Abstract
Purpose
To evaluate the impact of high estradiol (E2) levels on assisted reproductive technologies outcomes in high responders (≥12 oocytes retrieved) according to the controlled ovarian stimulation protocol (COS) used.
Methods
Clinical retrospective evaluation of total, clinical pregnancy and implantation rates in ART cycles performed in high responders according to the COS protocol used (long or antagonist) at Pathophysiology Unit of Human Reproduction and Sperm Bank of Pordenone from June 2000 to December 2010.
Results
In high responders total, clinical and implantation rates were significantly higher in long if compared with antagonist protocol with peak estradiol level ≤3,000 pg/ml; on the contrary there was a significantly higher implantation rate with antagonist than long protocol with peak estradiol >3,000 pg/ml. However in this subgroup of patients total and clinical pregnancy rates showed only a trend favouring antagonist possibly due to a statistical β error.
Conclusions
In high responders long protocol seems to work better than antagonist when peak E2 is lower than 3,000 pg/ml but the opposite may be true for cycles with higher E2 levels.
Keywords: Peak estradiol levels, Endometrium, Implantation rate, Pregnancy rate, GnRH agonists, GnRH antagonists
Introduction
After the introduction of in vitro fertilization by Edwards and Steptoe in 1978, much effort has been made to improve the clinical outcomes of assisted reproduction technologies (ART). Many protocols were implemented to improve the pregnancy rate in high, normal, and low ovarian responders. With the introduction of intracytoplasmic sperm injection in 1992, the chances of pregnancy were substantially improved as well as in severe male factor infertility. However, until now, the bottleneck for ART success is the low rate of embryo implantation. Even if embryos are produced in almost every cycle, at most only 20% successfully implant.
Embryo implantation is the result of a refined interplay between embryo quality and endometrial receptivity. The majority of authors agree that extremely high estradiol (E2) levels not only increase the risk of ovarian hyperstimulation, but also impair the reproductive outcome of ART [1–6]. Thus, in high responders, we need to pay attention to avoid excessive ovarian responses to controlled ovarian stimulation (COS), not only to prevent ovarian hyperstimulation syndrome (OHSS), but also to improve embryo implantation. However, notwithstanding cautious approaches to ovarian stimulation, some patients have an exaggerated response with extremely high peak E2 levels.
High oestrogen levels have been suggested to reduce ART efficacy by impairing oocyte quality [1] or endometrial receptivity [7–9]. Several studies have been published that endometrial gene expression is different during COS compared with the natural cycle [10].
Recently, it was suggested that an antagonist protocol, when compared with the long protocol, may more strictly resemble the natural cycle at the endometrial level [11] in terms of gene expression. GnRH antagonist use “per se” or combined with GnRH agonist induction of ovulation is also proposed to reduce the incidence of OHSS in high responders. However, to the best of our knowledge, there is no definitive agreement on which protocol produces the highest pregnancy rate in high responders.
The aim of present study was to retrospectively compare total, clinical pregnancy and implantation rates obtained with the long and antagonist protocols in high responders who had ≥12 oocytes retrieved and peak estradiol level lower or higher than 3,000 pg/ml.
Material and methods
ART cycles with fresh embryo transfers carried out at our centre in high responders (≥12 oocytes retrieved at pick up) from 2000 to 2010 were retrospectively analyzed. The total, clinical pregnancy and implantation rates in cycles with peak estradiol levels lower than and greater than 3,000 pg/ml according to the protocol (long or antagonist) used were calculated. Moreover, the total pregnancy rates for cycles with the long or antagonist protocol was also calculated in four subgroups of patients; those with peak E2 <2,000 pg/ml, >2,000 pg/ml, >3,000 pg/ml, and >4,000 pg/ml.
