Despite the low likelihood of nodal extension, a lack of identifiable clinical and pathologic features associated with a positive sentinel lymph node precludes selective use of sentinel lymph node biopsy.
Abstract
Background:
Axillary staging via sentinel node biopsy (SLNB) in patients with ductal carcinoma in situ with microinvasion (DCISM) is routinely performed but remains controversial with regard to the risk-benefit ratio.
Methods:
Retrospective single-institution review of patients with diagnosis of DCISM (invasive tumor ≤ 0.1 cm). Age, clinicopathologic data, and follow-up were recorded.
Results:
Of 90 patients, 33% were diagnosed by core needle biopsy (CNB), 37% by excisional biopsy, and 29% were upstaged from DCIS on CNB to DCISM at final operation. Three (10%) of 30 patients with DCISM on CNB were upstaged to invasive cancer on final pathology. Median age at diagnosis was 58.9 years (range: 30-89). Lumpectomy was performed in 45% of patients and mastectomy in 55%. Mean number of sentinel nodes was 2.59 (SE 0.17). Six (6.9%) of 87 patients with DCISM as final diagnosis had a positive SLNB (four lumpectomies, two mastectomies). There was no correlation with any clinicopathologic features, including palpable DCIS, DCIS grade/necrosis, or age at diagnosis. All six SLNB-positive patients had a complete axillary dissection; two had additional disease. Median follow-up time was 74.2 months (range: 2-169). In-breast recurrence was seen in three patients (5%), regardless of SLN status, DCIS grade, or necrosis. Two patients developed distant metastasis. Overall survival was 94.19% at 5 years for DCISM and 100% for DCISM with nodal disease.
Conclusion:
DCISM comprises 0.6% of breast cancer diagnoses at our institution. There is a low likelihood of nodal spread; however, a lack of identifiable clinicopathologic features associated with a positive SLNB limits selective SLNB use.
Introduction
Ductal carcinoma in situ (DCIS) accounts for 20% to 30%1–4 of all newly diagnosed breast cancer in the United States currently. This is considered a noninvasive malignancy, but it may also arise in association with invasive carcinoma. Current breast cancer staging and treatment recommendations are based primarily on the size and extent of an invasive component, if identified. However, when the invasive component is < 0.1 cm in its greatest extent, this is deemed “microinvasive” carcinoma. Treatment recommendations for this particular entity have yet to be standardized because of the rarity of this diagnosis.1,5–7
In invasive breast cancer, axillary staging via sentinel lymph node biopsy is routinely performed, but the role of sentinel lymph node biopsy in patients with DCIS alone or DCIS with microinvasion (DCISM) remains controversial because of the ill-defined risk-benefit ratio.2,4,5,7–10 Although sentinel lymph node biopsy is a low-risk procedure, it has been associated with multiple complications, including anaphylaxis to blue dye (0.7%),11 lymphedema (3% to 7%),12 and paresthesia (6% to 8%),13 among others. These risks should be balanced with the possibility of identifying a metastatic lymph node and the subsequent upstaging and alteration of treatment recommendations. The incidence of a positive sentinel lymph node in DCISM has been reported to be between 3% and 20%1,5,14–167,17 Our institutional experience, first reported in 2001, described an incidence of a positive sentinel lymph node of up to 20% for patients with DCISM.5 A follow-up study in 2005 demonstrated an incidence of 14%.18 However, the presence of isolated tumor cells (ITCs) in the sentinel node was included in the definition of a positive sentinel lymph node biopsy in both prior publications.5,18 According to the sixth edition of the American Joint Commission on Cancer (AJCC) breast cancer staging system, published in 2002, and the seventh edition, published in 2009, ITCs fail to represent a significant burden of nodal disease. Therefore, ITCs alone are not classified as nodal metastasis.6,19 The elimination of ITCs from the metastatic classification should lower the incidence of nodal disease reported in previous studies, although the presumed decrease has yet to be quantified.
The objective of this study was to redefine the incidence of true lymph node positivity associated with DCISM. We examined clinical and pathologic features at a single high-volume cancer center for an association with sentinel lymph node–positive disease, thereby guiding patient selection for sentinel lymph node biopsy in patients with this uncommon disease entity.
