Summary
The innate immune system detects viruses through molecular sensors that trigger the production of type I interferons (IFN-I) and inflammatory cytokines. Because viruses vary tremendously in size, structure, genomic composition, and tissue tropism, multiple sensors are required to detect their presence in various cell types and tissues. In this review, we summarize current knowledge of the diversity, specificity, and signaling pathways downstream of viral sensors and ask whether two distinct sensors that recognize the same viral component are complementary, compensatory, or simply redundant. We also discuss why viral sensors are differentially distributed in distinct cell types and whether a particular cell type dominates the IFN-I response during viral infection. Finally, we review evidence suggesting that inappropriate signaling through viral sensors may induce autoimmunity. The picture emerging from these studies is that disparate viral sensors in different cell types form a dynamic and integrated molecular network that can be exploited for improving vaccination and therapeutic strategies for infectious and autoimmune diseases.
Keywords: dendritic cells, viral, systemic lupus erythematosus, autoimmunity, diabetes, Toll-like receptors/pattern recognition receptors
The primary response to viral detection: type I interferons
Innate antiviral defense largely depends on type I interferons (IFN-I). These cytokines comprise IFNβ, multiple IFNα subtypes, as well as IFNκ and IFNω (called limitin in mouse) (1). IFN-I engage the IFNα receptor1/2 (IFNAR1/2) heterodimer on all cell types, inducing a signaling cascade that leads to the transcription of many IFN-stimulated genes (ISGs). ISG products block viral replication, establish an antiviral state in uninfected cells, and promote apoptosis of infected cells, ultimately limiting viral spreading. Key ISGs for blocking viral replication are the 2′-5′ oligoadenylate synthetases (2–5 OAS)/RNaseL system and RNA-activated protein kinase R (PKR). While the 2–5 OAS/RNaseL system induces RNA breakdown, preventing the translation of viral RNA (2, 3), PKR phosphorylates the eukaryotic translation initiation factor 2α (eIF2α), resulting in protein synthesis inhibition (4). IFN-I also enhance immune responses by inducing dendritic cell (DC) maturation, activation of monocytes and natural killer (NK) cells, as well as promoting T-cell responses and antibody production (5–7).
IFN-I secretion is triggered by the recognition of invading viruses. Viruses very enormously in terms of size, structure, and nucleic acid composition, which can be single stranded (ss) RNA, double stranded (ds) RNA, or DNA. Moreover, during infection, virions tend to localize in distinct intracellular compartments, including endosomes, the cytosol, and nucleus. To cope with this overwhelming diversity, the innate immune system detects invading viruses through germ-line encoded pattern recognition receptors (PRRs), which recognize common molecular features of pathogens, known as pathogen-associated molecular patterns (PAMPs) (8). Originally discovered in Drosophila (9), Toll-like receptors (TLRs) are the first and best characterized family of PRRs implicated in the recognition of viruses (10, 11). Several other types of PRRs involved in viral recognition have been subsequently identified. The diversity of antiviral PRRs enables recognition of disparate viral PAMPs. Moreover, different PRRs are expressed in distinct cellular compartments, enabling viral detection in multiple cellular locations. Finally, antiviral PRRs utilize diverse signaling pathways to induce the synthesis of IFN-I or inflammatory cytokines, enabling flexibility of antiviral responses. Following is a detailed description of PRR sensors that induce antiviral IFN-I responses.
TLRs that sense viral nucleic acids
TLRs are transmembrane proteins that consist of a luminal domain of leucine-rich repeats (LRRs) for pathogen sensing and a cytoplasmic Toll/interleukin-1 receptor homology (TIR) domain for downstream signaling (8). TLRs involved primarily in antiviral responses include TLR2, TLR3, TLR7, TLR8, and TLR9. While TLR2 is expressed on the cell surface and detects viral hemagglutinin and other unknown viral components (12–20), TLR3, TLR7, TLR8, and TLR9 detect viral nucleic acids in endosomal compartments (11, 21, 22). Upon viral infection, these TLRs are transported from the endoplasmic reticulum (ER) to a specialized endosomal compartment through an ER-associated twelve-membrane-spanning protein, UNC93B1 (23, 24). Viral nucleic acids gain access to TLR-containing endosomes following phagocytosis of virions or virally infected apoptotic cells. Moreover, some viruses are assembled in the endosomes during viral replication.
TLR3 detects dsRNA, which constitutes not only the genome of dsRNA viruses but also intermediates produced during replication of ssRNA and DNA viruses (25). Polyriboinosinic:polyribocytidylic acid [poly(I:C)], a synthetic dsRNA that mimics viral RNA, is often used as agonist of TLR3 (25, 26). TLR3 is expressed in macrophages, B cells and dendritic cells (DCs), particularly CD8α+ DCs, a conventional DC (cDC) subset that very effectively cross-presents antigens to CD8+ T cells (27). TLR3 is also expressed in non-immune cells such as fibroblasts, liver stellate cells, myofibroblasts, as well as some epithelial cells and endothelial cells. TLR3 signals through the TIR-domain containing adapter-inducing IFN-β protein (TRIF) (28, 29). TRIF interacts with tumor necrosis factor (TNF) receptor-associated factor 3 (TRAF3) and TRAF6 through a TRAF-binding motif (30, 31). TRAF3 activates inhibitor of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) kinase ε (IKKε) and TANK-binding kinase 1 (TBK1), which induce the phosphorylation and nuclear translocation of interferon regulatory factor-3 (IRF3). IRF3 activates the transcription of IFN-β. TRAF6 triggers a downstream signaling pathway that ultimately activates NF-κB and mitogen-activated protein kinases (MAPKs), resulting in the production of inflammatory cytokines, such as TNFα, interleukin-6 (IL-6), IL-12, and chemokine (C-C motif) ligand 2 (CCL2) (32).
In laboratory settings, murine TLR3 has been implicated in IFN-I responses to multiple RNA viruses. These include West Nile virus (WNV) and encephalomyocarditis virus (EMCV), a small non-enveloped (+) sense ssRNA enterovirus with tropism for the brain and heart (Fig. 1). In humans, however, TLR3 is essential for eliciting IFN-I and proinflammatory cytokines in response to the DNA virus herpes simplex virus 1 (HSV-1). Most likely, TLR3 detects RNA intermediates that are generated during HSV-1 replication (33). Genetically inherited mutations of UNC93B1, TLR3, or TRAF3 are associated with susceptibility to neonatal HSV-1 encephalitis (34–37). TLR3-deficient mice mount defective anti-HSV-1 CD8+ T-cell responses and are more susceptible to HSV-1 infection than wildtype (WT) mice (38). In addition to inducing IFN-I, TLR3 signaling results in the production of inflammatory cytokines. However, excessive TLR3-mediated inflammation in response to certain viruses has been shown to be rather detrimental to the host (39, 40).
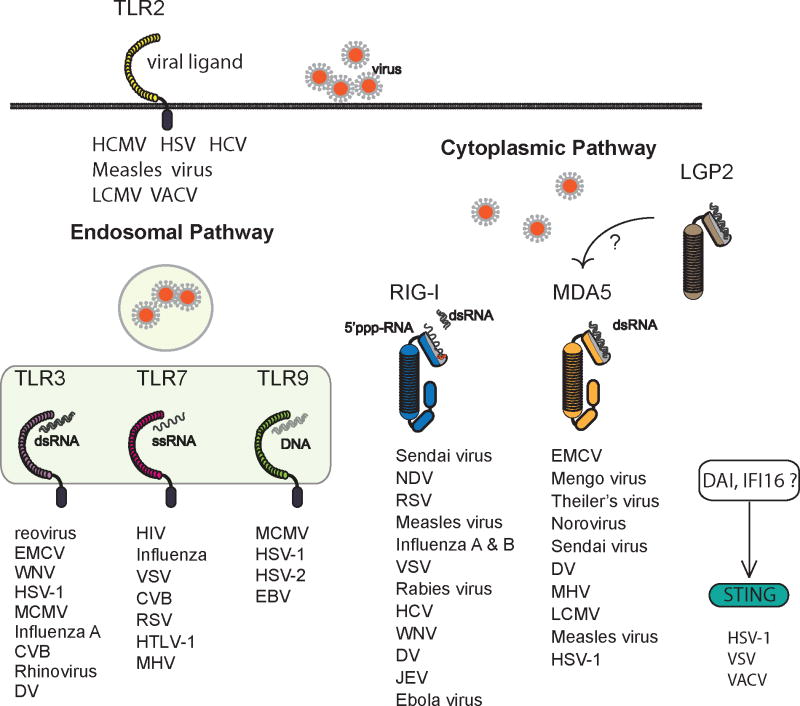
Fig. 1. Diversity and specificity of viral sensors.
