Abstract
Background
Circulating proteins may serve as biomarkers for the early diagnosis and treatment of shock. We have recently demonstrated that treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, significantly improves survival in a rodent model of lipopolysaccharide (LPS)-induced septic shock. Preliminary proteomic data showed that LPS-induced shock altered a number of proteins in circulation, including histone H3 (H3) and citrullinated histone H3 (Cit H3). The present study was designed to confirm these findings and to test whether the pro-survival phenotype could be detected by an early alteration in serum biomarkers.
Methods
Three experiments were performed. Exp 1: Western blotting was performed on serum samples from male C57B1/6J mice (n=9, 3/group) that belonged to following groups (a) LPS (20 mg/kg)-induced septic shock, (b) suberoylanilide hydroxanic acid (SAHA)-treated septic shock, and (c) sham (no LPS, no SAHA). Exp II: HL-60 granulocytes were cultured and treated with LPS (100 ng/ml) in the absence or presence of SAHA (10 μM). Sham (no LPS, no SAHA) granulocytes served as the control. The medium and cells were harvested at 3 h, and proteins were measured with Western blots. Exp III: LPS large dose (LD, 35 mg/kg) or small dose (SD, 10 mg/kg) was injected intraperitoneally into the C57B1/6J mice (n=10/group). Blood was collected at 3 h, and serum proteins were determined by Western blots or enzyme-linked immunosorbent assay (ELISA). All of the Western blots were performed with antibodies against H3, Cit H3, and acetylated H3 (Ac H3). ELISA was performed with antibody against tumor necrosis factor (TNF-α). Survival rates were recorded over 7 days.
Results
In Experiment I, intraperitoneal (i.p.) injection of LPS (20 mg/kg) significantly increased serum levels of H3, which was prevented by SAHA treatment. In Experiment II, LPS (100 ng/ml) induced expression and secretion of Cit H3 and H3 proteins in neutrophilic HL-60 cells, which was decreased by SAHA treatment. In Experiment III, administration of LPS (LD) caused a rise in serum H3 and Cit H3 but not Ac H3 at 3 h, and all of these animals died within 23 h (100% mortality). Decreasing the dose of LPS (SD) significantly reduced the mortality rate (10% mortality) as well as the circulating levels of Cit H3 (non detectable) and H3. An increase in serum TNF-α was found in both LPS (LD) and (SD) groups, but in a non dose-dependent fashion.
Conclusions
Our results reveal for the first time that Cit H3 is released into circulation during the early stages of LPS-induced shock. Moreover, serum levels of Cit H3 are significantly associated with severity of LPS-induced shock. Therefore, Cit H3 could serve as a potential protein biomarker for early diagnosis of septic shock, and for predicting its lethality.
Keywords: serum histone citrullinated H3, LPS, septic shock, biomarker
INTRODUCTION
Sepsis is a systemic inflammatory disorder and its progression to septic shock is a serious clinical problem with very high mortality. Early warning signs are frequently nonspecific and inconspicuous. Preceding symptoms can easily be mistaken as due to non-infected etiologies. The effectiveness of blood culture is usually considered a “gold standard” for diagnosing septicemia. However, a positive culture may take more than 48 hours of incubation and false negative results are very common due to the low density of blood bacteria at the early stage of infection. Alternative serum markers with higher sensitivity, specificity, and predictive value could help in the early detection and monitoring of sepsis progression, as well as the response to treatment. Therefore, identification of prognostic serum markers that predict risk of developing septic shock would help in the management.
Much research in the past decade has been focused on the exploration of one or a panel of inflammatory mediators for the diagnosis of systemic infection. 1 All these studies used the candidate biomarkers approach for selecting specific mediators in the inflammatory cascade or cell surface antigens that are either up- or down-regulated in response to pathogen invasion. This approach, however, has the disadvantage of limiting the search of potential biomarkers to only known proteins. Mass spectrometry-based proteomic profiling technologies, such as isobaric tag for relative and absolute quantitation (iTRAQ), have been used to identify host-response proteins for diagnosis of many diseases and pathologic conditions. Using iTRAQ technology, we have recently found that LPS-induced sepsis causes the rise of a panel of proteins in serum, including some histone proteins (un-published data).
Posttranslational modification of a histone protein, such as deimination (citrullination) and acetylation, can change its structure and function. Deimination/citrullination of histones by peptidyl arginine deiminase 4 (PAD4) can be induced by lipopolysaccharide (LPS). PAD4 is an enzyme previously known to convert protein arginine (Arg) to citrulline (Cit), a nonconventional amino acid in proteins. 2 This enzyme was first identified in human HL-60 leukemia cells upon differentiation along the granulocyte lineage 3 and is highly expressed in peripheral blood neutrophils. 4 It is known that the LPS-induced histone deimination/citrullination is an early response to inflammatory stimuli in neutrophils. 5 Moreover, citrullinated histone H3 (Cit H3) has been identified as a component of neutrophil extracellular traps (NETs) that are produced by degranulating neutrophils. This Cit H3 is released in the extracellular space as part of the neutrophiil response to infection. 6
The accumulated data point towards a link between citrullinated proteins and pathogenesis of diseases such as rheumatoid arthritis, multiple sclerosis and psoriasis. Lundberg et al showed that appearance and amounts of citrullinated proteins in arthritic joints of experimental animals are correlated the severity of inflammation. 7 However, these proteins reflect their relationship with autoimmune diseases. It remains unknown whether a citrullinated protein in neutrophils could serve as a serum biomarker to predict the severity of sepsis.
Recently, Xu et al reported their findings in Nature Medicine and stated that “extracellular histones, mainly H3 and H4, seem to be both biomarkers of disease progression and therapeutic targets in sepsis ...”. 8 In the present study, we measured histone H3 as a positive control, and additionally evaluated circulating levels of Cit H3 after lipopolysaccharide (LPS) injection in rodent model. We have already shown that treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, in rodent models of LPS-induced septic shock improves survival. 9, 10 First, we screened serum from these animals for circulating levels of histone proteins. Second, we assessed a possible source of the histone proteins in serum using a model of HL-60 granulocytes in vitro. Finally, we determined whether the serum Cit H3 protein associates with severity of sepsis (and outcome) using lethal and sub-lethal doses of LPS in vivo.
