Concerns about adverse effects and random assignment were the most common reasons cited by patients declining trial participation in four community oncology practices in New England.
Abstract
Purpose:
Less than 5% of patients with cancer participate in trials. Few studies have specifically addressed the role of cost to the patient as an influence on trial participation. Our main purpose was to determine the importance of added cost as a barrier to clinical trial participation in the community setting. Our secondary goal was to determine the most prevalent barriers to trial participation for patients.
Patients and Methods:
Four community practices in New England issued surveys to consecutive cohorts of patients with cancer. Patients were assessed for eligibility for clinical trials at their practice site. Trial-eligible patients who declined participation were asked to select reasons that contributed to their decision.
Results:
Surveys were issued to 1,755 patients. Seventy-one percent of all trial-eligible patients returned surveys. Forty-four percent of nonparticipating trial-eligible patients did not recall hearing about clinical trials from their provider. The most common reasons cited by trial-eligible patients for declining trial participation were fear of adverse effects (50%) and discomfort with random assignment (44%). Twenty-eight percent cited concerns about added cost, and 12% noted cost as the most important factor in their decision.
Conclusion:
Concerns about adverse effects and random assignment were the most common reasons cited by patients declining trial participation in four community oncology practices in New England. Cost considerations were important for a significant proportion of these patients. Many patients eligible for trial participation were not informed by their provider about the availability of research trials.
Introduction
Surveys of patients with cancer and physicians about their perceptions of clinical trials show that clinical studies are a highly valued component of quality cancer care.1–4 However, less than 5% of patients with cancer participate in clinical trials in community practices.5–8 Numerous studies have attempted to determine the barriers to trial participation among patients with cancer. Patient-related barriers include lack of awareness of trial availability, fear of adverse effects, cost of trial participation, distaste for random assignment, and added time to participate. Physician-related factors include availability of trials, time and cost of conducting trials, and medical disagreements about the choice of treatments mandated by studies.9–12
Over the past 10 years, the number of uninsured and underinsured patients has greatly increased in the United States. In 2009, 50.6 million Americans were uninsured13; in 2006, one third of individuals reported problems with medical bills or debt, and two thirds of low-income Americans were considered uninsured or underinsured.14–16 Many insurance products require sizeable deductible and coinsurance payments. These added costs for office visits, laboratory testing, imaging studies, and drugs could contribute to patients' decisions regarding participation in clinical trials. State and federal laws requiring trial coverage for National Cancer Institute–sponsored research trials do not specify that coinsurance or deductible payments be waived. In addition, diffusion of information regarding existing legislation is poor.17
Few studies have addressed the role of cost as a barrier to trial participation. In addition, most studies evaluating barriers to clinical trial participation have been performed in academic centers. A group of four community-based oncology practices in northern New England with established clinical trials programs conducted a survey study of patients to assess the role of cost and other barriers to clinical trial participation.
Patients and Methods
Between October 2008 and April 2010, four community oncology practices accrued patients for the survey trial. Eligible patients for the survey trial were new to the practices and had a solid tumor or hematologic malignancy. Patients with noninvasive processes (eg, ductal carcinoma in situ, superficial bladder cancer) or unclear diagnosis were not eligible for assessment. In addition, established patients who had disease progression or relapse and were identified as eligible for a clinical trial by the research nurse at each practice were included in the surveyed population.
A prospective continuous evaluation of new patients was performed by scanning new-patient schedules. Three to 4 weeks after the initial visit, research staff mailed a survey to the patient with a letter explaining the purpose of the survey (assessing barriers to trial participation). If patients did not return the survey within 2 weeks, they received a telephone call requesting that they consider completing the survey and returning it to research staff. The medical record of each patient eligible to be surveyed was reviewed 3 to 4 weeks after the patient's visit. A single reviewer (either principle investigator or lead research nurse) from each practice site assessed all patients for clinical trial eligibility at their local site. The reviewer established eligibility based on all inclusion and exclusion criteria for specific protocols open for enrollment at their practice. In addition, the reviewer recorded the patient's age, diagnosis, and Eastern Cooperative Oncology Group (ECOG) performance status as coded by the provider.
