM from egg extracts. Supernatants (2 μL) from the extracts that had been depleted with either anti-xBLM or control antibodies were analyzed by Western blot with the affinity-purified rabbit anti-xBLM antibody. Molecular markers are labeled as kilodaltons on the right side of panels A, C, and D.
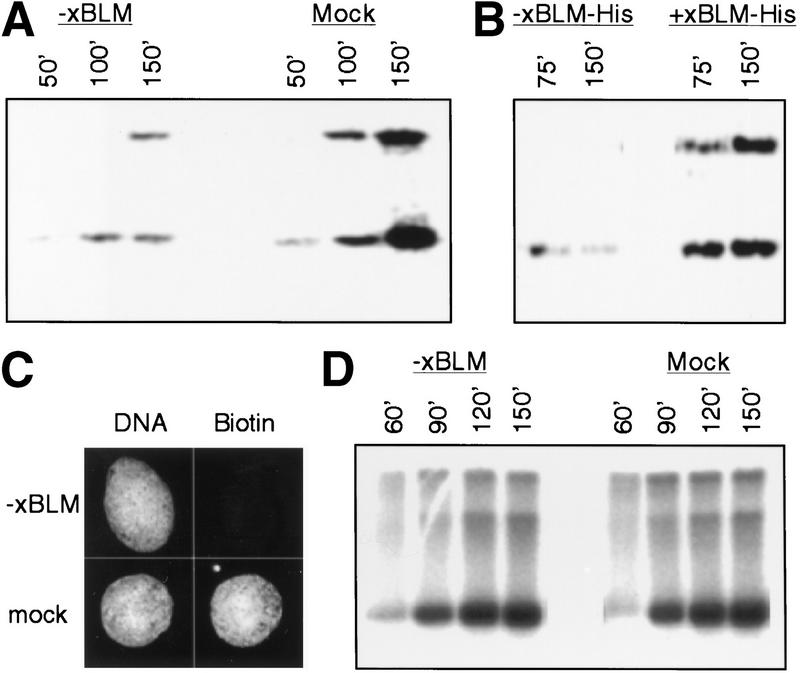
To investigate the role of xBLM in DNA replication, we performed a series of immunodepletion experiments. Interphase extracts were incubated with Affi-gel Protein A beads precoated with either anti-xBLM antibody or control antibody. This procedure effectively removed xBLM (>99%) from the anti-xBLM antibody-depleted extract but not the mock-depleted extract (Fig. 2D). To assay for DNA replication, the extracts were then incubated with demembranated sperm chromatin in the presence of [32P]dATP. Strikingly, DNA replication was severely inhibited (5–10-fold) in the xBLM-depleted extract (Fig. 3A). This effect was observed both with extracts reconstituted from frozen cytosol and membrane components and freshly made crude extracts. Moreover, replication was largely restored when the recombinant His-tagged xBLM protein was added back to the xBLM-depleted extract, suggesting that the inhibition is due solely to the removal of xBLM rather than some other cross-reacting protein (Fig. 3B). In addition, as shown in Figure 3C, nuclear formation, which is required for DNA replication, was normal in the absence of xBLM, suggesting that the inhibition is not an indirect effect on nuclear formation. Collectively these observations strongly suggest that xBLM plays an important role in DNA replication in reconstituted nuclei.
Figure 3.
Effect of xBLM depletion on DNA replication in interphase extracts. The reactions were continuously labeled with [32P]dATP, and samples were taken at the times indicated. The replication products were separated by electrophoresis on agarose gels. (A) Inhibition of DNA replication within the nuclei reconstituted around sperm chromatin in the xBLM-depleted extract. (B) Rescue of DNA replication by the recombinant xBLM-His protein. The purified His-tagged xBLM protein was added to the xBLM-depleted extract at the start of the nuclear reconstitution reaction (4 ng/μL final concentration). (C) Effect of xBLM depletion on nuclear assembly. Nuclei were reformed in the depleted extracts in the presence of biotin-dCTP. The incorporated biotin-dCTP was detected with Streptavidin Texas Red by immunostaining. DNA was stained with Hoechst dye. (D) Effect of xBLM depletion on m13 single-stranded DNA replication. m13 DNA (5 ng/μL final concentration) was incubated with the depleted cytosol (no membrane) in the presence of [32P]dATP.
