Abstract
Objective
To estimate the effect of assisted reproductive technology (ART) on major malformation (MM) rate in ART offspring independent of the effect of subfertility on MM.
Design
Meta-analysis.
Methods
This meta-analysis is based on our previously published meta-analysis of observational studies evaluating the relationship between ART treatment and MM rates, as well as recent research by Zhu et al. to estimate the impact of subfertility alone on MM in subfertile couples conceiving spontaneously.
Results
The overall odds ratio for MM in our original meta-analysis, in which all studies used apparently inappropriate control groups of “normal” populations, was 1.29 (95% CI 1.01–1.67). Here we attempted to estimate the risk of subfertility and used this estimate to perform an adjusted meta-analysis. Zhu et al. found that about 40% of the odds of MM was due to subfertility. When we took Zhu’s finding into account, the adjusted odds ratio in the meta-analysis was 1.01 (95% CI 0.82–1.23).
Conclusions
Our study suggests ART does not increase the risk of MM as much as previously reported. More research is needed to quantify the underlying risk of subfertility and separate it from the risk associated with ART. Physicians who counsel subfertile couples should recognize that previous studies of MM rates in ART patients probably overestimated the risk.
Keywords: Subfertility and major malformations, ART outcomes, Meta-analysis, IVF/ICSI outcomes
Introduction
The safety of IVF and ICSI, sometimes referred to as assisted reproductive technology (ART), is a matter of critical importance to patients and practitioners. To investigate one aspect of safety, we published a meta-analysis of observational studies in 2004 evaluating the relationship between in vitro fertilisation (IVF) and intra cytoplasmic sperm injection (ICSI) (together referred to herein as ART) and major malformations (MM) in the offspring [1]. The details of the meta-analysis—including the literature search, the study selection and the data extraction processes—are described there. We found 19 studies [2–20] for inclusion in the meta-analysis and found that ART increased the risk of MM with an odds ratio of 1.29 (95% CI 1.01–1.67). Our major conclusion was that the risk we found may be inflated by the fact that none of the studies used the most appropriate control group, namely subfertile couples conceiving spontaneously. Instead, each study used a control group of either a general population or a hospital specific “normal” cohort. Thus the risk found did not take into account the possibility that subfertile couples could be at an increased risk of MM in part because of the various underlying causes of their subfertility. Control groups made up of almost all normal couples do not consider this possibility. Lack of appropriate control groups in studies of risk associated with ART continues to be an issue. For example, as recently as 2009, the CDC published a report estimating the increased risk of certain congenital malformations in ART offspring at 2.0 to 4.0 [21]. The study lacked an appropriate control group [22].
While many studies have focused on the risks of ART treatment, actually separating that risk from the risk associated with the couples’ underlying subfertility has seldom been attempted for any outcomes in the offspring. However, many investigators [23–27] have suggested that underlying subfertility may have an impact on ART outcomes, separate from the putative impact of the ART treatment itself.
In 2006, Zhu et al. [28] conducted a study that responded to our study regarding inappropriate control groups. They examined the effect of subfertility on MM rates, using the Danish national birth cohort. They studied MM rates in couples who conceived after less than 6 months, 6 to 12 months, and more than 12 months of attempting to conceive. They collapsed the scale to a binary grouping: ≤12 and >12 months. This is consistent with the widely accepted view that subfertility is “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [29].
When Zhu et al. compared the fertile couples who conceived in 12 month or less to the subfertile couples, an increased risk of MM of 1.20 (95% CI 1.07–1.35) was found. When couples receiving IVF or ICSI (ART) were compared to the same group of fertile couples, an increased risk of MM of 1.50 was found (weighted average). This suggests that subfertility contributes close to 40% of the increased risk of MM that is observed in the offspring of ART-treated subfertile couples. Zhu et al. also observed that the overall prevalence of congenital malformations increased with increasing time to pregnancy (TTP). Zhu’s results support our suggestion and that of other investigators [30–32] that only subfertile couples are an appropriate control group for ART outcome studies because subfertility, with all its underlying causes, itself creates a risk of MM and other outcomes of concern.
Materials and methods
Since the Zhu study apparently gives the first estimate of the effect of the underlying subfertility on MM risk, that estimate was used to adjust our meta-analysis in a quantitative effort to account for the subfertility effect separate from the ART treatment effect. The literature was searched for other studies where estimates of MM rates were presented for subfertile couples. No studies were found, other than Zhu’s, which could be used to estimate the effect of underlying conditions on MM in subfertile couples. The literature was also searched to determine whether any other studies since 2006 had attempted to adjust MM odds ratios based on Zhu’s findings; none were found.
