Abstract
Imaging studies have shown an association between dopamine increases in striatum and cue induced craving in cocaine abusers. However, the extent to which dopamine increases reflect a primary rather than a secondary response to the cues remains unclear. Here we evaluated the extent to which dopamine increases by themselves can induce craving in cocaine abusers. Using PET and [11C]raclopride (D2 receptor radioligand sensitive to competition with endogenous dopamine) we show that in cocaine abusers (n = 20) oral methylphenidate (20 mg), which significantly increased dopamine in striatum, did not induce craving unless subjects were concomitantly exposed to cocaine-cues (video scenes of subjects self-administering cocaine). This suggests that dopamine increases associated with conditioned-cues are not primary responses but reflect downstream stimulation of dopamine cells (presumably glutamatergic afferents from prefrontal cortex and/or amygdala). Inasmuch as afferent stimulation of dopamine neurons results in phasic cell firing these findings suggest that “fast” dopamine increases, in contrast to the “slow” dopamine increases as achieved when using oral methylphenidate (mimicking tonic dopamine cell firing), are required for cues to trigger craving. The fact that methylphenidate induced craving only when given with the cocaine-cues highlights the context dependency of methylphenidate’s effects and suggests that its use for the treatment of ADHD subjects with co-morbid drug abuse should not increase craving.
Keywords: PET imaging, raclopride, addiction, caudate, putamen, conditioned responses, D2 receptors
In drug addicted subjects, exposure to places where they have taken the drug, to people with whom prior drug use occurred, or to paraphernalia used to administer the drug elicits intense drug craving that can trigger compulsive drug use. This phenomenon (cue-induced craving) is clinically relevant since it is a major contributor in the cycle of relapse in addiction (O’Brien et al., 1998). Dopamine (DA), which is a neurotransmitter involved with reward and with the prediction of reward (Schultz et al., 1997; Wise and Rompre, 1989), appears to be involved with cue-elicited drug craving. Indeed, in laboratory animals when neutral stimuli are paired with a rewarding drug they will, with repeated associations, acquire the ability to increase DA in nucleus accumbens (NAc) and in dorsal striatum, and these neurochemical responses are associated with drug-seeking behavior (Di Ciano and Everitt, 2004; Kiyatkin and Stein, 1996; Phillips et al., 2003; Vanderschuren et al., 2005; Weiss et al., 2000; Duvauchelle et al., 2000); though a few studies have failed to show this (Bradberry et al., 2000; Brown and Fibiger, 1992). Imaging studies in humans have also shown that in drug addicted subjects visual exposure to drug-cues (presented through a video) elicits DA increases in dorsal striatum and these increases are associated with subjective experiences of drug craving (Volkow et al., 2006; Wong et al., 2006).
Though striatal DA increases in cocaine abusers are associated with craving it is unclear if they reflect the primary signal or a downstream activation of DA cells by afferents from areas that processes the stimulus as salient. Since preclinical studies have shown that striatal DA increases induced by cues are dependent on the context of presentation (non-contingent presentation increases DA in NAc (Kiyatkin and Stein, 1996; Phillips et al., 2003) whereas contingent presentation increases DA in dorsal striatum (Ito et al., 2002), this would suggest that DA responses are modulated by regions/circuits that convey previously learned associations with the cue(s).
The purpose of this study was to test if DA increases associated with craving reflected primary or secondary responses. We reasoned that if striatal DA increases constituted a primary response for craving then DA increases without exposure to cocaine-conditioned cues would trigger craving; whereas if DA increases reflected a downstream response then increasing DA without the cocaine-conditioned cues would not result in craving. To test this we compared the effects of increasing striatal DA on drug craving in drug addicted subjects when tested with and without cocaine-conditioned cues. For this purpose we used methylphenidate (20 mg po) a stimulant drug that increases DA by blocking DA transporters (Volkow et al., 2002a). Methylphenidate was given concomitant to presentation of a cocaine-cue video (scenes of subjects smoking cocaine) and of the presentation of a neutral video (nature scenes) in 20 active cocaine abusers. Positron emission tomography (PET) and [11C]raclopride (DA D2 receptor radioligand sensitive to competition with endogenous DA (Volkow et al., 1994)) were used to measure the DA changes induced by MP for both conditions, which were compared against measures obtained after a placebo presented with a neutral video. Cocaine craving was measured using a brief version of the Cocaine Craving Questionnaire (CCQ) (Tiffany et al., 1993) that evaluates current cocaine craving (desire to use; intention and planning to use; anticipation of positive outcome; anticipation of relief from withdrawal or distressing symptoms; and lack of control over drug use). Our working hypothesis was that the increases in DA elicited by conditioned-cues are a downstream response from circuits that process the saliency values of the cue and that increasing DA without the cues would not elicit craving.
