Abstract
Studies have demonstrated that increased oxidative stress contributes to the pathogenesis and the development of pulmonary artery hypertension (PAH). Extracellular superoxide dismutase (SOD3) is essential for removing extracellular superoxide anions and it is highly expressed in lung tissue. However, it is not clear whether endogenous SOD3 can influence the development of PAH. Here we examined the effect of SOD3 knockout on hypoxia-induced PAH in mice and a loss-of-function SOD3 gene mutation (SOD3E124D) on monocrotaline (40 mg/kg)-induced PAH in rats. SOD3 knockout significantly exacerbated 2 weeks hypoxia-induced right ventricular (RV) pressure and RV hypertrophy, while RV pressure in SOD3 KO mice under normoxic conditions is similar to wild type controls. In untreated control rats at age of 8 weeks, there was no significant difference between wild type and SOD3E124D rats in RV pressure and the ratio of RV weight to left ventricular weight (0.25±0.02 in wild type rats vs. 0.25±0.01 in SOD3E124D rats). However, monocrotaline caused significantly greater increases of RV pressure in SOD3E124D rats (48.6±1.8 mmHg in wild type vs. 57.5±3.1 mmHg in SOD3E124D rats), of the ratio of RV weight to left ventricular weight (0.41±0.01 vs. 0.50±0.09, p<0.05), and of the percentage of fully muscularized small arterioles in SOD3E124D rats (55.2±2.3 % vs. 69.9 ±2.6 %, p<0.05). Together, these findings indicate that the endogenous SOD3 has no role in the development of PAH under control conditions, but plays an important role in protecting the lung from the development of PAH under stress conditions.
Keywords: pulmonary artery hypertension, right ventricular hypertrophy, oxidative stress, extracellular SOD
Introduction
Pulmonary artery hypertension (PAH) is a progressive disease with a very poor prognosis. PAH is characterized by a progressive elevation of pulmonary arterial pressure, ultimately inducing right ventricular (RV) hypertrophy and heart failure. Studies have demonstrated that increased oxidative stress, such as enhanced production of superoxide anions and other reactive oxygen species may contribute to the pathogenesis and the development of idiopathic PAH (IPAH) in patients and to PAH secondary to high pulmonary vascular flow in lambs.1,2 In addition, administration of antioxidants attenuates the development of PAH,3,4 suggesting that pulmonary oxidative stress regulates the development of PAH.
Superoxide dismutase (SOD) is a first line of defense against free radical attack. Three SOD isozymes have been identified, including a copper/zinc-containing SOD (SOD1), which is primarily cytosolic in location, a mitochondrial manganese SOD (SOD2), and an extracellular SOD (SOD3). SOD3 is a glycoprotein secreted into the extracellular fluid by fibroblasts that binds to sulfated polysaccharides, such as heparin and heparan sulfate,5,6 as well as to other matrix components.7,8 As a result, SOD3 binds to the surface of endothelial cells and the extracellular matrix, which has a high abundance of heparan sulfate.9 Lung is one of the organs with a relatively high SOD3 expression.5,10,11 Previous studies have demonstrated that overexpression of SOD3 attenuates hypoxia-induced PAH in mice11 and monocrotaline-induced PAH in rats.12 Overexpression of SOD3 also attenuates bleomycin-induced lung injury.13 However, because SOD3 has a minimal impact on total tissue SOD activity,10 it is uncertain whether endogenous SOD3 can influence the development of PAH. To address this question, we examined the effect of SOD3 knockout (KO) on hypoxia-induced PAH in mice, and the effect of SOD3 gene mutation (SOD3E124D) on monocrotaline-induced PAH in rats. Here we report that both SOD3E124D in rats or SOD3 knockout in mice had no effect on PAH and RV hypertrophy under control conditions but resulted in significantly greater increases of right ventricular pressure, pulmonary vascular remodeling, as well as greater RV hypertrophy in response to monocrotaline or chronic hypoxia. The findings indicate that the endogenous SOD3 plays an important role in protecting the lung from the development of PAH and RV hypertrophy under stress conditions.
Materials and Methods
SOD3 knockout Mice
SOD3 knockout mice and control wild type mice used in the present study are described previously.6,14,15 This study was approved by the Institutional Animal Care and Use Committee of University of Minnesota.