The COS was performed according to the long or antagonist protocol. In the long protocol, GnRH receptor downregulation was obtained with a single injection of the GnRH agonist depots Decapeptyl 3.75 (Ferring, Malmo, Sweden) or Enantone 3.75 (TAP Pharmaceuticals, Waukegan, IL). Antagonists (Cetrotide 0.25 mg SC, Serono; Orgalutran 0.25 NV, Organon) were introduced on the seventh day of ovarian stimulation with gonadotropins according to a fixed scheme. rFSH was almost exclusively used in both the long and antagonist cycles because of the good ovarian response of such patients. Final oocyte maturation and ovulation induction were induced with one vial of recombinant human chorionic gonadotropin (Ovitrelle 250 μg, Merk Serono Europe Ltd, London, UK) in all patients 36 h before pick up. In all patients, the luteal phase was supported with vaginal progesterone (Crinone 8 gel 90 mg u.i.d., Serono, Istanbul, Turkey; Proggefik 200 mg t.i.d., Effik Italia; Prometrium b.i.d., Rottapharm, MI, Italy) from the evening of pick up until the pregnancy test 14 days afterwards.
Prognostic factors such as female age, duration of infertility, FSH levels, number of oocytes retrieved, and number of embryos transferred in the two groups of patients (peak E2 levels ≤3,000 and >3,000 pg/ml) were compared (Tables 1, 2). The data were reported as the mean ± standard deviation (SD).
Table 1.
Prognostic factors in cycles with peak estradiol >3,000 pg/ml
| Agonist (long protocol) | Antagonist | |
|---|---|---|
| Female age (years)a | 33.8 ± 3.8 | 32.8 ± 4.4 |
| Infertility duration (years)a | 4.9 ± 3.3 | 4.7 ± 2.1 |
| Third day FSHa | 5.7 ± 1.6 | 5.1 ± 1.6 |
| Number of retrieved oocytesa | 19.8 ± 6.3 | 16.9 ± 3.8 |
| Number of embryos transferreda | 3 ± 1.1 | 2.9 ± 0.5 |
aData are reported as mean ± SD
FSH follicle stimulating hormone
Table 2.
Prognostic factors in cycles with peak estradiol ≤3,000 pg/ml
| Agonist (long protocol) | Antagonist | |
|---|---|---|
| Mean age (years)a | 33.4 ± 3.4 | 34.4 ± 3.2 |
| Infertility duration (years)a | 5.2 ± 3.6 | 5.1 ± 2.2 |
| Third day FSHa | 6.1 ± 1.7 | 6.2 ± 5.3 |
| Number of retrieved oocytesa | 16.2 ± 3.9 | 16.1 ± 3.4 |
| Number of embryos transferreda | 3.3 ± 0.9 | 2.9 ± 0.7 |
aData are reported as mean ± SD
FSH follicle stimulating hormone
The total pregnancy rate was defined as β HCG levels greater than 5 mUI/ml, the clinical pregnancy rate as an intrauterine sac with a fetal heart beat per oocyte retrieval, and the implantation rate as the ratio of the number of uterine sacs to the number of embryos transferred.
The comparison of overall clinical pregnancy and implantation rates in the subgroups was performed with χ2 test for independent samples. The difference between proportions was reported as significant or highly significant according to the P value (P < .05 or P < .01, respectively).
Results
Mean female age, duration of infertility, and third-day FSH levels were not significantly different between the agonist and antagonist protocols, with peak E2 levels ≤3,000 pg/ml or >3,000 pg/ml. The distribution of the different causes of infertility was comparable in both groups.
In cycles with peak E2 levels ≤3,000 pg/ml, overall mean pregnancy rates of 45.1% (216/478) and 30.4% (42/138) were observed with the long and antagonist protocols, respectively (P = 0.002). On the contrary, in cycles with peak E2 levels >3,000 pg/ml the overall mean pregnancy rate was 30.2% (29/96) and 44.2% (23/52) for the long and antagonist protocols, respectively (P = 0.12). A significant difference in pregnancy rates between the agonist and antagonist groups favouring the agonist group in patients with peak estradiol ≤3,000 pg/ml was observed. On the contrary, only a trend favouring the antagonist group in those with peak E2 levels >3,000 pg/ml was observed.