METHODS
Subjects
Twenty active cocaine-addicted subjects who responded to an advertisement were studied. Subjects fulfilled DSM- IV criteria for cocaine dependence and were active users for at least the prior 6 months (free-base or crack, at least “four grams” a week). Exclusion criteria included current or past psychiatric disease other than cocaine dependence; past or present history of neurological, cardiovascular or endocrinological disease; history of head trauma with loss of consciousness greater than 30 minutes; and current medical illness and drug dependence other than cocaine or nicotine. Table 1 provides demographic and clinical information on the subjects Written informed consent was obtained in all subjects.
Table 1.
Demographic and clinical characteristic of subjects
| Age | 43 ±6 years |
| Gender | 18 males, 2 females |
| Ethnicity | 16 African Americans, 4 Caucasians |
| Education | 13 ±2 years of education |
| Years of cocaine use | 16 ±7 years |
| Route of administration | 20 smoked cocaine |
| Dose used | 2.8 ±1.6 grams a day |
| Days since last cocaine use | 2.5 ±2 days (range 1 and 7 days) |
| Cigarette smokers | 15 current smokers |
Cardiovascular measures
Heart rate and blood pressure were monitored continuously throughout the procedure from which we computed the measures obtained 45 minutes prior to the video presentation (Pre) and those obtained 45 minutes after initiation of the video (Post). For the statistical comparisons we used the difference between the Pre and the Post measures and expressed these as percent change form the Pre measures.
Cocaine craving
Cocaine craving was assessed with the brief version of the CCQ, which evaluates current cocaine craving on a seven-point visual analogue scale. The average score was used as measure of cocaine craving (Tiffany et al., 1993). The CCQ was obtained prior to and at the end of the video presentations.
PET Scan
We used an HR+ tomograph (resolution 4.5×4.5×4.5 mm full width half-maximum, 63 slices) with [11C]raclopride using methods previously described (Volkow et al., 1993). Briefly, emission scans were started immediately after injection of 4-8 mCi (specific activity 0.5-1.5 Ci/μM at EOB). Twenty dynamic emission scans were obtained from time of injection up to 54 minutes. Arterial sampling was used to quantify total carbon-11 and unchanged [11C]raclopride in plasma. Subjects were scanned three times with [11C]raclopride on 2-3 different days under randomly ordered conditions (1) 60 minutes after oral placebo while watching a video of nature scenes (neutral condition), (2) 60 minutes after oral MP (20 mg) while watching a video of nature scenes and (3) 60 minutes after oral MP (20 mg) while watching a video that portrayed subjects smoking cocaine. Videos were started 15 minutes prior to injection of [11C]raclopride and were continued for 30 minutes after radiotracer injection. The neutral video featured non-repeating segments of nature stories and the cocaine-cue video featured non-repeating segments portraying scenes that simulated purchase, preparation, and smoking of cocaine (Childress, et al. 1999; Volkow, et al. 2006).
Image Analysis
For region identification, we summed the time frames from images taken from 10-54 minutes and resliced them along the intercommisural plane. Planes were added in groups of two to obtain 12 planes encompassing the caudate, putamen, ventral striatum, and the cerebellum, which were measured on 4, 3, 1, and 2 planes respectively. Right and left regions were averaged. These regions were projected to the dynamic scans to obtain concentrations of C-11 vs. time. These time- activity curves for tissue concentration, along with the time- activity curves for unchanged tracer in plasma, were used to calculate [11C]raclopride’s transfer constant from plasma to brain (K1) and the distribution volumes (DV), which corresponds to the equilibrium measurement of the ratio of tissue concentration to plasma concentration, in striatum and cerebellum, using a graphical analysis technique for reversible systems (Logan et al., 1990). The ratio of DV in striatum to that in cerebellum corresponds to (Bmax/Kd) +1 and is insensitive to changes in cerebral blood flow (Logan et al., 1994). The effects of MP on DA were quantified as percent change in Bmax/Kd with respect to the neutral conditions (placebo with neutral video).