SOD3E124D rats
SOD3E124D (SS-Sod3m1Mcwi) rats were identified as a mutation in an ethylnitrosourea (ENU) mutagenesis screen by the PhysGen Program in Genomic Applications (http://pga.mcw.edu), backcrossed to the Dahl/Salt Sensitive (Dahl/SS) strain, intercrossed and maintained as a homozygous colony. N2F7-F8 generation animals were provided as a gift by Transposagen Biopharmaceuticals. Since these rats were intentionally generated in the Dahl/SS background, provided by the Medical College of Wisconsin to Charles River Laboratory, Dahl/SS rats were used as controls.
Hypoxia-induced PAH in mice
Male SOD3 knockout mice and wild type control mice at age 10–14 weeks were exposed to hypobaric hypoxia as described by Hampl et al.16 Briefly, the pressure in the chamber was decreased progressively from 0.8 atm (16.9% O2) on Day 1 to 0.5 atm (10.5% O2) after Day 7, and was maintained at 10.5% O2 for 2 more weeks. The chamber was opened once every week for cleaning and feeding. After exposure to 10.5% O2 for 2 weeks, mice were removed from the hypoxia chamber for determination of RV pressure and hypertrophy. The sham mice were kept in normobaric conditions.
Induction of PAH in rats with monocrotaline (MCT)
Male SOD3E124D rats and wild type control rats (Dahl/SS) at age 5 weeks were given intraperitoneal injections of MCT (40 mg/kg, Sigma, St. Louis, MO) or an equivalent volume of vehicle as a control. The bodyweight of these rats were monitored weekly. Right ventricular (RV) and aortic pressure were then determined at 3 weeks after MCT or vehicle injection and samples were collected for related tests.
Having noticed that MCT-induced more PAH and pulmonary vascular remodeling in SOD3E124D rats, we further determined the rescue of the effect SOD mimetic, Mn(III)TMPyP (6mg/kg/day, Cayman Chemical, MI) on MCT-induced PAH in SOD3E124D rats. Mn(III)TMPyP treatment started immediately after injections of MCT.
Measurements of aortic pressure and RV hemodynamics
At the end of the study protocol, rats and mice were first anesthetized with 1.5% isoflurane Rats were intubated with a 16-gauge Teflon tube attached to a mechanical ventilator (Kent Scientific-Ventilator, ventilator settings: breathing frequency, 80 breaths per minute; pressures, 9/0 cm H2O; inspiratory/expiratory ratio, 1:1). Mice were intubated with a 20-gauge Teflon tube attached to MiniVent type 845 mouse ventilator (Hugo Sachs Elektronik).
A 1.2-F pressure catheter (Scisense Inc. Ontario Canada) was introduced through the right common carotid artery into the ascending aorta for measurement of systolic and diastolic blood pressures as described previously.14,15,17,18 For RV hemodynamics, open-chest RV catheterization was performed during anesthesia with 1.5% isoflurane.19 Data were collected when steady state was reached.
Sample Preparation
After the final hemodynamic assessment, the rats and the mice were euthanized by exsanguination, and the heart, lung, and other major organs were harvested. Lung weight was determined and the left lung was harvested and snap-frozen in liquid nitrogen for biochemical analysis. The airways of the top right lobe were subsequently perfused with and then fixed in 10% buffered formalin for histological analysis. The wet weight of RV and of left ventricle (LV) + septum (S) were weighed and the ratio of RV weight to LV + S were calculated as an index of RV hypertrophy.20
Histological staining, Semiquantification of Fibrosis, and Western Blots
For staining of smooth muscle α-actin, tissue sections (5µm) were deparaffinized, rehydrated, antigen recovered in Tris-EDTA buffer (pH=9.0) 30 minutes at 95–100°C, washed in PBS. The sections were incubated with 3% H2O2 in PBS for 20 minutes followed by 1% BSA solution for 1 hour. Sections were then incubated with a monoclonal primary antibody (1:400) against smooth muscle α-actin (Sigma-Aldrich) overnight at 4°C, and followed with Alexa Fluor 555 labeled secondary antibody against mouse Ig-G (1:1000) (Invitrogen).15 The slides were examined using a confocal microscope (Zeiss LSM510). Measurement of ventricular fibrosis and cardiac myocyte size were performed using the method described previously.17,21 For methods of Semiquantification of pulmonary vascular muscularization, fibrosis and Western Blots, please see http://hyper.ahajournals.org.