Clinical pregnancy rates in cycles where the peak estradiol was ≤3,000 pg/ml of 35.1% (168/478) and 19.5% (27/138) were observed in the agonist and antagonist groups, respectively. On the contrary, in cycles with peak estradiol levels >3,000 pg/ml, clinical pregnancy rates of 20.8% (20/96) and 34.6% (18/52) were observed in the agonist and antagonist groups, respectively. The difference in clinical pregnancy rate between the agonist and antagonist protocols was also highly significant, favouring the agonist group in cycles where the peak E2 level was ≤3,000 pg/ml (35.1% vs. 19.5%; P = 0.0007), but only a trend favouring the antagonist protocol was observed in cycles where the E2 levels were >3,000 pg/ml (20.8% vs. 34.6%; P = 0.1). However the differences in the implantation rates were significant in both subgroups, favouring the agonist group in cycles where the peak estradiol level was ≤3,000 pg/ml (agonist 16.6% vs. antagonist 9.5%; P = 0.0006) and in the antagonist group when the peak estradiol level was >3,000 pg/ml (antagonist 16.7% vs. agonist 8%) (Table 3). The small number of patients and the ongoing character of several pregnancies did not permit an analysis of the live birth rates to be performed.
Table 3.
ART outcomes in high responders according to COS protocol (long/antagonist)
| Protocol | Peak E2 ≤3,000 pg/ml | Peak E2 >3,000 pg/ml |
|---|---|---|
| Total pregnancy rate/o.p.u. | Total pregnancy rate/o.p.u. | |
| Agonist (long protocol) | 216/478 (45.1%) | 29/96 (30.2%) |
| Antagonist | 42/138 (30.4%) | 23/52 (44.2%) |
| P = 0.002 | P = 0.12 | |
| Clinical pregnancy rate/o.p.u. | Clinical pregnancy rate/o.p.u. | |
| Agonist (long protocol) | 168/478 (35.1%) | 20/96 (20.8%) |
| Antagonist | 27/138 (19.5%) | 18/52 (34.6%) |
| P = 0.0007 | P = 0.10 | |
| Implantation rate | Implantation rate | |
| Agonist (long protocol) | 255/1,528 (16.6%) | 23/287 (8%) |
| Antagonist | 38/396 (9.5%) | 25/149 (16.7%) |
| P = 0.0006 | P = 0.009 |
Total, clinical pregnancy and implantation rates in high responders (≥12 oocytes retrieved) according to the COS protocol (long vs. antagonist) and E2 peak levels (≤3,000 or >3,000 pg/ml)
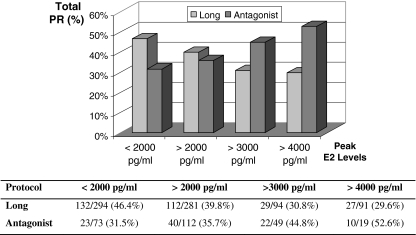
To further verify the impact of peak estradiol level on clinical outcome, we calculated the total pregnancy rate in subgroups of patients with peak estradiol levels <2,000, >2,000, >3,000, and >4,000 pg/ml in both the long and antagonist cycles. Total pregnancy rates of 31.5% (23/73), 35.7% (40/112), 44.8% (22/49), and 52.6% (10/19), respectively, for the antagonist, and 46.4% (132/284), 39.8% (112/281), 30.8% (29/94), and 29.6% (8/27) respectively, for the long protocol cycles were observed in these subgroups (Fig. 1). A clear opposite trend in the total clinical pregnancy rate in high responders was evident in the long and antagonist cycles : an increase of total pregnancy rates with greater peak estradiol levels was observed with GnRH antagonist but not agonist use.
Fig. 1.
PR, pregnancy rate; E2, estradiol. Total pregnancy rates in high responders (≥12 oocytes retrieved) in subgroups with different peak estradiol levels in the long and antagonist cycles
Discussion
Previous studies demonstrated a negative effect of high estradiol levels on the clinical outcomes of ART [1–6]. Heavy ovarian stimulation has previously been suggested to increase oocyte aneuploidies in comparison with milder one [12]. Profound alterations in endometrial genes expression have also been observed after COS compared with the natural cycle [10]. The great majority of such studies have been performed with agonists [8] and recent work seems to suggest that antagonists use may produce an endometrium more strictly resembling that observed during the natural cycle [11]. A recent study did not find any relationship between peak estradiol levels and pregnancy rates in antagonist cycles, but this study evaluated patients with relatively low estradiol levels [13]. The mechanism for possible detrimental effects of COS on endometrial receptivity is not yet clarified, but modified gene expression [14, 15], with an advancement [16] or delay [7] of endometrial development and a possible embryo-endometrial asynchrony have been suggested as pathogenic mechanisms.