To corroborate the location within the striatum where the DA changes occurred we also analyzed the DV ratio images using Statistical Parametric Mapping (SPM) (Friston et al., 1995). Paired t tests were performed to compare the MP conditions with the placebo-neutral condition and to compare the MP conditions when given with the cocaine-cue or the neutral videos (p < 0.01, uncorrected, threshold > 50 voxels).
Statistical Analysis
Differences between conditions on the behavioral, cardiovascular and PET measures were evaluated with repeated measure ANOVA. For the behavioral measures the repeated ANOVA included the 3 measures: (1) prior to placebo or MP and prior to video (pre-drug/pre-video); (2) 55 minutes post drug but prior to video (post-drug/pre-video) and (3) post drug and post video (post-drug/post-video). For the PET measures these included the three measures: (1) Placebo neutral-video condition, (2) MP cocaine-cue video condition and (3) MP neutral-cue video condition. Post hoc t tests were then used to determine which comparisons differed from one another. Product moment correlations were used to assess the correlation between striatal DA changes and cocaine craving (CCQ), plasma MP concentrations and MP-induced cardiovascular effects.
RESULTS
Concentration of MP in plasma
The plasma levels of MP did not differ between the two conditions of administration. These levels corresponded for the cocaine-cue and the neutral video conditions respectively to 3 ±1 and 4 ±3 ng/ml at 30 minutes; to 7 ±3 and 7 ±4 ng/ml at 60 minutes; to 7 ±2 and 8 ±3 ng/ml at 90 minutes; and to 5 ±2 and 6 ±2 ng/ml at 120 minutes after its administration. The correlation between plasma MP concentration and the cardiovascular, behavioral and DA measures were not significant.
Effects of MP on the cardiovascular measures when exposed to the cocaine-video versus the neutral video
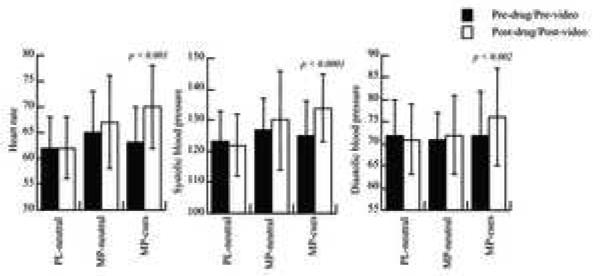
The changes in cardiovascular measures (pre – post / pre × 100) differed significantly between conditions for heart rate (Repeated ANOVA F=6, df 2, 59, p < 0.006), systolic (F= 11.6, p < 0.0001) and diastolic pressure (F=6.5 p < 0.004) (Figure 1). Post hoc t test revealed that when compared with the baseline condition MP increased the cardiovascular measures when it was given with the cocaine-cue video for heart rate (7 ±8% increases, p < 0.0008), systolic (7 ±6% increases, p < 0.0001) and diastolic pressure (6 ±7% increases, p < 0.002); whereas when MP was given with the neutral-cue video none of the cardiovascular effects were significant (Figure 1). Comparison between both MP conditions showed that MP’s cardiovascular effects were significantly greater when MP was given with the cocaine-cue than with the neutral-cue video for systolic (p < 0.01) and diastolic blood pressure (p < 0.03) but this difference did not reach significance for heart rate (p < 0.11).
Figure 1.
Cardiovascular measures for the placebo neutral-cue, the MP neutral-cue and the MP cocaine-cue conditions. Significant increases in heart rate and systolic blood pressure were observed only for the MP cocaine-cue condition. Values correspond to means and standard deviations.
Effects of MP on craving when exposed to the cocaine-video versus the neutral video
The repeated ANOVA for the MP cocaine-cue video condition (resulted in significant increases in craving (df 2, 38; F = 16.4, p < 0.0001) whereas MP when given with the neutral-cue video condition did not change craving (df 2, 38; F = 1.5, p < 0.24) (Figure 2). Post-hoc t tests for the MP cocaine-cue video condition revealed that craving was significantly greater for the post-MP/post-video measure than for the pre-MP/pre-video (df 19, t= 4.3, p < 0.0005) and the post-MP/pre-video measures (df 19, t= 4.2, p < 0.0005). The craving scores for the pre-MP/pre-video or post-MP/pre-video measures did not differ between the conditions (neutral-cue or the cocaine-cue video) (Figure 2), which indicates that oral MP by itself does not increase craving and that it’s the exposure to the video that accounts for the difference in craving.