MATERIALS AND METHODS
Antibodies and supplies
LPS (from S. typhosa, Cat# L6386, Lot# 038k4005) and dimethyl sulfoxide (DMSO) were purchased from the Sigma Aldrich, Co (St. Louis, MO). Suberoylanilide hydroxamic acid was purchased from Enzo Life Sciences International, Inc (Plymouth Meeting, PA). Primary antibody against acetyl-histone H3 at lysine 9 (Ac H3) was purchased from Upstate (Lake Placid, NY). Anti-citrulinated histone H3 (citrulline 2 + 8 + 17) antibody was purchased from abcam (Cambridge, MA). Anti-actin antibody was purchased from Sigma-Aldrich, Co. Anti-mouse and anti-rabbit IgG secondary antibodies were purchased from Amersham Biosciences (Piscataway, NJ). Protease Inhibitor Cocktail II was purchased from Calbiochem (San Diego, CA). RPMI 1640 medium, fetal bovine serum (FBS), and phosphate buffered saline (PBS) were from Gibco-BRL (Grand Island, NY). L-glutamine, and fetal calf serum (FCS) were from Invitrogen (Carlsbad, CA). All-trans retinoic acid (ATRA) was purchased from Acros Organics (Geel, Belgium). All other chemicals in this study were of analytical grade and obtained from the Sigma-Aldrich if not mentioned otherwise.
Animals
All the research was conducted in compliance with the Animal Welfare Act, and was approved by the Institutional Animal Care and Use Committee. Male C57B1/6J mice (6-8 weeks) weighing 25-30 g were purchased from Jackson Labs (Bar Harbor, ME). All animals were housed in plastic cages and had access to chow and water throughout the experiment. The animals were kept at room temperature (24 ± 2 °C) and exposed to alternative cycles of 12 h light and darkness. During the experiments the animals were monitored over 7 days, and survival rate was compared between the experimental and control groups.
Experimental protocols
In our previous study 9, mice were randomly divided into two groups: LPS control, and SAHA + LPS. Normal saline (10 μl/g body weight) or LPS (20 mg/kg) dissolved in normal saline was injected intraperitoneally. The mice were given DMSO (1 μl/g body weight) or SAHA (50 mg/kg) dissolved in DMSO intraperitoneally 2 hours before, and then soon after injection of LPS. Sham mice (no LPS, no SAHA) were used as the control. Serum separated from these blood samples was saved at -80°C and used for the current experiment. In the present study, large dose (35 mg/kg) or small dose (10 mg/kg) of LPS was administrated intraperitoneally. Survival rate was then recorded for over the next 7 days. Blood was collected from the orbital sinus 11 at 3 h after LPS injection.
Hemorrhagic shock protocol and blood sample collection
Male C57B1/6J mice (6-8 weeks) were anesthetized and instrumented with 3% isoflurane, and 1% bupivacaine was injected at the operative site to achieve local anesthesia. Isoflurane (0.7%) was used to maintain anesthesia during the experiment. Animals were allowed to breathe spontaneously under a nose cone scavenging system using a veterinary anesthesia delivery system (Kent Multi-Station Anesthesia Delivery System, Kent Scientific Corporation, Torrington, CT). With aseptic technique, the femoral artery and vein were cannulated with polyethylene catheters (PE10, Clay Adams, Sparks, MD). Blood pressure and heart rate were continuously monitored by a Ponemah Physiology Platform (Gould Instrument Systems, Valley View, OH). Each animal's estimated 30% blood loss volume was calculated using the formula: estimated blood volume (ml) = weight (g) × 0.07 (ml/g) × 30%. Three hours later, 0.5 ml of blood was collected from heart puncture, and serum was prepared and saved for biomarker analysis.
Cell culture and treatment
HL-60 cells obtained from American Type Culture Collection (ATCC) were maintained in Iscove's modified DMEM medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS). These cells were grown at 37 °C in a humidified incubator in 5% CO2 and 95% air, and were differentiated into granulocytes by culturing the cells in medium containing 1 μM ATRA for 3 days. The ATRA-differentiated HL-60 granulocytes were treated with 4 μM calcium ionophore in medium containing 1.5 mM calcium chloride, and then incubated with LPS (100 ng/ml) in the presence or absence of SAHA (10 μM) over 3 h. Following incubation, medium was collected, and cell lysates were prepared. The medium and the lysates were tested for H3, Ac H3, Cit H3 and actin (internal control) by Western blots.
Western blot analysis
Proteins (about 100 μg per lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on 15% polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked in 0.05% PBS-Tween (PBST) containing 5% milk (Bio-Rad Laboratories, Hercules, CA) and then incubated with the primary antibody at 4 °C overnight. The primary antibody was detected by incubation with horseradish peroxidase-coupled second antibody (1:3,000 in PBST with 5% milk) at room temperature for 2 h. The chemiluminescence detection was performed by using WesternLighting Chemiluminescence Reagent Plus (PerkinElmer LAS, Inc., Boston, MA). Films were developed using a standard photographic procedure and quantitative analysis of detected bands was carried out by densitometer scanning using VersaDoc Imaging System (BioRad Laboratories, Hercules, CA).
Evaluation of LPS-induced TNF-α in blood
Blood was collected from sham, LPS (SD), and LPS (LD) groups at 3 h after LPS injection (n=5/group). Quantitative determination of TNF-α in the blood of mice was made using Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) Kit (R&D Systems, Minneapolis, MN) according to manufacturer's instruction. The concentration of the cytokine was measured by optical densitometry at 450 nm in a SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA). All of the analyses were performed in triplicates.
Statistical analysis
Statistical differences were determined by Student t tests and ANOVA for two group and multiple group comparisons respectively (SPSS statistical software package, Chicago, Illinois). Kaplan-Meier survival curves were analyzed by using the MedCalc Statistical Software (Mariakerke, Belgium) for the comparison of LPS large dose (LD) and LPS small dose (SD) groups. Fisher's exact test was used to analyze association of serum protein biomarker with lethality of LPS-induced septic shock. Differences were considered to be statistically significant when p values were <0.05.
