Abstract
Objective
Perinatal iron deficiency results in persistent hippocampus-based cognitive deficits in adulthood despite iron supplementation. The objective of the present study was to determine the long-term effects of perinatal iron deficiency and its treatment on hippocampal anatomy and neurochemistry in formerly iron-deficient young adult rats.
Methods
Perinatal iron deficiency was induced using a low-iron diet during gestation and the first postnatal week in male rats. Hippocampal size was determined using volumetric magnetic resonance imaging at 8 weeks of age. Hippocampal neurochemical profile, consisting of 17 metabolites indexing neuronal and glial integrity, energy reserves, amino acids, and myelination, was quantified using high-field in vivo 1H NMR spectroscopy at 9.4 T (N = 11) and compared with iron-sufficient control group (N = 10).
Results
The brain iron concentration was 56% lower than the control group at 7 days of age in the iron-deficient group, but had recovered completely at 8 weeks. The cross-sectional area of the hippocampus was decreased by 12% in the formerly iron-deficient group (P = 0.0002). The hippocampal neurochemical profile was altered: relative to the control group, creatine, lactate, N-acetylaspartylglutamate, and taurine concentrations were 6–29% lower, and glutamine concentration 18% higher in the formerly iron-deficient hippocampus (P < 0.05).
Discussion
Perinatal iron deficiency was associated with reduced hippocampal size and altered neurochemistry in adulthood, despite correction of brain iron deficiency. The neurochemical changes suggest suppressed energy metabolism, neuronal activity, and plasticity in the formerly iron-deficient hippocampus. These anatomic and neurochemical changes are consistent with previous structural and behavioral studies demonstrating long-term hippocampal dysfunction following perinatal iron deficiency.
Keywords: Hippocampus, 1H NMR spectroscopy, Iron, Newborn, Perinatal iron deficiency
Introduction
Iron deficiency during the fetal and neonatal periods (i.e. perinatal period) is common in pregnancies complicated by maternal iron deficiency, diabetes mellitus, intrauterine growth restriction, and prematurity.1 Perinatal iron deficiency has deleterious effects on neurodevelopment in humans and rats.2 Within the developing brain, the hippocampus appears particularly vulnerable. Hippocampus-mediated recognition memory is impaired in iron-deficient newborn infants.3 Likewise, hippocampal energy metabolism, neurotransmission and myelination,4 dendritic structure, and elec-trophysiology5,6 are altered during the period of perinatal iron deficiency in developing rats.
Of further concern, the genomic, structural and functional deficits due to early iron deficiency persist despite iron supplementation in humans and rodents.2,7–10 Previous studies have demonstrated suppression of genes involved in plasticity,11 impaired long-term potentiation,5 and abnormal dendritic arborization6 in the hippocampus, as well as deficits on hippocampus-dependent cognitive tasks8,9 in formerly iron-deficient rats during early adulthood, despite the restoration of hippocampal iron concentration through iron supplementation.11 The anatomic and neurochemical underpinnings of these enduring deficits are not well understood. A previous in vivo 1H NMR spectroscopy study demonstrated that perinatal iron deficiency alters the neurochemical profile of the hippocampus in developing rats during the period of iron deficiency.4 Specifically, the neurochemical markers of energy homeostasis, neurotransmission, and myelination were affected,4 implying a metabolically hypoactive hippocampus during the period of iron deficiency. The efficacy of iron supplementation beginning early in postnatal life in reversing these neurochemical changes has yet to be established. The objective of the present study was to determine the long-term effects of perinatal iron deficiency and its treatment on hippocampal anatomy and neurochemistry using volumetric magnetic resonance imaging (MRI) and in vivo 1H NMR spectroscopy in formerly iron-deficient young adult rats. High-field in vivo 1H NMR spectroscopy is a sensitive method for simultaneously determining markers of neuronal and glial integrity, energy metabolism, membrane biosynthesis, and neurotransmitters in distinct brain regions of rats.4,12,13 Based on previous investigations, we hypothesized that formerly iron-deficient rats will demonstrate structural and neurochemical abnormalities in the hippocampus at young adulthood.