In this review of the 19 studies used in our original meta-analysis, one study was found to have a problem with the control group. After correspondence with the author about this problem, the study had to be excluded from our meta-analysis. Before this change, the OR with the 19 studies was 1.29 (95% CI 1.01–1.67). With the 18 remaining studies, using our original methodology, the corrected OR is 1.14 (95% CI .94–1.4).
Using the findings of Zhu’s study, the 18 studies in our meta-analysis were reanalyzed in order to derive an overall estimate of the results that might have been obtained if proper control groups had been used in those studies. Based on the fact that Zhu’s results showed ART increased the risk of MM about 50% and subfertility increased it 20%, it was assumed that subfertility is the cause of about 40% of the overall increased risk of MM observed in ART offspring. Therefore, the odds ratio in each study was reduced by 40%. For example, when the odds were 1.5, it was reduced to 1.30. When the odds were less than 1.0, which suggested a protective effect, an adjustment was obtained by subtracting the odds from 1.0, taking 40% of this difference, and then subtracting that difference from the observed odds ratio. For example, if the odds were .90, this was subtracted from 1.0, giving .10. Forty percent of .10, or .04, was subtracted from .90, giving an adjusted odds of .86.
The 95% confidence intervals (CIs) were obtained by the following method. For each study, the adjusted odds ratio was first obtained and then used to solve for the number of malformed infants in the ART group that would be expected if the number of infants with MM due to subfertility were eliminated from the numerator of the ART group. Using the adjusted numerator in the ART group, the adjusted 95% CIs were then calculated. The adjusted 95% CIs were very similar to the original 95% CIs in our meta-analysis. The random effects model was used in the meta-analysis. A fixed effect model was also used and the results were found to be almost identical to the results when the random effects model was used.
Reducing the odds ratio for each study by 40% is the conservative method of trying to adjust out the malformations as a result of subfertility. A less conservative method was also used; this entailed reducing the odds ratio for each study by the absolute value of .19. This is derived from the odds ratio of 1.19 for MM found in Zhu’s study for subfertile couples who conceived spontaneously. In this analysis, the random effects model was used, but the forest plot is not presented because it is almost identical to that obtained when the 40% reduction in the odds is used.
Results
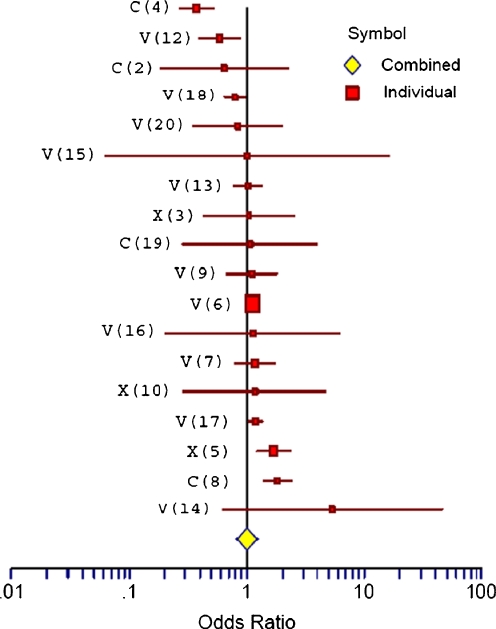
Figure 1 is a forest plot of the odds ratios and 95% CIs for the 18 studies in our original meta-analysis, after adjusting for the risk of subfertility by reducing the odds of each study by 40%.
Fig. 1.
Forest Plot of Odds shows the adjusted Odds Ratios and Confidence Intervals for the 18 studies. V: IVF Study; X: ICSI Study; C: Combined Results of ICSI and IVF; white diamond: Overall Odds Ratio. The reference number for each study is given in parentheses
Table 1 shows the unadjusted odds ratios in each of the 18 studies from our meta-analysis as well as the adjusted odds ratios for each study. The original overall odds ratio from our published meta-analysis of the 19 studies was 1.29 and statistically significant. When the one study with an error in the control group was eliminated, the odds ratio for the remaining 18 studies was 1.14 (95% CI 0.94–1.4).
Table 1.