Figure 2.
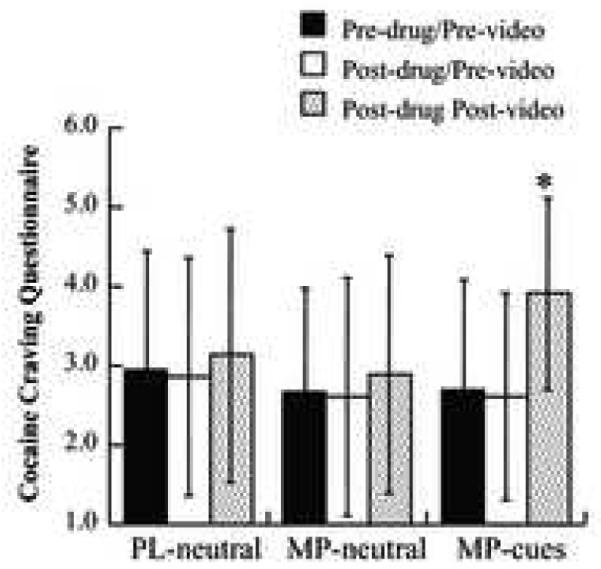
Average values on the Cocaine Craving Scale for the 3 conditions, before administration of the drug or placebo (Pre-drug/Pre-video), 55 minutes after administration of the drug but prior to video (Post-drug/Pre-video) and at the end of the video (Post-drug/Post-video). MP significantly increased craving only after watching the cocaine video. * Repeated ANOVA (F = 16.4, p < 0.0001); Post-hoc t tests for post-MP/post-video versus pre-MP/pre-video (p < 0.0005) and for post-MP/post-video versus post-MP/pre-video (p < 0.0005). Values correspond to means and standard deviations.
Effects of MP on [11C]raclopride binding when given with the cocaine-cue and the neutral videos
There were no differences between left and right regions for K1 or Bmax’/Kd’ and thus we report the results for the average scores in the left and right striatal and cerebellar regions. The K1 measure did not differ between conditions for any of the brain regions (Table 2). This indicates that the delivery of the tracer was not affected by MP (cocaine-cue or neutral-cue video conditions).
Table 2.
Measures of K1 and Bmax/Kd in cerebellum (CBL), caudate (CDT), putamen (PUT) and ventral striatum (VST) for the [11C]raclopride measures taken after placebo with the neutral video (PL-Neutral), after MP with the cocaine cue video (MP-Cue) and after MP with the neutral video (MP-Neutral). Comparisons correspond to repeated ANOVA (df 2,38) and show the F values and the significance level (p).
| K1 | Pl-Neutral | MP-Cue | MP-Neutral | ANOVA |
|---|---|---|---|---|
| CBL | 0.11 ±0.03 | 0.13 ±0.05 | 0.13 ±0.05 | F 1.6, p <0.23 |
| CDT | 0.10 ±0.01 | 0.10 ±0.02 | 0.10 ±0.02 | F 0.7, p <0.51 |
| PUT | 0.11 ±0.02 | 0.12 ±0.02 | 0.12 ±0.02 | F 1.7, p <0.20 |
| VST | 0.10 ±0.01 | 0.11 ±0.03 | 0.11 ±0.02 | F 1.2, p <0.33 |
| Bmax/Kd | ||||
| CDT | 2.32 ±0.27 | 2.21 ±0.25 | 2.13 ±0.37 | F 6.1, p < 0.006 |
| PUT | 2.75 ±0.33 | 2.61 ±0.37 | 2.54 ±0.39 | F 4.5, p < 0.02 |
| VST | 2.37 ±0.38 | 2.26 ±0.37 | 2.2 ±0.34 | F 2.5, p < 0.10 |
The Bmax’/Kd’ measures, which reflect D2 receptors that are not occupied by endogenous DA, differed significantly across conditions in caudate (F 6.1, p < 0.006), putamen (F 4.5, p < 0.02) and ventral striatum (F 3.9, p < 0.05). Post hoc t tests revealed that Bmax’/Kd’ measures when compared with the placebo-neutral condition were significantly reduced by MP in caudate both following the cocaine-cue (t = 2.5, df 19, p < 0.02) and the neutral-cue videos (t = 3.1, p < 0.006); in putamen following the neutral-cue (t = 2.8, p < 0.01) and showed a trend after the cocaine-cue video (t = 2.0, p < 0.06); and in ventral striatum after the neutral video (t = 2.4, p < 0.05) but did not differ for the cocaine-cue video. Comparison of Bmax/Kd measures between the cocaine–cue and the neutral-cue video conditions were not significant.
