Summary
The immune system plays an essential role in protecting the host against infections and to accomplish this task has evolved mechanisms to recognize microbes and destroy them. In addition, it monitors the health of cells and responds to ones that have been injured and die, even if this occurs under sterile conditions. This process is initiated when dying cells expose intracellular molecules that can be recognized by cells of the innate immune system. As a consequence of this recognition, dendritic cells are activated in ways that help to promote T-cell responses to antigens associated with the dying cells. In addition, macrophages are stimulated to produce the cytokine interleukin-1 that then acts on radioresistant parenchymal cells in the host in ways that drive a robust inflammatory response. In addition to dead cells, a number of other sterile particles and altered physiological states can similarly stimulate an inflammatory response and do so through common pathways involving the inflammasome and interleukin-1. These pathways underlie the pathogenesis of a number of diseases.
Keywords: DAMPs, IL-1, adjuvant, sterile inflammation
Introduction
Immune responses, both innate and adaptive, play an essential role in protecting vertebrates from microbes. In the absence of these defenses, individuals rapidly succumb to infection. This constant threat from microbes has imposed a strong selection pressure and indeed is thought to be the driving force in evolution that has shaped the immune system. As a consequence, the immune system has evolved numerous and diverse cellular and molecular mechanisms for detecting and responding to microbes and their products.
While the immune system has evolved to protect the host against infection, it is also clear that responses can be generated under absolutely sterile conditions and to molecules of non-microbial origin, even including ones from the host itself (1, 2). This is painfully evident to any reader who has experienced blunt trauma (e.g. banging a thumb with a hammer) after which the affected site rapidly becomes inflamed. This resulting inflammation is an innate immune response and one that is occurring without microbes having entered the host. Trauma, bleeding, cell injury, and irritant particles are among the many kinds of sterile stimuli that can trigger various kinds of immune responses, including both innate and adaptive ones.
Why should the immune system be concerned with non-infectious processes? One reason, which was well articulated by Matzinger (3, 4), is that some of these situations are potentially dangerous for the host and may even be harbingers of infection. Consider the example of cell death: if cells are dying by necrosis, then something is amiss and the host is losing functional components. By recognizing this process the immune system can rush defenses to the site and attempt to limit the damage, e.g. from microbes or other injurious agents. These responses can also be important to clear the cellular corpses and to stimulate tissue repair (5). A general theme that is emerging is that in addition to detecting microbes, the immune system is also involved in monitoring the host for the integrity of its tissues and cells and beyond this, maybe even physiological states, such as lipid metabolism and obesity (6, 7).
The mobilization of immune responses in sterile settings can have both positive and negative effects. On the one hand, injurious processes may be stopped or walled off and damage to the tissues repaired (5). On the other hand, the immune response may itself cause damage or produce mediators that lead to disease (5). Therefore, it is important to understand the mechanisms underlying these kinds of these responses and their role in health and disease.
In this article, we review our work on various facets of immune responses to non-microbial stimuli with an emphasis on responses to cell death and sterile irritant particles. We review primarily our work but put this into the context of other investigations in the field.
Adjuvants, cell death, and the stimulation of adaptive immune responses
Infection or immunization with microbes stimulates robust adaptive immune responses. However, immunization with highly purified proteins often does not stimulate immunity and instead may lead to tolerance (8). It has been known since the 1920s that to stimulate adaptive immune response to purified proteins, they must be admixed with adjuvants, which are immunostimulants often of microbial origin or made of irritant particles like alum (8, 9).
The situation was however different when individuals were immunized with cells instead of purified proteins. Inoculation with allogeneic cells that differed at major and/or minor histocompatibility antigens or autologous antigen-bearing cells (e.g. tumors) did elicit both antibody and T-cell responses and often quite strong ones. Perhaps because immunizations were occurring in a ‘black box’, with conditions being determined empirically, this paradox in the different requirements for immunization with proteins versus cells was not one that captured the attention of the field for many years.