Methods
Animal preparation
Male Sprague-Dawley rats (N = 31) from six separate litters were used in this study. Pregnant rats were purchased (Charles River Laboratories; Wilmington, MA, USA) and individually housed in a temperature and humidity-controlled room. Three dams were started on a low-iron diet (Formula TD 80396, Harlan-Teklad, Madison, WI, USA; elemental iron concentration: 3–6 mg/kg) upon arrival on gestational day 1–2. This low-iron regimen continued throughout the rest of gestation and until postnatal day (P) 7, followed by an iron-supplemented diet (Teklad 4% Mouse/Rat Diet 7001, Harlan-Teklad; elemental iron concentration: 198 mg/kg) until the day of the experiment (formerly iron-deficient group). This dietary model induces a similar degree of brain iron deficiency as found in iron-deficient human newborn infants at birth with complete replenishment of hippocampal iron concentration by adulthood.4,11 Three dams were maintained on the iron-supplemented diet throughout gestation and postnatal periods to produce the control group. Dams were allowed to deliver spontaneously and the litter size was limited to eight by culling on P2. Some rats in each litter (N = 5/group) were killed on P7 to determine brain iron concentration. The rest were weaned on P21 to the iron-supplemented diet and allowed to reach adulthood. A total of 21 rats were used for NMR studies on P56, followed by determination of brain iron concentration. All procedures were approved by the Institutional Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals.
Determination of brain iron concentration
Rats (N = 5/group on P7 and N = 7/group on P56) were deeply anesthetized using sodium pentobarbital (100 mg/kg IP). A blood sample was collected for determining hematocrit before in situ transcardial perfusion with normal saline. The brain was collected, flash frozen, and stored at −80 °C until analysis. Brain water content was determined by weighing the brain before and after drying for 72 hours. Brain iron concentration was assayed using atomic absorption spectroscopy.
MRI and in vivo 1H NMR spectroscopy
MRI and 1H NMR spectra were obtained from spontaneously breathing rats (N = 10, control group; N =11, formerly iron-deficient group) on P56 (i.e. 8 weeks of age) under inhalation anesthesia (gas mixture O2:N2O = 1:1, isoflurane 3% for induction, 1–2% for maintenance). All MR experiments were performed in a horizontal bore 9.4 T/31 cm magnet (Varian/Magnex Scientific, Yarnton, UK) interfaced to a Varian INOVA console (Varian, Inc., Palo Alto, CA, USA).4,12 Briefly, magnetic field homogeneity was adjusted using FASTMAP shimming technique. Multi-slice coronal brain images were acquired using RARE sequence (echo spacing = 15 ms, echo train length = 8, slice thickness = 1 mm). In vivo 1H NMR spectra were acquired from a 10–13 μl volume of interest (VOI) centered on the left hippocampus using ultra-short echo-time STEAM localization method (TE = 2 ms) combined with outer volume suppression and VAPOR water suppression.12
Determination of hippocampal cross-sectional area
The cross-sectional area of the hippocampus was measured from the coronal RARE images of the brain. The boundary of the hippocampus was outlined and the surface area was determined using the user-guided tools of the Image Browser software package (Varian, Inc.).
Quantification of metabolites
In vivo 1H NMR spectra were analyzed using LCModel with the spectrum of fast relaxing macromolecules included in the basis set.4,12 Unsuppressed water signal was used as internal reference. A brain water content of 80% was used in the calculation, based on the value determined in the harvested brains. The following 17 metabolites were quantified from 1H NMR spectra: alanine, ascorbate, aspartate, creatine (Cr), phosphocreatine (PCr), gamma-amino-butyric acid, glucose, glutamate, glutamine, glutathione, lactate, myo-inositol, N-acetylaspartate, N-acetylaspartylglutamate (NAAG), phosphoethanolamine, taurine, and sum of glycerophosphocholine and phosphocholine (GPC + PC). Total Cr (Cr + PCr), and PCr/Cr and glutamate/glutamine ratios were also determined.