Originally Publisheda and Adjusted Odds Ratios of the Association Between ART and Major Malformations
| Reference & study typeb | Originally published odds ratio | Significance | Adjusted odds ratio | Significance |
|---|---|---|---|---|
| 4 C | 0.54 | P < .05 | 0.38 | P < .05 |
| 12 V | 0.81 | NS | 0.59 | P < .05 |
| 2 C | 0.77 | NS | 0.64 | NS |
| 18 V | 0.86 | NS | 0.79 | P < .05 |
| 20 V | 0.90 | NS | 0.84 | NS |
| 15 V | 1.00 | NS | 1.00 | NS |
| 13 V | 1.04 | NS | 1.02 | NS |
| 3 X | 1.07 | P < .05 | 1.05 | NS |
| 19 C | 1.24 | NS | 1.07 | NS |
| 9 V | 1.21 | NS | 1.11 | NS |
| 6 V | 1.19 | P < .05 | 1.12 | NS |
| 16 V | 1.29 | NS | 1.13 | NS |
| 7 V | 1.25 | NS | 1.17 | NS |
| 10 X | 1.27 | P < .05 | 1.18 | P < .05 |
| 17 V | 1.38 | NS | 1.18 | NS |
| 5 X | 2.05 | P < .05 | 1.69 | P < .05 |
| 8 C | 2.23 | P < .05 | 1.82 | P < .05 |
| 14c V | 15.4 | NS | 5.4 | NS |
| Overall Odds Ratiod | 1.14e | P < .05 | 1.01 | NS |
a18 of the original studies are shown here; as explained in the text, one study has been removed.
bReference numbers refer to references in this publication, not the original publication. V: IVF Study; X: ICSI Study; C: Combined Results of ICSI and IVF.
cNo MM in control group; when .5 is substituted for zero, the adjusted OR is 5.4.
dSince the odds ratios in our meta-analysis were not significantly different for IVF versus ICSI and for singleton versus multiple, the data were pooled to obtain an overall odds ratio.
eOverall odds when 18 studies included.
When the odds ratio of each of the 18 studies was reduced by 40%, the adjusted overall odds ratio was 1.01 (95% CI 0.82–1.23). The rather low overall adjusted OR of 1.01 is due to the fact that some of the larger studies had a protective effect before adjustment and the analysis weights studies according to their sample size. This is our best estimate, at this time, of the overall risk of MM in ART patients when the effect of subfertility is removed. In an alternative approach to using the results from Zhu’s study, which found that subfertile couples who conceived spontaneously had an odds of 1.19 for MM, we subtracted the absolute value of .19 from each of the 18 studies. This resulted in an overall odds ratio of 0.93 (95% CI .73–1.18), suggesting a protective effect of ART.
Discussion
Risk associated with subfertility
Is it reasonable to think that subfertile patients would have underlying conditions that may predispose them to poor pregnancy outcomes? There is a great deal of evidence in the literature to support this view. While it is beyond the scope of this paper to catalog all prior efforts to answer this question, a few should be noted. Past investigators have looked at this issue with regard to preterm delivery (PTD), low birth weight (LBW) and perinatal mortality. Saunders et al. [23] appears to have been the earliest in the post IVF era. Their findings were published in a preliminary report on the first 2 years of statistics from the Australian IVF Register. They found that the PTD rate in singleton pregnancies both for IVF patients and for subfertile couples conceiving spontaneously while on the IVF waiting list (10.0%) exceeded that of the general population (6.2%). The incidence of LBW among singletons was also elevated in the IVF group (6.5%) and in the spontaneously conceiving group (8.7%) when compared to the general population (4.8%).
In 1992 Bhalla et al. [24], 1994 Joffe et al. [25], and 1997 Henriksen [26] made important additions to the efforts to quantify the risk of subfertility. Bhalla reported a significantly (p < 0.01) higher rate of PTD in a group of 112 subfertile (at least 2 years to conception) patients who conceived spontaneously (28.1%) when compared to normal controls (12.6%). Joffe reported in a population-based study that a delay in time to conception was a risk factor for poor obstetric outcomes, regardless of medical intervention. Pregnancies that ended in PTD among women who took more than 12 months to conceive tended to take 18% longer to conceive than other live births in the population-based cohort. Henriksen reported that when compared to women who conceived in 6 months or less, women who tried for more than 12 months and conceived spontaneously without infertility treatment had a significantly increased risk for PTD of 1.6 (95% CI 1.0–2.7).