RESULTS
Serum levels of histone H3 in the early stage of septic shock in mice treated with or without SAHA
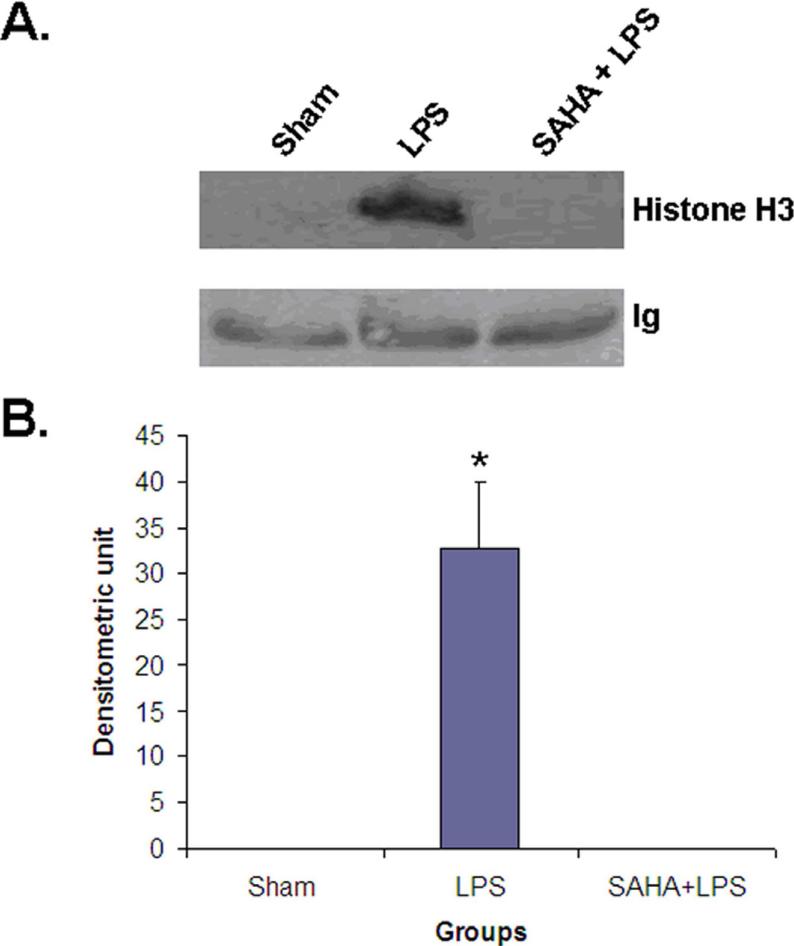
In screening biomarkers of septic shock and treatment, we used isobaric tag labeling for relative and absolute quantitation (iTRAQ) and found several potential serum biomarkers in LPS-induced septic shock and treatment. One of them is histone H3, a chromatin protein in nucleus. This protein was elevated in LPS-induced septic shock. Treatment with SAHA, a potent histone deacetylase inhibitor, decreased histone H3 in serum dramatically. To assure that the data were reliable, independent serum samples of sham (no LPS and no treatment), septic shock (3 h after LPS injection) and SAHA treatment (5 h after the first SAHA treatment, and 3 h after LPS injection) were collected from nine animals (n=3 /group) and analyzed by Western blot with anti-histone H3 antibody. In serum from sham and SAHA treatment groups, no histone H3 was detected. However, a histone H3 band was clearly observed from septic shock group (Fig 1). Our results demonstrated that septic shock significantly increased serum levels of histone H3, and SAHA treatment prevented this elevation. This raised several questions, such as the source of histone H3 in the circulation and whether serum levels could be used to assess the severity of sepsis.
Figure 1. Serum levels of histone H3 in the early stage of septic shock of mice treated with or without SAHA.
(A) Representative Western blotting of mouse serum with anti-histone H3 antibody (n=3) from animal groups of sham, LPS and SAHA +LPS. Blood was collected at 3 h after LPS injection. Sham (no LPS, and no SAHA treatment) animals serve as a control. (B) Specific histone H3 bands were quantified by densitometry and expressed as mean values ± SD (n = 3). The symbol * indicates significant difference from LPS group (p < 0.001).
Deiminated/citrullinated histone H3 and histone H3 in LPS-stimulated HL-60 granulocytes
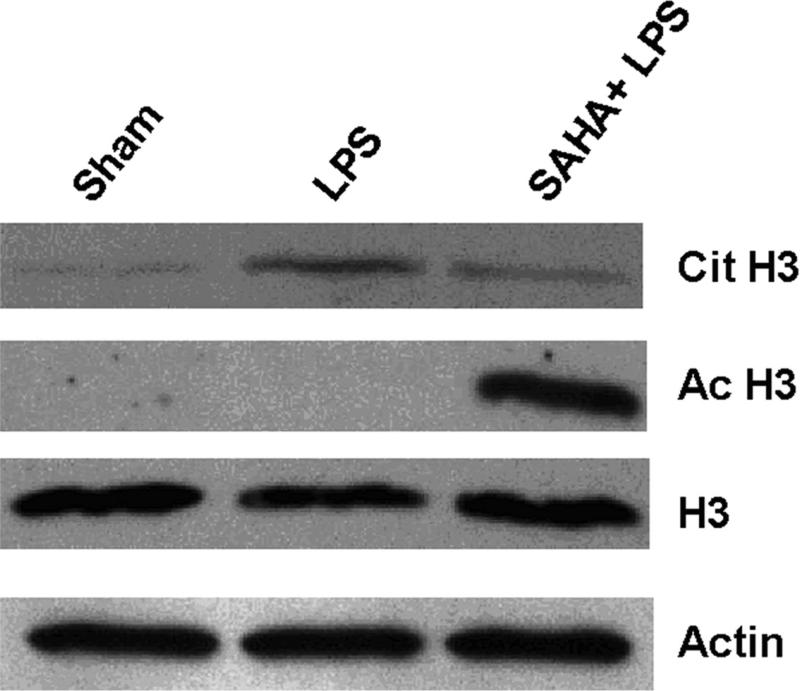
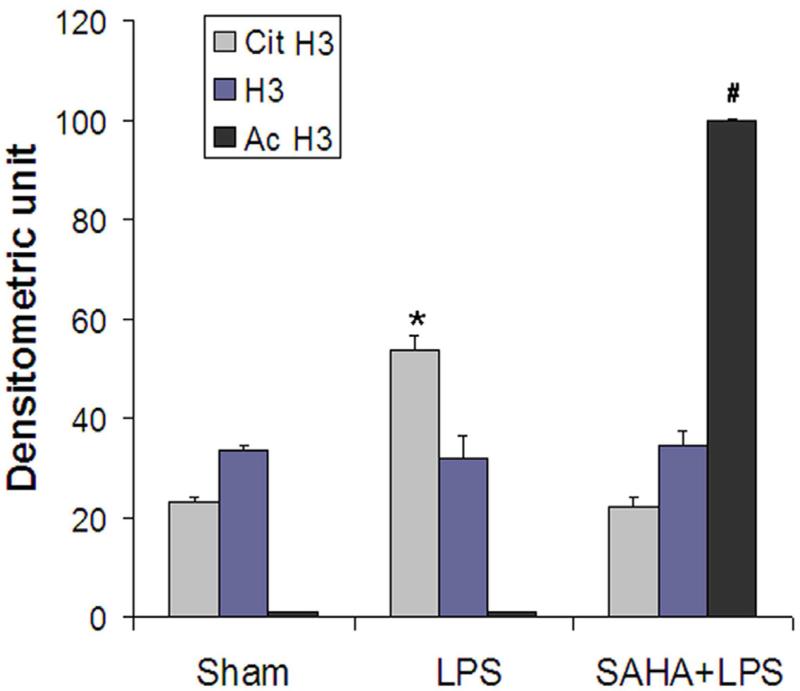
Previous studies have demonstrated that histone proteins can be deiminated/citrullinated by PAD4 in neutrophils in response to bacterial infection, and the histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. 