Statistical analysis
Data were analyzed using independent samples t-tests. Data are expressed as mean ± SEM. A P < 0.05 was considered statistically significant.
Results
The body weight, brain weight, brain water content, and hematocrit were comparable in the two groups on P56 (Table 1). The brain iron concentration in the iron-deficient group was 44% of the control group on P7 (2.44 ± 0.06 vs. 5.58 ± 0.30 μg/g, P = 0.0001), but had fully recovered on P56 (Table 1).
Table 1.
Body weight, hematocrit, brain weight, water content, and iron concentration on P56 rats in the control and formerly iron-deficient groups
| Parameter | Control group | Formerly iron-deficient group |
|---|---|---|
| Body weight (g) | 240 ± 17 | 223 ± 10 |
| Hematocrit (%) | 47 ± 1 | 48 ± 1 |
| Brain weight (mg) | 1.16 ± 0.02 | 1.04 ± 0.02 |
| Brain water content (%) | 80 ± 0.5 | 80 ± 0.5 |
| Brain iron concentration (μg/g wet tissue weight) | 7.94 ± 0.45 | 7.60 ± 0.20 |
Mean ± SEM, N = 10–11 per group for body weight, brain weight, and hematocrit; N = 7 per group for brain water and brain iron concentration. None of the parameters are significantly different between the groups.
MRI and 1H NMR spectroscopy
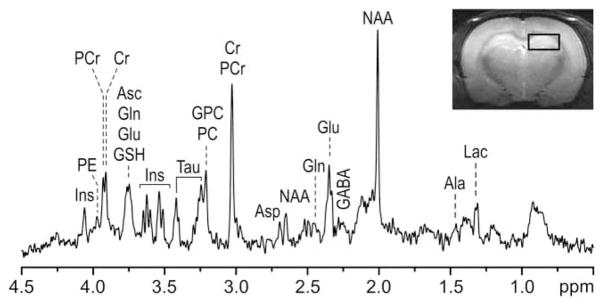
The coronal MR image and the representative in vivo 1H NMR spectrum acquired from the left hippocampus of a rat in the control group are shown in Fig. 1. The hippocampal cross-sectional area determined from the MRI was decreased by 12% in the formerly iron-deficient group relative to the control group (formerly iron-deficient: 5.57 ± 0.09 mm2 vs. control: 6.34 ± 0.13 mm2, P = 0.0002).
Figure 1.
In vivo 1H NMR spectraum from the hippocampus of a 56-day-old rat from the control group. The VOI on the left hippocampus for acquiring 1H NMR spectra is shown in the MRI (inset). Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; PCr, phosphocreatine; GABA, gamma-aminobutyric acid; Gln, glutamine; Glu, glutamate; GPC, glycerophosphocholine; Ins, myo-inositol; Lac, lactate; NAA, N-acetylaspartate; PC, phosphocholine; PE, phosphoethanolamine; Tau, taurine.