In 1999, Draper et al. [30] carried out a population-based case control study of perinatal deaths. They found that a history of subfertility in the index pregnancy, irrespective of treatment, increased the risk of perinatal death, with odds of 2.9 (95% CI 1.8–4.5). Compared to the infants of women without subfertility, the infants of women with untreated subfertility had an increased risk of perinatal death with odds of 3.3 (95% CI 1.6–6.8). In the treated subfertile group, there was also an increased risk of perinatal death, but the odds ratio was lower, 2.7, suggesting that treatment provided a protective effect. There are also, as described by Park et al. [33], genetic causes of subfertility in parents known to be associated with congenital anomalies in a baby, such as constitutional chromosomal rearrangements, including reciprocal and robertsonian translocations and inversions. Even with a normal karyotype there is the possibility of subtelomeric rearrangement or interstitial chromosomal deletions and duplications. Gonadal mosaicism in a parent is another possibility. Maternal stress could also play a role [34].
Time to pregnancy
Since increasing TTP is associated with a risk of adverse outcomes in the offspring, it is important to know where ART patients fall on the TTP spectrum. When reporting on ART treatment, TTP is seldom reported, but investigators often cite the duration of subfertility (DOS) in the presenting patients.
DOS is known to be significantly higher in patients achieving pregnancy through ART treatment. Several relatively recent studies from developed countries report this. Poikkeus reported a DOS of about 4 years in a Finnish IVF patient group [35]. Kupka reported a DOS of about 5 years in a large German study [36]. Thum reported a DOS of 3.3 years in an English patient group [37]. Boivin reported a DOS of 4.1 years among Danish couples undergoing IVF treatment [38].
It is fair to conclude that ART patients are rather far out on the TTP spectrum and by and large well beyond a TTP of 12 months. Therefore, our assumption that almost all ART couples would have conceived beyond 12 months is reasonable, and maybe a conservative one.
Protective effect
Does the protective effect of ART, as observed in 5 studies in our meta-analysis of 18 studies, have a basis in reality? There is some possibility that a protective effect may arise in the ART clinic laboratory where a variety of sperm and embryo selection processes occur. These range from pre-implantation genetic diagnosis (PGD) to various objective and subjective ways in which the embryologist attempts to identify the “best” gametes and embryos. For example, when male factors are present and ICSI is used, several criteria—including the proximity of appearance to normal in terms of morphology and motility, as well as other criteria—are used to select from among the available sperm. Poorer quality embryos may not develop to the blastocyst (6–8 cell) stage typically used for embryo transfer today. When a patient has multiple embryos available, those judged to be the “best” are transferred to the uterus first. Finally, inferior embryos that are cryopreserved at a patient’s request for use in a subsequent attempt to achieve pregnancy may be the ones that do not survive freezing and thawing.
Successful treatment may also provide protection, since it interrupts or cuts short the TTP for the subfertile couple, and thus avoids some delay. A prolonged TTP has been shown in several studies, in addition to Zhu’s, to increase risk. For example, Basso and Olsen [31] reported that the risk of neonatal death was significantly increased in all women with a TTP of greater than 12 months, OR 2.82 (95% CI 1.35–5.90), with the OR being 3.32 (95% CI 1.47–7.53) among those who had reported not receiving infertility treatment and 2.32 (95% CI 0.86–5.80) among those who reported treatment. Though the lower increased risk in the treated group is not statistically significant, it suggests the possibility of a protective effect.
Basso and Baird [32] found that an increasing TTP was associated with significantly increased risk of preterm (less than 37 weeks) delivery (PTD). In all primiparous women (treated or untreated) with a TTP of greater than 12 months, the OR for preterm birth was 1.38 (95% CI 1.14–1.69); in the untreated-only subset, the OR was very similar at 1.36 (95% CI 1.08–1.71). They concluded that the increased risk of preterm birth observed in this group could not be attributed solely to the effects of infertility treatment.
Furthermore, if TTP is longer, the patient will be older when conception occurs. Thus shortening TTP reduces the patient’s age at the time of conception. General population data in the U.S. have long shown the impact of maternal age on the rate of congenital defects. The incidence is approximately 2.7% at age 26 and by age 37 it is 3.35% [39]. Data from France also shows that as maternal age advances, the frequency of aneuploidy in the oocytes also increases [40].