DNA replication can be considered as two major steps: the unwinding of a double-stranded DNA template and the actual synthesis reaction on the resulting single-stranded DNA. To address at which of these steps xBLM functions, we determined the effect of xBLM depletion on the replication of m13 single-stranded DNA in the extract. This reaction does not require the unwinding of a double-stranded DNA template but rather requires the recruitment of multiple replication proteins to synthesize the complementary strand (Mechali and Harland 1982). As shown in Figure 3D, m13 DNA replicated equally well in xBLM-depleted and control-depleted extracts, suggesting that xBLM functions not in the synthesis step but rather in the unwinding step. This conclusion is consistent with the DNA helicase activity of xBLM.
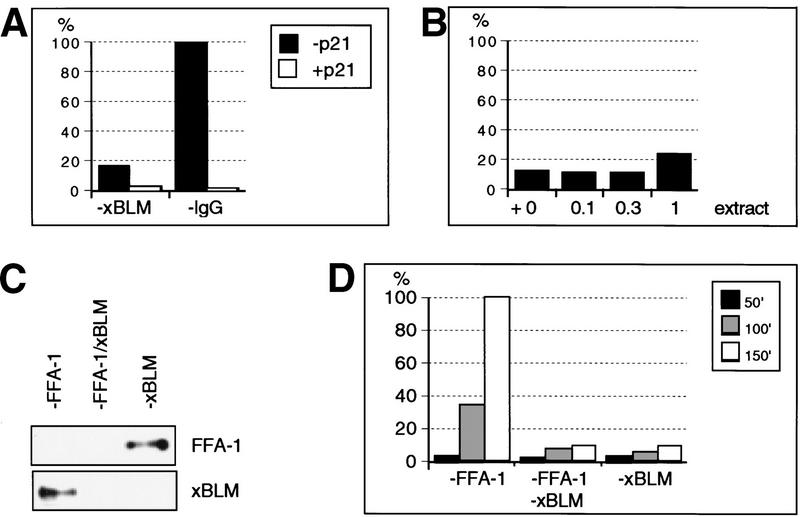
Although depletion of xBLM severely inhibited DNA synthesis in reconstituted nuclei, the inhibition was never complete. There are three likely explanations for the remaining DNA synthesis: (1) repair reaction; (2) incomplete depletion of xBLM; and (3) the presence of another functionally redundant protein(s). To differentiate replication from repair, we examined the sensitivity of the remaining DNA synthesis to the human p21 protein, which can specifically block DNA replication in Xenopus egg extracts by inhibiting Cdk2 kinase activity (Strausfeld et al. 1994; Jackson et al. 1995; Yan and Newport 1995). When p21 was added to a reaction with the xBLM-depleted extract, DNA synthesis was dramatically reduced to a level barely above background (Fig. 4A). These results suggest that the remaining DNA synthesis in the xBLM-depleted extract is still bona fide replication.
Figure 4.
Analysis of residual DNA synthesis in nuclei reconstituted in xBLM-depleted extracts. (A) Inhibition of residual DNA synthesis by p21. Reactions with either xBLM antibody-depleted or rabbit IgG-depleted extracts in the presence (0.4 μM) or absence of human p21 protein. [32P]dATP incorporation of the IgG-depleted reaction with no p21 is set at 100%. (B) Rescue of DNA replication. Reactions (10 μL) containing xBLM-depleted extracts were supplemented with various amounts of untreated extracts (in μL). Incorporation of [32P]dATP is plotted as percentages of that of the IgG-depleted reaction. (C) DNA replication in FFA-1 and xBLM single- or double-depleted extracts. (Left panel) Western analysis of the extracts that had been depleted with the indicated antibodies. Two blots were probed with affinity-purified rabbit anti-FFA-1 or anti-xBLM antibody. (Right panel) Incorporation of [32P]dATP of the three depleted reactions plotted against that of the IgG-depleted reaction at 150 min.
We then tested whether the remaining DNA synthesis is attributable to incomplete depletion of xBLM from the extract. Small amounts of untreated extract were added back to the xBLM-depleted extract to rescue DNA replication (recombinant xBLM was not used because the portion of properly folded protein could not be determined). Although we could detect xBLM in as little as 0.1 μL of extract (data not shown), this amount (1%) was insufficient to increase replication in a 10-μL reaction (Fig. 4B). Because the depletion of xBLM was at least 99% complete, these results suggest that the remaining DNA synthesis is not because of incomplete depletion.