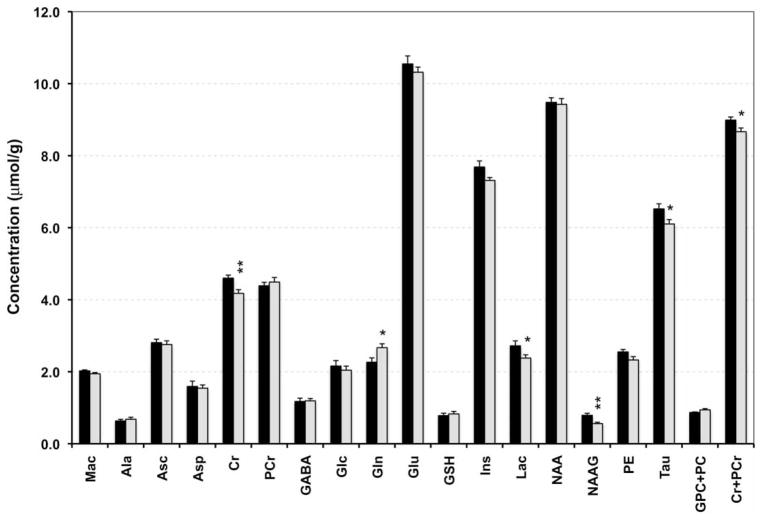
The spectral quality, consistently achieved in all experiments, enabled reliable quantification of 17 brain metabolites in both groups (Fig. 2). Compared with the control group, the concentrations of Cr (−9%), lactate (−13%), NAAG (−29%), taurine (−6%), and Cr + PCr (−4%) were lower, and that of glutamine (+18%) is higher, in the formerly iron-deficient group (P < 0.05, each; Fig. 2). The glutamate/glutamine ratio was 18% lower in the formerly iron-deficient group (formerly iron-deficient: 3.9 ± 0.5 vs. control: 4.8 ± 0.8, P = 0.009). In addition, there was a trend towards decreased myo-inositol (−5%, P = 0.05), phosphoethanolamine (−9%, P = 0.07) and increased GPC + PC (+8%, P = 0.08) concentrations (Fig. 2), and increased PCr/Cr ratio (formerly iron-deficient group: 1.09 ± 0.05 vs. control group: 0.96 ± 0.03, P = 0.06) in the formerly iron-deficient hippocampus. The rest of the neurochemicals were not affected by perinatal iron deficiency.
Figure 2.
Neurochemical profile of the hippocampus of 56-day-old rats in the control (black) and formerly iron-deficient (gray) groups. Values are mean ± SEM; N = 10 (control group) and N = 11 (formerly iron-deficient group). *P < 0.05 and **P < 0.01. Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; PCr, phosphocreatine; GABA, gamma-aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GSH, glutathione; Ins, myo-inositol; Lac, lactate; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PE, phosphoethanolamine; Tau, taurine: GPC + PC, sum of glycerophosphocholine and phosphocholine; Cr + PCr, sum of Cr and PCr (total creatine).
Discussion
In this study, we found decreased hippocampal size and altered neurochemical profile in iron-sufficient young adult rats exposed to iron deficiency during the fetal and neonatal periods. In spite of supplementing iron from the neonatal period and achieving brain iron repletion, anatomic and neurochemical abnormalities remained at young adulthood, suggesting a long-term and potentially irreversible effect of perinatal iron deficiency on the hippocampus.
The MRI showed a 12% decrease in hippocampal cross-sectional area in the formerly iron-deficient rats, a finding consistent with a previous study that demonstrated an approximately 17% decrease in hippocampal volume following gestational and lactational iron deficiency in mice.14 The discrepancy in hippocampal size between the two studies can be explained by the extended duration of iron deficiency in the latter study,14 where iron supplementation did not commence until P21 (i.e. 14 days later than the present study). Collectively, the two studies suggest a dose–response relationship between duration of perinatal iron deficiency and loss of hippocampal volume in adulthood. The lack of recovery of hippocampal size with iron rehabilitation is most likely a reflection of iron deprivation commencing from the fetal period. Prenatal undernutrition in rats results in smaller hippocampus at adulthood that is not amenable to recovery with postnatal nutritional and extranutritional (e.g. environmental enrichment) measures.15
Multiple factors likely account for decreased hippocampal size in the formerly iron-deficient rats. The period of brain iron deficiency in our model coincided with the period of neuronal proliferation and differentiation, onset of myelination, and synaptogenesis in the hippocampus.16 Any or all of these developmental processes may have been affected by iron deficiency and led to reduced hippocampal size at adulthood. Perinatal iron deficiency impairs the expression of neurotrophic factors that regulate neuronal differentiation in the hippocampus.11 The long-term sequelae of such dysregulated expression on neuronal number, size, and density in the adult hippocampus are not known. Similarly, iron deficiency during early postnatal life is associated with hypomyelination.4,17,18 A recent study using the same animal model demonstrated abnormal dendritic architecture, characterized by a proximal shift in peak branching, thinner third-generation branches and spine heads, and 25% fewer branches in the formerly iron-deficient hippocampus at adulthood.6 Additional studies are necessary to characterize the individual role of these factors in the smaller hippocampus at adulthood in perinatal iron deficiency.