Chemical Analysis
Oxidative stress marker TBARS (thiobarbituric acid reactive substances) content was determined as described previously.14 Total SOD activity and SOD2 activity of lung tissues were measured using a SOD activity kit (Cayman Chemical Company) according the manufacturer’s instructions. For relative lung SOD3 activity assay, a total of 2 µg primary antibody for SOD3 (Lifespan Biosciences) was added into 500µl tissue extract (2mg/ml) and then incubated for 1–2 hours at 4° C. Protein A/G-Agarose of 20 µl was then added to the mixture and incubated at 4° C on a rocker platform overnight. The immunoprecipitates were collected by centrifugation at 3,000 rpm for 30 seconds at 4° C. After gently washing with PBS 4 times, the pellets were resuspended in 400µl buffer for SOD activity assay with the commercial kit according to the manufacturer’s instructions. Control IgG was used as a negative control.
Total antioxidant capacity
Total antioxidant capacity of lung tissues was determined using an antioxidant power assay kit (Oxford Biomedical Research) according the manufacturer’s instructions.
Statistical Analysis
All values are expressed as mean ± standard error or median (± standard error). Data of two groups was compared with unpaired t-test. Two-way analysis of variance was used to test for differences between transgenic and wild type animals under control conditions and after MCT injection. If analysis of variance demonstrated a significant effect, post hoc pairwise comparisons were made using the Fisher least significant difference test. Statistical significance was defined as p<0.05.
Results
SOD3 knockout aggravated the hypoxia-induced increase of RV pressure and hypertrophy
To study whether SOD3 dysfunction can affect PAH in other experimental models, we determined the effect of SOD3 knockout on hypoxia-induced PAH in mice. RV pressure and the ratio of RV to LV + septum weight were not different between SOD3 knockout mice and wild type controls under control normoxic conditions. However, SOD3 knockout significantly exacerbated hypoxia-induced increases of RV pressure (Figure 1A) and RV hypertrophy as indicated by the ratio of RV to LV+S weight (Figure 1B). In addition, hypoxia caused increases of fully muscularized arterioles in both wild type controls and SOD3 knockout mice, while these increases were significantly greater in the SOD3 knockout mice than in the wild type controls (Figure 1C, Figure S1).
Figure 1.
SOD3 knockout in mice significantly exacerbated hypoxia-induced increases of RV pressure (A), of RV hypertrophy as indicated by the ratio of RV to LV+S weight (B), and of pulmonary vascular remodeling as indicated by significant increases of fully muscularized small arterioles (C). *p<0.05 vs sham control; #p<0.05 vs corresponding WT mice.
The SOD3E124D mutation had no significant effect on the animals’ growth but exacerbated the MCT-induced increase of RV pressure
SOD3E124D rats grew and developed normally. There were no significant differences in terms of body weight gain (Figure 2A), and left ventricular weight between SOD3E124D rats and wild type controls at age of 2 months (Table S1). In addition, systemic blood pressure, heart rate, RV pressure, and the ratio of RV to LV + septum weight were not different between SOD3E124D rats and wild type controls at age of 2 months (Table S1, Table S2, Figure 2B, 2C, 2D, Figure S2, S3). SOD3E124D had no detectable impact on lung SOD3 expression (Figure S4), but significantly reduced lung SOD3 activity ~60% in rats (0.31±0.02 in wild type rats versus 0.12±0.06 in SOD3E124D rats, p<0.05).
Figure 2.
SOD3E124D mutation in rats had no significant effect on growth but exacerbated MCT-induced increase of RV pressure. SOD3E124D mutation had no effect on growth under both control conditions and after MCT injection respectively (A). SOD3E124D mutation had no effect on RV pressure under control conditions but further elevated the MCT-induced increase of RV pressure, indicating more severe PAH in SOD3E124D rats (B,C). *p<0.05 vs sham control; #p<0.05 vs. corresponding WT rats.
MCT injection caused significant increase of RV pressure in both SOD3E124D rats and wild type rats (48.6±1.8 mmHg in wild type vs. 57.5±3.1 mmHg in SOD3E124D rats), while MCT caused a significantly greater increase of RV systolic pressure in SOD3E124D rats (Table S2, Figure 2B, 2D), indicating exacerbated pulmonary artery hypertension in SOD3E124D rats. MCT injection significantly attenuated the weight gain in both SOD3E124D and wild type rats (Figure 2A).
