Abstract
Introduction
Embryo selection can be carried out via morphological criteria or by using genetic studies based on Preimplantation Genetic Screening. In the present study, we evaluate the clinical validity of Preimplantation Genetic Screening with fluorescence in situ hybridization (PGS-FISH) compared with morphological embryo criteria.
Material and methods
A systematic review was made of the bibliography, with the following goals: firstly, to determine the prevalence of embryo chromosome alteration in clinical situations in which the PGS-FISH technique has been used; secondly, to calculate the statistics of diagnostic efficiency (negative Likelihood Ratio), using 2 × 2 tables, derived from PGS-FISH. The results obtained were compared with those obtained from embryo morphology. We calculated the probability of transferring at least one chromosome-normal embryo when it was selected using either morphological criteria or PGS-FISH, and considered what diagnostic performance should be expected of an embryo selection test with respect to achieving greater clinical validity than that obtained from embryo morphology.
Results
After an embryo morphology selection that produced a negative result (normal morphology), the likelihood of embryo aneuploidies was found to range from a pre-test value of 65% (prevalence of embryo chromosome alteration registered in all the study groups) to a post-test value of 55% (Confidence interval: 50–61), while after PGS-FISH with a negative result (euploid), the post-test probability was 42% (Confidence interval: 35–49) (p < 0.05). The probability of transferring at least one euploid embryo was the same whether 3 embryos were selected according to morphological criteria or whether 2, selected by PGS-FISH, were transferred. Any embryo selection test, if it is to provide greater clinical validity than embryo morphology, must present a LR-value of 0.40 (Confidence interval: 0.32–0.51) in single embryo transfer, and 0.06 (CI: 0.05–0.07) in double embryo transfer.
Discussion
With currently available technology, and taking into account the number of embryos to be transferred, the clinical validity of PGS-FISH, although superior to that of morphological criteria, does not appear to be clinically relevant.
Keywords: PGS-FISH, Embryo morphology, Embryo selection, Clinical validity
Introduction
For many years, the evaluation of embryos has been based on embryo morphology determined in accordance with criteria of embryo quality [1–3]. Nevertheless, other selection techniques have been proposed, to make a more direct assessment of the chromosomal complement of blastomeres. PGS-FISH is carried out for couples who do not have a known genetic defect but who appear to be at high risk of aneuploidy because of Advanced Maternal Age (AMA); Repeated Miscarriage (RM); Repeated Implantation Failure (RIF) or Male Factor (MF) [4–8]. It was assumed that screening for aneuploidy embryos and transferring only euploid embryos would reduce pregnancy losses and increase live birth rates. Several randomized controlled trials have shown, however, that PGS-FISH would appear not to be effective in improving live birth rates in IVF or intracytoplasmic sperm injection for these couples [9–19]. The methodology used in some of these clinical trials has been severely criticized, and their conclusions questioned [7, 8, 20, 21] thus giving rise to considerable controversy as to the true clinical validity of PGS-FISH. However, different authors have demonstrated the diagnostic validity of PGS-FISH, using as a gold standard the results obtained by subsequent rounds of FISH [22, 23] or otherwise by analysing the embryo at more advanced stages of development, using FISH [17, 24–28]. The good results thus obtained contradict those of the above-mentioned clinical trials.
One way to assess the usefulness of a screening test applied to embryo selection is to calculate the ratio of the probability of a given test producing a negative result for a euploid embryo to the probability of the same test producing a negative result for an aneuploid embryo. This is termed the negative likelihood ratio (LR-). The likelihood ratio is of enormous practical value, and it is becoming the preferred way of expressing and comparing the usefulness of different tests [29, 30]. In order to interpret the LR- it is necessary to determine the pre-test probability (the prevalence) of embryo aneuploidy. Moreover, before including such a test in daily practice, its clinical validity must be compared with that of other, existing tests. In the case of PGS-FISH for embryo selection, this comparison must be made with the embryo morphology method [31, 32].
Therefore, a PubMed search was performed and statistics of diagnostic efficiency calculated in order to compare the clinical validity of PGS-FISH with embryo morphology and thus determine whether the use of PGS-FISH would give more information than that obtained from embryo morphology. Furthermore, by applying the LR- thus obtained to a theoretical model based on hypergeometric probability statistics, we estimated the probability of transferring at least one chromosome-normal embryo, when the latter was selected either by morphological criteria or by the PGS-FISH method. The hypergeometric distribution is a discrete probability distribution that describes the number of successes in a sequence of n draws from a finite population without replacement, just as the binomial distribution describes the number of successes for draws with replacement. Perhaps the easiest way to understand this distribution is in terms of urn models. Suppose you are to draw n marbles without replacement from an urn containing N marbles in total, m of which are white. The hypergeometric distribution describes the distribution of the number of white marbles drawn from the urn. We calculated indeed the diagnostic performance to be required of a test of embryo selection for it to be considered of greater clinical validity than that obtained by the embryo morphology method.
Material and methods
To define the prevalences of chromosome abnormalities for the different groups (AMA, RM, RIF and MF) we have examined the review carried out by Donoso et al. [33]. We also have carried out an extensive review of other published studies. Furthermore, in order to compare the clinical validity of PGS-FISH and embryo morphology in embryo selection, we carried out a systematic search in PubMed, to enable the reconstruction of 2 × 2 tables (true positive, false positive, true negative and false negative).
To define the prevalence values, we took 16 papers from the review by Donoso et al. [33] 12 of which were finally included, 3 rejected because of a confounding of study groups within the population analysed and the other one rejected because of study group of women under 38 years studied. After consulting other information sources, via citations, a further 7 papers were included.
In the systematic search for relevant studies for the 2 × 2 table, the terms “FISH” and “PGD-AS”, and “CGH” and “PGD-AS” were combined (up to and including June 2009). Of the 23 potentially useful articles thus found, and of the 5 others located following an extensive review of the relevant bibliography, the only ones finally included were those that contained (or enabled the reconstruction of) 2 × 2 tables such that diagnostic efficiency statistics could be derived. Thus, a total of 8 articles were included. In the case of the studies that evaluated morphology as a selection criterion for embryo selection, the gold standard was held to be FISH, while for those studies evaluating FISH as a criterion for embryo selection, Comparative genomic hybridization (CGH) was taken as the gold standard. According to Standards for Reporting of Diagnostic Accuracy (STARD) an abnormal result (aneuploidies) is denominated positive [34].
In order to compare the clinical validity of PGS-FISH and embryo morphology in embryo selection, a statistical analysis was carried out as described below. The following diagnostic efficiency statistics were used for this comparison: sensitivity, specificity, LR+, LR- and DOR (Diagnostic odds ratio). In addition, the positive predictive value (PPV) and the negative predictive value (NPV) were calculated. To investigate all studies in a way that was standardised for predictive values, two strategies were used: (a) assuming unconditional predictive values (uPPV; uNPV) [35]; (b) fixing prevalence values (65%). The median value for chromosome abnormalities among all the groups was 65%, and so this was taken as the prevalence value (pre-test probability) of embryo aneuploidy for subsequent calculations.