Methods
The Don and Erika Wallace Comprehensive Breast Program at the H. Lee Moffitt Cancer Center and Research Institute offers all incoming patients with breast cancer participation in an institutional review board–approved, prospective database. This database contains only the information obtained at presentation via a patient questionnaire, such as age, race, presenting symptom, family history, type of biopsy, and so on. Institutional review board approval is obtained for each retrospective research study requiring access to the database. Follow-up information, such as type of surgery, adjuvant treatment recommendations, and disease status is added for applicable study patients from review of paper charts (before 2003) and electronic medical records (after 2003). All of the patients in the current study were surgically treated at the H. Lee Moffitt Cancer Center from January 1996 to June 2009 and had a diagnosis of DCISM. All patients had therapeutic breast surgery at Moffitt Cancer Center. Data collected included (1) patients' age and race; (2) personal and family history of breast cancer; (3) radiologic findings; (4) pathologic biopsy results; (5) histopathologic tumor characteristics; (5) type of breast surgery; (6) sentinel node biopsy results; and (7) follow-up data, including adjuvant treatment, recurrence, and mortality.
Pathologic evaluation of DCIS at our institution is composed of documentation of grade, presence of necrosis, absence of invasion, and histologic subtype (solid, cribiform, etc). The volume or extent of DCIS was not consistently recorded, nor was the presence of multifocality or multicentric disease. Similarly, over the course of the study period, assessment of estrogen receptor (ER) and progesterone receptor (PR) expression was not routinely performed until 2006 and therefore was not included in our data analysis.
At our institution, sentinel lymph node mapping consists of either peritumoral or subareolar injections (per surgeon choice) of both isotope (Technetium 99M filtered sulfur colloid; Cardinal Health, Tampa, FL) and blue dye (Isosulfan Blue; AnazaoHealth Corporation, Tampa, FL). Intraoperative node assessment is performed at the discretion of the operating surgeon if the preoperative diagnosis was DCIS. If performed, node assessment is done via frozen section or touch preparation of the sentinel nodes, a standard practice at this institution since 1995. All sentinel lymph nodes are sectioned at 0.2-cm intervals along the long axis and entirely submitted for histological evaluation. Two sections of each block are stained with hematoxylin and eosin and pancytokeratin (AE1/AE3, predilution; Ventana Medical Systems, Tucson, AZ,) immunohistochemistry, respectively. Intraoperative frozen sections of breast specimens for margin assessment are not routinely performed at our institution. Sentinel node positivity is defined as one or more metastatic deposits measuring < 0.2 mm in the lymph node, as specified by the seventh edition of the AJCC breast cancer staging manual.6
Descriptive statistics, Spearman correlation coefficient, logistic regression, and Cohen's kappa coefficient were used to correlate the clinical and pathologic data with the likelihood of sentinel lymph node positivity in patients with DCISM. Survival analyses were produced using SAS/STAT software (SAS, Cary, NC).
Results
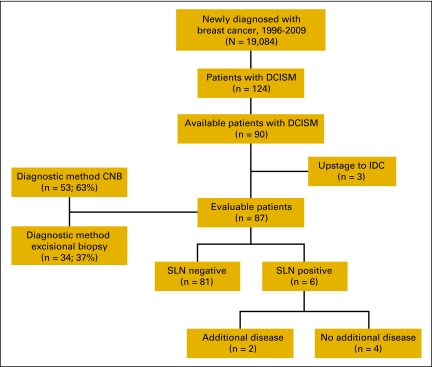
One hundred twenty-four patients with a diagnosis of DCISM were identified. Patients with incomplete records (n = 32) or no sentinel lymph node biopsy (n = 2) were excluded, leaving 90 evaluable patients (Figure 1). Patients were eliminated for incomplete records if (1) the original paper version of the pathology report from the definitive surgery was not transferred to the electronic medical record, or (2) other components of their care (ie, radiation or medical oncology) were performed outside of Moffitt Cancer Center without written documentation from outside providers scanned into the patient's electronic medical record.
Figure 1.
Patient inclusion in the study group. CNB, core needle biopsy; DCISM, ductal carcinoma in situ with microinvasion; IDC, invasive ductal carcinoma; SLN, sentinel lymph node biopsy.
The diagnosis of DCISM was made by core needle biopsy in 30 women (33%) and by excisional biopsy in 34 women (37%). An additional 26 women (29%) were initially diagnosed with DCIS on core needle biopsy but were upstaged to DCISM at definitive surgery. Similarly, three (10%) of the 30 women initially diagnosed with DCISM on core needle biopsy were upstaged to invasive cancer on final pathology and eliminated from additional analyses. Eighty-seven women ultimately had a final diagnosis of DCISM and make up the remainder of our study cohort.