The SOD3E124D mutation aggravated the MCT-induced increase of RV hypertrophy and fibrosis
In sham (no monocrotaline) rats at age of 2 months, there was no significant difference between WT and SOD3E124D rats in left ventricle (LV) + septum (S) weight (641±19.3 mg in wild type sham vs. 640±9.4 mg in SOD3E124D rats), right ventricle (RV) weight (162±6.7 mg in wild type sham vs. 157±2.8 mg in SOD3E124D rats), their ratio to body weight or tibia length, and ratio of RV weight to LV + septum weight (0.25±0.02 in wild type untreated sham rats vs. 0.25±0.01 in SOD3E124D untreated sham rats) (Table S1, Figure 2C). Consistent with the significantly greater increase of RV systolic pressure in SOD3E124D rats after MCT, SOD3E124D rats had significantly greater increases of RV weight (239±8.7 mg in wild type rats vs. 285±10.6 mg in SOD3E124D rats, p<0.05) and the ratio of RV to LV + septum (0.41±0.01 in wild type rats vs. 0.50±0.09 in SOD3E124D rats, p<0.05) in response to MCT (Table S1, Figure 2C), indicating that SOD3E124D mutation exacerbated MCT-induced RV hypertrophy. Histological analysis indicated that MCT caused a significantly greater increase of RV fibrosis (Figure S2, Figure S3A) and cardiac myocyte cross sectional area (Figure S2, Figure S3B), indicating severe RV remodeling in SOD3E124D rats after MCT.
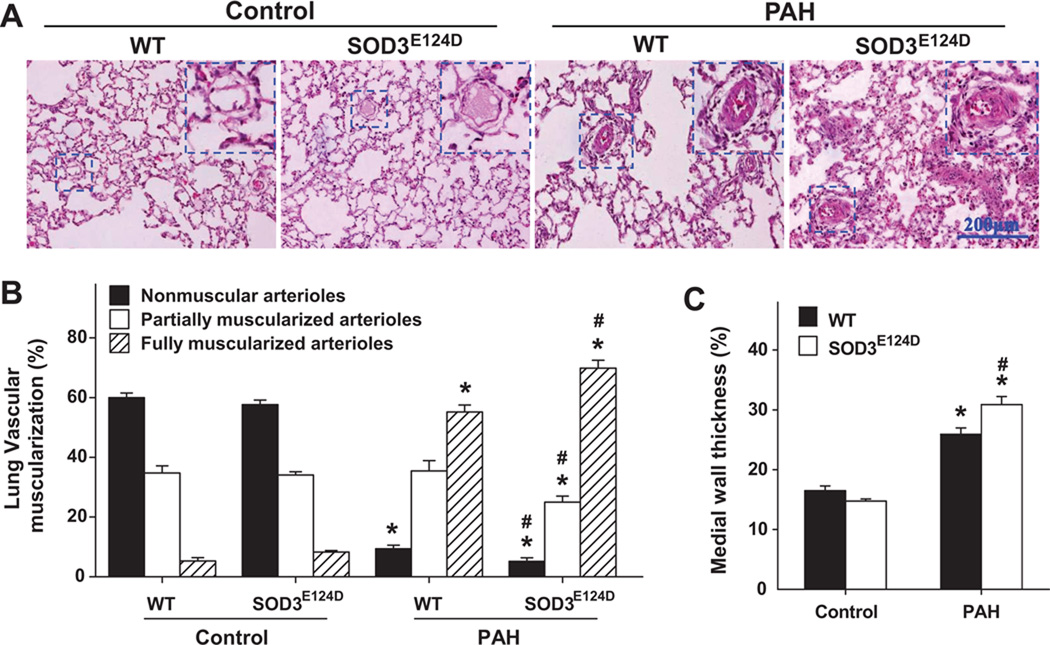
The SOD3E124D mutation exacerbated the MCT-induced pulmonary vascular remodeling
To determine the effect of SOD3E124D on pulmonary vascular remodeling, we determined the percentage of non-muscularized (NM), partially muscularized (PM), and fully muscularized small arterioles (FM) in wild type rats and SOD3E124D rats under sham conditions and 3 weeks after MCT injection (Figure 3A,B). MCT caused increases of fully muscularized small arterioles both in wild type rats and SOD3E124D rats (55.2 ± 2.3 % in wild type rats vs. 69.9 ± 2.6 % in SOD3E124D rats, p<0.05), but these increases were significantly greater in the SOD3E124D rats than in the wild type rats (Figure 3). Meanwhile, MCT also caused decreases of non-muscularized small arterioles both in wild type rats and SOD3E124D rats (9.4 ± 1.12 % in wild type rats vs. 5.2 ± 1.2 % in SOD3E124D rats, p<0.05), but these decreases were significantly greater in the SOD3E124D rats than in the wild type rats (Figure 3). In addition, SOD3E124D rats had significantly exacerbated MCT-induced medial wall thickness (Figure 3C) and medial area (Figure S5) of arteries of 50–200um. Together, these data indicate that SOD3E124D significantly exacerbated MCT-induced pulmonary vascular remodeling in rats.