Strengths and weaknesses of this study
One strength of our study is that our approach to the adjustment may be considered conservative compared to other adjustment methodologies. For example, we could have used the overall odds ratio of 1.29 from our meta-analysis and assumed that this 29% increase in the incidence of MM was the truth and that subfertility increased the incidence of MM 20%. Had we used this approach, we would have reduced the odds in each study in the original meta-analysis by 69% (.20 ÷ .29 = 69%) rather than 40%.
In support of the 40% risk reduction we have used here is the fact that it is in general agreement with the level of risk posed by subfertility alone as reported in other studies of risks both before and since Zhu’s paper. For example, Sun et al. reported that the risk of epilepsy was 1.71 in the ART group and 1.38 (95% CI 1.0–1.89) in the spontaneously conceiving subfertile group [41].
Our study also benefits from the strengths of Zhu’s study: namely a large cohort; the collection of TTP data prior to the birth; and the fact that, in Denmark, subfertile couples have access to up to three free IVF treatments, which eliminates most of the concern about skewing of the spontaneously conceiving group to lower socioeconomic status than the ART-treated group.
A weakness in our study stems from the fact that Zhu’s group only had data for spontaneous conceptions before and after a 12 month TTP. Thus using their estimate of the impact of underlying subfertility on the MM rate may underestimate the effect of duration of the underlying conditions causing infertility. In other words, couples who try for 24 or 36 or more months before conceiving spontaneously may be at greater risk of MM than those who conceive at, say, 18 months. If the distribution of couples in Zhu’s study did not represent the world experience—if, for example, it had an excess of couples who conceived between 12 to 15 months—using his results could underestimate the MM risk of underlying subfertility.
Another weakness in our study stems from the fact that the underlying causes of subfertility may differ to some extent between treated couples and those who manage to conceive spontaneously. For example, some infertile couples, with absent or blocked fallopian tubes who procreate through ART could never conceive spontaneously [42].
Conclusion
Our adjusted analysis suggests that the relative risk of MM in the offspring of ART treated couples is 1.01 and not statistically significant. It may be that with regard to MM, ART is safer than originally thought.
These results can also be described in terms of the actual incidence of MM. The risk of MM in the general U.S. population is 3% [43]. Our original (corrected) analysis, which showed an OR of 1.14, suggests an incidence of 3.42% for MM among ART offspring. Our adjusted analysis with an OR of 1.01 among ART treated patients suggests that the incidence of MM among ART treated patients is 3.03%. Though the change in incidence is small, a discussion of the increased risk with a patient can now be based on a study where an effort has been made to take into account the effect of subfertility on MM.
This analysis raises an important question about the exact magnitude of the effect of the underlying conditions in subfertile patients on the MM rate observed after ART treatment and points to the need for further studies. The literature contains studies with odds ratios that vary at least between .55 and 7.69. It is hoped that future well-designed studies will narrow the range and that the meta-analysis of such studies will give a better estimate of the effect of ART on MM.
Acknowledgement
This work was supported in part by the Department of Epidemiology and Biostatistics at Case Western University School of Medicine.
Footnotes
Capsule This study attempts to separate the risk of major malformation in ART offspring attributable to subfertility from the risk attributable to ART. After adjusting for subfertility, we found no increased risk.