The most likely explanation, therefore, is that some other protein may have an activity similar to (although much weaker than) that of xBLM and can partially compensate for the depletion of xBLM. This explanation is consistent with the observation that neither human nor mouse BLM is essential. A good candidate for the redundant protein is another RecQ helicase family member, FFA-1 (focus forming activity 1), the Xenopus homolog of Werner's syndrome protein (WRN; Yan et al. 1998). FFA-1 also functions in DNA replication, but its depletion from the extract does not affect DNA replication because of the presence of a redundant protein (data not shown). We depleted egg extracts with either anti-FFA-1 antibody or anti-xBLM antibody or both antibodies together. As shown in Figure 4C, the two antibodies did not cross-react with each other. To our surprise, double depletion of FFA-1 and xBLM did not further reduce the residual DNA synthesis when compared with the single depletion of xBLM (Fig. 4D). These results suggest that the redundant partner for xBLM is not FFA-1 but some other protein yet to be identified.
Discussion
We have shown that depletion of the Xenopus BLM homolog severely inhibits the replication of DNA in the reconstituted nuclei. Moreover, the inhibition can be reversed by the addition of the recombinant xBLM. Thus, it is the xBLM protein itself rather than some other codepleted protein that is required for efficient DNA replication. The depletion of xBLM, however, does not affect the replication of m13 single-stranded DNA, indicating that xBLM does not participate in DNA synthesis per se. This result, in combination with the helicase activity of xBLM, suggests that xBLM is involved in the unwinding of double-stranded DNA template. Finally, we show that double depletion of xBLM and another RecQ family member, FFA-1, does not cause further reduction in the efficiency of DNA replication, suggesting that these two proteins are not redundant to each other. This result is consistent with the different clinical and cellular phenotypes of Bloom's syndrome and Werner's syndrome (German and Ellis 1998; Schellenberg et al. 1998).
The marked reduction in DNA replication in xBLM-depleted Xenopus egg extracts is in contrast to the mild defect in DNA replication in human BS cells. One likely explanation for this discrepancy is that different assays were used in the two cases. Xenopus eggs are programmed for rapid DNA synthesis with an S phase of only 10 min, whereas somatic cells replicate DNA at a much lower rate with S phases of >8 h. As such the absence of xBLM may have a disproportional impact on our in vitro assay. Another explanation is that varying degrees of gene redundancy exist in different organisms. As support for this idea, null mutations of BLM cause embryonic lethality in mouse but not in human (Chester et al. 1998). It is possible that the redundant protein in Xenopus is poorly expressed or not very active in the unfertilized eggs. One way or the other, our experiments strongly suggest that xBLM plays an important role in DNA replication. A similar function in DNA replication for the human BLM protein may explain the replication defects and higher mutation rates of BS cells. Increased rates of recombination between homologous chromosomes and sister chromatids may be an indirect effect of abnormal replication.
Among the many potential functions suggested for the RecQ family of helicases, participation in DNA replication is emerging as a common theme. Mouse WRN protein copurifies with a large protein complex consisting of multiple replication proteins (Lebel et al. 1999). FFA-1, the Xenopus homolog of WRN, is involved in the assembly of replication foci (Yan et al. 1998). In the budding yeast Saccharomyces cerevisiae, double disruption of two helicase genes, Sgs1 (RecQ type) and Srs2 (non-RecQ type), blocks the cell cycle with an unreplicated genome (Lee et al. 1999). In the fission yeast Schizosaccharomyces pombe, cells carrying null mutations of the Rqh1 gene (RecQ type) are unable to recover from S phase arrest (Stewart et al. 1997), and double mutants of Rqh1 and several replication elongation genes are synthetically lethal (Murray et al. 1997). Even in E. coli, RecQ, the prototype family member, participates in the RecF recombination pathway, which is critical for the reinitiation of DNA replication after UV irradiation (Courcelle and Hanawalt 1999). Further characterization of the exact roles these proteins play in DNA replication and how these roles evolve with genome complexity will provide more insights into how cells maintain their genome.
Materials and methods
Egg extract preparation and nuclei reconstitution
Crude interphase extracts and cytosol and membrane components derived from them were prepared following the procedure of Smythe and Newport (1991). Both crude extracts and fractionated components were used and found to be similar in the experiments of this study. They were collectively referred to as extracts unless otherwise specified. Nuclei were reconstituted at room temperature by mixing either crude extract (1/2 of the final volume) or cytosol (1/3 of the final volume) and membrane (1/10 of the final volume) with ATP-regeneration system (2 mM ATP, 20 mM phosphocreatine, 50 μg/mL creatine kinase), demembranated sperm chromatin (1000/μL), and egg lysis buffer (250 mM sucrose, 2.5 mM MgCl2, 50 mM KCl, 1 mM DTT, 10 mM HEPES at pH 7.5).