Multiple viral sensors detect invading viruses. Genetic loss-of-function experiments have revealed the specificity of viral sensors in numerous viral infections. TLR2 is expressed on the cell surface and detects Human cytomegalovirus (HCMV), HSV-1, Hepatitis C virus (HCV), Measles virus, LCMV, and VACV virus (12–20). TLR3, TLR7, and TLR9 are located in endosomes. TLR3 recognizes RNA and DNA viruses such as reovirus (25), EMCV (123), West Nile virus (WNV) (234, 235), HSV-1 (35), MCMV (236), Influenza A (40), CVB (128), rhinovirus (237, 238), and Dengue virus (DV) (239). TLR7 recognizes ssRNA viruses such as human immunodeficiency virus (HIV) (240), Influenza virus (41, 42), VSV (43), CVB (241) and respiratory syncytial virus (RSV) (242), human T-cell leukemia virus (HTLV-1) (257) and mouse hepatitis virus (MHV) (258). TLR9 recognizes DNA viruses such as MCMV (243), HSV-1 (244), HSV-2 (245) and Epstein-Barr virus (EBV) (246). RIG-I and MDA5 sense replicating viruses in the cytosol. RIG-I recognizes a series of ssRNA viruses including paramyxoviruses such as Sendai virus (71, 247), Newcastle disease virus (NDV) (71, 247), RSV (248) and Measles virus (249, 250); Orthomyxovirus (Influenza A and B viruses (71)); Rhabdovirus (VSV (71, 247), Rabies virus (251)); Flavivirus (HCV (74), WNV (252), DV (239), Japanese encephalitis virus (JEV) (71)); and Filovirus (Ebola virus (95)). MDA5 detects picornaviruses such as EMCV, Mengo virus, and Theiler virus (71, 72), as well as calicivurses, such as norovirus (253). MDA5 has also been shown to recognize Sendai virus (75), DV (239), mouse hepatitis virus (MHV) (254), LCMV (233), Measles virus (249) and HSV-1 (255). DNA sensors such as DAI and IFI16 recognize cytosolic DNA viruses in a STING-dependent manner (99, 100, 103-106). STING is also involved in the recognition of RNA viruses such as VSV (105, 106).
TLR7 and TLR8 recognize ssRNA viruses (Fig. 1) and synthetic oligoribonuleotides (ORNs) (41–43), while TLR9 detects DNA viruses containing unmethylated CpG-rich DNA sequences (Fig. 1) and synthetic CpG oligodexoyribonucleotides (CpG ODNs) (44). TLR7 and TLR9 expression is chiefly restricted to immune cells, especially antigen-presenting cells (APCs), including cDCs, plasmacytoid DCs (pDCs), macrophages, and B cells. TLR8 was initially shown to sense viral ssRNA in human monocytes, and its murine homolog was thought to be non-functional (41). However, a recent study demonstrated that vaccinia viral (VACV) DNA activates murine TLR8 in pDCs, suggesting the possible involvement of TLR8 in sensing viral ssRNA in mice (45). TLR7, TLR8, and TLR9 transmit intracellular signals through the adapter protein myeloid differentiation primary response gene 88 (MyD88), which leads to production of both IFN-I and inflammatory cytokines. To induce IFN-I, MyD88 associates with Interleukin-1 receptor-associated kinase 1/4 (IRAK1/4), TRAF3/6, IKKα, and osteopontin, leading to the phosphorylation and activation of IRF7 (10, 22, 46–48). Phosphorylated IRF7 translocates into the nucleus, where it induces the transcriptional activation of IFN-I genes (49, 50). The PI3K-mTOR-p70S6K pathway positively regulates IRF7 activation (51), while interactions with 4E-BPs repress it at the translational level (52). To induce inflammatory cytokines, MyD88 associates with IRAK1/4, activating the TRAF6-TAK1 pathway that ultimately activates NF-κB and MAPK. MyD88 also recruits IRF5, which cooperates with NF-κB to activate inflammatory cytokine and chemokine gene transcription (53).
Whether engagement of TLR7/8/9 engenders IFN-I or inflammatory cytokines is dictated by the endosomal compartmentalization of the TLRs and their ligands (54, 55). Trafficking of DNA/RNA to specific compartments is regulated by physical-chemical properties, such that aggregated DNA/RNA localize in early endosomes, eliciting the production of IFN-I, while other DNA/RNA forms reach late endosomes, resulting in cytokine secretion and pDC maturation. To elicit IFN-I, TLR7 and TLR9 must translocate from the ER to a specialized lysosome-related organelle. This process requires AP-3 as well as Slc15a4, BLOC-1, and BLOC-2 (56, 57). TLR9 activation also requires proteolytic cleavage in the endosome and the presence of granulin as a cofactor (58–61). Initially it was thought that IFN-I induction via co-localization of TLR7/9 with their ligands in specialized endosomes occurred primarily in pDCs, whereas TLR7/9 signaling in non-pDCs resulted in the production of inflammatory cytokines. However, there is increasing data suggesting that cDCs are also capable of producing IFN-I in a TLR7/9 dependent manner (62, 63).
RIG-I like receptors
Retinoic acid inducible gene I (RIG-I)-like receptors (RLRs) are the cytosolic sensors for viral RNA (Fig. 1). There are currently three known family members: RIG-I, melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology-2 (LGP2) (8, 64). All three RLRs contain a conserved DExD/H box-containing RNA helicase domain that binds to RNA. MDA5 and RIG-I also contain two N-terminal caspase recruitment domains (CARD) involved in signaling (65–68). Both RIG-I and LGP2 contain an additional C-terminal repressor domain that inhibits signaling in the absence of ligands (68). RLRs recognize RNA structures that are highly specific to viral RNAs and distinct from endogenous 5′ capped mRNA. RIG-I preferentially binds to 5′-triphosphorylated ssRNA as well as short dsRNA (69). MDA5 recognizes long dsRNA like poly(I:C) and does not require 5′-triphosphorylation (69–73). The distinct ligand preference of each these two molecules enables recognition of disparate viruses (Fig. 1). For instance, MDA5 preferentially detects picornaviruses, such as EMCV, Theiler virus, and Mengo virus (71, 72). RIG-I detects a series of ssRNA viruses, including flaviviruses and orthomyxovirus (71, 74). Both MDA5 and RIG-I detect vesicular stomatitis virus (VSV) and Sendai virus (71, 75, 76). Recent evidence indicates that RIG-I can also detect certain DNA viruses with a genome rich in AT sequences. The RNA polymerase III transcribes AT-rich DNA into uncapped 5′-triphosphorylated ssRNA that triggers RIG-I pathway (77, 78).
