Abstract
Purpose
To assess the impact of embryonic stem cell culture medium (ESCM) on the pre- and post-implantation development of the mouse embryo, as a mammalian model, in comparison with the conventional culture medium, a potassium simplex optimized medium (KSOM).
Methods
Development in ESCM versus KSOM was compared in terms of embryo morphology, cleavage, cavitation, hatching, cell number, expression of TE and ICM transcription factors (Cdx2 and Oct4, respectively), implantation, and development in utero.
Results
An enriched medium like ESCM can be beneficial for in vitro embryo development when cultured from the 8-cell stage, as evidenced by promotion of blastocyst development with respect to cavity expansion, hatching, and cell division. Such benefits were not observed when embryos were cultured from the 2-cell stage.
Conclusions
ESCM may augment in vitro embryo development from the 8-cell stage. Using different culture media at different stages may be beneficial to achieve more effective human in vitro fertilization.
Keywords: Assisted reproductive technologies (ART), Culture media, Embryonic stem cell medium (ESCM), Potassium simplex optimized medium (KSOM), Preimplantation development
Introduction
Studying mammalian preimplantation development in vitro became a possibility in the 1950s [1–3], coinciding with a time when chemically defined media were being developed for culture of cell lines. In 1991, Simplex Optimized Medium (SOM) was developed to overcome the arrest of development at the two-cell stage [4, 5]. SOM was later modified by increasing the potassium ion concentration to formulate KSOM, which better supported in vitro blastocyst development [6]. Mouse studies using KSOM have made a significant impact on the design of culture media for human preimplantation embryos [7, 8]. Currently, media of chemical compositions that are nearly as simple as KSOM are routinely used in in vitro fertilization (IVF) clinics to culture human embryos.
The medical objective of perfecting a culture medium is to better treat infertility. Assisted reproductive technologies (ART) have helped many infertile couples have children, and ART babies today account for >1% of all births in the United States [9, 10]. However, the problem that persists is the high rate of developmental arrest of embryos in in vitro culture [11–16]. It is likely that high attrition rates have etiologies apart from culture media, such as intrinsic genetic abnormalities and damage incurred during collection and manipulation of gametes. However, the culture medium is one parameter that can and should be carefully reworked until it is optimal. Further, there is concern regarding the long-term effects of ART, particularly epigenetic alterations potentially caused by culture in vitro [17]. Two genomic imprinting diseases, Angelman syndrome and Beckwith-Weidemann syndrome, have a higher incidence in ART children [18–21], which may be attributed to suboptimal culture conditions of preimplantation embryos.
Embryonic stem (ES) cells have been derived from the blastocysts of mouse and human. ES cells can proliferate indefinitely while retaining developmental pluripotency [22–24]. The chemically defined ES cell culture media (ESCM) are formulated to support self-renewal of ES cells, which retain the developmental potential similar to ICM. ESCM is vastly more complex than KSOM, and contains various vitamins, antioxidants, and growth factors, such as BMP4 and LIF [25–27]. ESCM can maintain ES cells in culture for several years, after which ES cells are still capable of developing into all the tissues in the body, including gametes [25, 28, 29]. This suggests that ESCM can sustain developmental potential of embryonic cells without compromising their genetic and epigenetic integrities.
The goal of this study was to assess the impact of chemically defined ESCM on mouse preimplantation development. Because ESCM is more complex and able to sustain the integrity of ES cells, we suspected that ESCM could better support embryonic cells, particularly ICM, with which ES cells share many properties. If ESCM can promote ICM development without compromising trophectoderm (TE), then we would expect higher rates of developmental success in ART settings.
Materials and methods
Animals and embryo collection
The protocol for animal use was approved by the Institutional Animal Care and Use Committee. F1 (C57BL/6 × DBA/2; National Cancer Institute, Frederick, MD) and CD-1 (Charles River Laboratories, Wilmington, MA) mice were used. F1 females (8 ~ 12-weeks-old) were superovulated and mated with F1 males, as previously described [30]. Oviducts were removed from euthanized females, and 2-cell or 8-cell stage embryos were flushed using FHM HEPES Buffered Medium (Millipore, Chicago, IL). Those embryos that were fragmented or significantly delayed at the time of flushing were discarded and not used for experiments.
Embryo culture
Two- or eight-cell stage embryos were cultured in two different media: KSOM (KSOM with 1/2 amino acids, glucose and phenol red; Millipore), and ESGRO Complete Serum-Free Clonal Grade Medium (Millipore), which is manufactured as a serum-free and feeder-free medium to maintain mouse ES cells [25], and is referred to as Embryonic Stem Cell Medium (ESCM) in this study. Embryos in culture medium were covered with mineral oil and incubated at 37°C under 5% CO2, and imaged using Zeiss Axiovert 200 microscope with Hoffman Optics and MRm Digital Camera.
Immunohistochemistry and nuclear staining
Embryonic Day (E) 4.5 blastocysts were fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked in 5% bovine serum albumin in PBS. Primary antibodies used were monoclonal anti-Oct4 (C-10; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Cdx2 (CDX2-88; BioGenex, San Ramon, CA). Secondary antibody used was Alexa 488-goat anti-mouse (Invitrogen, Carlsbud, CA). Immunostained embryos were mounted in Vectashield medium with propidium iodide (Vector Laboratories, Burlingame, CA). Embryos were imaged at 1 μm intervals using Zeiss LSM5 PASCAL laser scanning confocal microscope. The same settings were used during imaging to allow comparison of expression levels among embryos.