12 Neutrophils might be a source of serum histone H3 during sepsis. To test whether histone H3 deimination/citrullination is induced as part of the neutrophil response to bacterial endotoxin, we exposed ATRA-treated HL-60 cells to LPS over 3 h and detected histone H3 deimination in cell lysates by immunoblotting. Medium from HL-60 cells was also analyzed for possibly secreted histone H3, Cit H3 and Ac H3. To ensure there was no false positive data that may be produced from the medium with dead cells, we carefully treated the cells and also set up a sham group as a control. As shown in Fig 2, an increase in Cit H3 was detected in the LPS group compared to the sham control. SAHA treatment significantly reduced the expression of Cit H3 along with an increase in acetylated H3. Moreover, more Cit H3 and H3 were observed in the cultured cell medium of LPS group (Fig 2C). By contrast, there were no detectable Cit H3 and H3 proteins in the medium from the SAHA + LPS group. Our results suggest that activation of granulocytes by LPS is likely one of the sources of H3 and Cit H3, and LPS-induced H3 citrullination could play an important role in the release of histone H3 into extracellular space. SAHA decreased H3 citrullination and reversed these alterations.
Figure 2. SAHA decreases expression and secretion of citrullinated histone H3 (Cit H3) protein in LPS-stimulated HL-60 granulocytes.
(A) Representative Western blotting of HL-60 cell lysates with antibodies against histone H3 (H3), acetyl histone H3 (Ac H3) and citrullinated histone H3 (Cit H3) from groups of sham, LPS and SAHA+LPS (n=3). Actin serves as an internal control. (B) Protein bands of Cit H3, H3 and Ac H3 were scanned, quantified by densitometry and expressed as mean values ± SD (n = 3). The symbol * indicates that a value significantly differs from sham and SAHA+LPS groups (p<0.05). The symbol # indicates that a value significantly differs from sham and LPS groups (p<0.001). (C) Medium of cultured HL-60 cells were separated on SDS-PAGE, and analyzed by Western blotting with antibodies against H3 and Cit H3.
Effect of different LPS doses on serum levels of Cit H3 and H3
It has been reported that the effects of LPS on lethality and protein expression are dose-dependent. 13, 14 To determine whether serum levels of H3, Cit H3 and acetylated H3 were dependent on the LPS dose, we injected the mice with a small dose (10 mg/ml) or a large dose (35 mg/ml). We then measured the serum levels of all three histone H3 and correlated these to mortality. Mice were randomly divided into three groups (n=10 / group): (1) sham group (no LPS), (2) LPS small dose (SD) group, and (3) LPS large dose (LD) group. Blood was collected at 3 h after LPS injection and serum was prepared for immunoblotting. All animals were observed for the survival rate.