In all cases, the point estimations of the diagnostic efficiency statistics and the asymptotic confidence intervals were calculated. For the case of uPPV and uNPV, bootstrap intervals were computed, because explicit expressions for standard errors of the estimates were not available [35]. When the 2 × 2 tables contained zero cells, reasonable estimates of some parameters (likelihood ratio, odds ratio, etc.) were not possible. In order to avoid these problems, 0.5 was added to all cells in the table [36]. Post-test probability was calculated using a likelihood ratio nomogram [37]. We anticipated that there would be considerable heterogeneity of results among the different studies. The heterogeneity of the diagnostic test properties was assessed by Cochran’s Q test [38], and was also quantified by the I2 value, i.e. the proportion of variability across studies that is due to heterogeneity rather than chance [39]. Very high values in this respect (above 0.5) reflect a high degree of heterogeneity and suggest the need for a more detailed study of the subgroups included. In our case, the small number of studies did not allow for a detailed exploration of the reasons for heterogeneity using meta-regression techniques. Finally, we performed a pooled estimation of the diagnostic efficiency statistics for each test and compared embryo morphology and PGS-FISH, using the method proposed by Dersimonian and Laird [40], which is affected only to a minor degree by heterogeneity among the studies. To calculate the pooled DOR (Diagnostic odds ratio) and LRs, a correction factor of 0.5 was added to all four cells in the 2 × 2 table, and logs were used in accordance with the recommendations of Gart and Zweiful [41]. The data for the different studies were analyzed using STATA (10.1) software (StataCorp LP, College Station, TX, USA).
By means of a hypergeometric distribution, we determined the probability of selecting at least one euploid embryo from a group of 6 morphologically normal embryos, selected by embryo morphology or by PGS-FISH, when one, two or three embryos were transferred. Using our model, we also determined the diagnostic performance (LR-) that should be required of any embryo selection test to ensure it would have greater clinical validity than that obtained by the embryo morphology method.
Results
The prevalence of embryo chromosome abnormalities in the different risk factor circumstances was as follows: 39.0–70.3% in AMA, 43.8–58.5% in RM among young women (<37 years), 63.2–75.0% in RM with AMA (≥37 years), 49.0–70.7% in RIF (irrespective of maternal age) and 52.5–93.3% in MF, depending on the pathology in question (Table 1).
Table 1.
Prevalence of abnormal embryos in Donor, AMA, RM, RIF and MF
| Study group | Author | Maternal age | Other characteristics | AEP |
|---|---|---|---|---|
| Donors | Kearns et al. [74] | 21–31 | – | 52.0 |
| Reis Soares et al. [75] | 23–31 | – | 56.5 | |
| Nelson et al. [76] | <30 | – | 28.0–83.0 | |
| Nagy and Chang [77] | <35 | – | 66.0 | |
| Munné et al. [78] | 18–35 | – | 0.0–100.0 | |
| AMA | Kahraman et al. [43] | ≥35 | – | 39.0 |
| Werlin et al. [79] | >38 | – | 53.7 | |
| Munné et al. [44] | 35–39 | – | 58.9 | |
| Munné et al. [44] | 40 | – | 65.1 | |
| Gianaroli et al. [80] | ≥38 | – | 63.0 | |
| Staessen et al. [17] | ≥37 | – | 63.2 | |
| Platteau et al. [42], | ≥37 | – | 65.3 | |
| Debrock et al. [10] | ≥35 | – | 69.7 | |
| Rubio et al. [81] | ≥38 | – | 70.3 | |
| RM | Miscarriage numbers | |||
| Vidal et al. [82] | ≤35 | ≥4 | 41.0 | |
| Platteau et al. [83] | <37 | ≥2 | 43.8 | |
| Munné et al. [84] | <35 | ≥3 | 57.0 | |
| Pellicer et al. [85] | ≤36 | ≥3 | 58.5 | |
| Simón et al. [86] | <35 | ≥2 | 58.9 | |
| Rubio et al. [81] | <37 | ≥2 | 63.5 | |
| Platteau et al. [83] | ≥37 | ≥2 | 66.9 | |
| Munné et al. [84] | ≥35 | ≥3 | 67.0 | |
| Werlin et al. [79] | – | ≥2 | 68.2 | |
| Garrisi et al. [87] | – | ≥2 | 69.3 | |
| Rubio et al.[88] | <37–≥37 | ≥2 | 70.7 | |
| Rubio et al. [81] | ≥37 | ≥2 | 72.7 | |
| RIF | Failure numbers | |||
| Kahraman et al. [43] | – | ≥2 | 49.0 | |
| Gianaroli et al. [80] | – | ≥2 | 57.0 | |
| Rubio et al. [81] | <37 | ≥3 | 61.2 | |
| Pehlivan et al. [89] | <37 | ≥3 | 65.4 | |
| Wilton et al. [46] | – | Yes | 67.0 | |
| Werlin et al. [79] | – | >2 | 67.9 | |
| Pehlivan et al. [89] | ≥37 | ≥3 | 70.7 | |
| Rubio et al. [81] | ≥37 | ≥3 | 71.5 | |
| MF | Factor | |||
| Rubio et al. [81] | – | Oligozoospermia | 43.2 | |
| Platteau et al. [90] | – | NOA | 52.5 | |
| Rubio et al. [81] | – | OA | 52.6 | |
| Rubio et al. [81] | – | Teratozoospermia | 55.9 | |
| Silber et al. [24] | ≤39 | Oligospermia | 58.0 | |
| Platteau et al. [90] | – | OA | 60.0 | |
| Rubio et al. [81] | – | NOA | 69.7 | |
| Silber et al. [24] | ≤39 | TESE | 78.0 | |
| Kahraman et al. [91] | – | Macrocephalic | 84.4 | |
| Kahraman et al. [91] | – | Absolute teratozoospermia | 93.3 |
AEP abnormal embryo prevalence; AMA advanced maternal age; RM repeated miscarriage; RIF recurrent implantation failure; MF male factor; NOA non-obstructive azoospermia; OA obstructive azoospermia; TESE testicular sperm extraction
Table 2 shows the characteristics of the studies included in the systematic review for calculating the diagnostic efficiency statistics.
Table 2.