Ligand binding to RLRs induces conformational changes leading to association with mitochondrial-associated interferon-β promoter stimulator 1 (IPS-1) (also known as MAVS, VISA, or Cardif) through card-card domain interactions (79–82). IPS-1 then recruits TRAF3, which activates TBK1 and IKKε (83). This leads to the phosphorylation and nuclear translocation of IRF3 and IRF7 resulting in the transcription of IFN-I genes (84, 85). IPS-1 also interacts with FAS-associated death domain protein (FADD) and receptor-interacting protein-1 (RIP-1) (79), which activate caspase-8 and caspase-10, resulting in NF-κB activation and production of inflammatory cytokines (86, 87). LGP2 lacks a CARD domain and was first thought to be a negative regulator for RIG-I and MDA5 (88). Accordingly, it was shown that LGP2-deficient mice produce more IFN-I in response to poly(I:C) stimulation and VSV infection than WT mice (89). However, a recent study provided evidence that LPG2 plays a positive role in antiviral responses: both RIG-I- and MDA5-mediated IFN-I responses to viral infections were impaired in mice lacking either LGP2 or the LGP2 ATP-binding site (90). Thus, the role of LGP2 in viral recognition and antiviral signaling remains unclear.
RLRs are expressed in virtually all cell types during an antiviral response. This ubiquitous expression enables both immune and non-immune cells to detect replicating viruses, produce IFN-I, and upregulate ISGs to block viral spreading. Importantly, MDA5 and RIG-I are themselves ISGs, as they are induced by IFN-I. Thus, their antiviral function is most likely delayed in comparison with other sensors that are constitutively expressed. The upregulation and activation of MDA5 and RIG-I also lead to the production of inflammatory cytokines and chemokines that recruit immune cells to sites of infection, as well as activating endothelium. The RLR signaling pathway is also tightly regulated. RIG-I activation requires ubiquitination of its CARD domains by an E3 ubiquitin ligase TRIM25 in a caspase-12 dependent manner (91, 92). Caspase-8 cleaves RIP-1 and negatively regulates RIG-I activation (93). A microRNA, mir-146a, has also been shown to negatively regulate RIG-I-mediated IFN-I production by macrophages in response to VSV (94). Moreover, viruses can counter RIG-I and MDA5 via multiple mechanisms. Ebola virus VP35 protein serves as competitor for dsRNA and disrupts RIG-I-mediated IFN-I production (95). Sendai virus V protein selectively binds to MDA5 and inhibits dsRNA-induced activation of the IFN-I genes (96).
Other viral sensors
The presence of viral DNA in the cytosol also leads to IFN-I production through cytoplasmic DNA sensors (97, 98). DNA-dependent activator of IFN-regulatory factors (DAI) (also known as ZBP1 or DLM-1) was the first DNA-binding protein shown to respond to cytosolic DNA (99, 100). However, the role of DAI in DNA sensing is highly cell type-specific and DAI-deficient mice respond normally to DNA-based vaccines (100–102). These data suggest that DAI is not solely responsible for the recognition of foreign DNA. IFI16 (p204 in mice), a member of PYHIN protein family, detects non-AT rich dsDNA like VACV DNA in vitro (103). The physiological importance of IFI16 is unclear at this point, due to the lack of any in vivo evidence for its impact on antiviral responses. An ER-associated protein, stimulator of interferon genes (STING), is required for the cytosolic DNA sensing pathway (104–106). HSV-1 and L. monocytogenes fail to induce IFN-I in DCs lacking STING and STING-deficient mice succumb to lethal HSV-1 infection due to abrogation of IFN-I responses (105, 106). Additionally, STING-deficient mice are exquisitely sensitive to VSV, which is normally detected by RIG-I, suggesting that STING is also involved in RIG-I signaling (105, 106). Given the close proximity between mitochondria and ER, it has been proposed that STING facilitates RIG-I recognition of viral RNA from ER-attached ribosomes and association with IPS-1 (97). High mobility group box (HMGB) proteins also have a role in sensing nucleic acids. Both intracellular DNA and poly(I:C)-mediated IFN-I production are impaired in cells lacking HMGB1, while only intracellular DNA mediated IFN-I production is defective in cells lacking HMGB2 (107). Moreover, a recent study suggested that a DExD/H box-containing helicase, DHX36, selectively binds to CpG-A and elicits IFN-I production in human pDCs (108). Finally, two cytosolic inflammasomes, AIM2 and NALP3, also detect viral DNA and RNA (109–118). These molecules are not involved in IFN-I production but trigger activation of caspase 1 and subsequent maturation of IL-1β (reviewed in 119–121).
Redundancy versus specialization of dsRNA sensors
TLR3 and MDA5 detect similar dsRNA structures, like poly(I:C), triggering IFN-I responses. This functional overlap may reflect molecular redundancy, so that one can substitute for the loss of the other. This redundancy may protect the organism from the deleterious effects of mutations that could otherwise abrogate detection of RNA viruses. However, there is increasing evidence that MDA5 and TLR3 only partially overlap and, in fact, may have distinct specialized functions.
A strain of EMCV, called EMCV-D, has tropism not only for the brain and heart but also replicates in the pancreas of mice causing extensive tissue damage and diabetes (122). Since EMCV replication generates dsRNA intermediates that trigger IFN-I responses through both MDA5 (71, 72) and TLR3 (123), this virus has been used to study the relationship between TLR3 and MDA5 in viral sensing in vivo. Both TLR3 and MDA5 are required for protection against EMCV-D infection (124). However, EMCV-D infection has very distinct pathological consequences in MDA5- and TLR3-deficient mice. MDA5-deficient mice have severe heart pathology marked by increased viral load in the heart and elevated troponin levels in the serum. In contrast, TLR3-deficient mice have only modestly augmented viral titers in the heart, suggesting that MDA5 plays a dominant role in protecting against EMCV-D induced myocarditis. In the pancreas, early IFN-I production mediated by TLR3 is essential for protecting insulin-producing β-cells from EMCV-D, as TLR3-deficient mice develop diabetes due to uncontrolled viral replication in the islets. MDA5 also functions in the pancreas, as MDA5+/− mice develop transient hyperglycemia. However, mice completely deficient for MDA5 succumbed to severe myocarditis and death before the development of diabetic symptoms. Finally, MDA5 and TLR3 seem to provide host anti-EMCV-D defense in different cell types. Bone marrow chimera experiments have shown that MDA5 acts predominantly in the radio-resistant ‘stromal’ compartment, whereas TLR3 acts predominantly in the radio-sensitive ‘hematopoietic’ cell compartment (124).
Coxsackievirus B (CVB), like EMCV, is a small non-enveloped (+) sense ssRNA enterovirus that has tropism for the heart and pancreas (125, 126, 127). TLR3 signaling in macrophages is required for the survival of mice following CVB infection; lack of TLR3 results in increased cardiac and liver damage during acute CVB infection (128). On the other hand, MDA-5 is not absolutely required for the induction of IFN-I in response to CVB but is essential for the production of maximal levels of systemic IFN-α after infection (129). Loss of MDA-5 enables the virus to replicate faster, resulting in increased liver and pancreas damage and heightened mortality. Taken together, these studies show that by acting in different tissues, cell types (hematopoietic cells vs. stromal), and cellular compartments (endosomes vs. cytosol), TLR3 and MDA5 play non-redundant but complementary roles in controlling infection by ssRNA viruses.
dsRNA as adjuvant in vaccination and immunotherapy
Poly(I:C) is a synthetic dsRNA that has long been recognized as a potential adjuvant for vaccination and anti-tumor immmunotherapy (130–132). Understanding how poly(I:C) promotes innate and adaptive immune responses has been the focus of recent investigations aimed at developing adjuvants that potentiate the immunogenicity of vaccines. Poly(I:C) chiefly engages two dsRNA sensors, MDA5 and TLR3, inducing robust production of both IFN-I and inflammatory cytokines (25, 71, 72, 133). Which of the dsRNA sensors and cytokine responses are essential for the adjuvant activity of poly(I:C)? These questions were recently addressed using a vaccination strategy where TLR ligands were administered in combination with antigen targeted to DC by conjugation with an anti-DC antibody. In these settings, poly(I:C) was a superior adjuvant compared to other TLR agonists in inducing CD4+ T-cell immunity (134, 135). Poly(I:C)-induced IFN-I was the predominant cytokine responsible for enhancing CD4+ T-cell immunity since the adjuvant effect of poly(I:C) was abrogated in mice lacking IFNAR1/2 (136). Bone marrow chimera experiments indicated that the adjuvant function of poly(I:C) on CD4+ T-cell responses relies primarily on MDA5 expression in both stromal and hematopoietic cells (Fig. 2A).