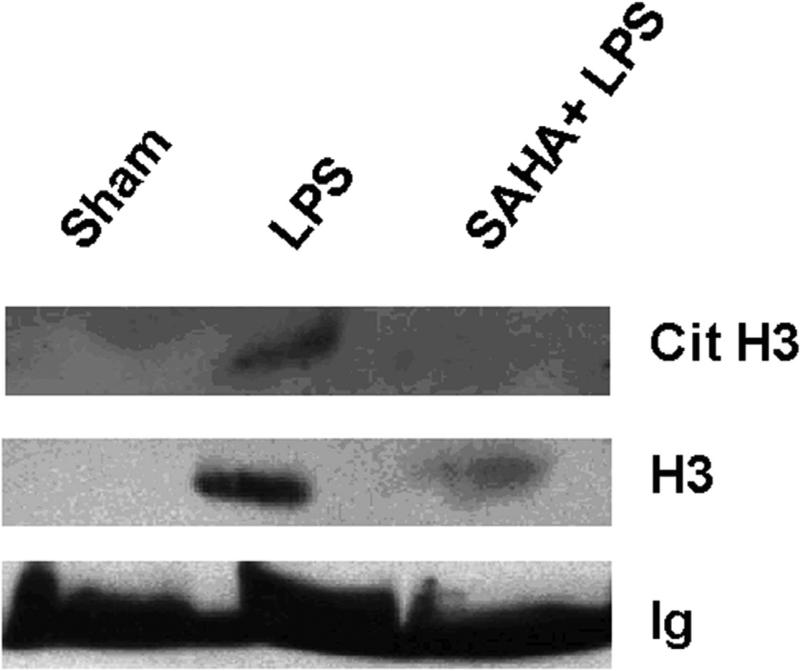
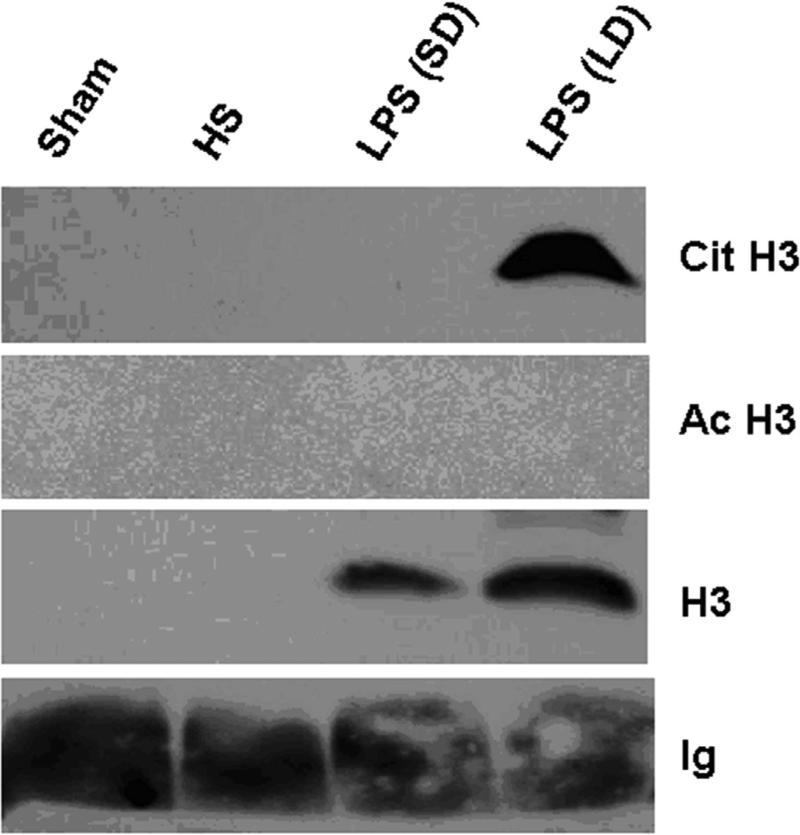
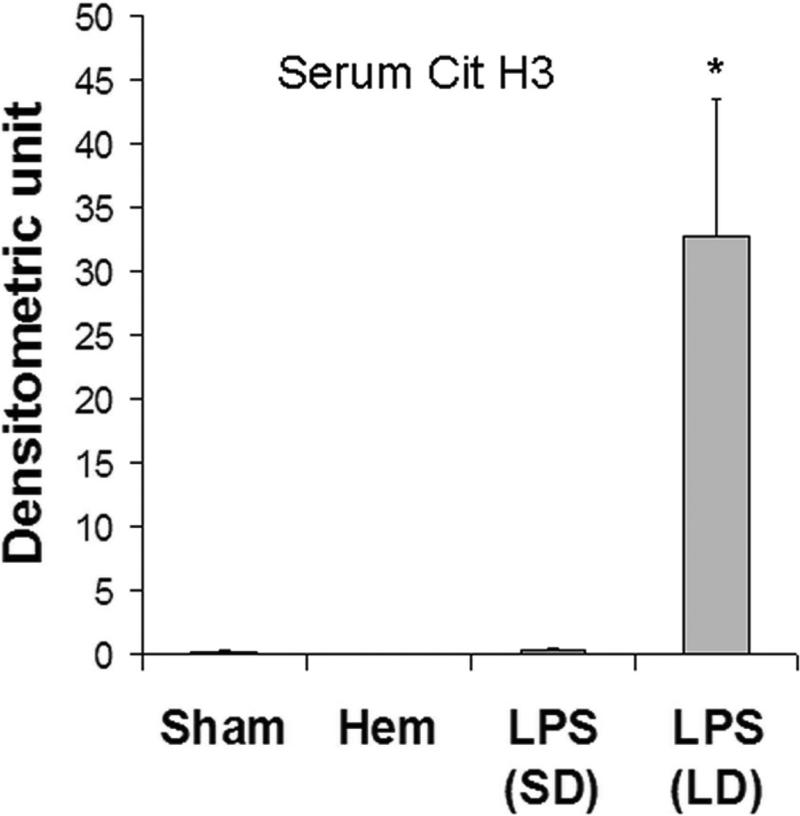
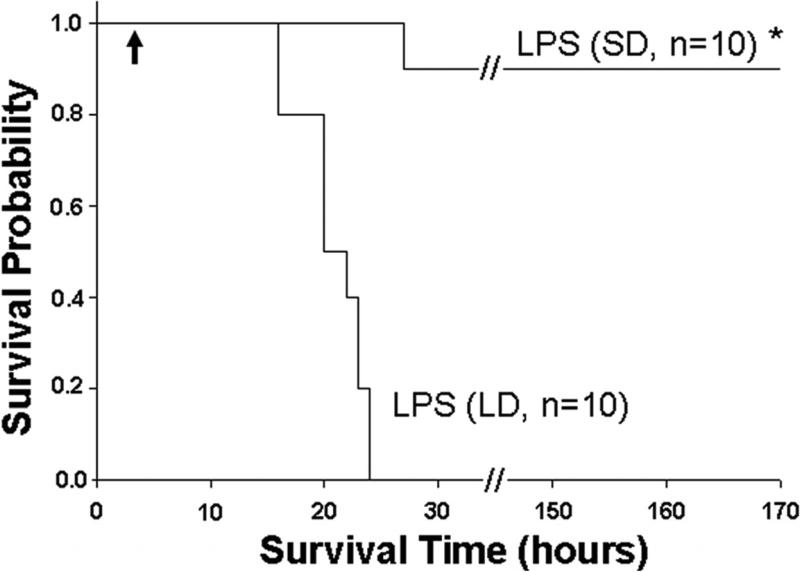
As shown in Fig 3, in our LPS-induced septic shock condition, all mice from LPS (LD) group died in less than 23 h. However, LPS (SD) animals displayed long term survival. Only one of ten mice died 27 h after LPS injection, while the rest survived for the entire 7 days observation period. The survival rate in the LPS (SD) group was significantly better compared to the LPS (LD) animals (90% vs. 0% respectively). To determine whether Cit H3 might be used as a biomarker to assess severity of LPS-induced septic shock, serum samples from each group were analyzed (n=5/group). Serums from mice that underwent hemorrhagic shock (HS) served as a control for the biomarker specificity. The Western blot data showed that serum levels of Cit H3 and H3 were high in the LD group mice, whereas these proteins were not detectable in the serum obtained from the sham and HS groups. An increase in serum H3 was found in LPS (SD) animals but not in sham (p < 0.038) and HS groups (p < 0.016), although the induction was lower than that from LPS (LD) group (p < 0.009). No Ac H3 was detected in all animals (Fig 3). These results indicate that lethal and sub-lethal doses of LPS induce very different serum profiles, with a dose dependent increase in the circulating Cit H3 and H3 levels. Also, these data imply that Cit H3 is a specific biomarker for severe LPS-induced septic shock among these groups.