Studies included in systematic review for calculating the diagnostic efficiency statistics
| Studies | Test | Morphological criteria | Gold Standard | Number of blastomeres analyzed | Chromosomes analyzed by FISH | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|
| Baltaci et al. [92] | Morphology | Veeck et al. [98] | FISH | 1 | X, Y, 13, 18, 21 | 150 | 115 | 274 | 437 |
| Magli et al. [93] | Morphology | Alikani et al. [99] | FISH | 1 | X, Y, 13, 15, 16, 18, 21, 22 | 2978 | 1106 | 531 | 490 |
| Munné et al. [94] | Morphology | cell number, fragmentation degree, blastomere sized | FISH | 1 | X, Y, 13, 16, 15, 17, 18, 21, 22, | 1832 | 651 | 2401 | 1170 |
| Rubio et al. [95] | Morphology | Alikani et al. [99] | FISH | 1 or 2 | X, Y, 13, 15, 16, 18, 21, 22 | 1895 | 762 | 1417 | 1637 |
| Ziebe et al. [96] | Morphology | cell number, fragmentation degree and location, blastomere sized, cytoplasmic appearance | FISH | Every blastomere | X, Y, 13, 16, 18, 21, 22 | 41 | 34 | 7 | 21 |
| Wilton et al. [46] | PGS-FISH | Not reported | CGH | 1 | X, Y, 13, 16, 18, 21, 22 | 29.5 | 0.5 | 19.5 | 50.5 |
| Keskintepe et al. [97] | PGS-FISH | Not reported | CGH | 1 | X, Y, 17, 16, 18, 21, 22 | 32.5 | 0.5 | 10.5 | 2.5 |
| Daphnis et al. [52] | PGS-FISH | Not reported | CGH | 1 or 2 | X, Y, 18 allways 3, 4, 6, 10, 11, 13, 16, 18, 22 | 17.5 | 0.5 | 10.5 | 3.5 |
TP True positive; FP False positive; FN False negative; TN True negative
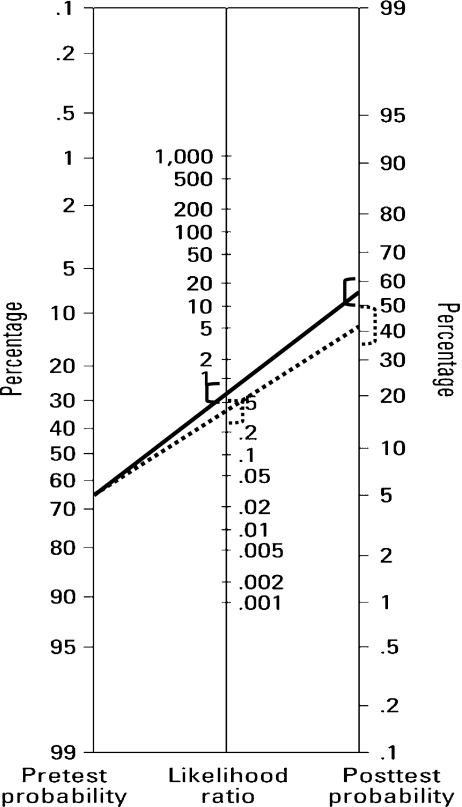
The values of the diagnostic efficiency statistics are shown in Tables 3 and 4. PGS-FISH provided significantly higher values for Specificity, PPV, uPPV, uNPV, PPV-65 and PNV-65 than did embryo morphology. The LR- value obtained for embryo morphology was 0.67 (CI: 0.53–0.84), while for PGS-FISH it was 0.38 (CI: 0.29–0.51) (p < 0.05). For a pre-test prevalence of embryo aneuploidy of 65%, the post-test probability after a negative result according to the embryo morphology was 55% (CI: 50–61%) and after a negative result of PGS-FISH it was 42% (CI: 35–49%) (Fig. 1).
Table 3.
Diagnostic efficiency statistics not related to prevalence for Morphology and PGS-FISH
| Sens | Spec | LR+ | LR- | DOR | ||
|---|---|---|---|---|---|---|
| Morphology | Baltaci et al. [92] | 0.35 (0.31, 0.40) | 0.79 (0.76, 0.82) | 1.70 (1.39, 2.08) | 0.82 (0.75, 0.88) | 1.59 (1.37, 1.84) |
| Magli et al. [93] | 0.85 (0.84, 0.86) | 0.31 (0.28, 0.33) | 1.22 (1.19, 1.27) | 0.49 (044, 0.54) | 0.92 (0.82, 1.05) | |
| Munné et al. [94] | 0.43 (0.42, 0.45) | 0.64 (0.62, 0.66) | 1.21 (1.13, 1.30) | 0.88 (0.84, 0.92) | 0.49 (0.45, 0.52) | |
| Rubio et al. [95] | 0.57 (0.55, 0.59) | 0.68 (0.66, 0.70) | 1.80 (1.68, 1.92) | 0.63 (0.60, 0.66) | 1.15 (1.07, 1.24) | |
| Ziebe et al. [96] | 0.85 (0.74, 0.94) | 0.38 (0.25, 0.52) | 1.38 (1.09, 1.79) | 0.38 (0.14, 0.81) | 2.87 (1.35, 7.80) | |
| pooled | 0.61 (0.41, 0.81) | 0.56 (0.39, 0.74) | 1.44 (1.19, 1.73) | 0.67 (0.53, 0.84) | 1.10 (0.67, 1.81) | |
| I2 | 99.8 | 99.5 | 96.5 | 97.0 | 99.0 | |
| PGS-FISH | Wilton et al. [46] | 0.60 (046, 0.74) | 0.99 (0.99, 0.99) | 61.62 (45.18, 82.96) | 0.40 (0.26, 0.54) | 2.59 (1.63, 4.68) |
| Keskintepe et al. [97] | 0.72 (0.63, 0.88) | 0.83 (0.75, 0.94) | 4.57 (1.45, 10.46) | 0.29 (0.14, 0.59) | 0.24 (0.04, 0.78) | |
| Daphnis et al. [52] | 0.63 (0.45, 0.81) | 0.87 (0.75, 0.95) | 5.04 (2.07, 11.83) | 0.42 (0.21, 0.69) | 0.33 (0.09, 1.18) | |
| pooled | 0.67 (0.57, 0.78) | 0.91 (0.79, 1.02) | 11.67 (1.59, 85.78) | 0.38 (0.29, 0.51) | 0.65 (0.12, 3.63) | |
| I2 | 33.5 | 87.4 | 95.8 | 0.0 | 86.9 | |
| P* | NS | p < 0.05 | NS | p < 0.05 | NS |
Sens sensitivity; Spec specificity; LR+ likelihood ratio positive; LR- likelihood ratio negative; DOR diagnostic odds ratio
*p value obtained from the comparison of the pooled estimation of diagnostic efficiency statistics of SCSA vs. CSP.
Table 4.
Diagnostic efficiency statistics related to prevalence for Morphology and PGS-FISH
| Prev | PPV | NPV | uPPV | uNPV | PPV-65 | NPV-65 | ||
|---|---|---|---|---|---|---|---|---|
| Morphology | Baltaci et al. [92] | 424/976 | 0.57 (051, 0.62) | 0.38 (0.35, 0.42) | 0.59 (0.55, 0.62) | 0.53 (0.52, 0.55) | 0.76 (0.72, 0.79) | 0.40 (0.38, 0.42) |
| Magli et al. [93] | 3509/5105 | 0.73 (0.72, 0.74) | 0.52 (0.49, 0.55) | 0.53 (0.53, 0.54) | 0.62 (0.60, 0.63) | 0.69 (0.69, 0.70) | 0.52 (0.50, 0.55) | |
| Munné et al. [94] | 4233/6054 | 0.74 (0.72, 0.75) | 0.67 (0.66, 0.69) | 0.53 (0.52, 0.54) | 0.52 (0.51, 0.53) | 0.69 (0.68, 0.71) | 0.38 (0.37, 0.39) | |
| Rubio et al. [95] | 3312/5711 | 0.71 (0.69, 0.73) | 0.46 (0.45, 0.48) | 0.60 (0.59, 0.61) | 0.58 (0.57, 0.58) | 0.77 (0.76, 0.78) | 0.46 (0.45, 0.47) | |
| Ziebe et al. [96] | 48/103 | 0.55 (0.43, 0.66) | 0.25 (0.11, 0.44) | 0.55 (0.51, 0.60) | 0.66 (0.54, 0.79) | 0.72 (0.67, 0.77) | 0.58 (0.40, 0.79) | |
| pooled | 0.69 (0.65, 0.72) | 0.47 (0.35, 0.59) | 0.56 (0.53, 0.59) | 0.57 (0.53, 0.61) | 0.73 (0.69, 0.76) | 0.45 (0.39, 0.50) | ||
| I2 | 90.3 | 99.1 | 96.5 | 97.8 | 96.8 | 97.8 | ||
| PGS-FISH | Wilton et al. [46] | 49/100 | 0.02 (0.01, 0.02) | 0.27 (0.17, 0.38) | 0.95 (0.93, 0.96) | 0.65 (0.60, 0.71) | 0.99 (0.99, 0.99) | 0.57 (0.50, 0.67) |
| Keskintepe et al. [97] | 43/46 | 0.01 (0.01, 0.02) | 0.83 (0.05, 0.94) | 0.73 (0.56, 0.83) | 0.70 (0.60, 0.81) | 0.89 (0.73, 0.95) | 0.65 (0.48, 0.79) | |
| Daphnis et al. [52] | 28/32 | 0.03 (0.02, 0.04) | 0.77 (0.07, 0.93) | 0.75 (0.62, 0.84) | 0.64 (0.57, 0.74) | 0.90 (0.79, 0.96) | 0.56 (0.44, 0.72) | |
| pooled | 0.02 (0.01, 0.02) | 0.58 (0.16, 1.00) | 0.82 (0.65, 0.98) | 0.65 (0.61, 0.70) | 0.94 (0.87, 1.02) | 0.58 (0.52, 0.65) | ||
| I2 | 67.8 | 79.9 | 90.6 | 0.0 | 73.0 | 0.0 | ||
| P* | p < 0.01 | NS | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
Prev Prevalence; PPV positive predictive value; NPV negative predictive value; uPPV unconditioned positive predictive value; uNPV unconditioned negative predictive value; PPV-65 estimated PPV according to 65% prevalence; PPN-65 estimated NPV according to 65% prevalence
*p value obtained from the comparison of the pooled estimation of diagnostic efficiency statistics of SCSA vs. CSP.