The correlations between DA changes and changes in cocaine craving as assessed with the CCQ were not significant for any of the MP conditions.
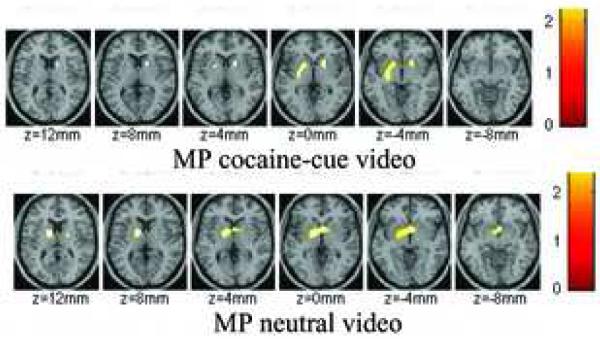
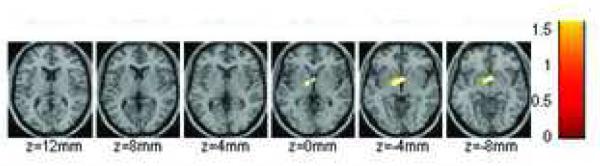
The SPM comparisons with the placebo neutral-cue video condition showed significant decreases (p < 0.01) in raclopride’s specific binding in dorsal striatum by oral MP both when given concomitantly with the neutral-cue video and when given with the cocaine-cue video (Figure 3). Also when MP was given with the neutral-cue video, but not with the cocaine-cue video, SPM revealed decreases in binding in the ventral striatum. Though the SPM comparison between both MP conditions did not reach significance at p <0.01 a lowering of significance to p < 0.05 revealed that MP-induced decreases in the ventral striatum were significantly greater when co-administered with the neutral-cue video than with the cocaine-cue video (Figure 4).
Figure 3.
SPM results for the areas where the distribution volume ratio (DVR) for [11C]raclopride was significantly decreased by methylphenidate both when given with the cocaine-cue video and when given with the neutral-cue video as compared to the placebo neutral-cue video condition (p < 0.01, uncorrected 100 pixels). Methylphenidate decreased [11C]raclopride’s specific binding in dorsal striatum for both conditions but decreased it in the ventral striatum only when given with the neutral-cue video.
Figure 4.
SPM results for the areas where the changes in the distribution volume ratio (DVR) differed when MP was given with the neutral-cue than with the cocaine-cue video. Changes were greater in ventral striatum when MP was given with the neutral than the cocaine-cue video (p < 0.05, uncorrected).
DISCUSSION
DA and craving
Here we show that MP significantly increased extracellular DA in striatum but that it induced craving only when administered with the cocaine-cue video. Moreover the craving measures were only elevated after the subjects had been exposed to the cocaine-cue video but not prior to it. Though prior studies in cocaine abusers had shown that cue-induced DA increases in striatum were associated with craving (Volkow et al., 2006; Wong et al., 2006), the current findings indicate that striatal DA increases (as induced by oral MP) while necessary, by themselves are insufficient to induce craving; craving required exposure to the conditioned-cues. Moreover, a comparison of the striatal DA changes we observed in a prior study (Volkow et al., 2006) when the cocaine-cue video was given without MP pretreatment revealed that the increases were similar in magnitude (caudate 6%; putamen 5%) to those we are reporting in the current study when the cocaine-cue video was given with MP (caudate 7%; putamen 5%) and to those when MP was given with the neutral-cue video (caudate 10%; putamen 8%). Despite the similar levels of striatal DA increases craving was observed only when the cocaine-cues were present (regardless of these being given with placebo or with MP), which is why we conclude that an additional pathway(s) is required to induce craving.