The patient survey is shown in Figure 1. Survey questions were selected by the principle investigators and research nurses at all four practice sites and were based on commonly reported barriers to trial participation in the academic setting. Questions about cost were included to pursue the primary objective of the study. The procedure for administering the surveys was developed during a 3-month pilot period. Surveys received during the pilot period are not included in the study data. Patients who were eligible for a clinical trial at their local practice site, had discussed trials with their physician, and did not participate in a trial were asked to select or write reasons for not participating. They were asked to select or write the most important reason contributing to their decision. Data recorded from the surveys and medical record reviews included the survey return rate, percentage of patients eligible for a clinical trial, number of clinical trial–eligible patients surveyed who declined participation, number of surveyed clinical trial–eligible patients who did not recall discussing trials with their physician, number of surveyed trial-eligible patients declining participation who selected specific reasons as contributors to their decision, and number of surveyed clinical trial–eligible patients who selected a specific reason as the most important contributor to their decision.
Figure 1.
Patient survey; trial-eligible patients who recalled discussing trial participation with their physician and declined participation were asked to complete this survey.
All four practice sites received institutional review board (IRB) approval for the administration of this survey study. Waiver of informed consent was provided by the IRBs for the trial. To receive waiver of informed consent, the codes linking patients' identifying information to their surveys were discarded once the surveys were returned.
Results
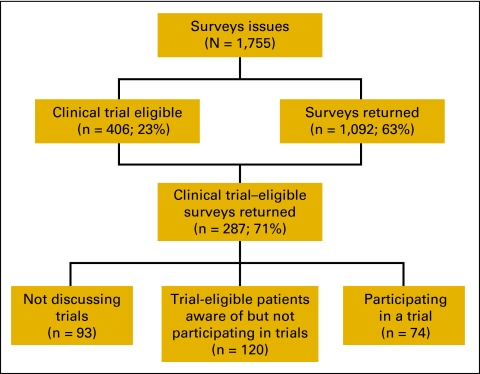
Between Ocober 2008 and April 2010, 1,755 patients received surveys and medical record reviews. Characteristics of the 1,092 patients returning surveys are listed in Appendix Table A1 (online only). With the notable exception of prostate cancer (which is seen less often in our practices relative to US incidence), the incidence of cancer diagnoses in these patients paralleled that of the US population.18 Most patients had ECOG performance status of 0 or 1. Of the 1,755 patients eligible for this survey trial, 406 (23%) were eligible to participate in a clinical trial at their local practice site. The percentage ranged from 11% to 30% at the different sites (Appendix Table A2, online only). Of patients eligible for clinical trials, 287 returned surveys (71% of patients eligible for clinical trials at all four sites; Fig 2).
Figure 2.
Flow sheet of survey responses; 71% of all clinical trial–eligible patients returned surveys; of respondents who did not participate, 44% had not discussed trials with their physician; 120 patients were aware of trial opportunities, were trial eligible, and declined participation; these patients responded to survey in Fig 1.
Two hundred thirteen of 287 trial-eligible patients who returned surveys did not participate in a clinical trial. Forty-four percent of these patients (93 of 213) did not recall discussing clinical trial opportunities with their physician. The percentage of surveyed trial-eligible patients who stated they did not discuss trials with their provider varied from 40% to 67% at the four practices. Of these patients, 48% were younger than 66 years of age and had ECOG performance score of 0 or 1 (Appendix Table A3, online only).
One hundred twenty patients returning surveys were eligible for a trial, discussed trials with their physicians, and declined trial participation. The reasons noted by these patients for declining trial participation are listed in Table 1. Concern about adverse effects (50%) and discomfort with random assignment (44%) were the reasons most often noted for declining trial participation. Concern about added cost was cited by 28% of these patients, and 12% selected cost as the most important reason for declining trial participation.
Table 1.
Reasons for Nonparticipation in Clinical Trials
| Reason | Listed As One Reason (%) | Listed As Most Important Reason (%)* |
|---|---|---|
| Possible side effects | 50 | 20 |
| Concern about random assignment | 44 | 18 |
| Cost/no insurance | 28 | 12 |
| Overwhelmed | 32 | 12 |
| Time | 32 | 12 |
| Physician recommended not participating | 5 | 5 |
| Other (surgical biopsy, moving, uncertainty of study treatment effect) | 9 | 8 |
NOTE. Patients who were offered trial participation but chose not to participate (n = 120) were asked to select or write in reasons for their decision. Percentage of patients selecting specific reasons contributing to decision to decline trial participation is listed.
Not all patients indicated most important reason.