Fig. 1.
Fagan nomogram using likelihood ratio and pre-test probability for PGD-AS and embryo morphology. Solid lines are embryo morphology test and dotted lines are PGD-AS. Confidence intervals in brackets. To use this tool, the probability or prevalence of embryo aneuploidies and the likelihood ratio for the diagnostic test has to be known. With this information, a line connecting the pre-test probability and the likelihood ratio is drawn and extended until it intersects with the post-test probability. The point of intersection is the new estimate of the probability of embryo aneuploidies
Both the embryo selection studies performed using morphology and the PGS-FISH studies produced a high degree of heterogeneity (I2 > 50%).
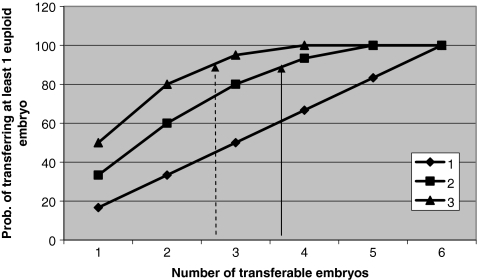
In the model of hypergeometric distribution (Fig. 2), on the basis of a post-test probability of selecting an euploid embryo by the embryo morphology method of 45% (CI: 39–50%), and by the PGS-FISH method of 58% (CI: 51–65), the following results were drawn. Firstly, the probability of transferring at least one normal embryo increases with: (a) the percentage of optimum embryos in the group (60% in double embryo transfer when the number of transferable embryos is 33% and 100% when the number of transferable embryos increases to 83%); (b) the number of embryos selected (50% in single embryo transfer, 80% in double embryo transfer y 95% in triple embryo transfer when the number of transferable embryos is 50%). This increase becomes steadily less pronounced above the level of 50% normal embryos in the group. Secondly, that the probability of transferring at least one normal embryo is the same (88%) whether 2 embryos selected by PGS-FISH or 3 embryos selected by embryo morphology are transferred (arrow in Fig. 2).
Fig. 2.
Probability of transferring at least one euploid embryo depending on the number of optimum embryos in a group of 6 transferable embryos and in accordance to the number of embryos transferred. Arrows indicate the probability of transferring at least one euploid embryo when 2 embryos are selected by PGS-FISH (solid arrow) or 3 embryos are selected by embryo morphology (dotted arrow)
For any embryo selection test to be of greater clinical validity than embryo morphology, it should have an LR- value of 0.40 (CI: 0.32–0.51) in SET and 0.06 in DET (CI: 0.05–0.07).
Discussion
Our systematic review revealed large differences among different studies concerning the pre-test probability of embryo aneuploidy. These differences concerned both the diverse clinical situations analysed (AMA, RM, RIF and MF) and the embryo studies based on donated eggs. The variations in the prevalence values may have been caused by various factors: firstly, by the different criteria used to define each study group. Thus, Staessen et al. [14] and Platteau et al. [42] defined AMA as patients with a maternal age ≥ 37 years, while Kahraman et al. [43] included women aged 35–39 years old and Munné et al. [44] and Debrock et al. [10] accepted patients aged 35 years and older. Secondly, by means of FISH, 7–12 chromosomes can be analysed, depending on the patient’s prior history, and those involved in the most common aneuploidies identified in spontaneous miscarriages can be included [45]. Thirdly, the considerable differences observed may have been caused by the controversial question of the reproducibility, accuracy and misdiagnosis rate of PGS-FISH [46, 47]. Among the causes of these controversies are the above-mentioned technical ones, in addition to those of a physiological nature. It is well documented that chromosomal mosaicism occurs in early human stage embryos [26, 48–51]. At least 40–50% of human embryos are chromosomally mosaic, while some present such high levels of abnormalities that they are considered to be completely chaotic [52]. This means that the blastomere biopsed for the PGS-FISH test may not represent the rest of the embryo, thus resulting in a false positive or a false negative diagnosis [53]. Mosaicism exists in embryos and cannot be corrected, and so this is an inherent limitation of the FISH technique when used in PGD [54]. Some laboratories biopsy and analyse two cells from each embryo in an effort to detect mosaicism. Although this provides some value, there may still be undetected mosaicism in the cells remaining in the embryo, and biopsying two cells is likely to produce a cost to the viability of the embryo [55, 56].
Vanneste et al. [57] recently observed, using CGH, that only 9% of early human stage embryos are chromosomally normal in all blastomeres. Some mosaic embryos could change into a euploid status by means of apoptosis, overgrowth of euploid cells or displacement towards trophectoderm lineage thus resulting in a viable embryo [58, 59]. These findings lead us to the question of whether any embryo is uniformly chromosomally normal at this early stage of human development and whether it is necessary for all blastomeres to be normal diploid at this early stage of development for a viable pregnancy to be achieved [52].
Although there are large differences in the prevalence of embryo aneuploidies among the studies reviewed, we found considerable similarity between our median value and that for embryo aneuploidies obtained by the European Society of Human Reproduction and Embryology Preimplantation Genetic Diagnosis Consortium [60] (65% vs. 64%). On comparing the prevalence values for RIF, RM and MF separately, our results were also found to be very similar (65% vs. 63%, 66% vs. 63% and 59% vs. 57% respectively). However, the prevalence values for AMA are more diverse: 63% in our study vs. the 72% obtained by the European Society of Human Reproduction and Embryology Preimplantation Genetic Diagnosis Consortium [60]. These discrepancies may be due to the above-mentioned differences in the definition of AMA.
In order to determine the diagnostic performance of PGS-FISH, we included only those studies that compared FISH with CGH. This was done because studies that used as a gold standard the results obtained from subsequent rounds of FISH [23, 24, 28] might be subject to incorporation bias. This distortion occurs when the result of the experimental test (first round of FISH) is combined with the result of the reference test and forms part of the gold standard (first round of FISH and subsequent rounds of FISH). Incorporation bias gives rise to an overestimation of test accuracy because the experimental and reference tests are partially identical. On the other hand, studies that analysed the embryo at more advanced stages of development [17, 25–27] were excluded because they could be subject to review bias, also known as “non blind diagnosis bias” or “non blind interpretation bias”. This problem is encountered when the reference test is interpreted with knowledge of the result of the experimental test. This may lead to overestimation of both sensitivity and specificity, especially if the interpretation of the result is to some extent subjective [61]. On the other hand, publication bias is a problem for all reviews in that studies with negative findings are less likely to be published than studies with positive findings. This could influence the outcome of the present study.