References
- 1.Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437–443. doi: 10.1007/s10815-004-8760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen JR, Gibson FL, Leslie GI, Saunders DM. Medical and developmental outcome at 1 year for children conceived by intracytoplasmic sperm injection. Lancet. 1998;351:1529–1534. doi: 10.1016/S0140-6736(98)10168-X. [DOI] [PubMed] [Google Scholar]
- 3.Sutcliffe AG, Taylor B, Saunders K, Thornton S, Lieberman BA, Grudzinskas JG. Outcome in the second year of life after in-vitro fertilisation by intracytoplasmic sperm injection: a UK case-control study. Lancet. 2001;357:2080–2084. doi: 10.1016/S0140-6736(00)05180-1. [DOI] [PubMed] [Google Scholar]
- 4.Palermo GD, Neri QV, Hariprashad JJ, Davis OK, Veeck LL, Rosenwaks Z. ICSI and its outcome. Semin Reprod Med. 2000;18:161–169. doi: 10.1055/s-2000-12555. [DOI] [PubMed] [Google Scholar]
- 5.Wennerholm UB, Bergh C, Hamberger L, Lundin K, Nilsson L, Wikland M, Källén B. Incidence of congenital malformations in children born after ICSI. Hum Reprod. 2000;15:944–948. doi: 10.1093/humrep/15.4.944. [DOI] [PubMed] [Google Scholar]
- 6.Ericson A, Källén B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504–509. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- 7.Anthony S, Buitendijk SE, Dorrepaal CA, Lindner K, Braat DDM, Ouden AL. Congenital malformations in 4224 children conceived after IVF. Hum Reprod. 2002;17:2089–2095. doi: 10.1093/humrep/17.8.2089. [DOI] [PubMed] [Google Scholar]
- 8.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 9.Isaksson R, Gissler M, Tiitinen A. Obstetric outcome among women with unexplained infertility after IVF: a matched case-control study. Hum Reprod. 2002;17:1755–1761. doi: 10.1093/humrep/17.7.1755. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig M, Katalinic A. Pregnancy course and health of children born after ICSI depending on parameters of male factor infertility. Hum Reprod. 2003;18:351–357. doi: 10.1093/humrep/deg048. [DOI] [PubMed] [Google Scholar]
- 11.Merlob P, Fisch B. Neonatal outcome and congenital malformations in children born after IVF. Hum Reprod. 2002;17:3004–3005. doi: 10.1093/humrep/17.11.3004-a. [DOI] [PubMed] [Google Scholar]
- 12.MRC Working Party. Rose G, Beral V, Davis JA, Edwards RG, Harper PS, O Loudon JD. Births in Great Britain resulting from assisted conception. BMJ. 1990;300:1229–1233. doi: 10.1136/bmj.300.6734.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westergaard HB, Johansen AMT, Erb K, Andersen AN. Danish National In-Vitro Fertilization Registry 1994 and 1995: a controlled study of births, malformations and cytogenetic findings. Hum Reprod. 1999;14:1896–1902. doi: 10.1093/humrep/14.7.1896. [DOI] [PubMed] [Google Scholar]
- 14.Souza SW, Rivlin E, Cadman J, Richards B, Buck P, Lieberman BA. Children conceived by in vitro fertilisation after fresh embryo transfer. Arch Dis Child Fetal Neonatal Ed. 1997;76(2):F70–F74. doi: 10.1136/fn.76.2.F70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verlaenen H, Cammu H, Derde MP, Amy JJ. Singleton pregnancy after in vitro fertilization: expectations and outcome. Obstet Gynecol. 1995;86:906–910. doi: 10.1016/0029-7844(95)00322-I. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe AG, Souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B. Minor congenital anomalies, major congenital malformations and development in children conceived from cryopreserved embryos. Hum Reprod. 1995;10:3332–3337. doi: 10.1093/oxfordjournals.humrep.a135915. [DOI] [PubMed] [Google Scholar]
- 17.Zádori J, Kozinszky Z, Orvos H, Katona M, Kaáli SG, Pál A. The incidence of major birth defects following in vitro fertilization. J Assist Reprod Genet. 2003;20:131–132. doi: 10.1023/A:1022682908307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinborg A, Loft A, Rasmussen S, et al. Neonatal outcome in a Danish national cohort of 3438 IVF/ICSI and 10362 non-IVF/ICSI twins born between 1995 and 2000. Hum Reprod. 2004;19:435–441. doi: 10.1093/humrep/deh063. [DOI] [PubMed] [Google Scholar]
- 19.Place I, Englert Y. A prospective longitudinal study of the physical, psychomotor and intellectual development of singleton children up to 5 years who were conceived by intracytoplasmic sperm injection compared with children conceived spontaneously and by in vitro fertilization. Fertil Steril. 2003;80:1388–1397. doi: 10.1016/j.fertnstert.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm UB, Albertsson-Wikland K, Bergh C, et al. Postnatal growth and health in children born after cryopreservation as embryos. Lancet. 1998;351:1085–1090. doi: 10.1016/S0140-6736(97)08247-0. [DOI] [PubMed] [Google Scholar]