Previous studies addressing the issue of the optimal oocytes number for ART success found that the relationship between pregnancy rate and number of retrieved oocytes plateaus around twelve oocytes [17]. Our retrospective evaluation of the effect of high estradiol levels on pregnancy rate was limited to cycles where at least 12 oocytes were retrieved. Moreover, some authors have suggested a detrimental effect of estradiol levels greater than 3,000 pg/ml on pregnancy rates [18]; therefore, this level was chosen as the cutoff for the statistical comparison of clinical outcomes.
Our data suggest that in cycles with high oocyte retrieval (≥12) the pregnancy rate differs according to the protocol used. A significant difference between the long and antagonist protocols in the total and clinical pregnancy was observed when the E2 levels were ≤3,000 pg/ml. For cycles with peak estradiol levels >3,000 pg/ml, only a trend favoring the antagonist protocol was observed. However, a significant difference in the implantation rates was also observed between the two protocols, favoring the agonist protocol when estradiol peak levels were ≤3,000 pg/ml and the antagonist protocol when the E2 levels were >3,000 pg/ml. Because of the limited number of observations of cycles with peak estradiol levels >3,000 pg/ml, we cannot exclude that, notwithstanding the significant differences in implantation rates, the lack of significance for pregnancy rates could be because of a β error. Even if the lower pregnancy rate with long protocol at higher E2 peak values, notwithstanding an higher number of retrieved oocytes, could be explained by a more frequent use of such protocol in polycystic ovarian syndrome this wasn’t the right explanation of our data due a more frequent use of antagonist than agonist in such patients. Moreover even if the use of long and antagonist protocol changed during the ten years experience, with an increasing use of antagonists in the last years, even analyzing separately the cycles from June 2000 to December 2005 and from January 2006 to December 2010 we find the same results in the two periods. So the different use of long and antagonist protocol over time seems not able to explain our observations.
Similar results have been recently obtained in a two-center study by Nelson et al. [19] in which higher pregnancy rates were obtained with the antagonist protocol compared with the agonist protocol in high responders (AMH level ≥15 pmol/l). However, because of the multicenter design of the study and the low number of patients included, the authors avoided any speculation about the difference in pregnancy rates observed with the two protocols. On the contrary, our preliminary results, albeit retrospective, were obtained at the same center. Considering the lack of evidence for a difference in embryo quality in high responders between the two protocols, we believe that different effects of the agonist and antagonist protocols on endometrial receptivity at various estradiol levels could better explain the differences in total, clinical and embryo implantation rates. Accordingly, recent studies demonstrated differential effects at the endometrial level with the GnRH agonist and antagonist protocols [8]. However, in contrast with our observations, some authors suggested that high E2 levels had detrimental effects on endometrial receptivity in both the agonist and antagonist protocols [20]. Previous studies also showed that a step-down long protocol may reduce the peak estradiol level in GnRH agonist cycles, reducing the detrimental effect on embryo implantation [18]. Similarly, cryopreservation and delayed thawed embryo transfer has been also suggested to avoid an impairment of the endometrial receptivity when the long protocol is used in high responders [21].
Our retrospective data, although underpowered for the subgroup with E2 levels >3,000 pg/ml, suggest that the detrimental effect of high peak estradiol levels is restricted or at least more evident in GnRH agonist cycles. On the contrary in antagonist cycles with the increase of peak E2 levels an improvement of the clinical outcome was observed. Our study suffers several limitations as the retrospective design or the possibility of uncontrolled variables related biases but, if confirmed by future prospective studies, could be a preliminary evidence that, in high responders, antagonists use could be not only safer by reducing OHSS incidence, but also more effective in limiting the detrimental effect of high estradiol levels on embryo implantation.