According to Dreesen et al. [62], an embryo selection test should have the lowest possible number of FN (abnormal embryo testing as normal), and therefore the highest NPV and the lowest LR-. This is because a negative test of embryo selection should provide the highest possible guarantee that the embryos transferred are normal. The inverse relation between NPV and prevalence, found in any screening test, might account for differences between the results (findings) of our study and those of other authors. On the one hand, and in view of the wide range of prevalence of embryo aneuploidies observed, this would account for the wide range of NPV found among the different studies. We have shown that these differences among studies are reduced when all studies were standardised for PV by calculating unconditional predictive values (uNPV and uPPV) or by fixing prevalence values (NPV-65 and PPV-65). On the other hand, it would also account for the observation made by various authors [17, 63] that PGS-FISH performs better for women with lower embryo aneuploidy rates (i.e. young women) than for women with higher embryo aneuploidy rates (i.e. AMA). Furthermore, the theoretical model proposed by Summers et al. [64] showed that the PGS-FISH gain is marginal with higher aneuploidy rates (>70%) even when there are large number of embryos available for biopsy.
The statistical differences revealed by our pooled analysis showed there to be greater efficiency in embryo selection by PGS-FISH (LR-: 0.38) than by embryo morphology (LR-: 0.67). Nevertheless, the diagnostic efficiency produced by PGS-FISH did not reach the level required in the theoretical model proposed by Los et al. [53] (LR-: 0.31). This could have been caused by the above-mentioned limitations of PGS-FISH, and could, to some extent, be overcome by using the alternative CGH analysis. CGH does not require the preparation of chromosomes from the sample and such a method would be extremely useful for gauging levels of aneuploidy and mosaicism in preimplantation embryos [65]. However, there are two factors that could limit the widespread incorporation of CGH testing of blastomeres into current practice in assisted reproduction: firstly, the fact that it takes several weeks to obtain comprehensive results from blastomeres precludes the performance of fresh blastocyst transfers; and secondly, CGH analysis is so technology and cost-intensive as to render its performance unaffordable for the vast majority of IVF centres. Given these caveats, the critical question is whether it is clinically feasible and beneficial to apply CGH screening to day 3 embryos and then selectively transfer, in a post-warming cycle, those ultimately determined to be normal [45, 59, 66].
Nevertheless, despite the potential advantage for PGD applications that array CGH provides (it takes less time and it makes possible to transfer the embryo on the fresh IVF cycle) [67], it should not be forgotten that, from a theoretical point of view, the 8-cell stage does not seem to be the most suitable level for PGS. This is due to the low rate of normal embryos and the high rate of abnormal and mosaic embryos that are present at this stage. According to Los et al. [53], an abnormal or mosaic biopsy reduces or even eliminates the limited mosaicism of the embryo, but decreases its transfer possibilities. In contrast, a normal biopsy aggravates the mosaicism in the embryo but increases the transfer possibilities. Thus, this aspect would lead to the paradoxical effect of an inverse relation between the developmental prospects of these embryos and their chances for transfer.
We have shown that the greater usefulness of PGS-FISH in the selection of euploid embryos decreases as higher numbers of embryos are transferred. Therefore, there is a very similar probability of transferring at least one euploid embryo whether three embryos selected by embryo morphology are transferred, or whether two selected by PGS-FISH are transferred. This inverse relation, between the number of embryos transferred and the advantages of PGS-FISH over embryo morphology, coincides with the findings of Donoso et al. [31]. These authors examined whether embryo selection in azoospermic men based only on developmental and morphological criteria would differ from selection based on PGS-FISH results. They concluded that in SET using only morphology criteria the probability of replacing a euploid embryo was 60%. But when two embryos are replaced, this probability increased to 80%, representing a reduction in the comparative advantage of the PGS-FISH method. These reductions in the benefit to be gained from PGS-FISH as the number of embryos to be transferred increases are similar to those reported in the present study.
Any new test of non-invasive embryo viability should achieve a high level of diagnostic performance in order to have greater clinical validity than the embryo morphology or PGS-FISH methods. A number of other non-invasive tests have been proposed, assessing protein, amino acid, soluble human leukocyte antigen G, oxygen consumption or birefringence measurements [68–72]. Our model makes it possible to estimate the diagnostic performance that would be required of any of these tests. Because in single embryo transfer (SET), the morphology method, assuming the LR- obtained in our own results, assures that 45% (CI: 39–50%) of the morphologically normal embryos selected are euploid, any new non-invasive test must present a LR- of at least 0.40 in order to guarantee a significant increase in the probability of selecting a euploid embryo in SET. With this LR-, the new test enables us to state that at least 57% (CI: 51–63%) of the normal embryos selected by the non-invasive test will be euploid. To date, the results obtained with these new tests do indeed achieve this yardstick for SET, as confirmed in the study by Seli et al. [73]. These authors analysed embryo culture media using Raman and near-infrared spectroscopy and obtained a sensitivity of 86% and a specificity of 76.5% with Raman spectroscopy, with a LR- value of 0.22. Near-infrared spectroscopy provided a sensitivity of 75% and a specificity of 83.3%, with a LR- value of 0.27.
However, these LR- values are far from the level required (LR-: 0.06) when the new test is to be used with respect to the transfer of two embryos, and when we wish to surpass the probability of transferring at least one euploid embryo that is provided by the embryo morphology method. As the LR- required of the new test is very low, we believe the new tests of embryo selection would have real clinical validity in the context of SET, because in transfers of two or more embryos they would be unlikely to have more clinical validity than that given by the embryo morphology method.
With current technology, and taking into account the number of embryos to be transferred, the clinical validity of PGS-FISH, although greater than that provided by morphological criteria, does not seem to be clinically relevant. In conclusion, until the utility can be better defined, the new tests of embryo selection should be considered experimental, and the procedure only conducted under study conditions and with appropriate consent.
Footnotes
Capsule Taking into account PGS-FISH technology and the number of embryos to be transferred, the clinical validity of PGS-FISH does not appear to be clinically relevant.