Discussion
In this multisite study performed at community oncology practices, we conducted a prospective evaluation of a continuous cohort of patients over an 18-month period. To our knowledge, this study represents the largest sample of clinical trial–eligible patients treated in the community setting who were surveyed to assess barriers to trial participation. Trial eligibility was established by detailed medical record review performed by a single individual at each practice site. The survey return rate was high, capturing 71% of all clinical trial–eligible patients visiting the practices during the study period. We believe that this is the first study to specifically address patients' concern about cost associated with clinical trial participation.
In the study, concerns about adverse effects and the random assignment process were the reasons most often cited by patients for declining trial participation. These factors are among the most often cited barriers in other survey studies.9,10,12 Concern about added cost incurred by trial participation was cited by 28% of patients, with 12% of patients noting cost as the most important reason for declining participation. Markman et al19 reported that 19% of surveyed patients with cancer experienced a large amount of distress from the financial cost of care. Twenty-five percent of respondents with yearly income less than $40,000 decided to forgo a recommended cancer treatment because of expense.
New Hampshire, Maine, and Vermont have unemployment rates below the national average. New Hampshire has the third lowest unemployment rate in the United States.20 In this study, 87% of clinical trial–eligible patients providing reasons for declining to participate in a trial were treated at practices in New Hampshire. Uninsured patients comprise less than 2% of patients seen at the practices participating in this study. Therefore, the predominant patient population evaluated in this study faces more favorable economic conditions than those faced by the average patient in the United States. Cost is likely to be an even greater barrier to trial participation in regions with less favorable economic conditions.
New Hampshire, Vermont, and Maine have state legislation addressing clinical trial coverage. New Hampshire SB 409 requires coverage of any medically necessary care administered in the trial setting subject to the terms and conditions of a patient's policy.21 Therefore, a policy can stipulate no trial coverage. All deductible and coinsurance requirements are subject to the patient's policy. Maine Title 24-A mandates coverage for an enrollee who has a life-threatening illness for which no standard treatment is effective.22 It is unclear whether this statement precludes adjuvant trials or trials of advanced cancers for which palliative treatments exist. Neither the Vermont nor Maine legislation specify which procedures should be covered for eligible patients or whether copayments and deductibles still apply.23 It is not possible to determine the impact that legislation had on limiting insurance denial of trial coverage in this study. However, there are persisting cost considerations for patients beyond insurance coverage of routine care in a clinical trial setting. One opportunity to reduce patient concern about added cost incurred by trial participation would be to advocate for legislation to cover the cost of deductible and coinsurance payments for standard care administered in the setting of a qualifying clinical trial. In addition, authors and committees developing clinical trials should consider potential patient cost when designing trials.
At community oncology practices with established clinical trial programs, inadequate trial availability is a significant barrier to patient participation.6 Less than 15% of patients evaluated at two of our four practices were eligible for a clinical trial open at their institution during the study period. Possible reasons for limited trial availability at community practices are inadequate reimbursement for research activities, absence of or insufficient IRB support, lack of experienced research staff, lack of an appropriate patient population, and inadequate physician commitment to clinical research.
Although the primary objective of the study was to assess patient barriers to trial participation in the community setting, we clearly identified that physicians remain a significant barrier to trial participation.24,25 Forty-four percent of nonparticipating patients who were eligible for a trial did not recall hearing about trial opportunities. Of these patients, 48% were younger than 66 years of age with a good performance status. At the practice with the largest research department and highest rate of trial accrual, 40% of trial-eligible patients who did not participate in a trial were unaware of trial opportunities during the 18-month course of the study.
In conclusion, concerns about random assignment, concerns about added cost, trial availability, and physician bias are potentially remediable barriers identified in this study. A report by the Clinical Trials Working Group highlights challenges such as the bureaucracy of trial development, scientific quality of trials, and inadequate support for trialists.26 The results of our study inform us that important patient-related barriers and physician commitment to clinical trials need to be addressed to increase accrual to available trials. Continued efforts to expand trial access in the community setting and novel strategies to enhance physician commitment to clinical trials are required. An effort to clarify the random assignment process and its importance in gaining knowledge may reduce patient reluctance to participate in randomized trials. Lastly, gaining an understanding of the impact of cost in other regions of the United States could provide stronger impetus to advocate for more comprehensive insurance coverage of clinical trials.