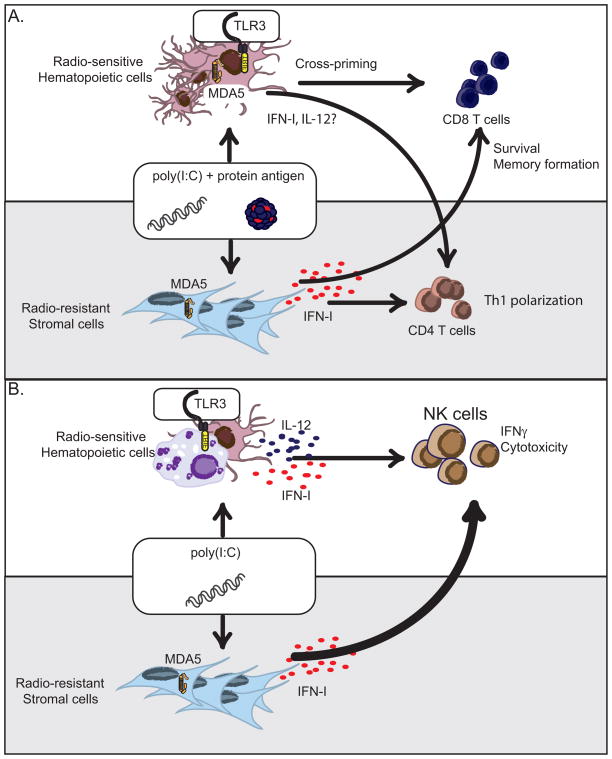
Fig. 2. Hematopoietic and stromal contributions to poly(I:C)-mediated immunity.
(A). Poly(I:C) promotes CD4+ and CD8+ T cell immunity through the production of IFN-I and other cytokines. Poly(I:C)-mediated IFN-I responses are dependent on stromal expression of MDA5. However, adjuvant effects of poly(I:C) on CD4+ T cell immunity require MDA5 expression in both hematopoietic and stromal compartments. Poly(I:C) triggers the TLR3 pathway in CD8α+ DCs to promote cross-presentation to CD8+ T cells while signaling through MDA5 pathway in stromal cells promotes CD8+ T cell survival and memory formation. (B). Poly(I:C) promotes NK cell activation through the production of IFN-I and IL-12. NK cell activation via IFN-I depends on MDA5-expressing radio-resistant stromal cells while TLR3+ hematopoietic cells promote NK cell activation through IL-12 secretion. The MDA5 pathway plays a dominant role in NK cell responses to poly(I:C) while the TLR3 pathway has a secondary effect.
Poly(I:C) also enhances CD8+ T-cell responses to antigens. It was first shown that poly(I:C) triggers the TLR3 pathway in CD8α+ DCs, promoting the ability of these cells to cross-present antigens to CD8+ T cells (26). Subsequently, it was shown that poly(I:C) enhances CD8+ T-cell responses by activating not only the TLR3-TRIF pathway but also the MDA5-IPS-1 pathway (81, 137). Specifically, it was demonstrated that TLR3 activation is essential for the induction of primary CD8+ T-cell responses by enhancing antigen cross-presentation, whereas MDA5 is essential for memory CD8+ T-cell responses by inducing systemic release of IFN-I that promotes the survival of primed CD8+ T cells. This result is consistent with original studies showing that IFN-I provides an important survival signal to CD8+ T cells during viral infections (139–141). Bone marrow chimera experiments indicated that stromal expression of MDA5 is sufficient to promote systemic IFN-I release and survival of activated CD8+ T cells (137), whereas MDA5 expression in hematopoietic cells is dispensable (Fig. 2A). Altogether, these studies indicate that the adjuvant capacity of poly(I:C) in CD8+ T-cell responses is mediated by both TLR3 and MDA5, although these dsRNA sensors act in different accessory cells and mediate distinct functions.
Poly(I:C) can also promote NK cell-mediated antitumor activity. Human NK cells express TLR3 and can directly respond to poly(I:C) stimulation in vitro (142–145). Conversely, mouse NK cells do not express TLR3 and poly(I:C)-mediated NK cell activation requires the participation of additional cell types. It was first reported that poly(I:C)-mediated NK cell activation requires myeloid DCs in a TRIF-dependent manner (146). A subsequent report demonstrated that poly(I:C) triggers both the TRIF and IPS-1 pathways in CD8α+ DCs, which in turn activate NK cells (147), suggesting that both TLR3 and MDA5 are important for poly(I:C)-mediated NK cell responses. A recent study showed that in vivo MDA5 plays a dominant role in NK cell activation by promoting systemic IFN-I. In contrast, TLR3 has a secondary impact that becomes evident only in the absence of MDA5 (133). Moreover, NK cell activation via IFN-I depends on MDA5-expressing radio-resistant stromal cells, while TLR3+ hematopoietic cells promote NK cell activation through IL-12 secretion. Therefore, poly(I:C) stimulates mouse NK cells indirectly through the induction of both IFN-I and IL-12 by distinct dsRNA sensors expressed in various cell types (Fig. 2B).
These studies indicate that the poly(I:C)-mediated adjuvant effect involves both TLR3 and MDA5, although their functions are distinct. TLR3 is important for activating antigen-presenting cells, DCs, and possibly macrophages, promoting cross-presentation of antigen to CD8+ T cells and systemic release of IL-12. MDA5 is essential for release of systemic IFN-I by stromal cells, which is essential for enhancing CD4+ T-cell responses, CD8+ T-cell survival, and NK activation (Fig. 2). Even though bone marrow chimera experiments suggest that MDA5-expressing radio-resistant cells (i.e. stromal cells) are primarily responsible for the production of IFN-I, one cannot exclude that some radio-resistant cells within the immune system such as Langerhans cells and subsets of dermal DCs and macrophages (148, 149) may contribute to poly(I:C)-mediated IFN-I responses. Given this, the cells responsible for poly(I:C)-mediated IFN-I production have not been unequivocally identified.
TLR7/9-equipped pDCs: what is their impact on IFN-I response in vivo?
pDCs are a specialized DC subset that produces large quantities of IFN-I in response to bacterial and viral stimulation in vitro (150–155). Unlike cDCs, pDCs do not express TLR2, TLR4, or TLR3 but detect both RNA and DNA viruses through TLR7 and TLR9, respectively. Engagement of TLR7 and TLR9 with viral products leads to the production of both IFN-I and inflammatory cytokines such as IL-12, IL-6, and TNF-α as well as pDC maturation. pDCs constitutively express IRF7 and hence are refractory to viral replication. Therefore, although both are present, neither RIG-I nor MDA5 contribute significantly to viral recognition in pDCs (156, 157).