References
- 1.Royen E, Mangelschots K, Neubourg D, Laureys I, Ryckaert G, Gerris J. Calculating the implantation potential of day 3 embryos in women younger than 38 years of age: a new model. Hum Reprod. 2001;16(2):326–32. doi: 10.1093/humrep/16.2.326. [DOI] [PubMed] [Google Scholar]
- 2.Holte J, Berglund L, Milton K, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22(2):548–57. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- 3.Placido G, Wilding M, Strina I, et al. High outcome predictability alter IVF using a combined score for zygote and embryo morphology and growth rate. Hum Reprod. 2002;17(9):2402–9. doi: 10.1093/humrep/17.9.2402. [DOI] [PubMed] [Google Scholar]
- 4.Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S. Preimplantation genetic diagnosis for aneuploidy screening in repeated implantation failure. Reprod Biomed Online. 2005;10:381–8. doi: 10.1016/S1472-6483(10)61800-7. [DOI] [PubMed] [Google Scholar]
- 5.Gianaroli L, Magli MC, Ferraretti AP, et al. The beneficial effects of preimplantation genetic diagnosis for aneuploidy support extensive clinical application. Reprod Biomed Online. 2005;10(5):633–40. doi: 10.1016/S1472-6483(10)61671-9. [DOI] [PubMed] [Google Scholar]
- 6.Donoso P, Devroey P. PGD for aneuploidy screening: an expensive hoax? Best Pract Res Clin Obstet Gynaecol. 2007;21(1):157–68. doi: 10.1016/j.bpobgyn.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Harper J, Sermon K, Geraedts J, et al. What next for preimplantation genetic screening? Hum Reprod. 2008;23(3):478–80. doi: 10.1093/humrep/dem424. [DOI] [PubMed] [Google Scholar]
- 8.Kuliev A, Verlinsky Y. Impact of preimplantation genetic diagnosis for chromosomal disorders on reproductive outcome. Reprod Biomed Online. 2008;16(1):9–10. doi: 10.1016/S1472-6483(10)60550-0. [DOI] [PubMed] [Google Scholar]
- 9.Blockeel C, Schutyser V, Vos A, et al. Prospectively randomized controlled trial of PGS in IVF/ICSI patients with poor implantation. Reprod Biomed Online. 2008;17(6):848–54. doi: 10.1016/S1472-6483(10)60414-2. [DOI] [PubMed] [Google Scholar]
- 10.Debrock S, Melotte C, Spiessens C, et al. Preimplantation genetic screening for aneuploidy of embryos after in vitro fertilization in women aged at least 35 years: a prospective randomized trial. Fertil Steril. 2010;93(2):364–73. doi: 10.1016/j.fertnstert.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 11.Hardarson T, Hanson C, Lundin K, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23:2806–12. doi: 10.1093/humrep/den217. [DOI] [PubMed] [Google Scholar]
- 12.Jansen RP, Bowman MC, Boer KA, Leigh DA, Lieberman DB, McArthur SJ. What next for preimplantation genetic screening (PGS)? Experience with blastocyst biopsy and testing for aneuploidy. Hum Reprod. 2008;23(7):1476–8. doi: 10.1093/humrep/den129. [DOI] [PubMed] [Google Scholar]
- 13.Mastenbroek S, Twisk MV, Sikkema-Raddatz B, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 14.Mersereau JE, Pergament E, Zhang X, Milad MP. Preimplantation genetic screening to improve in vitro fertilization pregnancy rates: a prospective randomized controlled trial. Fertil Steril. 2008;90(4):1287–9. doi: 10.1016/j.fertnstert.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Meyer LR, Klipstein S, Hazlett WD, Nasta T, Mangan P, Karande VC. A prospective randomized controlled trial of preimplantation genetic screening in the “good prognosis” patient. Fertil Steril. 2009;91(5):1731–8. doi: 10.1016/j.fertnstert.2008.02.162. [DOI] [PubMed] [Google Scholar]
- 16.Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92(1):157–62. doi: 10.1016/j.fertnstert.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Staessen C, Platteau P, Assche E, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849–58. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 18.Staessen C, Verpoest W, Donoso P, et al. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod. 2008;23(12):2818–25. doi: 10.1093/humrep/den367. [DOI] [PubMed] [Google Scholar]
- 19.Stevens J, Wale P, Surrey ES, Schoolcraft WB. Is aneuploidy screening for patients aged 35 or over beneficial? A prospective randomized trial. Fertil Steril. 2004;82(2):249. doi: 10.1016/j.fertnstert.2004.07.664. [DOI] [Google Scholar]
- 20.Cohen J, Grifo JA. Multicentre trial of preimplantation genetic screening reported in the New England Journal of Medicine: an in-depth look at the findings. Reprod Biomed Online. 2007;15(4):365–6. doi: 10.1016/S1472-6483(10)60358-6. [DOI] [PubMed] [Google Scholar]
- 21.Munné S, Gianaroli L, Tur-Kaspa I, et al. Substandard application of preimplantation genetic screening may interfere with its clinical success. Fertil Steril. 2007;88(4):781–4. doi: 10.1016/j.fertnstert.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Colls P, Escudero T, Cekleniak N, Sadowy S, Cohen J, Munné S. Increased efficiency of preimplantation genetic diagnosis for infertility using “no result rescue”. Fertil Steril. 2007;88(1):53–61. doi: 10.1016/j.fertnstert.2006.11.099. [DOI] [PubMed] [Google Scholar]
- 23.Munné S, Magli C, Bahçe M, et al. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: XY, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn. 1998;18(13):1459–66. doi: 10.1002/(SICI)1097-0223(199812)18:13<1459::AID-PD514>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munné S. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 2003;79(1):30–8. doi: 10.1016/S0015-0282(02)04407-2. [DOI] [PubMed] [Google Scholar]
- 25.Baart EB, Opstal D, Los FJ, Fauser BC, Martini E. Fluorescence in situ hybridization analysis of two blastomeres from day 3 frozen-thawed embryos followed by analysis of the remaining embryo on day 5. Hum Reprod. 2004;19(3):685–93. doi: 10.1093/humrep/deh094. [DOI] [PubMed] [Google Scholar]
- 26.Baart EB, Martini E, Berg I, et al. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 2006;21(1):223–33. doi: 10.1093/humrep/dei291. [DOI] [PubMed] [Google Scholar]
- 27.Michiels A, Assche E, Liebaers I, Steirteghem A, Staessen C. The analysis of one or two blastomeres for PGD using fluorescence in-situ hybridization. Hum Reprod. 2006;21(9):2396–402. doi: 10.1093/humrep/del186. [DOI] [PubMed] [Google Scholar]
- 28.DeUgarte CM, Li M, Surrey M, Danzer H, Hill D, DeCherney AH. Accuracy of FISH analysis in predicting chromosomal status in patients undergoing preimplantation genetic diagnosis. Fertil Steril. 2008;90(4):1049–54. doi: 10.1016/j.fertnstert.2007.07.1337. [DOI] [PubMed] [Google Scholar]
- 29.Greenhalgh T. How to read a paper. Papers that report diagnostic or screening tests. BMJ. 1997;315(7107):540–3. doi: 10.1136/bmj.315.7107.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical epidemiology: A basic science for clinical medicine. Boston: Williams and Wilkins; 1991. [Google Scholar]
- 31.Donoso P, Platteau P, Papanikolaou EG, Staessen C, Steirteghem A, Devroey P. Does PGD for aneuploidy screening change the selection of embryos derived from testicular sperm extraction in obstructive and non-obstructive azoospermic men? Hum Reprod. 2006;21(9):2390–5. doi: 10.1093/humrep/del177. [DOI] [PubMed] [Google Scholar]
- 32.Moayeri SE, Allen RB, Brewster WR, Kim MH, Porto M, Werlin LB. Day-3 embryo morphology predicts euploidy among older subjects. Fertil Steril. 2008;89(1):118–23. doi: 10.1016/j.fertnstert.2007.01.169. [DOI] [PubMed] [Google Scholar]