Figure 3.
SOD3E124D mutation caused significantly greater pulmonary vascular remodeling. Distribution of nonmuscular, partially muscular, and fully muscularized small arterioles in WT rats and MCT-induced PAH rats (A,B). SOD3E124D significantly aggravated MCT-induced pulmonary vascular muscularization (B). SOD3E124D mutation significantly exacerbated MCT-induced relative medial wall thickness of larger arteries in rats (C). *p<0.05 vs sham control; #p<0.05 vs corresponding WT rats.
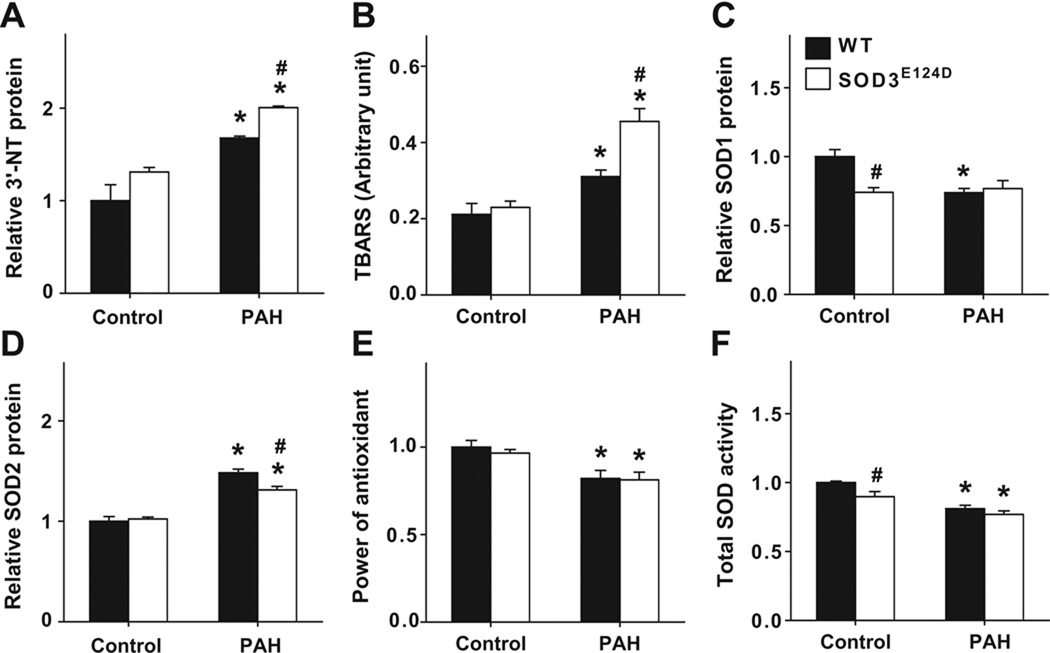
The SOD3E124D mutation exacerbated the MCT-induced pulmonary oxidative stress
MCT also caused increases of pulmonary 3’-nitrotyrosine, and TBARS both in wild type rats and SOD3E124D rats, but these increases were significantly greater in the SOD3E124D rats than in the wild type rats (Figure 4A, 4B, Figure S7), indicating a greater degree of pulmonary oxidative stress in SOD3E124D rats than in wild type rats after MCT. Expression of lung SOD1 and SOD2 were determined (Figure 4C, 4D). SOD3E124D had no detectable impact on lung overall antioxidant capacity as indicated by the power of antioxidants (Figure 4E). Lung total SOD activity was significantly lower in SOD3E124D rats only under control conditions and the difference was small (Figure 4F). Lung SOD2 activity was unchanged in SOD3E124D rats (Figure S8).