Scoring of blastocyst development and measurement of cell number and cavity volume
Blastocysts were scored based on the following three categories: an unhatched (uh) blastocyst contains a distinct blastocyst cavity without any TE protruding through the zona pellucida (ZP); a partly hatched (ph) blastocyst exhibits various extent of TE protrusion through the ZP; and a fully hatched (fh) blastocyst is completely outside of the ZP. The total cell number of each embryo was measured based on the nuclear staining with propidium iodide. Confocal optical sections were captured along the entire thickness (z-axis) of the embryo at 1 μm interval, and were viewed using the Zeiss LSM Image Browser software to count the number of both interphase and mitotic phase nuclei with the assistance of the overlay tool of the software. To measure the volume of blastocyst cavity, digital images of embryos were captured using a Zeiss Axiovert 200 microscope and MRm camera, which was operated through the AxioVision software. When a blastocyst cavity appeared spherical, the diameter (d) was measured using the “measure length” tool of the software to calculate the volume of cavity (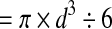 ). When a blastocyst cavity was ellipsoidal, the longest diameter (ld) and shortest diameter (sd) were measured, and the cavity volume was estimated accordingly (
). When a blastocyst cavity was ellipsoidal, the longest diameter (ld) and shortest diameter (sd) were measured, and the cavity volume was estimated accordingly (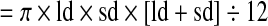 ). In a partly hatched blastocyst, the volume of cavity that was situated within the ZP and the volume of the cavity outside of the ZP were measured separately according to the above formulas, and the total volume of cavity was calculated as their sum.
). In a partly hatched blastocyst, the volume of cavity that was situated within the ZP and the volume of the cavity outside of the ZP were measured separately according to the above formulas, and the total volume of cavity was calculated as their sum.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA extraction from blastocysts, cDNA synthesis, and real-time PCR using the specific β-actin, Gapdh, Oct4 and Cdx2 primers were performed as previously described [31].
Embryo transfer
E4.5 blastocysts were transferred in pseudopregnant CD-1 females that had been mated with vasectomized CD-1 males the previous three nights. Six to seven blastocysts were transferred per uterine horn. Numbers of implantation sites and fetuses and the morphology of fetuses were recorded on Day 14.5 ~ 15.5 of gestation. These days were chosen to provide information on the extent of fetal development as well as the frequency of embryonic loss after implantation, which can be detected by the presence of residual dark tissues, or implantation scar, in the uterus. Although we did not assess development to term in this study, normal fetuses at Day 14 of pregnancy rarely fail to develop to full term [32, 33]. Furthermore, full-term birth occasionally results in accidental loss of pups due to cannibalization by the mother, which would impede quantitative comparison of the efficiency of fetal development between the KSOM and ESCM groups. Also, in this study, we did not transfer embryos that were cultured in ESCM from the 2-cell stage. This is because many of the embryos did not develop into blastocysts by E4.5, and those were unlikely to implant if transferred to surrogate mothers.
Statistical analysis
All experiments were repeated at least three times using independent collections of embryos. For morphological assessment of embryo development, the average and standard deviation were calculated for each developmental stage category, and compared between the KSOM and ESCM groups by Student’s t-test. For the analyses of cell numbers and cavity volume, all the data were compiled, and compared between the KSOM and ESCM groups by Student’s t-test. For gene expression analyses, the average and standard deviation of relative expression levels were calculated from the three biological replicates, and compared between the KSOM and ESCM groups by Student’s t-test.
Results
Blastocyst development is impaired when embryos are cultured in ESCM from the 2-cell stage
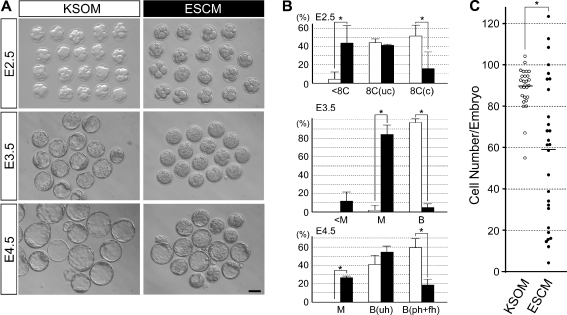
Two-cell stage embryos (E1.5) were divided into two groups: one group was cultured in KSOM and the other was in ESCM. Morphologies of embryos were assessed daily until E4.5 (Fig. 1a). When cultured in KSOM, most embryos reached the 8-cell stage in 24 h (E2.5). Although many embryos in ESCM also reached the 8-cell stage, a distinct number of embryos were still at the 4 ~ 7-cell stage (Fig. 1a,b). In 48 h (E3.5), many embryos in KSOM formed a blastocyst cavity, whereas most embryos in ESCM did not (Fig. 1a,b). The embryos in ESCM appeared to be at morula stage, although they occupied most of the space in the zona pellucida (ZP), thus appearing larger than a typical morula in KSOM (Fig. 1a). In 72 h (E4.5), most embryos in KSOM became blastocysts with an expanded cavity. Many embryos in ESCM also became blastocysts in 72 h. However, the number of embryos that formed a blastocyst cavity was lower in ESCM than in KSOM (Fig. 1b). Furthermore, the number of blastocysts that were partly or fully hatched from ZP was significantly lower in ESCM (Fig. 1b). Thus, the culture of 2-cell stage embryos in ESCM impaired various aspects of development, namely cleavage, cavitation, and hatching.