The hippocampal neurochemical profile was also affected in the formerly iron-deficient rats. The demonstrated neurochemical changes are not the result of hippocampal volume loss, since the VOI selected for 1H NMR spectroscopy was adjusted based on hippocampal volume and the reported values are tissue concentrations and not content. A previous study had demonstrated alterations in seven metabolites in the hippocampus during the period of iron deficiency.4 The concentrations of five metabolites remained altered in the present study, in spite of normalization of brain iron concentration. The magnitude of these neurochemical changes is comparable to the 5–23% concentration differences observed in the hippocampus and striatum during the period of iron deficiency in this model.4,13 Whereas some of the neurochemical changes likely represent residual effects of previous iron deficiency, others potentially represent a programming effect of perinatal iron deficiency on the hippocampus or toxicity from iron therapy. These possibilities are not mutually exclusive. Interestingly, the persistent neurochemical changes in the hippocampus contrast with the response of the striatum to iron therapy in perinatal iron deficiency, where the neurochemical profile normalizes by P37.13 The dissimilar responses of the brain regions subserving different modalities of cognitive function to iron deficiency and supplementation may explain the unbalanced development of memory systems following perinatal iron deficiency.9,19,20
The neurochemical changes in the formerly iron-deficient hippocampus are consistent with biochemical processes likely to be affected by iron deficiency. Two of the neurochemical changes index decreased energy metabolism. Decreased Cr and Cr + PCr concentrations imply reduced flux capacity for high-energy phosphoryl transfer from PCr to ATP in the formerly iron-deficient hippocampus.21 The decreased steady-state concentration of lactate likely indicates decreased oxidative glucose metabolism.22 Collectively, these changes suggest a hypometabolic state in the formerly iron-deficient hippocampus. The decreased concentration of taurine further supports this possibility, since there is a positive correlation between brain taurine concentration and cerebral metabolic rate.23 Suppression of hippocampal energy metabolism has been demonstrated during the period of iron deficiency.4 This is not surprising, given that most of the mitochondrial enzymes involved in the oxidative production of ATP contain iron and are affected by iron deficiency.24 However, energy metabolism remained perturbed in the formerly iron-deficient hippocampus in the present study, despite the resolution of iron deficiency. This implies a potential programming effect of perinatal iron deficiency on hippocampal energy homeostasis. Previous studies have demonstrated long-term dysregulation of critical genes, including energy-related genes in the hippocampus due to perinatal iron deficiency.10,11,25 This is dissimilar to the effect of iron deficiency on energy metabolism in non-brain tissues, such as the skeletal muscle and intestinal mucosa, and non-hippocampal brain regions, such as the striatum, where iron supplementation rapidly normalizes tissue energy metabolism.13,24
The increased glutamine concentration was likely responsible for the decreased glutamate/glutamine ratio in the present study and suggests that glutamate–glutamine cycling between neuron and glia may be disrupted in the formerly iron-deficient hippocampus. Typically, glutamate released from the neurons into the extracellular space is taken up by nearby astrocytes, converted to glutamine through the action of glutamine synthase enzyme, and trafficked back to the neurons for reconversion to glutamate, thereby completing the so-called glutamate–glutamine cycle.26,27 The decreased glutamate/glutamine ratio and increased glutamine in the present study implies dysregulated glutamatergic neurotransmission in the formerly iron-deficient hippocampus.28,29 Decreased taurine and NAAG, which modulate N-methyl-D-aspartate glutamate receptor function,30,31 in the formerly iron-deficient hippocampus is concordant with this interpretation. Collectively, these neurochemical changes corroborate the previously demonstrated decreased long-term potentiation and synaptic plasticity in the formerly iron-deficient hippocampus at adulthood.6,10,11,32 Alternately, reactive astrocytosis also may be responsible for increased glutamine in the formerly iron-deficient hippocampus.33 A previous study demonstrated upregulation of epidermal growth factor, which is known to induce glutamine synthase activity and promote astrocytosis, in the iron-deficient hippocampus.32