Figure 3. Serum levels of citrullinated histone H3 (Cit H3) and histone H3 (H3) in mice underwent different insults.
(A). Representative Western blotting of mouse serum with antibodies against H3, Ac H3 and Cit H3 from animal groups of sham, hemorrhagic shock (Hem), LPS small dose (SD) and LPS large dose (LD) (n=5/group). Immunoglobulin (Ig) serves as an internal control. (B). Specific Cit H3 bands were scanned, quantified by densitometry and expressed as mean values ± SD (n = 5/group). The symbol * indicates that a value significantly differs from sham and LPS (SD) groups (p<0.001). (C). Specific H3 bands were scanned, quantified by densitometry and expressed as mean values ± SD (n =5/group). The symbol * indicates that a value significantly differs from Sham, Hem and LPS (SD) groups (p<0.009). The symbol # indicates that a value significantly differs from Sham (p < 0.038), and Hem groups (p<0.016). (D). Kaplan-Meier curves were used for the comparison of survival between the LPS (LD) and LPS (SD) groups. Mice were intraperitonially injected with LPS (SD) (10 mg/kg) or LPS (LD) (35 mg/kg) (n=10 per group). Survival rates were recorded over 168 hours (7 days) after LPS insult and statistic analysis was performed. The symbol “arrow” indicates blood-drawing time (3 h after LPS injection). The symbol * indicates that a value significantly differs from the LPS (LD) group (p < 0.01).
Serum levels of Cit H3 are associated with severity of LPS-induced septic shock
The average survival times were about 20 h and 146 h for the LD and SD LPS groups respectively. The earliest time of death was ~16 h and 27 h, whereas the longest survival times were 23 h and 168 h for LD and SD groups respectively. The only mouse that died in the SD group still survived longer (27 h) than the best survivors (23 h) in the LD group. Most importantly, serum Cit H3 was high in the LD group and undetectable in the SD group (Table 1). Statistical analysis with Fisher's exact test showed that the survival difference was significant between LPS (SD) and LPS (LD) groups (p=0.00006, n=10). These results indicate that an early increase in circulating Cit H3 protein is associated with high lethality in this model of LPS-induced shock.
Table 1.
Serum Cit H3 and H3 associate with severity of LPS-induced sepsis
| LPS (LD, n=10) | LPS (SD, n=10) | Fisher's Exact Test | |
|---|---|---|---|
| Serum H3 | + | +/- | |
| Serum Cit H3 | + | - | * p = 0.00006 |
| Mice died within 23 h | 10 | None | |
| Mice died at 27 h | N/A | 1 | |
| Mice survived over 168 h | N/A | 9 |
Serum levels of TNF-α protein in mice insulted by different dose of LPS in vivo
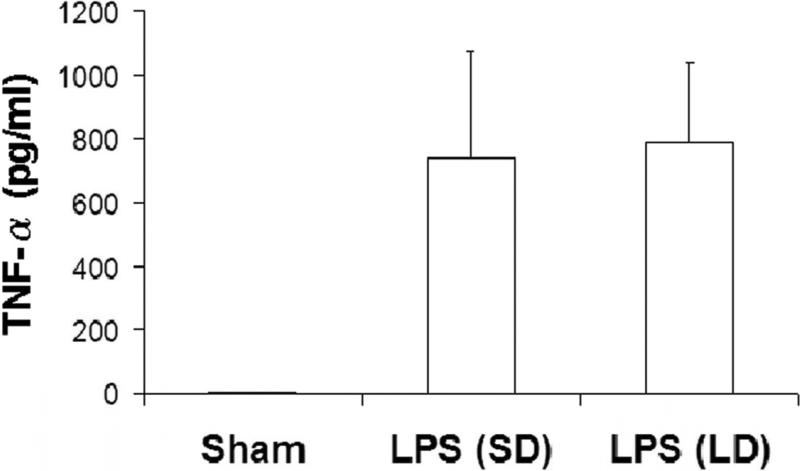
There is general agreement that the main pathogenic mediator in lethal septic shock is TNF-α. 15 To determine whether TNF-α can predict lethality of LPS-induced shock as well as Cit H3, blood was collected from sham, LPS (SD), and LPS (LD) groups at 3 h after LPS injection (n=5/group). TNF-α protein concentration was measured using an ELISA kit. Serum TNF-α protein was hardly detected in the normal mice, but LPS injection increased the circulating level of TNF-α protein. At 3 h after endotoxin insult, TNF-α increased to 741 pg/ml in LPS (SD) group and 792 pg/ml in LPS (LD) group. Statistical analysis showed that there was no significant difference in serum TNF-α levels between these two groups (Fig 4; p=0.77). These results demonstrate that TNF-α release in response to LPS occurs in a non-dose-dependent fashion. Together with the data from Fig 3, our studies indicate that serum Cit H3 does not simply mimic the levels of TNF-α, but may actually better reflect the severity of shock.
Figure 4. No significant difference of serum TNF-α between LPS (SD) and LPS (LD) groups.
Blood was collected from sham, LPS (SD), and LPS (LD) groups at 3 h after LPS injection (n=5/group) and serums were prepared from these blood samples. The level of TNF-α protein was analyzed by ELISA (described in materials and methods). All analyses were performed in triplicate. No significant difference was found between LPS (SD) and LPS (LD) groups (p=0.77).