Fig. 1.
Development of mouse preimplantation embryos when cultured from the 2-cell stage in KSOM or ESCM. a Morphologies of embryos after 24 h (E2.5), 48 h (E3.5) and 72 h (E4.5) of culture. Scale bar = 50 μm. b Progression of embryo development at E2.5, E3.5, and E4.5. The average values and standard deviations are based on the analyses of three independent sets of experimental specimens. Significantly fewer number of embryos reached the compacted 8-cell stage at E2.5 in the ESCM group than in the KSOM group (asterisks indicate p < 0.05; Student’s t-test). A total of 42 and 44 embryos were examined for the KSOM (white bar) and ESCM (black bar) groups, respectively. <8C: 4–7 cells; 8C(uc): uncompacted 8 cells; 8C(c): compacted 8 cells; <M: 8 or fewer cells; M: morula; B: blastocyst; B(uh): unhatched blastocyst; B(ph + fh): partly or fully hatched blastocyst. c The total cell number per embryo, assessed by observation of serial confocal optical sections of embryos stained with propidium iodide (n = 27 for KSOM and n = 28 for ESCM). Each circle represents the total cell number of an individual embryo. The average cell number for each group is represented by a horizontal bar. The average cell number of KSOM-cultured embryos is significantly higher than that of ESCM-cultured embryos (asterisk indicates p < 0.01; Student’s t-test)
We scored the total cell number of each embryo at E4.5, and compared between KSOM and ESCM cultures. On average, the embryo in KSOM contained 89.5 cells (n = 27), while that in ESCM contained 59.2 cells (n = 28) (Fig. 1c), confirming that ESCM impaired cleavage (Fig. 1a). Notably, the cell numbers in ESCM were markedly variable among embryos, ranging from 4 to 124, and some embryos in ESCM had a higher cell count than any of the embryos in KSOM (Fig. 1c). This suggests that ESCM is not entirely detrimental to embryo development, although it impaired embryo development on average.
Expansion of blastocyst cavity and hatching are enhanced when embryos are cultured in ESCM from the 8-cell stage
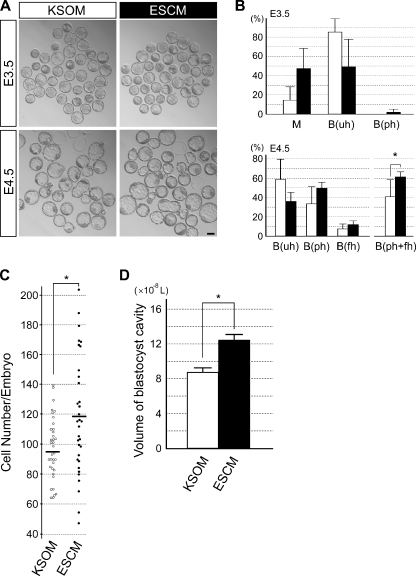
We then examined the effects of ESCM when embryos were cultured from the 8-cell stage (E2.5). After the collection from the oviducts, 8-cell stage embryos were cultured either in KSOM or ESCM. The number of embryos that developed into blastocysts within 24 h of culture (E3.5) were higher in KSOM than in ESCM (Fig. 2a,b). However, by 48 h (E4.5), the number of blastocysts that either partly or fully hatched from ZP was higher in ESCM than in KSOM (Fig. 2a,b). The average cell number at E4.5 was significantly higher in embryos cultured in ESCM (118.3 cells; n = 33) than in KSOM (96.8 cells; n = 36) (Fig. 2c). Furthermore, the average size of the blastocyst cavity was significantly larger in embryos in ESCM than in KSOM (Fig. 2d). Therefore, when cultured from the 8-cell stage, ESCM promotes blastocyst development with respect to cell division, hatching, and cavity expansion.
Fig. 2.
Development of mouse preimplantation embryos when cultured from 8-cell stage in KSOM or ESCM. a Morphologies of embryos after 24 h (E3.5) and 48 h (E4.5) of culture. Scale bar = 50 μm. b Progression of embryo development at E3.5 and E4.5. The average values and standard deviations are based on the analyses of six independent sets of experimental specimens. A total of 161 and 167 embryos were examined for the KSOM (white bar) and ESCM (black bar) groups, respectively. Significantly higher number of embryos reached the hatched blastocyst stages at E4.5 in the ESCM groups than in the KSOM group (asterisks indicate p < 0.05; Student’s t-test). M: morula; B(uh): unhatched blastocyst; B(ph): partly hatched blastocyst; B(fh): fully hatched blastocyst. c The total cell number per E4.5 embryo, assessed by observation of serial confocal optical sections of embryos stained with propidium iodide (n = 36 for KSOM and n = 33 for ESCM). Each circle represents the total cell number of an individual embryo. The average cell number for each group is represented by a horizontal bar. The average cell number of ESCM-cultured embryos is significantly higher than that of KSOM-cultured embryos (asterisk indicates p < 0.01; Student’s t-test). d The volume of blastocyst cavity, estimated based on the diameters of the cavity of photographed embryos (n = 112 for KSOM and n = 110 for ESCM). The graphs show the average value and standard deviation. The average cavity volume of ESCM-cultured embryos is significantly higher than that of KSOM-cultured embryos (asterisk indicates p < 0.01; Student’s t-test)
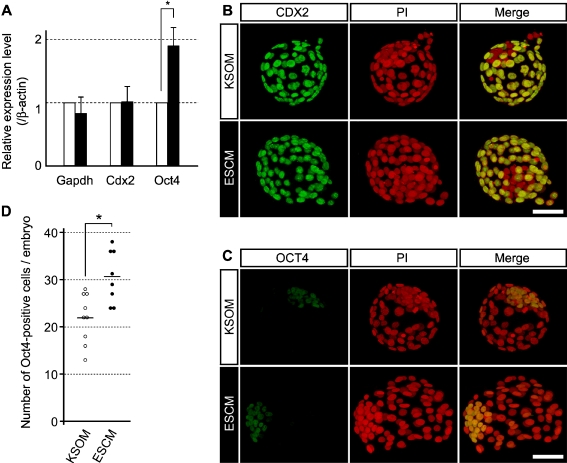