Figure 4.
SOD3E124D mutation caused significantly greater increase of lung oxidative stress. Increases of oxidative stress markers 3’-nitrotyrosine and TBARS (A,B) were observed in SOD3E124D rats. Changes were also noted in the expression of SOD1 protein (C), SOD2 protein (D), and total SOD activity (E) in SOD3E124D and wild type rats under control conditions or after MCT injection. No change was seen in the overall antioxidant capacity (F) *p<0.05 vs sham control; #p<0.05 vs corresponding WT rats.
SOD mimetic Mn(III)TMPyP rescued SOD3E124D rats from MCT-induced pulmonary artery hypertension: To further determine the impact of lung oxidative stress on the development of PAH, we determined the effect of Mn(III)TMPyP treatment (6mg/kg/day). Mn(III)TMPyP significantly reduced MCT-induced RV pressure (Figure 5A), the ratio of RV weight to LV + septum weight (Figure 5B), and lung vascular remodeling in SOD3E124D rats (Figure 5C,D).
Figure 5.
SOD mimetic Mn(III)TMPyP significantly rescued SOD3E124D rats from MCT-induced increase of RV pressure (A), RV hypertrophy (B) and pulmonary vessel remodeling (C). *p<0.05 vs rats with MCT injection alone.
Discussion
SOD3 binds to the surface of endothelial cells and the extracellular matrix and plays a critical role in removing extracellular free radical species. SOD3 is highly expressed in the lung. However, as SOD3 has a minimal impact on total tissue SOD activity,10 it is uncertain whether endogenous SOD3 can influence the development of PAH. In the present study, we report that SOD3E124D had no effect on overall pulmonary oxidative stress, PAH and RV hypertrophy under control conditions but resulted in more severe pulmonary hypertension, more remodeling of the pulmonary arteries and more right ventricular hypertrophy and fibrosis in the setting of MCT-induced pulmonary hypertension. In addition, SOD3 knockout aggravated hypoxia-induced PAH in mice. The findings indicate that endogenous SOD3 plays an important role in protecting the lung from the development of PAH under stress conditions.
The over-expression of extracellular SOD3 reduces both hypoxia- and monocrotaline-induced PAH.11,12 Similarly, an increase in SOD3 activity decreases hypoxic pulmonary vasoconstriction in bovine pulmonary artery rings.22 Loss of SOD3 could result in increased levels of O2− and down-stream radicals such as peroxynitrite, decreased H2O2 in extracellular space, and reduced intercellular diffusion of endothelial NO to surrounding cell types. Decreased NO bioavailability enhances the development of PAH, while increased NO or increase of its down-stream product cGMP by inhibition of PDE5 attenuate the development of PAH.23 Thus it is possible that a contributing factor to the exacerbated PAH in SOD3E124D rats and SOD3 knockout mice is increased scavenging of NO by superoxide and a subsequent reduction in NO/cGMP bioavailability.
The role of endogenous SOD3 in pulmonary vascular physiology and pathophysiology has not been clear. In these studies we show that loss of SOD3 function does not affect the tone or structure of the pulmonary arteries under normoxic control conditions. The finding that loss of function mutation in SOD3E124D rats and SOD3 knockout in mice results in more severe pulmonary hypertension and more RV hypertrophy after MCT or hypoxia (but not under control conditions) is conceptually consistent with previous studies that loss of SOD3 function exacerbated infarction or pressure overload-induced left ventricular maladaptive remodeling.14,24 Thus, O2− and possibly related down-stream radicals, play a detrimental role in the pathophysiology of PAH as well as other pathological conditions such as ventricular remodeling.25,26
The overall role of SOD and oxidative stress are of particular interest in the pulmonary vasculature because the expression of SOD2 is found to be reduced in IPAH patients and in fawn-hooded rats that spontaneously develop PAH.27 The decrease in SOD2 precedes the development of PAH in the fawn-hooded rats. The use of an SOD mimetic prevents pulmonary hypertension and reduces right ventricular hypertrophy in rats exposed to chronic hypoxia28 and in fawn-hooded rats.4 Similar to the effects of SOD3 depletion, a reduction in SOD2, should also increase O2− levels and down-stream radicals such as peroxynitrite, and decrease levels of H2O2. SOD2 and SOD3 thus appear to play a major role in protecting against the development of PAH through decreasing O2− and increasing H2O2 and NO bioavailability.29 These results suggest that SOD mimetics or treatments that increase endogenous SOD2 or SOD3 may have therapeutic value in PAH.