The functional significance of the MRI and neurochemical changes was not assessed in the present study. Nevertheless, the results provide potential mechanisms for the long-term hippocampal dysfunction due to perinatal iron deficiency. Loss of hippocampal volume is associated with cognitive impairments in humans and rodents.14,34,35 While a 12% decrease in hippocampal size may appear modest, a similar degree (6–11%) of hippocampal atrophy is associated with significant cognitive impairments in preterm infants34 and adults with early stage Alzheimer’s disease.35 Likewise, a comparable magnitude of neurochemical changes in other brain regions is associated with behavioral deficits in iron-deficient developing rats13 and cognitive impairments in adult humans with neurodegenerative disorders.36–38 The neurochemical evidence of suppressed energy metabolism, cellular activity, and plasticity corroborate the previously demonstrated similar effects, such as suppressed brain-derived neurotrophic factor activity, and decreased postsynaptic density protein-95, calmodulin-dependent protein kinase II-alpha, and structural guidance protein expressions in the formerly iron-deficient hippocampus,6,10,11 and explain the altered long-term potentiation5 and perturbed dendritic cytoskeletal assembly6 in this structure. It is noteworthy that the decreased glutamate/glutamine ratio and taurine and NAAG concentrations demonstrated in the present study are considered as biomarkers of schizophrenia.28,39–42 Maternal iron deficiency during gestation is considered as a potential risk factor for schizophrenia in the offsping.43
While perinatal iron deficiency was most likely responsible for our results, iron toxicity during iron supplementation may also have played a role. Perinatal iron deficiency upregulates iron transporters in the hippocampus.44 Thus, there is a potential for increased susceptibility to oxidant-mediated injury during iron supplementation. Neurodegeneration due to excess iron supplementation during development has been demonstrated in mice.45 Additional studies using different doses and durations of iron supplementation are necessary to address this possibility. The animal model used in the present study mimics perinatal iron deficiency in human newborn infants due to maternal gestational diabetes mellitus or intrauterine growth restriction.1 Hence, our results are relevant to those clinical conditions. Future research incorporating an un-supplemented iron-deficient group is necessary to determine the effects of chronic iron deficiency (i.e. during prenatal and postnatal periods) on the hippocampus. Finally, as our study period was limited to young adulthood, additional studies are also warranted to determine whether the demonstrated structural and neurochemical changes improve or worsen over time.
In summary, perinatal iron deficiency is associated with reduced size and altered neurochemical profile of the hippocampus at young adulthood in rats. The ability to detect these changes attests to the sensitivity and reliability of high-field 1H NMR spectroscopy for such investigations. The non-invasiveness of the method also suggests its potential use in human infants and children with iron deficiency. The enduring and deleterious nature of the hippocampal deficits underscores the importance of preventing perinatal iron deficiency in populations at risk for this condition.
Acknowledgments
This work was supported by NIH grants RO1 HD29421, P41 RR008079 and P30 NS057091, the Keck Foundation and by a NSF Graduate Research Fellowship and Facilitation Award.
Footnotes
Disclosure of conflicts of interest
None of the authors have conflicts of interest to disclose.
Author contributions
Rao: study design, data acquisition and analysis, manuscript preparation; Tkac: data acquisition and analysis, manuscript preparation; Schmidt: Data interpretation, manuscript preparation; Georgieff: study concept, data interpretation, manuscript preparation.