Although the effective cavity expansion and hatching indicate the epithelial integrity of TE, other characteristics of TE may be compromised by ESCM, which is formulated to culture ES cells. Thus, we investigated the expressions of Cdx2 and Oct4, which are the key transcription factors for the formation of TE and ICM, respectively [34–36]. Quantitative RT-PCR analysis of embryos at E4.5 showed that the Cdx2 mRNA level was similar between the KSOM and ESCM groups (Fig. 3a). In contrast, the Oct4 mRNA level was significantly elevated in the ESCM group compared to the KSOM group (Fig. 3a). This is consistent with the activity of ESCM, which is to maintain ICM-like features in ES cells. However, it raises a concern that ESCM may cause ectopic expression of Oct4 in TE. Thus, we examined the spatial expressions of CDX2 and OCT4 proteins by immunocytochemistry. In both KSOM and ESCM groups, CDX2 protein was localized to the nuclei of the outer layer but not of the internal cell population, indicating that CDX2 was specifically expressed in TE (Fig. 3b). Likewise, in both groups, OCT4 protein was enriched in the nuclei of the internal cell population (Fig. 3c). These results suggest that ESCM does not impair the spatial expression patterns of the lineage-specific transcription factors. Notably, the average number of OCT4-positive cells per embryo was significantly higher when cultured in ESCM (30.6 cells; n = 8) than in KSOM (21.9 cells; n = 9) (Fig. 2d). Thus, the increase in the number of OCT4-positive cells may be contributing to the increase in the Oct4 mRNA level (Fig. 3a) in embryos cultured in ESCM.
Fig. 3.
Expression of TE-specific and ICM-specific transcription factors, CDX2 and OCT4, at E4.5 in embryos cultured from the 8-cell stage in KSOM or ESCM. a qRT-PCR analysis of Cdx2 and Oct4 mRNA levels. Relative expression levels of Gapdh, Cdx2, and Oct4 are normalized with that of β-actin. In this graph, the relative expression level of each gene in KSOM-cultured embryos is presented as 1. The average values and standard deviations are based on the analyses of three independent sets of experimental specimens. The expression level of Oct4 is significantly higher in ESCM-cultured embryos than in KSOM-cultured embryos (asterisk indicates p < 0.01; Student’s t-test). b,c) Projected confocal optical images of representative embryos that are stained for CDX2 (b) or for OCT4 (c), shown in green. Nuclei are stained with propidium iodide (PI), shown in red. Scale bars = 50 μm. d The number of OCT4-positive cells per embryo, assessed by observation of serial confocal optical sections of embryos stained with anti-OCT4 antibody (n = 9 for KSOM and n = 8 for ESCM). Each circle represents OCT4-positive cell number of an individual embryo. The average OCT4-positive cell number for each group is represented by a horizontal bar, which is significantly higher in ESCM-cultured embryos than in KSOM-cultured embryos (asterisk indicates p < 0.01; Student’s t-test)
ESCM does not compromise the efficiencies of implantation and fetal development
Although the blastocysts that developed from the 8-cell stage in ESCM appeared intact, their ability to support implantation and fetal development needed to be verified. Thus, we transferred blastocysts (E4.5), which were cultured in either KSOM or ESCM from the 8-cell stage, into the uteri of surrogate females at 3.5 days post coitum (d.p.c.), and their development was examined at 14.5 ~ 15.5 d.p.c. The numbers of implantation sites and fetuses were not significantly different between the KSOM and ESCM groups (Table 1). Also, the size and gross morphology of fetuses were not evidently different between the two groups. These results suggest that the culture of embryos in ESCM from the 8-cell to the blastocyst stages does not impair post-implantation development, specifically the efficiency of implantation and fetal development.
Table 1.
Efficiency of implantation and postimplantation development of cultured blastocysts
| Culture Medium | No. of blastocysts transferred (No. of surrogates) | No. of implantationa (% of blastocyst transferred) | No. of abnormal fetus | No. of normal fetus (% of implantation) |
|---|---|---|---|---|
| KSOM | 55 (5) | 49 (89.0%) | 1b | 28 (57.1%)c |
| ESCM | 62 (6) | 52 (83.9%) | 0 | 25 (48.1%)c |
a Total number of residual implantation marks, and abnormal and normal fetus
b The fetus was evidently small in size and white in color, possible sign of necrosis
c Comparison by chi-square test did not yield significant difference (p = 0.36)
Discussion
We investigated the effect of ESCM on the development of mouse preimplantation stage embryos, with a scope to improve the compositions of embryo culture media. The impact of ESCM on development was strikingly different between cultures from the 2-cell stage and from 8-cell stage. The former slowed down cell divisions, whereas the latter promoted cell division and the development of blastocyst, particularly of ICM. Importantly, culturing from the 8-cell stage in ESCM did not impair the expression patterns of key transcription regulators for TE and ICM formation, and neither did it significantly compromise the rates of implantation and fetal development. Although this study alone is not sufficient to conclude that ESCM is superior to KSOM for embryo culture, it raises the possibility that culture media with more complex chemical composition than KSOM could enhance the developmental potential of embryos. Other complex media that have been used for mouse ES cells may also exhibit similar beneficial effect on the 8-cell stage embryos. However, conventional ES cell culture media contain undefined components, such as animal serum, whereas the ESCM used in the present study is chemically defined and does not contain animal serum. In light of potential application to human ART, it would be critical to employ chemically defined media for safety as well as consistency.