We hypothesize that these may be prefrontal-striatal, prefrontal-mesencephalic and/or amygdalar-mesencephalic glutamatergic pathways since they regulate DA cell firing and DA release (Sesack and Carr, 2002; Grace et al., 2007, Geisler et al., 2007, Georges and Aston-Jones, 2002). Moreover imaging studies have shown that activation of prefrontal regions and amygdala is associated with craving in cocaine abusers (Volkow et al., 1991; Grant et al., 1996; Breiter et al., 1997; Maas et al., 1998; Volkow et al., 1999a; Childress et al., 1999; Wang et al., 1999; Garavan et al., 2000; Kilts et al., 2001; Bonson et al., 2002) and studies in laboratory animals reveal a critical role of fronto-striatal, fronto-mesencephalic and amygdalo-mesencepahalic pathways in cue-induced reinstatement (Kalivas and Volkow, 2005, Kalivas and McFarland, 2003, McFarland et al., 2004). Specifically inhibition of the prefrontal cortex or the amygdala blocks stress induced reinstatement of cocaine seeking (McFarland et al., 2004). This would suggest that without activation of prefrontal and/or amygdalar pathways increases in striatal DA would not result in craving. Supporting this hypothesis are our prior findings that intravenous MP, which increases striatal DA with much faster kinetics than oral MP and is highly rewarding to cocaine abusers (Volkow et al 1995), induced craving only in the cocaine abusers in whom it increased prefrontal metabolism (orbitofrontal cortex and cingulate gyrus) (Volkow et al., 1999a). Moreover, in controls in whom intravenous MP also increases striatal DA but does not produce craving it decreased metabolism in these prefrontal regions (Volkow et al., 2005).
In the current study increasing DA, which is implicated in mediating the motivational drive towards a reward (Cagniard et al., 2006a), without the conditioned-cue did not result in motivational engagement. This is consistent with results obtained in inducible DA transporter (DAT) knockdown mice in whom DA levels are elevated; these animals performed double the work to receive the preferred reinforcer (chocolate flavored pellets) but did not differ on their behavior when there were no salient choices (Cagniard et al., 2006b).
Failure to induce craving with MP may reflect the slow increases in DA induced by oral MP (Volkow and Swanson, 2003), which differ from the fast DA increases induced by conditioned cues (Phillips et al., 2003; Stuber et al., 2005). Indeed, intravenous MP, which induces fast DA increases (Volkow and Swanson, 2003), significantly increases cocaine craving in cocaine abusers even when administered in a laboratory setting devoid of cocaine cues (Volkow et al., 1997). The craving in this case may occur because the fast and large DA increases induced by intravenous MP are equivalent to the DA increases induced by intravenous cocaine (Volkow et al., 1999b), producing an internal state that is a powerful and recognizable cocaine-related cue.
The dynamic characteristics of the DA increases are known to modulate the reinforcing effects of stimulant drugs, fast DA increases are associated with reward whereas slow increases are not (Volkow and Swanson, 2003). This is consistent with the involvement of “phasic” DA cell firing (induces a large transient DA increase), rather than “tonic” DA cell firing (induces stable DA levels), in encoding for reward and reward prediction (Grace et al., 2007). It is also possible that fast DA changes (increases followed by decreases) are involved in craving responses. Indeed studies in rodents using microdialysis have shown that falling striatal DA concentrations following cocaine-induced DA increases trigger operant responses for the drug (Wise et al., 1995) and studies using voltammetry have shown that lever pressing for the drug is maximal following a decline in cocaine-induced DA transient frequency (Stuber et al., 2005). In this respect cue-induced activation of prefrontal or amygdalar glutamatergic pathways, which modulate phasic DA cell firing (Gariano et al., 1988; Murase et al., 1993, and Aston-Jones, 2001), may be the mechanism through which cues triggers craving.
In the current study we did not see a correlation between striatal DA increases and cocaine craving when MP was given concomitant to the cocaine-cues, which differs from previous findings reporting an association between cue-induced DA increases in dorsal striatum and drug craving in cocaine addicted subjects (Volkow et al., 2006, Wong et al., 2006). This discrepancy is likely to reflect the fact that in the prior studies the increases in DA were driven by the cues whereas in the current study DA increases are likely to be a function not just of the cues but also of the pharmacological effects of MP and/or their interaction. Moreover as previously described the dynamics of the DA increases are likely to differ, fast increases with exposure to cues and slow increases with exposure to oral MP.