References
- 1.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 4.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 5.Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 6.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci. 2010;32:238–48. doi: 10.1159/000314341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youdim MB. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14:45–56. doi: 10.1007/BF03033574. [DOI] [PubMed] [Google Scholar]
- 8.McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–82. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 10.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 11.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–8. doi: 10.1203/PDR.0b013e31819d90a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo1H NMR spectroscopy. Magn Reson Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- 13.Ward KL, Tkac I, Jing Y, Felt B, Beard J, Connor J, et al. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutr. 2007;137:1043–9. doi: 10.1093/jn/137.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranade SC, Rose A, Rao M, Gallego J, Gressens P, Mani S. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience. 2008;152:859–66. doi: 10.1016/j.neuroscience.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Katz HB, Davies CA, Dobbing J. Effects of undernutrition at different ages early in life and later environmental complexity on parameters of the cerebrum and hippocampus in rats. J Nutr. 1982;112:1362–8. doi: 10.1093/jn/112.7.1362. [DOI] [PubMed] [Google Scholar]
- 16.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–24. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr. 2000;130:2821–30. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- 18.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci. 2003;25:308–15. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 19.Carlson ES, Fretham SJB, Unger E, O’Connor M, Petryk A, Schallert T, et al. Hippocampus specific iron deficiency alters competition and cooperation between developing memory systems. J Neurodevelop Disord. 2010;2:133–43. doi: 10.1007/s11689-010-9049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt AT, Ladwig EK, Wobken JD, Grove WM, Georgieff MK. Delayed alternation performance in rats following recovery from early iron deficiency. Physiol Behav. 2010;101:503–8. doi: 10.1016/j.physbeh.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jost CR, Van Der Zee CE, In’t Zandt HJ, Oerlemans F, Verheij M, Streijger F, et al. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15:1692–706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 22.Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–63. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- 23.van Gelder NM. Brain taurine content as a function of cerebral metabolic rate: osmotic regulation of glucose derived water production. Neurochem Res. 1989;14:495–7. doi: 10.1007/BF00964908. [DOI] [PubMed] [Google Scholar]
- 24.Dallman PR, Schwartz HC. Myoglobin and cytochrome response during repair of iron deficiency in the rat. J Clin Invest. 1965;44:1631–8. doi: 10.1172/JCI105269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard J, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006:173–96. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- 26.Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S–31S. doi: 10.1093/jn/130.4.1026S. [DOI] [PubMed] [Google Scholar]
- 27.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 28.Gaisler-Salomon I, Miller GM, Chuhma N, Lee S, Zhang H, Ghoddoussi F, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology. 2009;34:2305–22. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol. 2003;65:401–27. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- 30.Suarez LM, Solis JM. Taurine potentiates presynaptic NMDA receptors in hippocampal Schaffer collateral axons. Eur J Neurosci. 2006;24:405–18. doi: 10.1111/j.1460-9568.2006.04911.x. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–57. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- 32.Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr. 2008;138:2495–501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123–42. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 34.Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47:713–20. doi: 10.1203/00006450-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 2009;19:1055–64. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 36.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384:23–8. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 37.Chawla S, Wang S, Moore P, Woo JH, Elman L, McCluskey LF, et al. Quantitative proton magnetic resonance spectroscopy detects abnormalities in dorsolateral prefrontal cortex and motor cortex of patients with frontotemporal lobar degeneration. J Neurol. 2010;257:114–21. doi: 10.1007/s00415-009-5283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28:1330–9. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Do KQ, Lauer CJ, Schreiber W, Zollinger M, Gutteck-Amsler U, Cuenod M, et al. gamma-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem. 1995;65:2652–62. doi: 10.1046/j.1471-4159.1995.65062652.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsai SJ. Central N-acetyl aspartylglutamate deficit: a possible pathogenesis of schizophrenia. Med Sci Monit. 2005;11:HY39–45. [PubMed] [Google Scholar]
- 41.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M. Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naive schizophrenic patients. BMC Psychiatry. 2005;5:6. doi: 10.1186/1471-244X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, et al. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010;49:2783–90. doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry. 2008;65:1136–44. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddappa AJ, Rao RB, Wobken JD, Casperson K, Leibold EA, Connor JR, et al. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53:800–7. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 45.Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, et al. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2007;28:907–13. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]