One of the components in ESCM that may have impaired development of 2-cell stage embryos is the glucose in high concentrations: KSOM contains 0.2 mM, whereas ESCM contains 17.5 mM. Several studies have suggested that a high glucose concentration is detrimental to embryo development, specifically when cultured before the 8-cell stage [37–41]. This is likely a reflection of the dynamic change in carbohydrate utilization during preimplantation development [37, 42–45]. However, other studies suggest that the inhibitory effect of high glucose concentration can be alleviated by the addition of non-essential amino acids, glutamine and alanine [46, 47]. Considering that ESCM contains these amino acids, it is unlikely that the high glucose concentration alone impaired development. Another potential aspect of ESCM that might have impaired the development of 2-cell stage embryos is the lack of EDTA. EDTA is included in various chemically defined culture media for mouse and human embryos, and it has been shown to promote development from one-cell stage [48, 49]. The action of EDTA to enhance embryo development is not fully understood, but it apparently involves activities other than its action to chelate metal ions [50]. It is of particular interest to test whether the addition of EDTA to ESCM could eliminate its detrimental effect on the 2-cell stage embryos.
ESCM promoted the formation of the blastocyst when cultured from the 8-cell stage. It is difficult to pinpoint specific components that contributed to this potentially beneficial effect. However, it is worth noting that ESCM contains several growth factors, namely insulin, BMP4, and LIF, all of which are absent in KSOM. Currently, growth factors are not included in commercial culture media for mouse or human embryos because many feel the current culture media work adequately and fear that growth factors may cause unexpected developmental anomalies [51]. However, numerous mouse studies have shown that supplementation with growth factors, such as epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), transforming growth factor-alpha (TGF-α) and transforming growth factor-beta (TGF-β) improves in vitro development. For example, EGF, TGF-α, and TGF-β all promote development to the blastocyst stage, increase cell numbers [52], and stimulate trophoblast outgrowth [53]. Insulin-like growth factor 1 (IGF-1) has been shown to stimulate ICM growth in particular [54]. Furthermore, both mouse and human female reproductive tracts secrete various growth factors, such as IGF-1, EGF, vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), and the embryo itself expresses receptors for these growth factors [55–60]. Also, the cumulus, granulosa cells, and embryos themselves secrete growth factors [61–67]. LIF in particular may be important in early embryo-maternal dialogue: LIF is crucial for implantation in mouse embryos [68], and dysregulation of LIF has been suggested as a cause for human infertility [69].
Although mimicking the actual microenvironment of the female reproductive tract may not necessarily improve embryo culture media [7], it seems intuitive that in vitro conditions ought to reflect the natural environment for optimal embryo development. Such attempts are most exemplified by a “two-step protocol”, which uses two sequential media to mimic the physiological changes that occur when embryos move from the oviduct to the uterus [70]. However, complete agreement on the superiority of the use of sequential media is still lacking because successful pregnancies have been achieved in both single medium and sequential media protocols [71–75]. The present study is supportive of potential benefit of sequential media protocols, because the response of embryos to ESCM dramatically changed between the 2-cell and the 8-cell stages. However, it is important to point out that the 8-cell stage embryos used in the present studies were collected from the oviducts (i.e., in vivo embryos), which may behave differently from the 8-cell stage embryos that develop in culture medium after in vitro fertilization (i.e., in vitro embryos). Further studies are essential to compare the responses of in vivo and in vitro 8-cell stage embryos to ESCM in light of potential application to develop better two-step protocols.
Recent studies show that developmental paucity of experimentally manipulated embryos can be “rescued” by culturing from the 8-cell stage in ESCM [31, 76]. Such manipulations include the knockdown of Pard6b and Cdx2 genes by injection of RNA interference agents, which severely compromises embryo development in KSOM. These studies support the notion that ESCM can potentiate development of cultured embryos.
Our data show that ESCM increased the level of Oct4 mRNA and the number of Oct4-positive cells in the embryo, which is consistent with the activity of ESCM to maintain various features of ICM in ES cell culture. However, an alternative explanation is that the embryos in ESCM were simply further along in development, and cells of the ICM had proliferated, therefore yielding this elevated level of Oct4. ESCM indeed increased the total cell number, the size of blastocyst cavity, and the efficiency of hatching. All these observations are consistent with the idea that ESCM essentially speeds up development. However, the qRT-PCR study indicated that the level of Cdx2 was essentially the same between the KSOM and ESCM groups (Fig. 3a), suggesting that cells of TE did not proliferate as much as cells of ICM in ESCM. Thus, certain features of TE, i.e., cavity expansion and hatching, were enhanced in ESCM, whereas the other feature, i.e., Cdx2 expression, was not. Interestingly, Cdx2 is important for the differentiation of trophoblast to enable implantation and placenta formation, but it is dispensable for the blastocyst cavity formation [36, 77]. Thus, it is possible that the impact of ESCM on blastocyst development may be more selective than generally speeding up development.