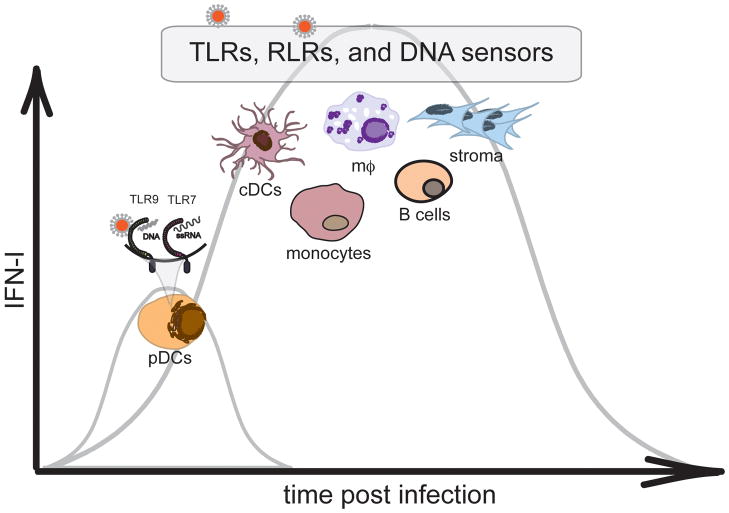
pDCs are thought to be the first line of defense against viral infection because of their immediate capacity to produce IFN-I and other factors that prime innate and adaptive immune responses. Until recently, the contribution of pDCs to antiviral responses in vivo was examined using antibodies to deplete this subset (158). Although these studies were highly informative, the antibodies used to eliminate pDCs also bind to and deplete other cell types. Within the past few years, genetically engineered mice have become available that more specifically lack pDCs, either constitutively or by inducible depletion (159, 160). pDC are constitutively absent in mice lacking the basic helix-loop-helix transcription factor E2-2/Tcf4, which is essential for pDC development (159). In BDCA2-DTR transgenic mice, which selectively express the diphtheria toxin receptor on pDCs, administration of diphtheria toxin results in the transient but specific depletion of pDCs (160). By infecting BDCA2-DTR mice with VSV or MCMV, it was demonstrated that pDCs provide an initial but limited source of IFN-I and can control viral burden only when relatively low amounts of virus are inoculated. However, in contrast to typical experimental settings that involve inoculation with high doses of virus, many natural infections occur from contact with low doses of virus. Therefore, under physiological conditions, pDCs may effectively control viral replication. Furthermore, early pDC-derived IFN-I may be important for initiating the establishment of an antiviral state by augmenting expression of numerous antiviral ISGs that render cells resistant to viral infections (1, 161, 162). Additionally, early pDC-derived IFN-I contributes to the induction of other viral sensors in both immune and stromal cells, resulting in further amplification of IFN-I responses (Fig. 3).
Fig. 3. Cellular sources of IFN-I during viral infections.
TLR7- and TLR9-expressing pDCs detect RNA and DNA viruses and provide an initial source of IFN-I. However, pDC-mediated IFN-I production is limited and transient, indicating that the majority IFN-I required for controlling viral replication is derived from additional cellular sources such as cDCs, macrophages, monocytes, B cells, and stromal cells that utilize TLRs, RLRs, and DNA sensors for viral detection. The expression of RLRs and DNA sensors is often low but highly inducible in response of IFN-I, suggesting that initial IFN-I by pDCs may be essential for RLR- and DNA sensor-mediated responses.
IFN-I and pDCs in autoimmune diseases
While IFN-I is critical for limiting viral replication and promoting protective immunity, excessive IFN-I production has also been linked to autoimmunity. Chronic IFN-I production has been implicated in systemic lupus erythematosus (SLE) (5, 6, 47, 163–169). Patients treated with long term IFN-I therapy for tumors, multiple sclerosis (MS), or chronic viral infections acquire SLE-like syndromes (170–173), corroborating a pathogenic role for IFN-I in the development of SLE. pDC infiltration into SLE skin lesions has also been reported (174). While pDCs are usually absent in healthy skin, under pathological circumstances including injury, viral infection, or inflammatory disorders, they can accumulate. What attracts pDCs to the skin is somewhat unclear, but chemoattractants produced by resident skin cells in aggravated areas are likely candidates (175). Prochemerin is an inactive precursor protein constitutively released in the skin by dermal endothelial cells, fibroblasts, and keratinocytes. After perturbation, prochemerin is cleaved into its active form, chemerin, by serine proteases. pDCs express the G protein-coupled receptor ChemR23 and hence migrate into lymphoid tissues and inflamed skin in response to chemerin (175, 176). CXCR3 ligands may also be important for recruiting pDCs to skin lesions (177, 178). Additional factors that could attract pDCs to injured skin are adenosine, FPRL2 ligand (F2L), C3a, and C5a (175).
In skin lesions of SLE patients, self-DNA/RNA immunocomplexes are internalized through Fc receptors and stimulate IFN-I secretion by pDCs through TLR7 and TLR9, resulting in increased serum IFN-I levels and IFN signatures in the transcriptome of peripheral blood mononuclear cells (179–184). Recently, it was reported that in SLE lesions, neutrophils are recruited to inflamed skin where they undergo cell death releasing neutrophil extracellular traps (NETs) upon exposure to SLE-derived anti-ribonucleoprotein antibodies (185, 186). These NETs contain self-DNA complexed with the antimicrobial peptide LL37 and HMGB1. LL37 converts inert self-DNA into aggregated, condensed structures that are delivered to and retained within early TLR9+ endocytic compartments, where they trigger IFN-I production by pDCs (187). Additionally self-DNA also can be delivered in a complex with HMGB1 through the RAGE receptor (188). Steroids, such as glucocorticoids (GCs), are often used to treat inflammatory conditions, because they induce apoptosis of several immune cell types. However, stimulation of pDCs with TLR7/9 ligands confers resistance to GC-induced apoptosis (189, 190). Thus, corticosteroids are not effective in SLE. However, blocking the TLR9 pathway with chloroquine has been shown to prevent IFN-I production by pDCs following exposure to DNA/immunocomplexes (183). Thus, elimination of pDCs or blocking their secretion of IFN-I may be viable alternative strategies for treating SLE.
IFN-I-producing pDCs are present in psoriatic skin lesions. The recruitment of pDCs to psoriatic skin is thought to be mediated by chemerin (175, 191). In a xenograft model for psoriasis, blockade of pDC-derived IFN-I hindered the development of psoriasis, but disease progressed after reconstitution with IFNα, indicating that pDCs and IFN-I are necessary for the pathogenesis of psoriasis in vivo (192). Furthermore, treatment of psoriatic lesions with Aldara cream, which contains the TLR7/8 agonist imiquimod, exacerbates disease by recruiting pDCs to lesions and inducing IFN-I production (193). Within psoriatic skin lesions, pDCs internalize self-DNA complexed with LL37 and secrete IFN-I.
Although pDC accumulation in SLE and psoriatic skin lesions appears to be problematic, their recruitment to the skin can have positive consequences in other situations. Aldara cream is often used to treat skin lesions induced by human papilloma virus (HPV), molluscum contagiosum virus (MCV), and certain skin cancers. How Aldara cream induces regression of skin lesions is not well understood, although hallmarks of treatment include pDC accumulation and IFN-I production (194, 195), Two groups (196, 197) demonstrated recently that pDCs are recruited to the skin, produce IFN-I, and promote wound healing following injury by tape stripping or chemical insult. However, such injury and subsequent pDC responses in a lupus-prone background results in the development of SLE-like lesions. Thus, whether pDC infiltration and IFN-I production in the skin is harmful or protective depends largely on the context.
IFN-I and pDCs have been implicated in autoimmune neuroinflammation and multiple sclerosis (MS); however, their roles remain controversial. Avonex, or IFNβ-1a, is used to prevent symptoms and slow the development of disease in patients with relapsing-remitting MS, suggesting that IFN-I plays a beneficial role in MS. A number of groups have explored the role of pDCs and IFN-I in a mouse model for MS, experimental autoimmune encephalomyelitis (EAE), and provide evidence that IFN-I and pDCs are protective in this model. IFN-I constrains T-helper 17 (Th17)-mediated autoimmune inflammation (198), while IFNAR deficiency in myeloid cells leads to severe disease with an enhanced effector phase and increased lethality (199). Depletion of pDCs with mPDCA-1 anitbody during the acute or relapsing phase of EAE also augments disease severity (200). In addition, mice lacking MHC class II expression on pDCs develop exacerbated EAE due to enhanced priming of encephalitogenic CD4+ T-cell responses in secondary lymphoid tissues (201). In WT mice, pDCs apparently curb the autoimmune T-cell response by promoting the expansion of myelin-specific T-regulatory cells through MHC class II-dependent interactions with CD4+ T cells. Alternatively, it has been reported that depletion of pDCs at the time of immunization ameliorates the clinical symptoms of EAE by reducing the numbers of myelin-specific Th17 T cells (202). Thus, the contribution of pDCs to EAE induction and progression is unclear at this time; further work in mice specifically lacking pDCs should help clarify this issue.