The negative effects of high response are increased risk of OHSS, impaired embryo implantation, and possibly the recently reported detrimental effects on perinatal outcomes. Avoiding fresh embryo transfer in such patients could be a promising way to prevent late OHSS and, perhaps, any detrimental effect on the embryo implantation and perinatal outcome. However, frozen embryo transfer to postpone pregnancy could have a negative psychological impact on the patient and possibly reduce the pregnancy rate by increasing pregnancy loss [22] as a consequence of impaired embryo developmental potential. Moreover, even by avoiding fresh embryo transfer, a substantial risk of early OHSS after ovulation induction with HCG persists with both the agonist and antagonist protocols. Our retrospective data showing the possible prevention of the detrimental effect on embryo implantation with use of the antagonist protocol could be particularly interesting in light of the recently implemented approach of triggering ovulation with a GnRH agonist. Even if GnRH agonist triggering of final oocyte maturation was considered to be detrimental for pregnancy rates in the past, both donor [23] and frozen-thawed homologous cycles [24] seem to suggest that this effect is mainly related to a defective luteal phase. Accordingly, GnRH agonist ovulation triggering coupled with aggressive luteal support seem able to save the fresh embryo transfer with extremely interesting pregnancy rates and extremely low or no risk at all of both early and late OHSS [25]. If a residual detrimental effect of GnRH agonist triggering on pregnancy rates in fresh cycles with high response [26] could be completely avoided by embryo freezing/vitrification and delayed thawed/warmed embryo transfer on unstimulated cycles, it should be addressed by future studies. So far, preliminary data show high pregnancy rates with a clinical approach that combines GnRH agonist triggering, pronuclear zygotes vitrification, and delayed warmed embryo transfer [27, 28].
In conclusion our retrospective experience suggests that an increase of pregnancy rate with increasing peak E2 level can be observed with antagonist but not long protocol. Due to the lack of difference in the quality of transferred embryos, if confirmed in prospective studies, our data could suggest a different effect of the two protocols on endometrial receptivity at least at extreme ovarian response. We are aware that our observations, even if interesting and original, need substantial confirmations due to the possibility of biases related to uncontrolled variables.
Conflicts of Interest
Authors do not declare any conflict of interest.
Financial support There were no financial supports to the study.
Footnotes
Capsule
High estradiol levels could impair endometrial receptivity. Our retrospective data seem to suggest that antagonist use can prevent this detrimental endometrial effect in high responders (≥12 oocytes retrieved).
The study was not previously presented in any meeting or congress.
References
- 1.Valbuena D, Martin J, Pablo JL, Remohi J, Pellicier A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because the directly affect the embryo. Fertil Steril. 2001;76:962–968. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 2.Mitwally MFM, Bhakoo HS, Crickard K, Sullivan M, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–596. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 3.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of HCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod. 2004;19:2446–2453. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 4.Lee FK, Lai TH, Lin TK, Horng SG, Chen SC. Relationship of progesterone/estradiol ratio on day of HCG administration and pregnancy outcomes in high responders undergoing in vitro fertilization. Fertil Steril. 2009;92:1284–1289. doi: 10.1016/j.fertnstert.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. Dynamics of early estradiol production may be associated with outcomes of in vitro fertilization. Fertil Steril. 2010;94:2868–2870. doi: 10.1016/j.fertnstert.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 6.Arslan M, Bocca S, Arslan EO, Duran HE, Stadtmauer L, Oehninger S. Cumulative exposure to high estradiol levels during the follicular phase of IVF cycles negatively affects implantation. J Assist Reprod Genet. 2007;24:111–117. doi: 10.1007/s10815-006-9101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Domingues F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–4510. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 8.Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, Vos J, et al. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocols effect. Biol Reprod. 2010;82:679–686. doi: 10.1095/biolreprod.109.081299. [DOI] [PubMed] [Google Scholar]