A recent concern for ART is that in vitro culture may cause epigenetic alterations that impact the health of animals in the long-term. Model animal studies show that culture of preimplantation embryos up to the blastocyst stage in a simple, defined medium causes significant changes in gene expression and DNA methylation patterns when compared to development in vivo [78–82]. While the physiologic impact of these epigenetic alterations is still unknown, mice that are derived from in vitro cultured embryos exhibit several behavioral abnormalities [83, 84]. Thus, culture in a simple medium may cause subtle yet significant health problems. ESCM has been used to culture ES cells for long periods of time without significantly altering their genetic and epigenetic states, suggesting that the epigenetic integrity of embryonic cells is relatively stable in ESCM. Thus, more complex media, like ESCM, may be more suited for ART, as they could potentially minimize epigenetic alterations during in vitro culture. Thus, future studies are to focus on the potential role of ESCM, particularly in sequential media using zygotes generated from IVF. A move towards more complex media in a sequential culture protocol may be the next step in the improvement of embryo culture media.
Acknowledgement
This work was supported by NIH grants (P20RR024206 to VBA; G12RR003061 and P20RR016453 to the JABSOM Imaging Core).
Footnotes
Capsule
Blastocyst development is enhanced when embryos are cultured from the 8-cell stage in the embryonic stem cell culture medium, compared to conventional KSOM medium.
References
- 1.Whitten WK. Culture of tubal ova. Nature. 1956;177:96. doi: 10.1038/177096a0. [DOI] [PubMed] [Google Scholar]
- 2.McLaren A, Biggers JD. Successful development and birth of mice cultivated in vitro as early embryos. Nature. 1958;182:877–878. doi: 10.1038/182877a0. [DOI] [PubMed] [Google Scholar]
- 3.Chang MC. Fertilization of rabbit ova in vitro. Nature. 1959;193:466–467. doi: 10.1038/184466a0. [DOI] [PubMed] [Google Scholar]
- 4.Lawitts JA, Biggers JD. Overcoming the 2-cell block by modifying standard components in a mouse embryo culture medium. Biol Reprod. 1991;45:245–251. doi: 10.1095/biolreprod45.2.245. [DOI] [PubMed] [Google Scholar]
- 5.Lawitts JA, Biggers JD. Optimization of mouse embryo culture media using simplex methods. J Reprod Fertil. 1991;91:543–556. doi: 10.1530/jrf.0.0910543. [DOI] [PubMed] [Google Scholar]
- 6.Lawitts JA, Biggers JD. Culture of preimplantation embryos. Meth Enzymol. 1993;225:153–164. doi: 10.1016/0076-6879(93)25012-Q. [DOI] [PubMed] [Google Scholar]
- 7.Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DK, Lane M. Embryo culture system. In: Gardner DK, editor. In vitro fertilization: a practical approach. New York: Informa Healthcare USA Inc.; 2007. [Google Scholar]
- 9.Schultz RM, Williams CJ. The science of ART. Science. 2002;296:2188–2190. doi: 10.1126/science.1071741. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2008 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2010.
- 11.Gardner DK, Lane M, Schoolcraft WB. Culture and transfer of viable blastocysts: a feasible proposition for human IVF. Hum Reprod. 2000;15(Suppl 6):9–23. [PubMed] [Google Scholar]
- 12.Milki AA, Hinckley MD, Fisch JD, Dasig D, Behr B. Comparison of blastocyst transfer with day 3 embryo transfer in similar patient populations. Fertil Steril. 2000;73:126–129. doi: 10.1016/S0015-0282(99)00485-9. [DOI] [PubMed] [Google Scholar]
- 13.Barratt CL. St John JC, Afnan M. Clinical challenges in providing embryos for stem-cell initiatives. Lancet. 2004;364:115–118. doi: 10.1016/S0140-6736(04)16649-X. [DOI] [PubMed] [Google Scholar]
- 14.Hardy K, Spanos S, Becker D, Iannelli P, Winston RML, Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci USA. 2001;98:1655–1660. doi: 10.1073/pnas.98.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behr B. Blastocyst culture and transfer. Hum Reprod. 1999;14:5–6. doi: 10.1093/humrep/14.1.5. [DOI] [PubMed] [Google Scholar]
- 16.Jun SH, Choi B, Westphal L, Behr B, Reijo Pera RA, Wong WH, et al. Defining human embryo phenotypes with cohort-specific prognostic factors in in vitro fertilization. PLoS ONE. 2008;3:e2562. doi: 10.1371/journal.pone.0002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ. 2006;174:341–348. doi: 10.1503/cmaj.050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox CF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of L1T1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Bouc Y. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 23.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 25.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/S0092-8674(03)00847-X. [DOI] [PubMed] [Google Scholar]
- 26.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 27.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 29.Amit M, Carpenter MK, Inokuma MS, Chiu C, Harris CP, Maknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 30.Alarcon VB, Marikawa Y. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol Reprod. 2003;83:347–358. doi: 10.1095/biolreprod.110.084400. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon VB. Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biol Reprod. 2010;69:1208–1212. doi: 10.1095/biolreprod.103.018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. Proc Natl Acad Sci USA. 2001;98:13501–13506. doi: 10.1073/pnas.241517598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward MA, Kaneko T, Kusakabe H, Biggers JD, Whittingham DG, Yanagimachi R. Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol Reprod. 2003;69:2100–2108. doi: 10.1095/biolreprod.103.020529. [DOI] [PubMed] [Google Scholar]