Cloning of xBLM
Two degenerate primers were used for the cloning of xBLM: 5′-CGGGATCCTGGGGICAYGAYTTYMG-3′ (upstream; translated from WGHDFR) and 5′-GGGGTACCDATICCCATICCRAANGC-3′ (downstream; translated from ALTATA). The enzyme was AmpliTaq Gold (Perkin Elmer), and the template DNA was purified from a Xenopus oocyte lambda gt11 cDNA library. A touchdown protocol was used for PCR: 10 sec at 94°C, 4 min at 47°C (−0.5°C per cycle for 16 cycles and then 4 min at 37°C for 35 cycles), and 25 sec at 72°C. The carboxyl terminus was cloned by a 3′ RACE reaction from the same DNA template, whereas the amino terminus was cloned by 5′ RACE from the total RNA isolated from oocytes.
Expression and purification of recombinant xBLM
The entire xBLM gene was cloned between the NdeI and Xho I sites of expression vector pET-29b (Novagen). The recombinant xBLM expressed by pET-29b vector has no additional amino acid added at the amino terminus and only two amino acids (from the XhoI site) plus six histidines at the carboxyl terminus. The recombinant xBLM was first purified on a HiTrap Chelating column (Pharmacia) following the procedure suggested by Novagen. The imidazole-eluted fractions containing recombinant xBLM were pooled and further purified on a Pharmacia HiTrap SP column. Specifically, the column was first washed with 40 column volumes of 400 mM NaCl, then eluted with a linear gradient of 450 mM to 650 mM NaCl in a buffer containing 25 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM DTT, and 10% glycerol. Typically, the recombinant xBLM was eluted in 500–600 mM NaCl fractions.
DNA helicase assay
The substrate for DNA helicase assays is a 5′-32P-labeled 48mer oligonucleotide hybridized to circular m13 single-stranded DNA. The xBLM-His protein was incubated with the substrate at the final concentration of 2 ng/μL and 0.8 ng/μL, respectively, in egg lysis buffer. After 30 min at room temperature, the reactions were terminated by mixing with an equal volume of stop buffer (0.5% SDS, 20 mM EDTA) and separated on a 12% polyacrylamide/TAE gel.
Antibody production and purification
A glutathione S transferase (GST) fusion protein containing amino acids 1–671 of xBLM was purified from SDS-PAGE and then injected into rabbits. The resulting polyclonal antibodies were purified following the published procedure (Yan et al. 1993). Briefly, they were first purified on an affinity column of a GST fusion protein containing amino acids 1–173 of xBLM. The anti-GST antibodies were then removed by an affinity column of GST tag alone.
Immunodepletion
Immunodepletion was performed by incubating extracts (diluted twofold with egg lysis buffer) with Affi-gel Protein A beads (Bio-Rad) that had been precoated with either the affinity-purified rabbit anti-xBLM antibody or rabbit IgG. After 1 h at 4°C, the beads were removed by low-speed centrifugation, and the supernatants were collected for replication assays. The efficiency of depletion was assessed by Western blot with the appropriate antibodies.
Replication assays
Radioactive assays of nuclear DNA replication were performed by including [32P]dATP in reactions containing sperm chromatin and interphase extracts or reactions containing m13 single-stranded DNA and cytosol. Samples were taken at various time points, processed, and separated on 1% agarose TAE gels as described previously (Smythe and Newport 1991). The dried gels were exposed to X-ray film and a PhosphorImager (Fuji). For visual assays of DNA replication, 10 μM biotin-dCTP was added to the reaction and detected by Streptavidin-Texas Red (Calbiochem) following the published procedure (Yan and Newport 1995). The GenBank accession no. for xBLM is AF30784.
Acknowledgments
We thank Dr. Yoshihiro Matsumoto for providing the purified recombinant human p21 protein and Drs. John Taylor and Robert Perry for critically reading the manuscript. This work was supported by grants from the V Foundation, Ellison Medical Foundation, and National Institutes of Health (R01 GM57962-02) to H.Y. and NIH training grant (CA-09035-25) to S.L.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Hong_Yan@fccc.edu; FAX (215) 728-3565.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.822400.