Effects of MP on DA in striatum
Here we show that a therapeutic dose of oral MP (20 mg) significantly increased extracellular DA in striatum. However, the magnitude of the DA increases did not differ when MP was given with the neutral-cue than with the cocaine-cue videos. This differs from our previous findings in non drug-abusing controls in whom we showed that MP-induced striatal DA increases were larger when MP was given with a salient stimulus (visual food stimuli when food deprived, money) than when given with a neutral stimuli (Volkow et al., 2002b; Volkow et al., 2004). We had interpreted these results to reflect the context dependent effects of MP (DA increases result from DAT blockade and spontaneous DA release); so that greater DA increases occurred when exposed to a salient stimulus that increases DA cell firing.
This was particularly surprising since we predicted that the effects of the cues would be greater in cocaine abusers since cocaine-cues are highly salient to cocaine abusers and by themselves can increase DA in dorsal striatum (Volkow et al., 2006; Wong et al., 2006). The reason why we are not observing the amplification of the DA increases from MP with the salient stimuli (cocaine-cues) in cocaine abusers is unclear. A likely possibility is that while MP may amplify the effects of relatively weak reinforcing stimuli it may not do so for stronger ones. However, because chronic cocaine has been associated with supersensitivity of DA D2 autoreceptors (Davidson et al., 2000; Jones et al., 1996; King et al., 2002), it is also possible that the slow and small DA increases induced by MP could be sufficient to inhibit DA release attenuating the “phasic” DA cell firing associated with the cues. Alternatively since prior imaging studies have shown decreased activity of DA neurons in cocaine abusers (Volkow et al., 1997; Martinez et al., 2007) failure to observe an amplification of the DA signal may reflect a ceiling effect.
In this study oral MP decreased binding in ventral striatum in the cocaine abusers when coupled with the neutral-cue video but not when coupled with the cocaine-cue video. This could be interpreted to indicate that the cocaine-cue video may have inhibited MP-induced DA increases in ventral striatum. Because DA cell firing decreases when an expected reward does not occur (Schultz et al., 1997) the finding could reflect the failed expectations of the cocaine abusers to receive cocaine when exposed to cocaine-cues, which they have previously associated with experiencing the effects of the drug.
Implications for the treatment of cocaine addiction
The use of MP has been proposed as a potential treatment for cocaine addiction. However while the initial studies showed positive responses these appeared to be driven by the inclusion of undiagnosed cases of ADHD since in subsequent trials that excluded co-morbid subjects, MP was not therapeutically beneficial (Gawin et al., 1985). Subsequent studies in cocaine abusers with co-morbid ADHD showed mixed results with one study documenting that MP decreased cocaine choice (Collins et al., 2006) and another failing to show benefit on cocaine use or craving (Schubiner et al., 2002). In our study the measures of craving obtained when the cocaine-cues were given with MP were similar to those we previously reported in cocaine abusers using the same questionnaire and the same cocaine-cue video but without MP (Volkow et al., 2006). This indicates that at this dose oral MP does not attenuate craving in non ADHD co-morbid cocaine abusers.
On the other hand because MP blocks DAT amplifying weak DA responses one may have predicted that MP would have amplified the effects of the cue in inducing craving. The fact that it did not is clinically relevant since there is concern that treating ADHD subjects who have drug abuse co-morbidity could promote further drug use. Failure to see an enhancement of craving by MP suggests that when properly prescribed this medication by itself will not increase craving in drug addicted subjects.
Study Limitations
The poor temporal resolution of the PET [11C]raclopride method allowed us to detect DA changes over a 20-30 minute period, limiting our ability to detect short-lasting DA increases as reported for cocaine-cues with voltammetry (Phillips et al., 2003). The [11C]raclopride method is best suited to detect DA release in regions of high D2 receptor density such as striatum, but not low receptor density such as the amygdala, where animals studies have shown cue-elicited DA increases (Weiss et al., 2000). Moreover, the limited spatial resolution of the PET methodology constrained us to measure ventral striatum rather than the NAc and within this nucleus to distinguish between the core and the shell, which serve different roles in drug responses and in conditioning. Also while the changes in [11C]raclopride binding are related to extracellular DA, the precise relationship with extracellular DA is not understood (Gjedde et al., 2005).