Median age at diagnosis was 58.9 years (range: 30-89). Eighty-seven percent of participants were White, whereas other races accounted for far fewer percentages (Hispanic 6%, Black 5%, and Asian 4%). Fifty-one percent had left-sided breast cancer. Seventeen percent of the patients had a previous personal history of breast cancer; the majority (73%) of these prior cancers were in the contralateral breast. An additional 13% also had a history of other cancers, most commonly melanoma and nonmelanoma skin cancers (50%).
DCIS typically presents with no physical findings on breast examination but frequently is detected on routine mammographic screening by the presence of abnormal microcalcifications. Similarly, in our series, only 10% of patients presented with palpable abnormalities; all of these correlated with abnormalities seen on imaging. The most common mammographic finding for these patients was suspicious microcalcifications (92%). Of the eight women who presented with a palpable mass, seven (88%) also had a focused breast ultrasound. The more common sonographic findings were a corresponding hypoechoic mass (n = 4), no sonographic abnormality (n = 2), or sonographic architectural distortion (n = 1). A small proportion (six patients, 7%) of the study cohort also had breast magnetic resonance imaging to determine the extent of disease for surgical planning purposes.
Breast-conserving surgery was the initial procedure for 59 (68%) of the 87 patients. Twenty (34%) of the 59 women who initially underwent lumpectomy ultimately went on to mastectomy for residual positive surgical margins. Overall, 39 (45%) of women had successful breast conservation. All women had successful lymphatic mapping and subsequent sentinel lymph node biopsy as part of their definitive operation.
Most women had high- or moderate-grade DCIS (67% and 24%, respectively), and only 9% had low-grade disease. Sixty-seven percent of the women had associated comedonecrosis. In all cases of DCISM, attempts were made to determine the receptor status (estrogen and progesterone) on the small invasive component; however, this was not always possible because of the paucity of invasive disease. The PR was positive in 25%, negative in 32%, and unobtainable in 43%. ER was positive in 23%, negative in 34%, and unobtainable in 43%. As mentioned in the Methods section, receptor status was rarely performed on the DCIS component, and only if there was insufficient tissue to make the determination on the invasive component. Of the 37 patients (43%) with unobtainable receptors on the area of microinvasion, receptor status was performed on the DCIS component in three patients (8%). One patient's DCIS was ER/PR positive and the other two patients were both ER/PR negative.
The mean number of sentinel lymph nodes retrieved per patient was 2.59 (SE 0.17). Six (6.9%) of 87 patients with DCISM as a final diagnosis had a positive sentinel lymph node (four of those patients had lumpectomy, and two had mastectomy as the final surgical procedure). Micrometastatic lymph node disease (a metastatic deposit > 0.2 mm but < 2 mm)19 was identified in two of the six women and involved only one of the sentinel nodes if more than one sentinel lymph node was retrieved. Macrometastatic lymph node disease (a metastatic deposit > 2 mm)19 was identified in the other four women and again involved one or two of the sentinel lymph nodes if more than one was retrieved. In all six node-positive patients, the foci of nodal disease were detected on final pathology, and not identified by intraoperative cytology. All six women subsequently returned to the operating room for a completion axillary lymph node dissection; two had additional macrometastatic nodal disease. Both of these women initially had macrometastatic disease in the positive sentinel lymph node. Three (3.4%) of the 87 patients had ITCs as the only finding on sentinel lymph node biopsy.
Although the presence of metastatic disease in the axillary lymph nodes with DCISM was low in our study cohort, treatment management was altered. Node-negative patients with DCISM did not receive adjuvant chemotherapy. However, all patients with a positive sentinel lymph node biopsy, including those with ITC-only disease, received chemotherapy. As a result of the low burden of axillary disease in this series, patients who required whole breast irradiation did not have their radiation fields extended to encompass the nodal basins. In addition, no patients received comprehensive chest wall radiation solely for the presence of nodal disease.
Univariate analyses failed to demonstrate a significant correlation between nodal disease and any clinical or pathologic features, including palpable mass (P = .55), grade (P = .504), necrosis (P = .17), or age at diagnosis (P = .059) (Table 1).
Table 1.
Univariate Analysis for Different Clinicopathologic Factors and Sentinel Lymph Node Positivity
| Factor | SLN Status (P) |
|---|---|
| DCIS grade | .54 |
| Necrosis | .171 |
| Age | .059 |
| Diagnostic method, core versus excisional | .18 |
| Size of DCISM | .688 |
| Palpable mass | .55 |
| ER status | .1895 |
| PR status | .072 |
Abbreviations: DCIS, ductal carcinoma in situ; DCISM, ductal carcinoma in situ with microinvasion; ER, estrogen receptor; PR, progesterone receptor; SLN, sentinel lymph node biopsy.