A role for IFN-I, pDCs, and dsRNA sensors in autoimmune diabetes
Type I diabetes (T1D) is an autoimmune disease characterized by destruction of the pancreatic β-cells. Dysregulation of the adaptive immune response has been implicated in T1D by early findings of autoantibodies against a variety of islet antigens in human patients, and animal models, particularly the non-obese diabetic (NOD) mouse, have been crucial for defining the loss of T-cell tolerance in T1D (203). More recently, innate immune defects involving activation of viral sensors and subsequent IFN-I responses have also been implicated in the pathogenesis of T1D; emphasis has been placed on RLRs as well as TLR7 and 9 in pDCs (172, 204). However, experimental results from a number of systems have proved confusing and somewhat contradictory; depending on the model system and specific pathogen used, viruses and IFN-I have been shown to be both protective and pathogenic during T1D.
A large number of studies support the general concept that detection of viral infection and perhaps excessive subsequent IFN-I responses can lead to onset of T1D. RNA virus infections have been associated with the pathogenesis of T1D. Viruses with tropism for the pancreas can induce T1D by directly destroying β-cells or by causing immune cell-mediated β-cell death (204). Enterovirus family members such as CVB have been linked to human T1D, and EMCV-D has been used to study T1D in animal models. In the BioBreeding rat model, infection with Kilham’s rat virus induces T-cell-mediated diabetes (205). In mice, CVB and EMCV-D can replicate in the pancreas causing extensive tissue damage and diabetes (122, 129). T1D onset in mice infected with CVB has been attributed to bystander damage of β-cells leading to inflammation, tissue damage, and the release of sequestered islet antigen, resulting in the restimulation of resting autoreactive T cells (206). In contrast, diabetes induced by EMCV-D seems to be a consequence of direct β-cell destruction due to the cytopathic effect of viral infection and macrophage-mediated phagocytosis of apoptotic cells rather than T-cell-mediated insulitis (207). Clearly, initiation and progression of disease vary considerably in these studies, and hypotheses for the link between viral infection with the development of T1D reflect this diversity. Perhaps the most widely known hypotheses are molecular mimicry, bystander activation, and viral persistence (127)
In addition to direct viral infection, dsRNA agonists such as poly(I:C) can stimulate T1D development in animal models (208–210). This observation suggests that dsRNA detection by MDA5 and perhaps TLR3 during viral infection may affect T1D progression in an IFN-I-dependent fashion. IFN-I production has been associated with the progression of T1D, (203, 204, 211). One report demonstrated that transgenic mice expressing IFN-I in β-cells develop diabetes and islet inflammation (212). A more recent study revealed that antibody blockade of IFNAR1 in NOD mice delayed the onset of T1D (213). Further work by this group has demonstrated that early antibody-mediated depletion of pDCs in NOD mice results in decreased incidence of T1D (214). Although these studies were done in mice, expansion of IFN-I-producing pDCs has also been observed in human patients with T1D around the time of diagnosis (215). These studies support a role for viral sensors and pDCs in promoting a pathogenic IFN-I response during T1D.
In contrast, another set of studies, more in line with the hygiene hypothesis, indicate that viral detection and IFN-I responses may be beneficial in the setting of autoimmunity because they induce T-cell tolerance. Consistent with this theory, NOD mice have delayed or complete protection from the onset of diabetes following infection with a variety of viral pathogens (216–218). A number of mechanisms have been proposed for the prevention or amelioration of autoimmunity consequent to viral infection, including enhanced T-regulatory cell activity and the upregulation of inhibitory molecules and cytokines (218–221).
IFN-I and viral sensors have also been mechanistically linked to the prevention of T1D caused by viral infection. Treatment of NOD mice with IFN-I or dsRNA can be protective and reduce the incidence of T1D depending on the time of administration (222–224). Interestingly, human genetic analyses have demonstrated that polymorphisms in the MDA5 gene are linked with resistance to T1D. Importantly, the ‘protective’ polymorphisms either reduce expression or functional dsRNA-mediated IFN-I responses of MDA5 (225–229). These salient studies have provided a potential mechanism by which viruses, through recognition by viral sensors and the production of IFN-I can contribute to the pathogenesis of T1D. As we have learned from previous studies, the combination of viruses, viral sensors, and IFN-I can have both pro-inflammatory and tolerogenic effects, and further work is needed to dissect how their interactions lead to autoimmunity.
This conundrum was recently addressed in a mouse model of viral induced diabetes (124). In this study, TLR3 was critical for protecting pancreatic islets from viral infection; TLR3 knockout animals developed severe hyperglycemia after infection with EMCV-D. Similarly, mice lacking one allele of MDA5 developed transient hyperglycemia. The unresolved hyperglycemia observed in TLR3-deficient mice was a direct result of hematopoietic cells failing to mount an early IFN-β response that protects β-cells from virus-induced damage. These data demonstrate that IFN-I responses mediated by MDA5 and TLR3 serve to limit the pathology of virus-induced diabetes when caused by direct infection of the islets (Fig. 4). The role of these sensors in the pathogenesis of autoimmune diabetes remains to be seen.
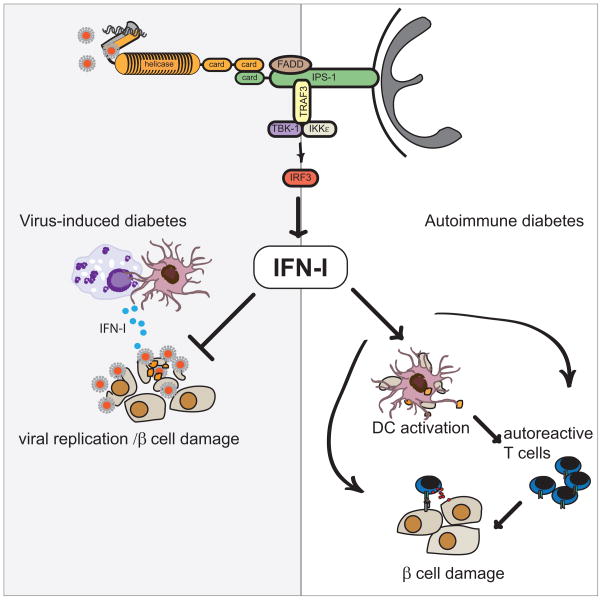
Fig. 4. MDA5 and IFN-I in virus-induced and autoimmune diabetes.
Viruses such as EMCV and CVB cause diabetes in mice. Polymorphisms in MDA5, a sensor for β-cell tropic viruses such as CVB and EMCV-D, have been linked to the development of human T1D. In virus-induced diabetes, activation of the MDA5 pathway in DCs and macrophages triggers IFN-I responses, which inhibits viral replication and prevents βcell injury. On the other hand, in autoimmune diabetes, MDA5 signaling might elicit excessive IFN-I production that could induce apoptosis of β-cells and promote presentation of β-cell antigens to autoreactive T cells by islet-resident DCs (256). IFN-I is also capable of activating autoreactive T cells directly in an antigen non-specific fashion.
Cumulatively, these studies suggest that the beneficial or detrimental effect of sensors and IFN-I depends on the pathogenesis of diabetes and therefore viral sensors may play multiple roles in the spectrum of human T1D.
Concluding remarks and future perspectives
Clearly multiple cell types and sensors are required for protective responses to viral infection. Although most cell types are capable producing IFN-I, pDCs provide an immediate burst of IFN-I in response to viruses. However, the pDC IFN-I response is limited in both magnitude and time, and other cells play a critical role in containing most viruses (160). Both splenic and hepatic macrophages are important for IFN-I production and control of viral replication in LCMV-infected mice (230, 231). Molecularly, LCMV-mediated IFN-I production requires both TLR and RLR pathways (19, 232, 233). Restriction of VACV infection by IFN-I requires TLR2 and inflammatory monocytes (20). Furthermore, MDA5-expressing stromal cells and TLR3-expressing DCs are critical sources of IFN-I during poly(I:C)-mediated immunity and EMCV infection (72, 124, 136). Although the pDC contribution to IFN-I production is limited, it is likely that the initial burst of pDC-derived IFN-I drives the expression of RLRs, amplifying IFN-I responses. Altogether, these findings indicate that in vivo IFN-I is derived from diverse molecular and cellular sources that probably depend on the nature of the pathogens and their tropisms.