In this study we can not determine the extent to which the DA increases observed when the cocaine-cue video was preceded by MP reflect the effects of the cocaine-cues, the effects of MP or their interaction. Similarly we cannot determine the extent to which the increases in cardiovascular measures in the MP cocaine-cue condition reflect the effects of the cocaine-cues or the interaction between MP and the cocaine-cues.
Finally in this study we used MP to block DA transporters but MP also blocks the norepinephrine transporters. Thus MP’s noradrenergic effects may have contribute to the current findings.
Conclusion
Here we show that slow increases in DA as achieved with oral MP were not associated with craving when there was no concomitant presentation of conditioned cues. This suggests that DA increases associated with conditioned cues reflect secondary responses to activation of pathway(s) that modulate DA cell firing and release (i.e fronto-striatal and fronto-mesencephalic glutamatergic pathways). Moreover fast DA changes as triggered by phasic DA cell firing may underlie craving rather than the slow DA changes produced by oral MP. Inasmuch as MP is being proposed as a potential treatment for cocaine abusers with co-morbid ADHD the failure to see an enhancement of craving by MP suggests that when properly prescribed this medication by itself will not increase craving in drug addicted subjects.
Acknowledgements
We thank David Alexoff, Pauline Carter, Barbara Hubbard, Lisa Muench, Kith Pradhan, Colleen Shea, David Schlyer, Michael Schueller, Paul Vaska, Don Warner, Youwen Xu, Karen Apelskog and Linda Thomas for their contributions. Research supported by NIH’s Intramural Research Program (NIAAA), NIDA (DA06278-15), GCRC (MO1RR10710) and by DOE (DE-AC01-76CH00016) and for Dr Childress (VA VISN 4 MIRECC and the DANA Foundation).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J. Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience. 1992;48:621–629. doi: 10.1016/0306-4522(92)90406-r. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006a;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006b;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–167. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Davidson C, Ellinwood EH, Lee TH. Altered sensitivity of dopamine autoreceptors in rat accumbens 1 and 7 days after intermittent or continuous cocaine withdrawal. Brain Res. Bull. 2000;51:89–93. doi: 10.1016/s0361-9230(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castaneda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav. Neurosci. 2000;114:1156–1166. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical Parametric Maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Gawin F, Riordan C, Kleber H. Methylphenidate treatment of cocaine abusers without attention deficit disorder: a negative report. Am. J. Drug Alcohol Abuse. 1985;11:193–197. doi: 10.3109/00952998509016861. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21(16):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int. Rev. Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl. Acad. Sci. U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Lee TH, Wightman RM, Ellinwood EH. Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: In vitro voltammetric assessment. Psychopharmacology. 1996;126:331–338. doi: 10.1007/BF02247384. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- King GR, Pinto G, Konen J, Hillburn C, Tran S, Love W, Cayse R, Castro G. The effects of continuous cocaine duration on the induction of behavioral tolerance and dopamine autoreceptor function. Eur. J. Pharmacol. 2002;446:111–118. doi: 10.1016/s0014-2999(02)01822-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Conditioned changes in nucleus accumbens dopamine signal established by intravenous cocaine in rats. Neurosci. Lett. 1996;211:73–76. doi: 10.1016/0304-3940(96)12731-2. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb, Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Logan J, Volkow ND, Fowler JS, Wang GJ, Dewey SL, MacGregor R, Schlyer D, Gatley SJ, Pappas N, King P. Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J. Cereb. Blood Flow Metab. 1994;14:995–1010. doi: 10.1038/jcbfm.1994.132. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am. J. Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am. J. Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci. Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp. Clin. Psychopharm. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Stuber GG, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J. Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am. J. Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Dewey SL, Schlyer D, MacGregor R, Logan J, Alexoff D, Shea C, Hitzemann R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J. Nucl. Med. 1993;34:609–613. [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, Wolf A. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am. J. Psychiatry. 1999a;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang G-J, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A, Pappas N, King P. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999b;31:59–66. doi: 10.1002/(SICI)1098-2396(199901)31:1<59::AID-SYN8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Franceschi D, Maynard L, Ding YS, Gatley SJ, Gifford A, Zhu W, Swanson JM. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002a;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002b;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am. J. Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Telang F, Maynard L, Logan J, Gatley SJ, Pappas N, Wong C, Vaska P, Zhu W, Swanson JM. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am. J. Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J. Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc. Natl. Acad. Sci. U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Ann. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl.) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]