Acknowledgment
Supported by an American Society of Clinical Oncology State Society Grant and by the Northern New England Clinical Oncology Society. Presented in poster format at the 46th Annual Meeting of ASCO, June 4-8, 2010, Chicago, IL; at the 2010 Annual Meeting of the Northern New England Clinical Oncology Society, October 29-30, 2010, Stowe, VT; and at the Annual Investigators Meeting of the MD Anderson Cancer Center Community Clinical Oncology Program, March 3-4, 2011, Houston, TX.
Appendix
Table A1.
Characteristics of All Patients Who Returned Survey (N = 1,092)
| Characteristic | No. of Patients |
|---|---|
| Practice site | |
| Maine Center for Cancer Medicine | 246 |
| New Hampshire Oncology Hematology | 616 |
| Seacoast Cancer Center | 81 |
| Vermont Center for Cancer Medicine | 149 |
| Malignancy | |
| Breast | 343 |
| Lung | 140 |
| Colorectal | 119 |
| Hematologic | 105 |
| Prostate | 88 |
| Genitourinary, nonprostate | 59 |
| Head and neck | 42 |
| Gynecologic | 40 |
| Gastric/esophageal | 26 |
| CNS | 26 |
| Pancreas | 22 |
| Other | 82 |
| ECOG performance status | |
| 0 | 549 |
| 1 | 288 |
| 2-4 | 96 |
| Unknown | 159 |
| Age, years | |
| < 45 | 70 |
| 45-55 | 224 |
| 56-65 | 294 |
| 66-75 | 266 |
| > 75 | 238 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table A2.
Trial-Eligible Patients by Practice Site
| Practice Site | Clinical Trial Eligible |
Returned Surveys From Trial Eligible (No.) | |
|---|---|---|---|
| No. | % | ||
| New Hampshire Oncology Hematology | 281 of 931 | 30 | 217 |
| Maine Center for Cancer Medicine | 39 of 353 | 11 | 31 |
| Vermont Center for Cancer Medicine | 63 of 295 | 21 | 28 |
| Seacoast Cancer Center | 23 of 176 | 13 | 11 |
Table A3.
Trial-Eligible Patients Who Did Not Recall Discussing Trial Participation With Physician
| Characteristic | Returned Surveys: Trial Eligible/Not Participating | No Discussion of Trial With Physician |
|
|---|---|---|---|
| No. | % | ||
| Total No. of patients | 213 | 93 | 44 |
| Age > 65 years or PS > 1 | 52 | ||
| Age < 66 years or PS < 2 | 48 | ||
| New Hampshire Oncology Hematology | 167 | 66 | 40 |
| Maine Center for Cancer Medicine | 17 | 8 | 47 |
| Vermont Center for Cancer Medicine | 20 | 13 | 65 |
| Seacoast Cancer Center | 9 | 6 | 67 |
Abbreviation: PS, performance status.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Douglas J. Weckstein, Christian A. Thomas, Ivette F. Emery, Barbara F. Shea, Alison P. Fleury, Margaret E. White
Administrative support: Douglas J. Weckstein, Christian A. Thomas, Alison P. Fleury, Margaret E. White, Elizabeth Chase, Cindy Robinson, Stacey Frazier, Christine Pilar
Provision of study materials or patients: Douglas J. Weckstein, Christian A. Thomas, Ivette F. Emery, Barbara F. Shea
Collection and assembly of data: Douglas J. Weckstein, Christian A. Thomas, Alison P. Fleury, Margaret E. White, Elizabeth Chase, Cindy Robinson, Stacey Frazier, Christine Pilar
Data analysis and interpretation: Douglas J. Weckstein, Margaret E. White, Elizabeth Chase
Manuscript writing: Douglas J. Weckstein, Christian A. Thomas, Ivette F. Emery, Barbara F. Shea, Alison Fleury, Margaret E. White, Elizabeth Chase, Cindy Robinson, Stacey Frazier, and Christine Pilar
Final approval of manuscript: Douglas J. Weckstein, Christian A. Thomas, Ivette F. Emery, Barbara F. Shea, Alison Fleury, Margaret E. White, Elizabeth Chase, Cindy Robinson, Stacey Frazier, and Christine Pilar
References
- 1.Townsley CA, Chan KK, Pond GR, et al. Understanding the attitudes of the elderly towards enrollment into cancer clinical trials. BMC Cancer. 2006;6:34. doi: 10.1186/1471-2407-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JM, Nyhof-Young J, Moric J, et al. Identifying motivations and barriers to patient participation in clinical trials. J Cancer Educ. 2006;21:237–242. doi: 10.1080/08858190701347838. [DOI] [PubMed] [Google Scholar]
- 3.Somkin CP, Altschuler A, Ackerson L, et al. Organizational barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005;11:413–421. [PubMed] [Google Scholar]