- 34.Nichols J, Zevnick B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/S0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 35.Niwa H, Miyazaki J, Smith A. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2004;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 36.Strumpf D, Mao C, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 37.Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biggers JD, Gardner DK, Leese HJ. Control of carbohydrate metabolism in preimplantation mammalian embryos. In: Rosenblum IY, Heyner S, editors. Regulation of growth in development. Boca Raton: CRC Press; 1989. pp. 19–32. [Google Scholar]
- 39.Kim JH, Funahashi H, Niwa K, Okuda K. Glucose requirement at different developmental stages of in-vitro fertilised bovine embryos cultured in semi-defined medium. Theriogenology. 1993;39:875–886. doi: 10.1016/0093-691X(93)90425-5. [DOI] [PubMed] [Google Scholar]
- 40.Conaghan J, Handyside AH, Winston RML, Leese HJ. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J Reprod Fertil. 1993;99:87–95. doi: 10.1530/jrf.0.0990087. [DOI] [PubMed] [Google Scholar]
- 41.Gardner DK. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology. 1998;49:83–102. doi: 10.1016/S0093-691X(97)00404-4. [DOI] [PubMed] [Google Scholar]
- 42.Brinster RL. Studies on the development of mouse embryos in vitro IV. Interaction of energy sources. J Reprod Fertil. 1965;10:227–240. doi: 10.1530/jrf.0.0100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner DK, Leese HJ. Non-invasive measurement of nutrient uptake by single cultured preimplantation mouse embryos. Hum Reprod. 1986;1:25–27. doi: 10.1093/oxfordjournals.humrep.a136336. [DOI] [PubMed] [Google Scholar]
- 44.Hardy K, Hooper MAK, Handyside AH, Rutherford AJ, Winston RML, Leese HJ. Noninvasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum Reprod. 1989;4:188–191. doi: 10.1093/oxfordjournals.humrep.a136869. [DOI] [PubMed] [Google Scholar]
- 45.Gardner DK, Lane M, Batt PA. The uptake and metabolism of pyruvate and glucose by individual pre-attachment sheep embryos developed in vivo. Mol Reprod Dev. 1993;36:313–319. doi: 10.1002/mrd.1080360305. [DOI] [PubMed] [Google Scholar]
- 46.Lane M, Gardner DK. Amino acids and vitamins prevent culture-induced metabolic perturbations and associated loss of viability of mouse blastocysts. Hum Reprod. 1998;13:991–997. doi: 10.1093/humrep/13.4.991. [DOI] [PubMed] [Google Scholar]
- 47.Barnett DK, Bavister BD. Inhibitory effect of glucose and phosphate on the second cleavage division of hamster embryos: is it linked to metabolism? Hum Reprod. 1996;11:177–183. doi: 10.1093/oxfordjournals.humrep.a019013. [DOI] [PubMed] [Google Scholar]
- 48.Abramczuk J, Solter D, Koprowski H. The beneficial effect of EDTA on development of mouse one-cell embryos in chemically defined medium. Dev Biol. 1977;61:378–383. doi: 10.1016/0012-1606(77)90308-6. [DOI] [PubMed] [Google Scholar]
- 49.Gardner DK, Lane M. Alleviation of the 2-cell block and development to the blastocyst of CF1 mouse embryos: role of amino acids, EDTA and physical parameters. Hum Reprod. 1996;11:2703–2712. doi: 10.1093/oxfordjournals.humrep.a019195. [DOI] [PubMed] [Google Scholar]
- 50.Matsukawa T, Ikeda S, Imai H, Yamada M. Alleviation of the two-cell block of ICR mouse embryos by polyaminocarboxylate metal chelators. Reproduction. 2002;124:65–71. doi: 10.1530/rep.0.1240065. [DOI] [PubMed] [Google Scholar]
- 51.Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol. 2008;20:292–304. doi: 10.1097/GCO.0b013e3282fe743b. [DOI] [PubMed] [Google Scholar]
- 52.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Dev Biol. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haimovici F, Anderson DJ. Effects of growth factors and growth factor-extracellular matrix interactions on mouse trophoblast outgrowth in vitro. Biol Reprod. 1993;49:124–130. doi: 10.1095/biolreprod49.1.124. [DOI] [PubMed] [Google Scholar]
- 54.Harvey MB, Kaye PL. Insulin-like growth factor-1 stimulates growth of mouse preimplantation embryos in vitro. Mol Reprod Dev. 1992;31:195–199. doi: 10.1002/mrd.1080310306. [DOI] [PubMed] [Google Scholar]
- 55.Chia CM, Winston RM, Handyside AH. EGF, TGF-alpha and EGFR expression in human preimplantation embryos. Development. 1995;121:299–307. doi: 10.1242/dev.121.2.299. [DOI] [PubMed] [Google Scholar]
- 56.Sharkey AM, Dellow K, Blayney M, Macnamee M, Charnock-Jones S, Smith SK. Stage-specific expression of cytokine and receptor messenger ribonucleic acids in human preimplantation embryos. Biol Reprod. 1995;53:974–981. doi: 10.1095/biolreprod53.4.974. [DOI] [PubMed] [Google Scholar]
- 57.Smotrich DB, Stillman RJ, Widra EA, Gindoff PR, Kaplan P, Graubert M, et al. Immunocytochemical localization of growth factors and their receptors in human pre-embryos and Fallopian tubes. Hum Reprod. 1996;11:184–190. doi: 10.1093/oxfordjournals.humrep.a019014. [DOI] [PubMed] [Google Scholar]