Median follow-up time was 74.2 months (range: 2-169). An in-breast recurrence was identified in three patients (3.5%) regardless of original sentinel lymph node status (P = .17) or correlation with grade or necrosis of in situ disease (P = .16 and .78, respectively). There were no axillary recurrences. Two patients developed distant metastasis; one died of metastatic breast cancer attributed to a previously diagnosed contralateral invasive ductal carcinoma. Overall survival given by Kaplan-Meier product limit method was 94.2% at 5 years for DCISM without nodal involvement and 100% for DCISM with nodal disease (P = .97).
Discussion
DCISM comprises 0.6% of breast cancer diagnoses at our institution. This is consistent with other reports in the literature,5,14–16 making this diagnosis unusual but frequent enough to raise clinical questions regarding appropriate management. On the basis of the most recent AJCC stage classification, the incidence of lymph node involvement is lower (6.9%) than previously reported at this institution, however, and is similar to the published incidence at other institutions.1,15,16 This study failed to identify any significant clinical or pathologic features predictive of lymph node metastasis in patients with DCISM, including (1) palpable mass, (2) extent of microinvasive component, (3) DCIS grade, (4) receptor status, or (5) comedonecrosis. Age at diagnosis approached statistical significance (P = .059); however, there is not sufficient evidence to use age as a determinant of sentinel lymph node biopsy utilization in conjunction with a DCISM diagnosis.
Murphy et al20 studied the correlation between axillary disease in DCISM and local recurrence and did not find an increased risk of local recurrence in patients with a positive sentinel lymph node. However, this study did demonstrate a correlation between local recurrence and tumor grade/necrosis. Our results concur with this study in the absence of increased risk of local disease in sentinel lymph node–positive DCISM; however, we did not identify any relationships between either grade or necrosis and in-breast tumor recurrence.
In 2002, de Mascarel et al16 published the largest series of patients with DCISM (n = 243) and divided these patients in two distinct pathologic groups: type 1 with isolated cells, and type 2 with clusters of cells. Thus the clinical behavior of the type 1 subgroup was similar to DCIS without microinvasion, and the type 2 subgroup resembled invasive ductal carcinoma associated with large areas of DCIS. However, this study included cases with a > 0.1 cm focus of invasion in the type 2 subgroup; given the current classification system, these cases would not be considered microinvasive disease. Furthermore, this pathologic classification has not been widely adopted, nor is it used at our institution. Our series failed to demonstrate a relationship between the extent of the microinvasive component and clinical behavior. This difference is likely multifactorial and related not only to sample size, but also the inclusion of T1a tumors in de Mascarel's type 2 subgroup. Our positive sentinel lymph node rate of 6.9% is lower than that reported for de Mascarel's type 2 subgroup (10.1%) and higher than that reported for the type 1 subgroup (0%); it is likely that our series reflects a combination of the two pathologic subsets.
In our series, a diagnosis of DCISM was associated with high overall survival and low in-breast, regional, and systemic recurrence. This favorable clinical outcome after a median 72-month follow-up is comparable to those of prior series.1 Our findings may reflect the aggressive clinical management of our patients, with 55% of them undergoing mastectomy as the final surgical procedure and completion lymph node dissection for all the patients with a true-positive sentinel lymph node.
Although our data did not identify parameters for the selective use of sentinel lymph node biopsy in patients with DCISM, we believe that in the future, with a larger data set and development of molecular markers and assays, selection criteria will be defined. Clinicians should be wary of a preoperative DCISM diagnosis by core needle biopsy because of subsequent risk of upstaging to more extensive invasive disease on surgical excision. As we expand our clinical series and follow-up for patients with DCISM, we may be able to better tailor the treatment for each one of our patients with this uncommon diagnosis.