- 33.Donoso P, Staessen C, Fauser BC, Devroey P. Current value of preimplantation genetic aneuploidy screening in IVF. Hum Reprod Update. 2007;13(1):15–25. doi: 10.1093/humupd/dml043. [DOI] [PubMed] [Google Scholar]
- 34.Bossuyt PM, Reitsma JB, Bruns DE, et al. Standards for reporting of diagnostic accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49(1):7–17. doi: 10.1373/49.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Jialiang L, Fine JP, Safdar N. Prevalence-dependent diagnostic accuracy measures. Stat Med. 2007;26(17):3258–73. doi: 10.1002/sim.2812. [DOI] [PubMed] [Google Scholar]
- 36.Zhou XH, Obuchowski NA, McClish DK. Statistical methods in diagnostic medicine. New York: Wiley; 2002. [Google Scholar]
- 37.Fagan TJ. Nomogram for Bayes theorem. N Engl J Med. 1975;293(5):257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 38.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2001;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 41.Gart JJ, Zweiful JR. On the bias of various estimators of the logit and its variance with applications to quantal bioassay. Biometrika. 1967;54(1):181–7. [PubMed] [Google Scholar]
- 42.Platteau P, Staessen C, Michiels A, Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84(2):319–24. doi: 10.1016/j.fertnstert.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Kahraman S, Bahçe M, Samli H, et al. Healthy births and ongoing pregnancies obtained by preimplantation genetic diagnosis in patients with advanced maternal age and recurrent implantation failure. Hum Reprod. 2000;15(9):2003–7. doi: 10.1093/humrep/15.9.2003. [DOI] [PubMed] [Google Scholar]
- 44.Munné S, Sandalinas M, Escudero T, Márquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reprod Biomed Online. 2002;4(3):223–32. doi: 10.1016/S1472-6483(10)61810-X. [DOI] [PubMed] [Google Scholar]
- 45.Preimplantation genetic testing: a Practice Committee Opinion. Fertil Steril. 2007;88:1497–504. [DOI] [PubMed]
- 46.Wilton L, Voullaire L, Sargeant P, Williamson R, McBain J. Preimplantation aneuploidy screening using comparative genomic hybridization or fluorescence in situ hybridization of embryos from patients with recurrent implantation failure. Fertil Steril. 2003;80(4):860–8. doi: 10.1016/S0015-0282(03)01162-2. [DOI] [PubMed] [Google Scholar]
- 47.Gleicher N, Weghofer A, Barad D. Preimplantation genetic screening: “established” and ready for prime time? Fertil Steril. 2008;89(4):780–8. doi: 10.1016/j.fertnstert.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 48.Ruangvutilert P, Delhanty JD, Serhal P, Simopoulou M, Rodeck CH, Harper JC. FISH analysis on day 5 post-insemination of human arrested and blastocyst stage embryos. Prenat Diagn. 2000;20(7):552–60. doi: 10.1002/1097-0223(200007)20:7<552::AID-PD871>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Merino E, Emiliani S, Vassart G, Bergh M, Vannin AS, Abramowicz M, et al. Incidence of chromosomal mosaicism in human embryos at different developmental stages analyzed by fluorescence in situ hybridization. Genet Test. 2003;7(2):85–95. doi: 10.1089/109065703322146768. [DOI] [PubMed] [Google Scholar]
- 50.Coonen E, Derhaag JG, Dumoulin JC, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Human Reprod. 2004;19(2):316–24. doi: 10.1093/humrep/deh077. [DOI] [PubMed] [Google Scholar]
- 51.Bielanska M, Jin S, Bernier M, Tan SL, Ao A. Diploid-aneuploid mosaicism in human embryos cultured to the blastocyst stage. Fertil Steril. 2005;84(2):336–42. doi: 10.1016/j.fertnstert.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 52.Daphnis DD, Fragouli E, Economou K, et al. Analysis of the evolution of chromosome abnormalities in human embryos from Day 3 to 5 using CGH and FISH. Mol Hum Reprod. 2008;14(2):117–25. doi: 10.1093/molehr/gam087. [DOI] [PubMed] [Google Scholar]
- 53.Los FJ, Opstal D, Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update. 2004;10(1):79–94. doi: 10.1093/humupd/dmh005. [DOI] [PubMed] [Google Scholar]
- 54.Wilton L, Thornhill A, Traeger-Synodinos J, Sermon KD, Harper JC. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;24(5):1221–8. doi: 10.1093/humrep/den488. [DOI] [PubMed] [Google Scholar]
- 55.Cohen J, Wells D, Munné S. Removal of 2 cells from cleavage stage embryos is likely to reduce the efficacy of chromosomal tests that are used to enhance implantation rates. Fertil Steril. 2007;87(3):496–503. doi: 10.1016/j.fertnstert.2006.07.1516. [DOI] [PubMed] [Google Scholar]
- 56.Goossens V, Rycke M, Vos A, et al. Diagnostic efficiency, embryonic development and clinical outcome after the biopsy of one or two blastomeres for preimplantation genetic diagnosis. Hum Reprod. 2008;23(3):481–92. doi: 10.1093/humrep/dem327. [DOI] [PubMed] [Google Scholar]
- 57.Vanneste E, Voet T, Caignec C, et al. Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15(5):577–83. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 58.Derhaag JG, Coonen E, Bras M, et al. Chromosomally abnormal cells are not selected for the extra-embryonic compartment of the human preimplantation embryo at the blastocyst stage. Hum Reprod. 2003;18(12):2565–74. doi: 10.1093/humrep/deg485. [DOI] [PubMed] [Google Scholar]
- 59.Trussler JL, Pickering SJ, Ogilvie CM. Investigation of chromosomal imbalance in human embryos using comparative genomic hybridization. Reprod Biomed Online. 2004;8(6):701–11. doi: 10.1016/S1472-6483(10)61652-5. [DOI] [PubMed] [Google Scholar]
- 60.Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Rij M, Harper JC. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod. 2009;24(8):1786–810. doi: 10.1093/humrep/dep059. [DOI] [PubMed] [Google Scholar]
- 61.Knotternus JA, Weel C. General introduction: Evaluation of diagnostic procedures. In: Knottnerus JA, editor. The evidence base of clinical diagnosis. London: BMA Books; 2002. [Google Scholar]
- 62.Dreesen J, Drüsedau M, Smeets H, et al. Validation of preimplantation genetic diagnosis by PCR analysis: genotype comparison of the blastomere and corresponding embryo, implications for clinical practice. Mol Hum Reprod. 2008;14(10):573–9. doi: 10.1093/molehr/gan052. [DOI] [PubMed] [Google Scholar]
- 63.Twisk M, Mastenbroek S, Hoek A, et al. No beneficial effect of preimplantation genetic screening in women of advanced maternal age with a high risk for embryonic aneuploidy. Hum Reprod. 2008;23(12):2813–7. doi: 10.1093/humrep/den231. [DOI] [PubMed] [Google Scholar]
- 64.Summers MC, Foland AD. Quantitative decision-making in preimplantation genetic (aneuploidy) screening (PGS) J Assist Reprod Genet. 2009;26(9–10):487–502. doi: 10.1007/s10815-009-9352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wells D, Delhanty JD. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod. 2000;6(11):1055–62. doi: 10.1093/molehr/6.11.1055. [DOI] [PubMed] [Google Scholar]
- 66.Sher G, Keskintepe L, Keskintepe M, Maassarani G, Tortoriello D, Brody S. Genetic analysis of human embryos by metaphase comparative genomic hybridization (mCGH) improves efficiency of IVF by increasing embryo implantation rate and reducing multiple pregnancies and spontaneous miscarriages. Fertil Steril. 2009;92(6):1886–94. doi: 10.1016/j.fertnstert.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 67.Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod. 2008;14(12):703–10. doi: 10.1093/molehr/gan062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brison DR, Hollywood K, Arnesen R, Goodacre R. Predicting human embryo viability: the road to non-invasive analysis of the secretome using metabolic footprinting. Reprod Biomed Online. 2007;15(3):296–302. doi: 10.1016/S1472-6483(10)60342-2. [DOI] [PubMed] [Google Scholar]