Clinical Perspective.
Pulmonary artery hypertension (PAH) is a progressive disease with a very poor prognosis. PAH is characterized by a progressive elevation of pulmonary arterial pressure, ultimately inducing right ventricular (RV) hypertrophy and heart failure. Studies have demonstrated that increased oxidative stress may contribute to the pathogenesis and the development of idiopathic PAH. Extracellular superoxide dismutase (SOD3) plays an important role in attenuating superoxide anion in the extracellular space. However, the effect of the endogenous SOD3 on the development of PAH has not been clear. Here we report that both SOD3 knockout in mice or SOD3E124D mutation in rats resulted in significantly greater increases of RV pressure, RV hypertrophy and pulmonary vascular remodeling in response to hypoxia (in mice) or monocrotaline (in rats). The findings indicate that endogenous SOD3 plays an important role in protecting against the development of PAH and subsequent RV hypertrophy under stress conditions. These results suggest that SOD mimetics or treatments that increase endogenous SOD3 may have therapeutic value in PAH.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by NHLBI Grants R21HL102597 (YC), R21HL098719 (YC), R21HL098669 (YC), HL079168 (RJB) and HL65322 (EKW) from the National Institutes of Health, GRNT2260175 (YC) and a Scientist Development Award from American Heart Association (XH), and VA Research funding (EKW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Conflict of Interest Disclosures: Dr. Eric Ostertag has served as the Chief Executive Officer of Transposagen Biopharmaceuticals; Dr. Aron Geurts has served as a consultant for Transposagen Biopharmaceuticals. The other authors report no conflicts.
References
- 1.Bowers RP, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 2.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 3.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–H1047. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, Enghild JJ. Extracellular superoxide dismutase (EC-SOD) binds to type I collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–13710. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 8.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 9.Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- 10.Sentman ML, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 11.Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 13.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;282:L719–L726. doi: 10.1152/ajplung.00058.2001. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampl V, Tristani-Firouzi M, Nelson DP, Archer SL. Chronic infusion of nitric oxide in experimental pulmonary hypertension: pulmonary pressure-flow analysis. Eur Respir J. 1996;9:1475–1481. doi: 10.1183/09031936.96.09071475. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, Schwedhelm E, Lüneburg N, Böger RH, Zhang P, Chen Y. Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine. Circulation. 2009;120:2222–2229. doi: 10.1161/CIRCULATIONAHA.108.819912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handoko ML, de Man FS, Happé CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation. 2009;120:42–49. doi: 10.1161/CIRCULATIONAHA.108.829713. [DOI] [PubMed] [Google Scholar]
- 20.Urboniene D, Haber I, Fang YH, Thenappan T, Archer SL. Validation of High-Resolution Echocardiography and Magnetic Resonance Imaging Versus High-Fidelity Catheterization in Experimental Pulmonary Hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L401–L412. doi: 10.1152/ajplung.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y. PGC-1alpha Regulates Expression of Myocardial Mitochondrial Antioxidants and Myocardial Oxidative Stress After Chronic Systolic Overload. Antioxid Redox Signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad M, Zhao X, Kelly MR, Kandhi S, Perez O, Abraham NG, Wolin MS. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecSOD. Am J Physiol Heart Circ Physiol. 2009;297:H1453–H1461. doi: 10.1152/ajpheart.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, Bache RJ, Duncker DJ, Chen Y. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44:1305–1313. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA. Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L36–L43. doi: 10.1152/ajplung.00283.2001. [DOI] [PubMed] [Google Scholar]
- 26.Herst PM, Tan AS, Scarlett DJ, Berridge MV. Cell surface oxygen consumption by mitochondrial gene knockout cells. Biochim Biophys Acta. 2004;1656:79–87. doi: 10.1016/j.bbabio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 28.Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol. 2004;141:105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.