- 9.Strowitzki T, Germeyer A, Popovici R, Wolff M. The human endometrium as fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 10.Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Conejero JA, Simon C, Pellicer A, Horcajadas JA. Is ovarian stimulation detrimental to the endometrium? Reprod Biomed Online. 2007;15:45–50. doi: 10.1016/S1472-6483(10)60690-6. [DOI] [PubMed] [Google Scholar]
- 12.Rubio C, Mercader A, Alama P, Lizan C, Rodrigo L, Labarta E, et al. Prospective cohort study in high responder oocyte donors using two hormonal stimulation protocols: impact on embryo aneuploidy and development. Hum Reprod. 2010;25:2290–2297. doi: 10.1093/humrep/deq174. [DOI] [PubMed] [Google Scholar]
- 13.Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, Landuyt L, et al. Does the estradiol level on the day of human chorionic gonadotropin administration have an impact on pregnancy rates in patient treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24:2902–2909. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- 14.Makkar G, Ng EHY, Yeung WSB, Ho PC. Reduced expression of interleukin-11 and interleukin-6 in the periimplantation endometrium of excessive ovarian responders during in vitro fertilization treatment. J Clin Endocrinol Metab. 2006;91:3181–3188. doi: 10.1210/jc.2006-0180. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EHY, Yeung WSB, et al. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKKI in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010;15:479–490. doi: 10.1093/humrep/dep429. [DOI] [PubMed] [Google Scholar]
- 16.Bodri D, Sunkara SK, Coomarasamy A. Gonadotropin-releasing hormone agonists versus antagonists for controlled ovarian hyperstimulation in oocyte donors: a systematic review and meta-analysis. Fertil Steril. 2010 Aug 3 [Epub ahead of print]. [DOI] [PubMed]
- 17.Gaast MH, Eijkemans MJ, Net JB, Boer EJ, Burger CW, Leeuwen FE, et al. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13:476–480. doi: 10.1016/S1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 18.Simon C, Garcia Velasco JJ, Valbuena D, Peinado JA, Moreno C, Remohi J, et al. Increasing uterine receptivity by decreasing estradiol levels during preimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;75:525–531. doi: 10.1016/s0015-0282(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 19.Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, et al. Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24:867–875. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 20.Orvieto R, Meltzer S, Rabison J, Zohav E, Anteby EY, Nahum R. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertil Steril. 2008;90:1294–1296. doi: 10.1016/j.fertnstert.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27:357–363. doi: 10.1007/s10815-010-9412-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Brandes M, Verzijden JCM, Hamilton CJCM, de Weys NPC, de Bruin JP, Bots RSGM et al. Is the fertility treatment itself a risk factor for early pregnancy loss? Reprod Biomed Online. 2010 [Epub ahead of print]. [DOI] [PubMed]
- 23.Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. GnRH agonist versus recombinant HCG in an oocyte donation programme: a randomized, prospective, controller, assessor-blind study. Reprod Biomed Online. 2009;19:486–492. doi: 10.1016/j.rbmo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Griesinger G, Kolibianakis EM, Papanikolau EG, Diedrich K, Steirteghem A, Devroey P, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–621. doi: 10.1016/j.fertnstert.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Humaidan P, Bredkjær HE, Westergaard LG, Andersen CY. 1500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril. 2009;93:847–854. doi: 10.1016/j.fertnstert.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Youssef M, Van der Veen F, Al-Inhany HG, Griesinger G, Mochtar MH, van Wely M. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles (Review). Cochrane. 2010 issue 11. [DOI] [PubMed]
- 27.Griesinger G, Berndt H, Schultz L, Depenbush M, Schultze-Mosgau A. Cumulative live birth rates after GnRH-agonist triggering of final oocyte maturation in patients at risk of OHSS: a prospective, clinical cohort study. Eur J Obstet Gynecol Reprod Biol. 2010;149:190–194. doi: 10.1016/j.ejogrb.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Herrero H, Pareja S, Losada C, Cobo AC, Pellicer A. Avoiding the use of human chorionic gonadotropin combined with oocyte vitrification and GnRH agonist triggering versus coasting. A new strategy to avoid ovarian hyperstimulation syndrome. Fertil Steril. 2010 [Epub ahead of print]. [DOI] [PubMed]