- 69.Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15(5):271–7. doi: 10.1093/molehr/gap012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopes AS, Greve T, Callesen H. Quantification of embryo quality by respirometry. Theriogenology. 2007;67(1):21–31. doi: 10.1016/j.theriogenology.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Montag M, Schimming T, Köster M, et al. Oocyte zona birefringence intensity is associated with embryonic implantation potential in ICSI cycles. Reprod Biomed Online. 2008;16(2):239–244. doi: 10.1016/S1472-6483(10)60580-9. [DOI] [PubMed] [Google Scholar]
- 72.Scott L, Berntsen J, Davies D, Gundersen J, Hill J, Ramsing N. Symposium: innovative techniques in human embryo viability assessment. Human oocyte respiration-rate measurement–potential to improve oocyte and embryo selection? Reprod Biomed Online. 2008;17(4):461–9. doi: 10.1016/S1472-6483(10)60232-5. [DOI] [PubMed] [Google Scholar]
- 73.Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88(5):1350–7. doi: 10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- 74.Kearns WG, Pen R, Graham J, et al. Preimplantation genetic diagnosis and screening. Semin Reprod Med. 2005;23(4):336–47. doi: 10.1055/s-2005-923391. [DOI] [PubMed] [Google Scholar]
- 75.Reis Soares S, Rubio C, Rodrigo L, et al. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertil Steril. 2003;80(3):656–657. doi: 10.1016/S0015-0282(03)00787-8. [DOI] [PubMed] [Google Scholar]
- 76.Nelson JR, Potter DA, Wilcox JG, Frederick JL, Kolb BA, Behr BR. Preimplantation genetic diagnosis in embryos created from oocytes donation. Fertil Steril. 2005;84(1):328–9. doi: 10.1016/j.fertnstert.2005.07.859. [DOI] [Google Scholar]
- 77.Nagy ZP, Chang CC. Current advances in artificial gametes. Reprod Biomed Online. 2005;11(3):332–9. doi: 10.1016/S1472-6483(10)60841-3. [DOI] [PubMed] [Google Scholar]
- 78.Munné S, Ary J, Zouves C, et al. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod Biomed Online. 2006;12(3):340–6. doi: 10.1016/S1472-6483(10)61007-3. [DOI] [PubMed] [Google Scholar]
- 79.Werlin L, Rodi I, DeCherney A, Marello E, Hill D, Munné S. Preimplantation genetic diagnosis as both a therapeutic and diagnostic tool in assisted reproductive technology. Fertil Steril. 2003;80(2):467–8. doi: 10.1016/S0015-0282(03)00605-8. [DOI] [PubMed] [Google Scholar]
- 80.Gianaroli L, Magli MC, Munné S, Fiorentino A, Montanaro N, Ferraretti AP. Will preimplantation genetic diagnosis assist patients with a poor prognosis to achieve pregnancy? Hum Reprod. 1997;12(8):1762–7. doi: 10.1093/humrep/12.8.1762. [DOI] [PubMed] [Google Scholar]
- 81.Rubio C, Rodrigo L, Pérez-Cano I, et al. FISH screening of aneuploidies in preimplantation embryos to improve IVF outcome. Reprod Biomed Online. 2005;11(4):497–506. doi: 10.1016/S1472-6483(10)61146-7. [DOI] [PubMed] [Google Scholar]
- 82.Vidal F, Giménez C, Rubio C, et al. FISH preimplantation diagnosis of chromosome aneuploidy in recurrent pregnancy wastage. J Assist Reprod Genet. 1998;15(5):310–3. doi: 10.1023/A:1022552713015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Platteau P, Staessen C, Michiels A, Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in patients with unexplained recurrent miscarriages. Fertil Steril. 2005;83(2):393–7. doi: 10.1016/j.fertnstert.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 84.Munné S, Chen S, Fischer J, et al. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertil Steril. 2005;84(2):331–5. doi: 10.1016/j.fertnstert.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 85.Pellicer A, Rubio C, Vidal F, et al. In vitro fertilization plus preimplantation genetic diagnosis in patients with recurrent miscarriage: an analysis of chromosome abnormalities in human preimplantation embryos. Fertil Steril. 1999;71(6):1033–9. doi: 10.1016/S0015-0282(99)00143-0. [DOI] [PubMed] [Google Scholar]
- 86.Simón C, Rubio C, Vidal F, et al. Increased chromosome abnormalities in human preimplantation embryos after in-vitro fertilization in patients with recurrent miscarriage. Reprod Fertil Dev. 1998;10(1):87–92. doi: 10.1071/R98030. [DOI] [PubMed] [Google Scholar]
- 87.Garrisi JG, Colls P, Ferry KM, Zheng X, Garrisi MG, Munné S. Effect of infertility, maternal age, and number of previous miscarriages on the outcome of preimplantation genetic diagnosis for idiopathic recurrent pregnancy loss. Fertil Steril. 2009;92(1):288–95. doi: 10.1016/j.fertnstert.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 88.Rubio C, Simón C, Vidal F, et al. Chromosomal abnormalities and embryo development in recurrent miscarriage couples. Hum Reprod. 2003;18(1):182–8. doi: 10.1093/humrep/deg015. [DOI] [PubMed] [Google Scholar]
- 89.Pehlivan T, Rubio C, Rodrigo L, et al. Impact of preimplantation genetic diagnosis on IVF outcome in implantation failure patients. Reprod Biomed Online. 2003;6(2):232–7. doi: 10.1016/S1472-6483(10)61715-4. [DOI] [PubMed] [Google Scholar]
- 90.Platteau P, Staessen C, Michiels A, et al. Comparison of the aneuploidy frequency in embryos derived from testicular sperm extraction in obstructive and nonobstructive azoospermic men. Hum Reprod. 2004;19(7):1570–4. doi: 10.1093/humrep/deh306. [DOI] [PubMed] [Google Scholar]
- 91.Kahraman S, Sertyel S, Findikli N, et al. Effect of PGD on implantation and ongoing pregnancy rates in cases with predominantly macrocephalic spermatozoa. Reprod Biomed Online. 2004;9(1):79–85. doi: 10.1016/S1472-6483(10)62114-1. [DOI] [PubMed] [Google Scholar]
- 92.Baltaci V, Satiroglu H, Kabukçu C, et al. Relationship between embryo quality and aneuploidies. Reprod Biomed Online. 2006;12(1):77–82. doi: 10.1016/S1472-6483(10)60984-4. [DOI] [PubMed] [Google Scholar]
- 93.Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87(3):534–41. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- 94.Munné S, Chen S, Colls P, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online. 2007;14(5):628–34. doi: 10.1016/S1472-6483(10)61057-7. [DOI] [PubMed] [Google Scholar]
- 95.Rubio C, Rodrigo L, Mercader A, et al. Impact of chromosomal abnormalities on preimplantation embryo development. Prenat Diagn. 2007;27(8):748–56. doi: 10.1002/pd.1773. [DOI] [PubMed] [Google Scholar]
- 96.Ziebe S, Lundin K, Loft A, et al. FISH analysis for chromosomes 13, 16, 18, 21, 22, X and Y in all blastomeres of IVF pre-embryos from 144 randomly selected donated human oocytes and impact on pre-embryo morphology. Hum Reprod. 2003;18(12):2575–81. doi: 10.1093/humrep/deg489. [DOI] [PubMed] [Google Scholar]
- 97.Keskintepe L, Sher G, Keskintepe M. Reproductive oocyte/embryo genetic analysis: comparison between fluorescence in-situ hybridization and comparative genomic hybridization. Reprod Biomed Online. 2007;15(2):303–9. doi: 10.1016/S1472-6483(10)60343-4. [DOI] [PubMed] [Google Scholar]
- 98.Veeck LL. An Atlas of Human Gametes and Conceptuses. London: Parthenon; 1998. [Google Scholar]
- 99.Alikani M, Cohen J, Tomkin G, et al. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42. doi: 10.1016/S0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]