In summary, this is the second largest series of consecutive patients diagnosed with DCISM.16 Our data suggest that despite the low likelihood of nodal extension, a lack of identifiable clinical and pathologic features associated with a positive sentinel lymph node precludes the use of selective sentinel lymph node biopsy. Thus, until further data are generated and validated, a sentinel lymph node biopsy should be performed on all patients with DCISM diagnosed either on core needle biopsy or after a definitive resection.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Jose M. Pimiento, M. Catherine Lee, Christine Laronga
Financial support: Jose M. Pimiento
Administrative support: Christine Laronga
Provision of study materials or patients: Jose M. Pimiento, M. Catherine Lee
Collection and assembly of data: Jose M. Pimiento, M. Catherine Lee, Nicole N. Esposito
Data analysis and interpretation: Jose M. Pimiento, M. Catherine Lee, Nicole N. Esposito, John V. Kiluk, Nazanin Khakpour, Gang Han, Christine Laronga
Manuscript writing: Jose M. Pimiento, M. Catherine Lee, Nicole N. Nicosia Esposito, John V. Kiluk, Nazanin Khakpour, W. Bradford Carter, Christine Laronga
Final approval of manuscript: Jose M. Pimiento, M. Catherine Lee, Nicole N. Esposito, John V. Kiluk, Nazanin Khakpour, W. Bradford Carter, Gang Han, Christine Laronga
References
- 1.Vieira CC, Mercado CL, Cangiarella JF, et al. Microinvasive ductal carcinoma in situ: Clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol. 2008;73:102–107. doi: 10.1016/j.ejrad.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 2.Sakr R, Barranger E, Antoine M, et al. Ductal carcinoma in situ: Value of sentinel lymph node biopsy. J Surg Oncol. 2006;94:426–430. doi: 10.1002/jso.20578. [DOI] [PubMed] [Google Scholar]
- 3.Guth AA, Mercado C, Roses DF, et al. Microinvasive breast cancer and the role of sentinel node biopsy: An institutional experience and review of the literature. Breast J. 2008;14:335–339. doi: 10.1111/j.1524-4741.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 4.Camp R, Feezor R, Kasraeian A, et al. Sentinel lymph node biopsy for ductal carcinoma in situ: An evolving approach at the University of Florida. Breast J. 2005;11:394–397. doi: 10.1111/j.1075-122X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 5.Cox CE, Nguyen K, Gray RJ, et al. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): Why map DCIS? Am Surg. 2001;67:513–519. discussion 519-521. [PubMed] [Google Scholar]
- 6.Greene FL. New York, NY: Springer; 2010. AJCC Cancer Staging Manual; pp. 347–376. [Google Scholar]
- 7.Rosner D, Lane WW, Penetrante R. Ductal carcinoma in situ with microinvasion. A curable entity using surgery alone without need for adjuvant therapy. Cancer. 1991;67:1498–1503. doi: 10.1002/1097-0142(19910315)67:6<1498::aid-cncr2820670606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Klauber-DeMore N, Tan LK, Liberman L, et al. Sentinel lymph node biopsy: Is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol. 2000;7:636–642. doi: 10.1007/s10434-000-0636-2. [DOI] [PubMed] [Google Scholar]
- 9.Sakr R, Bezu C, Raoust I, et al. The sentinel lymph node procedure for patients with preoperative diagnosis of ductal carcinoma in situ: Risk factors for unsuspected invasive disease and for metastatic sentinel lymph nodes. Int J Clin Pract. 2008;62:1730–1735. doi: 10.1111/j.1742-1241.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 10.Buttarelli M, Houvenaeghel G, Martino M, et al. [Interest of sentinel lymph node biopsy for the staging of ductal carcinoma in situ] Ann Chir. 2004;129:508–512. doi: 10.1016/j.anchir.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley MC, Hansen N, McMasters KM. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg. 2004;188:49–61. doi: 10.1016/j.amjsurg.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Zavagno G, Belardinelli V, Marconato R, et al. Sentinel lymph node metastasis from mammary ductal carcinoma in situ with microinvasion. Breast. 2007;16:146–151. doi: 10.1016/j.breast.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Intra M, Zurrida S, Maffini F, et al. Sentinel lymph node metastasis in microinvasive breast cancer. Ann Surg Oncol. 2003;10:1160–1165. doi: 10.1245/aso.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 16.de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, et al. Breast ductal carcinoma in situ with microinvasion: A definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer. 2002;94:2134–2142. doi: 10.1002/cncr.10451. [DOI] [PubMed] [Google Scholar]
- 17.Zavotsky J, Hansen N, Brennan MB, et al. Lymph node metastasis from ductal carcinoma in situ with microinvasion. Cancer. 1999;85:2439–2443. doi: 10.1002/(sici)1097-0142(19990601)85:11<2439::aid-cncr19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Wilkie C, White L, Dupont E, et al. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg. 2005;190:563–566. doi: 10.1016/j.amjsurg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Rubin P, Hansen JT. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. TNM Staging Atlas; pp. 169–178. [Google Scholar]
- 20.Murphy CD, Jones JL, Javid SH, et al. Do sentinel node micrometastases predict recurrence risk in ductal carcinoma in situ and ductal carcinoma in situ with microinvasion? Am J Surg. 2008;196:566–568. doi: 10.1016/j.amjsurg.2008.06.011. [DOI] [PubMed] [Google Scholar]