References
- Chester N, Kuo F, Kozak C, C.D. O'Hara CD, Leder P. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes & Dev. 1998;12:3382–3393. doi: 10.1101/gad.12.21.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcelle J, Hanawalt PC. RecQ and RecJ process blocked replication forks prior to the resumption of replication in UV-irradiated Escherichia coli. Mol Gen Genet. 1999;262:543–551. doi: 10.1007/s004380051116. [DOI] [PubMed] [Google Scholar]
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Ehmann UK, Williams JI. Human diseases associated with DNA repair. Adv Rad Biol. 1979;8:85–174. [Google Scholar]
- German J, Ellis NA. Bloom Syndrome. In: Vogelstein B, Kinzler KW, editors. The Genetic Basis of Human Cancer. New York: McGraw-Hill; 1998. pp. 301–315. [Google Scholar]
- Hand R, German J. A retarded rate of DNA chain growth in Bloom's syndrome. Proc Natl Acad Sci. 1975;72:758–762. doi: 10.1073/pnas.72.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, Yeh ET, Strauss JF, III, Maul GG. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK, Chevalier S, Philippe M, Kirschner MW. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FB, Lombard DB, Neff NF, Mastrangelo MA, Dewolf W, Ellis NA, Marciniak RA, Yin Y, Jaenisch R, Guarente L. Association of the Bloom syndrome protein with topoisomerase IIIα in somatic and meiotic cells. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- Karow JK, Chakraverty RK, Hickson ID. The Bloom's syndrome gene product is a 3′-5′ DNA helicase. J Biol Chem. 1997;272:30611–30614. doi: 10.1074/jbc.272.49.30611. [DOI] [PubMed] [Google Scholar]
- Lebel M, Spillare EA, Harris CC, Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J Biol Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- Lee SK, Johnson RE, Yu SL, Prakash L, Prakash S. Requirement of yeast SGS1 and SRS2 genes for replication and transcription. Science. 1999;286:2339–2342. doi: 10.1126/science.286.5448.2339. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Lonn U, Lonn S, Nylen U, Winblad G, German J. An abnormal profile of DNA replication intermediates in Bloom's syndrome. Cancer Res. 1990;50:3141–3145. [PubMed] [Google Scholar]
- Mechali M, Harland RM. DNA synthesis in a cell-free system from Xenopus eggs: Priming and elongation on single-stranded DNA in vitro. Cell. 1982;30:93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Murray JM, Lindsay HD, Munday CA, Carr AM. Role of Schizosaccharomyces pombe RecQ homolog, recombination, and checkpoint genes in UV damage tolerance. Mol Cell Biol. 1997;17:6868–6875. doi: 10.1128/mcb.17.12.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff NF, Ellis NA, Ye TZ, Noonan J, Huang K, Sanz M, Proytcheva M. The DNA helicase activity of BLM is necessary for the correction of the genomic instability of bloom syndrome cells. Mol Biol Cell. 1999;10:665–676. doi: 10.1091/mbc.10.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. Nuclear reconstitution in vitro: Stages of assembly around protein-free DNA. Cell. 1987;48:205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- Ray JH, German J. The cytogenetics of the chromosome-breakage syndromes. In: German J, editor. Chromosome Mutation and Neoplasia. New York: Alan R. Liss; 1983. pp. 135–167. [Google Scholar]
- Schellenberg GD, Miki T, Yu CE, Nakura J. Werner Syndrome. In: Vogelstein B, Kinzler KW, editors. The Genetic Basis of Human Cancer. New York: McGraw-Hill; 1998. pp. 347–359. [Google Scholar]
- Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Meth Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Stewart E, Chapman CR, Al-Khodairy F, Carr AM, Enoch T. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 1997;16:2682–2692. doi: 10.1093/emboj/16.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld UP, Howell M, Rempel R, Maller JL, Hunt T, Blow JJ. Cip1 blocks the initiation of DNA replication in Xenopus extracts by inhibition of cyclin-dependent kinases. Curr Biol. 1994;4:876–883. doi: 10.1016/s0960-9822(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes & Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID. The Bloom's syndrome gene product interacts with topoisomerase III. J Biol Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- Yan H, Newport J. An analysis of the regulation of DNA synthesis by cdk2, Cip1, and licensing factor. J Cell Biol. 1995;129:1–15. doi: 10.1083/jcb.129.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Merchant AM, Tye BK. Cell cycle-regulated nuclear localization of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes & Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]
- Yan H, Chen CY, Kobayashi R, Newport J. Replication focus-forming activity 1 and the Werner syndrome gene product [see comments] Nat Genet. 1998;19:375–378. doi: 10.1038/1263. [DOI] [PubMed] [Google Scholar]
- Yankiwski V, Marciniak RA, Guarente L, Neff NF. Nuclear structure in normal and bloom syndrome cells. Proc Natl Acad Sci. 2000;97:5214–5219. doi: 10.1073/pnas.090525897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Hu P, Ye TZ, Stan R, Ellis NA, Pandolfi PP. A role for PML and the nuclear body in genomic stability. Oncogene. 1999;18:7941–7947. doi: 10.1038/sj.onc.1203367. [DOI] [PubMed] [Google Scholar]