- 21.Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. Assisted reproductive technology and major structural birth defects in the United States. The National Birth Defects Preventive Study. Hum Reprod. 2009;24:360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- 22.Rimm AA, Katayama AC, Katayama KP. ART and major structural birth defects in the United States. Hum Reprod. 2009;24:1765. doi: 10.1093/humrep/dep095. [DOI] [PubMed] [Google Scholar]
- 23.Saunders DM, Mathews M, Lancaster PAL. The Australian Register: current research and future role. Ann NY Acad Sci. 1988;541:7–21. doi: 10.1111/j.1749-6632.1988.tb22237.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhalla AK, Sarala G, Dhaliwal L. Pregnancy following infertility. Aust NZ Obstet Gynecol. 1992;32:249–251. doi: 10.1111/j.1479-828X.1992.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 25.Joffe M, Li Z. Association of time to pregnancy and the outcome of pregnancy. Fertil Steril. 1994;62:71–75. doi: 10.1016/s0015-0282(16)56818-6. [DOI] [PubMed] [Google Scholar]
- 26.Henriksen TB, Baird DD, Olsen J, Hedegaard M, Secher NJ, Wilcox AF. Time to pregnancy and preterm delivery. Obstet Gynecol. 1997;89:594–599. doi: 10.1016/S0029-7844(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 27.Kovalevsky G, Rinaudo P, Coutifaris C. Do assisted reproductive technologies cause adverse fetal outcomes? Fertil Steril. 2003;79:1270–1272. doi: 10.1016/S0015-0282(03)00397-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility treatment, and congenital malformations: Danish national birth cohort. BMJ 2006;doi:10.1136/BMJ38919.495718.af. [DOI] [PMC free article] [PubMed]
- 29.Zegers-Hochschild F, Adamson GD, Mouson J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary of ART Terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Draper ES, Kurinczuk JJ, Abrams KR, Clarke M. Assessment of separate contributions to perinatal mortality of infertility history and treatment: a case-control analysis. Lancet. 1999;353:1746–1749. doi: 10.1016/S0140-6736(98)08500-6. [DOI] [PubMed] [Google Scholar]
- 31.Basso O, Olsen J. Subfecundity and neonatal mortality: longitudinal study within the Danish national birth cohort. BMJ doi:10.1136/bmj.38336.616806.8F (4 February 2005). [DOI] [PMC free article] [PubMed]
- 32.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18:2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Mathur R, Smith GCS. Congenital anomalies after treatment for infertility. BMJ. 2006;333:665–666. doi: 10.1136/bmj.38982.702581.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barid DD, Wilcox AJ, Kramer MS. Why might infertile couples have problem pregnancies. Lancet. 1999;353:1724–1725. doi: 10.1016/S0140-6736(99)90050-8. [DOI] [PubMed] [Google Scholar]
- 35.Poikkeus P, Unkila-Kallio L, Vilska S, Repokari L, et al. Impact of infertility characteristics and treatment modalities on singleton pregnancies after assisted reproduction. Reprod Biomed Online. 2006;13(1):135–144. doi: 10.1016/S1472-6483(10)62027-5. [DOI] [PubMed] [Google Scholar]
- 36.Kupka MS, Dorn C, Richter O, Felberbaum R, Ven H. Impact of reproductive history on in vitro fertilization and intracytoplasmic sperm injection outcome: evidence form the German IVF Registry. Fertil Steril. 2003;80(3):508–516. doi: 10.1016/S0015-0282(03)00760-X. [DOI] [PubMed] [Google Scholar]
- 37.Thum MY, Gafar A, Wren M, Faris R, Ogunyemi B, et al. Does egg-sharing compromise the chance of donors or recipients achieving a live birth? Hum Reprod. 2003;18(11):2363–2367. doi: 10.1093/humrep/deg464. [DOI] [PubMed] [Google Scholar]
- 38.Boivin J, Schmidt L. Infertility—related stress in men and women predicts treatment outcome 1 year later. Fertil Steril. 2005;83:1745–1752. doi: 10.1016/j.fertnstert.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Croen LA, Shaw GM. Young maternal age and congenital malformations: A population-based study. Am J Pub Health. 1995;85:710–713. doi: 10.2105/AJPH.85.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111(3–4):206–212. doi: 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Vestergaard M, Christensen J, Zhu JL, Bech BH, Olsen J. Epilepsy and febrile seizures in children of treated and untreated subfertile couples. Hum Reprod. 2007;22:215–220. doi: 10.1093/humrep/del333. [DOI] [PubMed] [Google Scholar]
- 42.Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. Reply: ART and major structural birth defects in the USA. Hum Reprod. 2009;24:1766. doi: 10.1093/humrep/dep097. [DOI] [PubMed] [Google Scholar]
- 43.CDC Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR. 2008;57(1):1–5. [PubMed] [Google Scholar]