DISCUSSION
In this study, we have demonstrated that improved survival in LPS injected animals that are treated with SAHA is associated with a reduction in serum H3 protein levels. We further showed that LPS stimulates histone H3 deimination/citrullination in HL-60 granulocytes and in an in-vivo mouse model of septic shock. Moreover, H3 deimination induced by LPS can enhance HL-60 neutrophil secretion of histone proteins (H3 and Cit H3) into the extracellular space, suggesting that Cit H3 could at least in part initiate the formation of NETs and lead to an increase in serum histome proteins. More importantly, we found for the first time that serum levels of Cit H3 associate with the severity of LPS-induced sepsis, which indicates that early measurement of circulating Cit H3 protein can be helpful in predicting survival in lethal septic shock.
Our studies also suggest some possible answers to other questions such as where the serum histones come from, how they are released into the blood stream, why an early rise in the circulating Cit H3 associates with survival in this model of shock, and whether Cit H3 is more useful than conventional biomarker/mediator(s). Some of these issues are discussed in the following paragraphs.
Our findings show that alteration in serum Cit H3 is associated with H3 in vivo when animals are injected with large dose of LPS (Fig 3A). Also, it seems that these two proteins are secreted together into the medium from the HL-60 cells in vitro (Fig 2C). It is not clear where the histone proteins come from during sepsis. 16, 17 However, neutrophils are an attractive possibility, as they are the most abundant white blood cells and they are actively involved in post-septic immune response. 16 NETs formation is a conceivable action for neutrophils to release the histone proteins into the blood circulation. Conventionally, two strategies are considered for neutrophils to contain and clear bacterial infection: phagocytosis and degranulation. Recently, a third strategy has been proposed, i.e., formation of NETs. 18 NETs arise from the release of neutrophil nuclear contents into the extracellular space. They are composed of decondensed chromatin that is decorated with granular as well as cytoplasmic proteins. Neutrophils use these three strategies to combat and clear microbes, but they operate over different timescales. Phogocytosis takes about 10 min, degranulation releases antimicrobial molecules in 30 min, and NETs usually need 2-3 h to secrete the nuclear proteins. 19 In our studies, we did not find H3 and Cit H3 in the extracellular space (serum and the cultured cell medium) until about 3 h (data not shown), suggesting that NETs may be the major origin of the histone proteins.
Many researchers have found that histone deimination/citrullination is an important molecular mechanism for NET formation. 19 Experiments performed on the netrophilic HL-60 cell line also suggest that NET formation depends on histone deimination/citrullination, which is catalyzed by PAD4. 12 Neutrophils express high levels of PAD4 enzyme. 4 In response to various stimuli, including pathogen infection and inflammatory response, PAD4 in neutrophils rapidly hydrolyzes quanidino group of histones to ureido group and ammonia so that histones become deiminated/citrullinated histones. These mediate chromatin decondensation and NET formation. Using the HL-60 granulocytes as a model system, Wang et al. have recently shown that activated PAD4 and deiminated/citrullinated histone initiate chromatin decondensation, and that global histone hypercitrullination regulates the unfolding of chromatin structures during NET formation. 12 Neeli et al. found that histone deimination in neutrophils represents a rapid and robust reaction to signals arising in a microbial infection or inflammatory stimuli. 5 This data, together with our current findings, clearly support the concept that histone deimination/citrullination plays a role in the generation of the circulating Cit H3 and H3.
To date, two models describing the release of NETs have been proposed: a DNA extrusion mechanism from intact cells, and a cell death mechanism (Fig 5). Addressing the question of how neutrophils form NETs, Fuchs et al. further monitored the individual cells via live video microscopy which confirmed NET formation. 20 In these experiments, they demonstrated that ex vivo, activated neutrophils enter a program where the nuclear and granular membranes dissolve and the nuclear contents decondense into the cytoplasm. Then the plasma membrane ruptures and chromatin decorated with granular proteins is released into the extracellular space. This mechanism may reflect NET formation occurring upon direct stimulation by pathogens. In contrast, another study described that in the presence of bacterial LPS and platelets, neutrophils can generate NETs within minutes. 21 This process has been implicated in sepsis-associated microvascular thrombosis and involves indirect neutrophil stimulation mediated by platelets in the environment of slow blood flow or coagulation. 19 In our studies, we did not find an elevation in the serum histone proteins within a few minutes after LPS injection. The explanation could be that although LPS-mediated neutrophil activation needs a short time, it may not be initiated until the later stages of sepsis. At the time when we collected blood, the inflammatory process and shock were still in an early stage without any evidence of a coagulation disorder such as disseminated intravascular coagulation (DIC). With phorbol myristate acetate (PMA), Staphylococcus aureus, or Candida albicans as stimuli, Fuchs et al. reported that the entire process of NET formation takes between 2-3 hours. 20 Based on their finding, we incubated the HL-60 cells with LPS for 3 h to ensure complete histone deimination and NET formation. In our in vivo experiments, we designed the time for blood collection at 3 h after LPS injection, which confirmed that sufficient/adequate time had passed for detection of serum histone proteins. In addition, from the survival experiments we observed that animals were still in good condition at 3 h after ip administration of LPS. They did not display signs of severe sepsis such as reduced mobility, conjunctivitis, diarrhea, and fur ruffling within 3 h. In the lethal LPS (LD) group, mice began to die at 16 h and all were dead within 23 h. Thus, three hours is fairly early in the time course of sepsis. The fact that serum Cit H3 was detectable as early as 3 h in animals that subsequently died suggests that this circulating protein can predict the severity of shock.
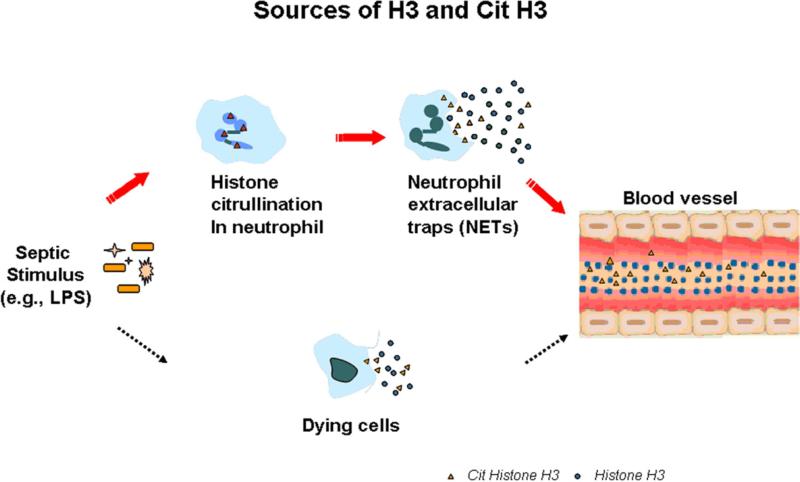
Figure 5. Possible sources of serum citrullinated histone H3 (Cit H3) and histone H3 (H3).