- 4.Finn R. Surveys identify barriers to participation in clinical trials. J Natl Cancer Inst. 2000;92:1556–1558. doi: 10.1093/jnci/92.19.1556. [DOI] [PubMed] [Google Scholar]
- 5.Umutyan A, Chiechi C, Beckett LA, et al. Overcoming barriers to cancer clinical trial accrual: Impact of a mass media campaign. Cancer. 2008;112:212–219. doi: 10.1002/cncr.23170. [DOI] [PubMed] [Google Scholar]
- 6.Go R, Frisby K, Lee J, et al. Clinical trial accrual at a community based cancer center. Cancer. 2006;106:426–433. doi: 10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- 7.Crosson K, Eisner E, Brown C, et al. Primary care physicians' attitudes, knowledge, and practices related to cancer clinical trials. J Cancer Educ. 2001;16:188–192. doi: 10.1080/08858190109528771. [DOI] [PubMed] [Google Scholar]
- 8.Frisby K, Lee J, Mathiason C, et al. Clinical trial accrual patterns and barriers among newly diagnosed adult patients at a community cancer center: A prospective study. J Clin Oncol. 2005;23(suppl):532s. abstr 6017. [Google Scholar]
- 9.Melisko M, Hassin F, Metzroth L, et al. Patient and physician attitudes toward breast cancer clinical trials: Developing interventions based on understanding barriers. Clin Breast Cancer. 2005;6:45–54. doi: 10.3816/CBC.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 10.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: A meta analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 11.Solomon MJ, Page CK, Young JM, et al. Patient entry into randomized controlled trials of colorectal cancer treatment: Factors influencing participation. Surgery. 2003;133:608–613. doi: 10.1067/msy.2003.119. [DOI] [PubMed] [Google Scholar]
- 12.Meropol N. Barriers to clinical trial participation as perceived by patients and oncologists. J Natl Compr Canc Netw. 2007;5:655–664. doi: 10.6004/jnccn.2007.0067. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Commerce. US Census Bureau: Income, poverty, and health insurance coverage in the United States, 2009. http://www.census.gov/prod/2010pubs/p60-238.pdf.
- 14.Davis K. Learning from high performance health systems around the globe. http://www.commonwealthfund.org/content/publications/testimonies/2007/Jan/learning-from-high-performance-health-systems-around-the-globe.aspx.
- 15.Cunningham P. The growing financial burden of health care, national and state trends 2001-2006. Health Aff (Millwood) 2010;29:1037–1044. doi: 10.1377/hlthaff.2009.0493. [DOI] [PubMed] [Google Scholar]
- 16.USA Today/Kaiser Family Foundation/Harvard School of Public Health. National survey of households affected by cancer. http://www.kff.org/kaiserpolls/upload/7591.pdf.
- 17.Kelahan A. Dissemination of information on legislative mandates and consensus-based programs addressing payment of the costs of routine care in clinical trials through the world wide web. Cancer. 2004;100:1238–1245. doi: 10.1002/cncr.20066. [DOI] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer statistics 2010. http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010.
- 19.Markman M, Luce R. Impact of the cost of cancer treatment: An internet based survey. J Oncol Pract. 2010;6:69–73. doi: 10.1200/JOP.091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau of Labor Statistics. Overview of BLS statistics on unemployment. http://www.bls.gov/bls/unemployment.htm.
- 21.New Hampshire Senate Bill 409-FN, ch 264. http://www.gencourt.state.nh.us/legislation/2000/sb0409.html.
- 22.Maine Rev Stat Title 24-A, ch 56. http://www.mainelegislature.org/legis/statutes/24-a/title24-ach56-a.pdf.
- 23.Vermont House Bill 6. http://www.leg.state.vt.us/docs/legdoc.cfm?url=/docs/2006/acts/act003.htm.
- 24.Kemeny M, Peterson B, Kornblith A, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 25.Moffitt K, Brogan F, Brown C, et al. Statewide cancer clinical trial navigation service. J Oncol Pract. 2010;6:127–132. doi: 10.1200/JOP.200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical Trials Working Group. Restructuring the national cancer clinical trials enterprise. http://transformingtrials.cancer.gov/files/ctwg-report.pdf.