- 58.Osterlund C, Wramsby H, Pousette A. Temporal expression of platelet-derived growth factor (PDGF)-A and its receptor in human preimplantation embryos. Mol Hum Reprod. 1996;2:507–512. doi: 10.1093/molehr/2.7.507. [DOI] [PubMed] [Google Scholar]
- 59.Lighten AD, Hardy K, Winston RM, Moore GE. Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol Reprod Dev. 1997;47:134–139. doi: 10.1002/(SICI)1098-2795(199706)47:2<134::AID-MRD2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 60.Moller B, Rasmussen C, Lindblom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod. 2001;7:65–72. doi: 10.1093/molehr/7.1.65. [DOI] [PubMed] [Google Scholar]
- 61.Watson R, Anthony F, Pickett M, Lambden P, Masson GM, Thomas EJ. Reverse transcription with nested polymerase chain reaction shows expression of basic fibroblast growth factor transcripts in human granulosa and cumulus cells from in vitro fertilisation patients. Biochem Biophys Res Commun. 1992;187:1227–1231. doi: 10.1016/0006-291X(92)90434-M. [DOI] [PubMed] [Google Scholar]
- 62.Blasio AM, Vigano P, Cremonesi L, Carniti C, Ferrari M, Ferrari A. Expression of the genes encoding basic fibroblast growth factor and its receptor in human granulosa cells. Mol Cell Endocrinol. 1993;96:R7–R11. doi: 10.1016/0303-7207(93)90111-V. [DOI] [PubMed] [Google Scholar]
- 63.Muttukrishna S, Groome N, Ledger W. Gonadotropic control of secretion of dimeric inhibins and activin A by human granulosa-luteal cells in vitro. J Assist Reprod Genet. 1997;14:566–574. doi: 10.1023/A:1022524516824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piccinni MP, Scaletti C, Mavilia C, Lazzeri E, Romagnani P, Natali I, et al. Production of IL-4 and leukemia inhibitory factor by T cells of the cumulus oophorus: a favorable microenvironment for preimplantation embryo development. Eur J Immunol. 2001;31:2431–2437. doi: 10.1002/1521-4141(200108)31:8<2431::AID-IMMU2431>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 65.Svalander PC, Holmes PV, Olovsson M, Wikland M, Gemzell-Danielsson K, Bygdeman M. Platelet-derived growth factor is detected in human blastocyst culture medium but not in human follicular fluid - a preliminary report. Fertil Steril. 1991;56:367–369. [PubMed] [Google Scholar]
- 66.Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S, et al. Cytokine involvement in oocytes and early embryos. Fertil Steril. 1991;56:265–272. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
- 67.Hemmings R, Langlais J, Falcone T, Granger L, Miron P, Guyda H. Human embryos produce transforming growth factors alpha activity and insulin-like growth factors II. Fertil Steril. 1992;58:101–104. doi: 10.1016/s0015-0282(16)55144-9. [DOI] [PubMed] [Google Scholar]
- 68.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 69.Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, et al. Interleukin-11, IL-11 receptor alpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69:53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–3440. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- 71.Biggers JD, Racowsky C. The development of fertilized human ova to the blastocyst stage in KSOMAA medium: is a two-step protocol necessary? Reprod Biomed Online. 2002;5:133–140. doi: 10.1016/S1472-6483(10)61615-X. [DOI] [PubMed] [Google Scholar]
- 72.Macklon NS, Pieters MHEC, Hassan MA, Jeucken PHM, Eijkemans MJC, Fauser BCJM. A prospective randomized comparison of sequential versus monoculture systems for in-vitro human blastocyst development. Hum Reprod. 2002;17:2700–2705. doi: 10.1093/humrep/17.10.2700. [DOI] [PubMed] [Google Scholar]
- 73.Biggers JD, Summers MC. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90:473–483. doi: 10.1016/j.fertnstert.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling embryo study. Fertil Steril. 2009;92:1783–1786. doi: 10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Paternot G, Debrock S, D’Hooghe TM, Spiessens C. Early embryo development in a sequential versus single medium: a randomized study. Reprod Biol Endocrin. 2010;8:83. doi: 10.1186/1477-7827-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu G, Gentile L, Do JT, Cantz T, Sutter J, Psathaki K, et al. Efficient Derivation of Pluripotent Stem Cells from siRNA-Mediated Cdx2-Deficient Mouse Embryos. Stem Cells Dev 2011;20:485–93. [DOI] [PMC free article] [PubMed]
- 77.Ralston A, Rossant J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev Biol. 2008;313:614–629. doi: 10.1016/j.ydbio.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 78.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 79.Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WMIII, Biniszkiewicz D, et al. Epigenetic instability in ES cells and cloned mice. Science. 2001;293:95–97. doi: 10.1126/science.1061402. [DOI] [PubMed] [Google Scholar]
- 80.Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod. 2001;64:918–926. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- 81.Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- 82.Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18(20):3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, et al. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandez-Gonzalez R, Moreira PN, Perez-Crespo M, Sanchez-Martin M, Ramirez MA, Pericuesta E, et al. Long term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–772. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]