The diversity of viral sensors is essential to mount effective IFN-I responses against invading pathogens. Distinct sensors differ in their specificity for viral products, permitting recognition of a plethora of viruses. Viral sensors also differ in their tissue distribution enabling a given organ to effectively respond to a specific virus. For EMCV-D, MDA5 expression is essential in the heart, while both TLR3 and MDA5 are required for controlling EMCV-mediated β-cell damage in the pancreas. Furthermore, within the same organ, different sensors might be required at different time points during infection. Because MDA5 is an ISG, its expression is negligible before infection, whereas TLR3 is constitutively expressed. Therefore, MDA5-mediated responses to EMCV may require early IFN-I production induced by TLR3 signaling (66, 124). In conclusion, pathogen specificity, tissue distribution, and the timing of viral sensor expression are all essential parameters that shape IFN-I responses during viral infections. Depending on the nature of the virus and the particular tissue or cell types infected, viral sensors can have either complementary or redundant roles and may synergize or antagonize each other.
While IFN-I is critical for limiting viral replication and eliciting protective immunity, excessive IFN-I production can promote autoimmune responses. However, IFN-I can also ameliorate autoimmunity. Thus, the beneficial or detrimental effect of sensors and IFN-I depends largely on disease pathogenesis. Given this, viral sensors may play multiple roles in the spectrum of autoimmune disease. Undoubtedly, understanding the interplay among these viral sensors will ultimately help us design better vaccines and therapies for infectious and autoimmune diseases.
Acknowledgments
We thank Susan Gilfillan for helpful comments. These studies were supported by the Juvenile Diabetes Research Foundation (JDRF) grant 24-2007-420 and National Institutes of Health grant CA109673 (to M. Colonna). Y. Wang was supported by Pulmonary and Critical Care Training Grant 2T32HL007317 from the National Heart, Lung, and Blood Institute (NHLBI). M. Swiecki was supported by the NRSA training grant 5T32DK007296 from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). S.A. McCartney was supported by the NRSA training grants F30HL096354 from NHLBI and T32DK007296 from NIDDK.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 2.Malathi K, et al. A transcriptional signaling pathway in the IFN system mediated by 2′-5′-oligoadenylate activation of RNase L. Proc Natl Acad Sci USA. 2005;102:14533–14538. doi: 10.1073/pnas.0507551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber GN, Wambach M, Wong ML, Dever TE, Hinnebusch AG, Katze MG. Translational regulation by the interferon-induced double-stranded-RNA-activated 68-kDa protein kinase. Proc Natl Acad Sci USA. 1993;90:4621–4625. doi: 10.1073/pnas.90.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 10.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 13.Compton T, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurt-Jones EA, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leuk Biol. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 17.Bieback K, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S, et al. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 20.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JM, Iwasaki A. Toll-like receptors regulation of viral infection and disease. Adv Drug Deliv Rev. 2008;60:786–794. doi: 10.1016/j.addr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 26.Schulz O, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 27.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 29.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 31.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 32.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 33.Jacquemont B, Roizman B. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J Virol. 1975;15:707–713. doi: 10.1128/jvi.15.4.707-713.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 36.Perez de Diego R, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sancho-Shimizu V, et al. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 38.Davey GM, Wojtasiak M, Proietto AI, Carbone FR, Heath WR, Bedoui S. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243–2246. doi: 10.4049/jimmunol.0903013. [DOI] [PubMed] [Google Scholar]
- 39.Gowen BB, et al. TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J Immunol. 2006;177:6301–6307. doi: 10.4049/jimmunol.177.9.6301. [DOI] [PubMed] [Google Scholar]
- 40.Le Goffic R, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 42.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 43.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez J, Huang X, Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci USA. 2010;107:6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 47.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 48.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 50.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 53.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 54.Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007;19:24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blasius AL, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–62. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. The Journal of experimental medicine. 2011;208:643–51. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park B, et al. Granulin Is a Soluble Cofactor for Toll-like Receptor 9 Signaling. Immunity. 2011;34:505–513. doi: 10.1016/j.immuni.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 62.Mancuso G, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 63.Hoshino K, et al. Critical role of IkappaB Kinase alpha in TLR7/9-induced type I IFN production by conventional dendritic cells. J Immunol. 2010;184:3341–3345. doi: 10.4049/jimmunol.0901648. [DOI] [PubMed] [Google Scholar]
- 64.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 65.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 66.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovacsovics M, Martinon F, Micheau O, Bodmer JL, Hofmann K, Tschopp J. Overexpression of Helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation. Curr Biol. 2002;12:838–843. doi: 10.1016/s0960-9822(02)00842-4. [DOI] [PubMed] [Google Scholar]
- 68.Saito T, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato H, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito T, Gale M., Jr Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med. 2008;205:1523–1527. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 72.Gitlin L, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zust R, et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loo YM, et al. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proc Natl Acad Sci USA. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gitlin L, et al. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 2010;6:e1000734. doi: 10.1371/journal.ppat.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furr SR, Chauhan VS, Sterka D, Jr, Grdzelishvili V, Marriott I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol. 2008;14:503–513. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 80.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 81.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Saha SK, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 86.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 88.Rothenfusser S, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 89.Venkataraman T, et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 90.Satoh T, et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I- mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 92.Wang P, et al. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol. 2010;11:912–919. doi: 10.1038/ni.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajput A, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 94.Hou J, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 95.Cardenas WB, et al. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrejeva J, et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 99.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci USA. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 102.Lippmann J, et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 103.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yanai H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 108.Kim T, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 110.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 112.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 116.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 117.Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 119.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 121.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the Antimicrobial Response by NLR Proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Yoon JW, McClintock PR, Onodera T, Notkins AL. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980;152:878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hardarson HS, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 124.McCartney SA, et al. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J Clin Invest. 2010;121:1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6:279–289. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 126.Richer MJ, Horwitz MS. Coxsackievirus infection as an environmental factor in the etiology of type 1 diabetes. Autoimmun Rev. 2009;8:611–615. doi: 10.1016/j.autrev.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 127.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Richer MJ, Lavallee DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One. 2009;4:e4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huhn MH, McCartney SA, Lind K, Svedin E, Colonna M, Flodstrom-Tullberg M. Melanoma differentiation-associated protein-5 (MDA-5) limits early viral replication but is not essential for the induction of type 1 interferons after Coxsackievirus infection. Virology. 2010;401:42–8. doi: 10.1016/j.virol.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 130.Levine AS, Sivulich M, Wiernik PH, Levy HB. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-L-lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39:1645–1650. [PubMed] [Google Scholar]
- 131.Levine AS, Levy HB. Phase I-II trials of poly IC stabilized with poly-L-lysine. Cancer Treat Rep. 1978;62:1907–1912. [PubMed] [Google Scholar]
- 132.Robinson RA, DeVita VT, Levy HB, Baron S, Hubbard SP, Levine AS. A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patieonts with leukemia or solid tumors. J Natl Cancer Inst. 1976;57:599–602. doi: 10.1093/jnci/57.3.599. [DOI] [PubMed] [Google Scholar]
- 133.McCartney S, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trumpfheller C, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stahl-Hennig C, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar H, et al. Cutting edge: cooperation of IPS-1 and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–687. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation associated protein 5 expressed in stromal cells. J Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 139.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 140.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le Bon A, et al. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 142.Sivori S, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidt KN, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 144.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 145.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 146.Akazawa T, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miyake T, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 148.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Klein I, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 151.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 152.Ronnblom L, Forsgren A, Alm GV. Characterization of interferons induced by bacteria and interferon-producing leukocytes in human peripheral blood. Infect Immun. 1983;40:126–132. doi: 10.1128/iai.40.1.126-132.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Perussia B, Fanning V, Trinchieri G. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4:120–137. [PubMed] [Google Scholar]