Serum H3 and Cit H3 could come from dying cells and neutrophil exacellular traps (NETs). We hypothesize that the circulating histone proteins likely result from formation of NETs during LPS-induced sepsis (see text). At beginning of the sepsis, LPS stimulates histone citrullination catalyzed by PAD4 in neutrophils. These neutrophils enter a program where the nuclear and granular membranes dissolve, and the nuclear contents decondense into the cytoplasm. Then the plasma membrane ruptures and chromatin is released into the extracellular space.
In the current study, we have compared Cit H3, histone H3 and TNF-α in terms of effectiveness as biomarkers. Histone H3 has been proposed as a biomarker in sepsis by Xu et al recently.8 This biomarker was detected in the circulation of baboons challenged with E. Coli, and an increase in the histone H3 levels was accompanied by the onset of renal dysfunction.8 In our experiments, we confirmed that the circulating H3 protein was induced by LPS, a component of the outer cell wall membrane of Gram-negative bacteria. But this did not correlate with the dose or lethality of the LPS. We observed that increase in circulating H3 was not only found in LPS (LD) group but also in LPS (SD) group. Similarly, TNF-α, a main pathogenic mediator in septic shock, was detected in the serum from both the LPS (LD) and LPS (SD) groups (p = 0.77). Given that Cit H3 was only noted in the LPS (LD) group, and that all of these animals subsequently died, it may be a better marker to discriminate between mild sepsis and severe sepsis. Moreover, our studies suggest that Cit H3 does not simply mirror changes in TNF-α, and actually appears to be more useful than TNF-α in monitoring the severity of LPS-induced shock. We also discovered that Cit H3 was not detected in mice that had undergone hemorrhagic shock (Fig. 3A). Based on our hypothesis that major source of Cit H3 is the neutrophil extracellular traps (NETs) after infection (Fig. 5), serum Cit H3 appears to be specific for severe sepsis.
This study has certain limitations that must be acknowledged. SAHA, a pan-histone deacetylase inhibitor, can induce histone acetylation. However, whether acetylation of histone H3 by SAHA interferes with deimination of histone H3 and its mechanism remain unknown. Also, it is not clear how many citrullination sites are involved in histone H3, since we used an antibody that can only detect citrullinated H3 at citrulline 2 + 8 + 17. Recently, Stensland et al performed liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis with alternating collision-induced dissociation (CID) and electron transfer dissociation (ETD) to specifically detect and characterize citrullinated peptides. 22 The combination of LC/MS/MS with CID and ETD might be a useful technique to help us to solve these problems in the future. Finally, we used LPS to induce shock in this model, which clearly does not replicate all the facets of a poly-microbial infection. However, this was a proof of concept study exploring the mechanistic aspects of the process. An ongoing clinical study has shown an increase in circulating Cit H3 levels in patients with ventilator associated pneumonia due to multiple organisms. We have also used a cecal ligation and puncture model to verify these findings.
In summary, we have demonstrated for the first time that citrullinated histone H3 can be released into the extracellular space in vivo (blood) and in vitro (medium of the cell culture) as early as 3 h after LPS insult. In addition, we have shown that serum levels of citrullinated histone H3 and histone H3 are associated with the severity and lethality of sepsis. Compared to serum H3 and TNF-α, citrullinated histone H3 better reflects the severity of LPS-induced shock, and could potentially predict outcome of severe sepsis.
Acknowledgement
Supported by NIH RO1 GM084127 (to HBA) and a generous research endowment by the Polsky family. Data presented at the 6th Annual Surgical Academic Congress, Huntington Beach, CA (February, 2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opinin Pediatr. 2006;18(2):125–131. doi: 10.1097/01.mop.0000193293.87022.4c. [DOI] [PubMed] [Google Scholar]
- 2.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: gene, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima K, Hagiwara T, Ishigami A, Nagata S, Asaga H, Kuramoto M, Senshu T, Yamada M. Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3). J Biol Chem. 1999;274(39):27786–27792. doi: 10.1074/jbc.274.39.27786. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277(51):49562–49568. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 5.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180(3):1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 6.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1(3):194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, Zendman AJ, Harris HE. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7(3):R458–467. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, Kheirbek T, Finkelstein RA, Velmahos GC, deMoya M, Hales CA, Alam HB. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32(5):517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein R, Chong W, Jin G, Lu J, deMoya M, Velmahos GC, Alam HB. Survival lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148(2):246–254. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoff J. Methods of blood collection in the mouse. Lab Animal. 2000;29(10):47–53. [Google Scholar]
- 12.Wang Y, Li M, Stadler S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis D, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton BE, Jackson JV. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61(4):1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque R, Umstead TM, Ahn K, Phelps D, Floros J. Effect of low doses of lipopolysaccharide prior to ozone exposure on bronchoalveolar lavage. Pneumon. 2009;22(2):143–155. [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 16.Chaput C, Zychlinsky A. Sepsis: the dark side of histone. Nat Med. 2009;15(11):1245–1246. doi: 10.1038/nm1109-1245. [DOI] [PubMed] [Google Scholar]
- 17.Warner RL, Bhagavathula N, Nerusu KC, Lateef H, Younkin E, Johnson KJ, Varani J. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp Mol Pathol. 2004;76(3):189–195. doi: 10.1016/j.yexmp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 19.Papaynnopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends in Immunol. 2009;30(11):513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs TA, Abed U, Goosman C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark SR, Ma AC, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 22.Stensland M, Holm A, Klehne A, Fleckenstein B. Targeted analysis of protein citrullination using chemical modification and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(17):2754–2762. doi: 10.1002/rcm.4185. [DOI] [PubMed] [Google Scholar]