- 154.Fitzgerald-Bocarsly P, Feldman M, Mendelsohn M, Curl S, Lopez C. Human mononuclear cells which produce interferon-alpha during NK(HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J Leukoc Biol. 1988;43:323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- 155.Ferbas JJ, Toso JF, Logar AJ, Navratil JS, Rinaldo CR., Jr CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J Immunol. 1994;152:4649–4662. [PubMed] [Google Scholar]
- 156.Coccia EM, et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 157.Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting edge: TLR-dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{alpha} production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 158.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cisse B, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagano Y, Kojima Y. [Immunizing property of vaccinia virus inactivated by ultraviolets rays] C R Seances Soc Biol Fil. 1954;148:1700–1702. [PubMed] [Google Scholar]
- 162.Lindenmann J, Burke DC, Isaacs A. Studies on the production, mode of action and properties of interferon. Br J Exp Pathol. 1957;38:551–562. [PMC free article] [PubMed] [Google Scholar]
- 163.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci USA. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ronnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23:113–121. doi: 10.1016/j.smim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 165.Ronnblom L, Eloranta ML, Alm GV. Role of natural interferon-alpha producing cells (plasmacytoid dendritic cells) in autoimmunity. Autoimmunity. 2003;36:463–472. doi: 10.1080/08916930310001602128. [DOI] [PubMed] [Google Scholar]
- 166.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol. 2008;20:401–407. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 168.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21:471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 169.Stetson DB. Connections between antiviral defense and autoimmunity. Curr Opin Immunol. 2009;21:244–250. doi: 10.1016/j.coi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rizvi R, Hojjati M. Interferon-alpha induced lupus in a patient with chronic hepatitis C virus. J Clin Rheumatol. 2011;17:152–153. doi: 10.1097/RHU.0b013e31821557e7. [DOI] [PubMed] [Google Scholar]
- 171.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 172.Bonaci-Nikolic B, Jeremic I, Andrejevic S, Sefik-Bukilica M, Stojsavljevic N, Drulovic J. Anti-double stranded DNA and lupus syndrome induced by interferon-beta therapy in a patient with multiple sclerosis. Lupus. 2009;18:78–80. doi: 10.1177/0961203308093550. [DOI] [PubMed] [Google Scholar]
- 173.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha ⁄ beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 176.Vermi W, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen SC, et al. Expression of chemokine receptor CXCR3 by lymphocytes and plasmacytoid dendritic cells in human psoriatic lesions. Arch Dermatol Res. 2010;302:113–123. doi: 10.1007/s00403-009-0966-2. [DOI] [PubMed] [Google Scholar]
- 178.Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J Invest Dermatol. 2006;126:507–509. doi: 10.1038/sj.jid.5700052. [DOI] [PubMed] [Google Scholar]
- 179.Han GM, Chen SL, Shen N, Ye S, Bao CD, Gu YY. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003;4:177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- 180.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 183.Means TK, et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Savarese E, et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 185.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 188.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 189.Guiducci C, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lepelletier Y, et al. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid predendritic cells (pDCs) Blood. 2010;116:3389–3397. doi: 10.1182/blood-2010-05-282913. [DOI] [PubMed] [Google Scholar]
- 191.Albanesi C, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gilliet M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 194.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vermi W, et al. Spontaneous regression of highly immunogenic molluscum contagiosum virus (MCV)-induced skin lesions is associated with plasmacytoid dendritic cells and IFN-DC infiltration. J Invest Dermatol. 2011;131:426–434. doi: 10.1038/jid.2010.256. [DOI] [PubMed] [Google Scholar]
- 196.Gregorio J, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prinz M, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 200.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Irla M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Isaksson M, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39:2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 203.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 204.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 205.Guberski DL, et al. Induction of type I diabetes by Kilham’s rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254:1010–1013. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 206.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 207.Baek HS, Yoon JW. Direct involvement of macrophages in destruction of beta-cells leading to development of diabetes in virus-infected mice. Diabetes. 1991;40:1586–1597. doi: 10.2337/diab.40.12.1586. [DOI] [PubMed] [Google Scholar]
- 208.Devendra D, et al. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- 209.Moriyama H, et al. Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci USA. 2002;99:5539–5544. doi: 10.1073/pnas.082120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lang KS, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 211.Castano L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 212.Stewart TA, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan NJ. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 213.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Q, McDevitt HO. The role of interferon alpha in initiation of type I diabetes in the NOD mouse. Clin Immunol. 2011;140:3–7. doi: 10.1016/j.clim.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 215.Allen JS, et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes. 2009;58:138–145. doi: 10.2337/db08-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179:7325–7333. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
- 217.Feillet H, Bach JF. On the mechanisms of the protective effect of infections on type 1 diabetes. Clin Dev Immunol. 2004;11:191–194. doi: 10.1080/17402520400004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boettler T, von Herrath M. Protection against or triggering of Type 1 diabetes? Different roles for viral infections. Expert Rev Clin Immunol. 2011;7:45–53. doi: 10.1586/eci.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Diana J, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 221.Diana J, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med. 2010;208:729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Serreze DV, Hamaguchi K, Leiter EH. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989;2:759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 223.Tanaka-Kataoka M, et al. Oral use of interferon-alpha delays the onset of insulin-dependent diabetes mellitus in nonobese diabetes mice. J Interferon Cytokine Res. 1999;19:877–879. doi: 10.1089/107999099313398. [DOI] [PubMed] [Google Scholar]
- 224.Sobel DO, Ahvazi B. Alpha-interferon inhibits the development of diabetes in NOD mice. Diabetes. 1998;47:1867–1872. doi: 10.2337/diabetes.47.12.1867. [DOI] [PubMed] [Google Scholar]
- 225.von Herrath M. Diabetes: A virus-gene collaboration. Nature. 2009;459:518–519. doi: 10.1038/459518a. [DOI] [PubMed] [Google Scholar]
- 226.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smyth DJ, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 228.Shigemoto T, Kageyama M, Hirai R, Zheng J, Yoneyama M, Fujita T. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J Biol Chem. 2009;284:13348–13354. doi: 10.1074/jbc.M809449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Downes K, et al. Reduced expression of IFIH1 is protective for type 1 diabetes. PLoS One. 2010;5:e12646. doi: 10.1371/journal.pone.0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Louten J, van Rooijen N, Biron CA. Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection. J Immunol. 2006;177:3266–3272. doi: 10.4049/jimmunol.177.5.3266. [DOI] [PubMed] [Google Scholar]
- 231.Lang PA, et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52:25–32. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
- 232.Jung A, et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou S, Cerny AM, Zacharia A, Fitzgerald KA, Kurt-Jones EA, Finberg RW. Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J Virol. 2010;84:9452–9462. doi: 10.1128/JVI.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 235.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tabeta K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang Q, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Slater L, et al. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6:e1001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis. 2011;5:e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 242.Davidson S, et al. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol. 2011 doi: 10.4049/jimmunol.1002635. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 244.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 245.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fiola S, Gosselin D, Takada K, Gosselin J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J Immunol. 2010;185:3620–3631. doi: 10.4049/jimmunol.0903736. [DOI] [PubMed] [Google Scholar]
- 247.Yoneyama M, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 248.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ikegame S, Takeda M, Ohno S, Nakatsu Y, Nakanishi Y, Yanagi Y. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. J Virol. 2010;84:372–379. doi: 10.1128/JVI.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One. 2007;2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 252.Fredericksen BL, Gale M., Jr West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol. 2006;80:2913–2923. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Colonna M. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Roth-Cross JK, Bender SJ, Weiss SR. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Melchjorsen J, et al. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J Virol. 2010;84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Colisson R, et al. Free HLTV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115:2177–2185. doi: 10.1182/blood-2009-06-224741. [DOI] [PubMed] [Google Scholar]
- 258.Cervantes-Barragan L, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]