Our foray into this issue of adjuvants and how dead cells influenced adaptive immune responses started with an unexpected observation in the laboratory. We were studying how particulate antigens were ‘cross presented’ on major histocompatibility complex (MHC) class I molecules in vivo in ways that would stimulate CD8+ T-cell responses (10, 11). We wanted to study the effect on these responses of manipulating the cytokine milieu at the site of antigen presentation in vivo, and to do this, we injected antigen together with irradiated cell lines that were engineered to secrete particular cytokines. We found that such cell lines strongly augmented the response to the injected antigen, but to our surprise, this occurred whether or not the cells had been transduced with cytokine genes (Fig. 1). What we came to appreciate was that dying or dead cells would provide a strong adjuvant activity to antigens in and around them (12). This ‘endogenous’ adjuvant activity (i.e. coming from autologous cells rather than foreign/external materials) augmented the priming of both CD4+ and CD8+ T-cell responses (12) and could help explain why immunization to cellular antigens is often successful without adjuvants. Other groups have reported similar findings (4).
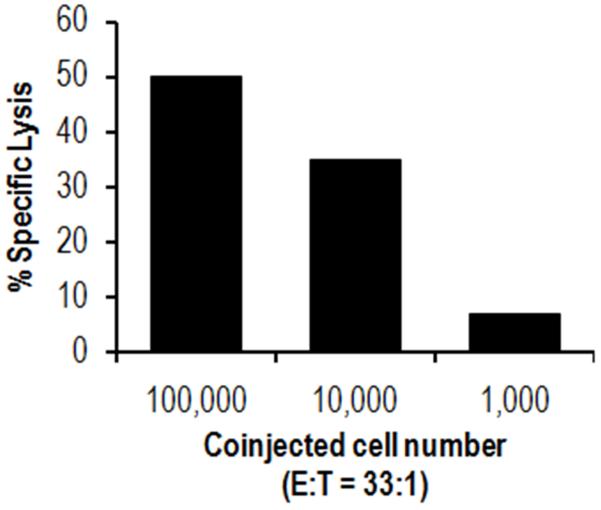
Fig. 1. Cells contain an endogenous adjuvant activity that augments the priming of CD8+ T-cell responses.
A limiting amount of antigen (5 μg ovalbumin-conjugated beads) was co-injected into mice together with either 105, 104, or103 mitomycin C-treated syngeneic GL261 cells. Seven days later, splenocytes were harvested and stimulated with ovalbumin-transfected EG7 cells. CTL activity was measured against EG7 cells 5 days later in a 51Cr release assay. E:T ratio= 33:1. The data show that the GL261 cells, which lack antigen, contain an activity that markedly augments the priming of CD8+ T-cell responses to the co-injected antigen.
We found that endogenous adjuvant activity was present to lesser or greater extents in all cells we examined, including both cultured and primary ones (12). The adjuvant activity was constitutively present in these cells under normal basal condition, and its levels increased in cells that were stressed by heat shock or inhibition of protein translation (12). Within normal or stressed cells, the adjuvant activity was present in the cytosol, and simple mechanical rupture of the plasma membrane released it into the extracellular fluids (12).
These findings indicated that in living cells, endogenous adjuvant molecules are sequestered by the plasma membrane and therefore not visible to the immune system. However when cells undergo necrosis for any reason, they invariably lose integrity of their plasma membrane, and when this occurs, cytosolic contents are released. It appears that the immune system has evolved the capacity to detect the release of some of these intracellular molecules in ways that stimulate the generation of adaptive immune responses to antigen in and around the dying cells (Fig. 2). Through this mechanism, living cells are ignored and dying ones are rapidly detected and investigated.
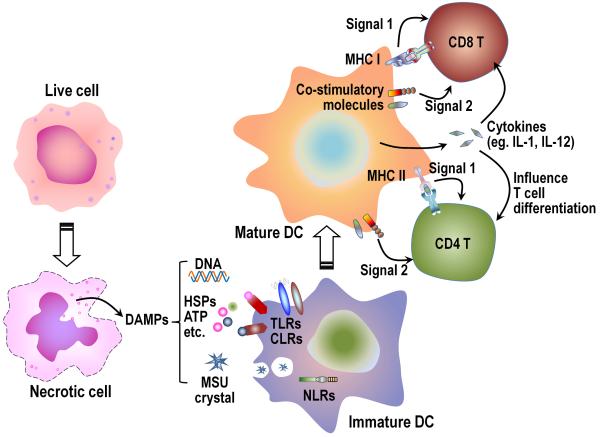
Fig. 2. Adjuvant effect of DAMPs in stimulating dendritic cells.
When cells undergo necrosis intracellular DAMPs (such as DNA, HSPs, MSU, etc.) are released into the extracellular milieu. Some of these DAMPs can act as adjuvants to stimulate dendritic cells (DCs), through pattern recognition receptors, such as TLRs, CLRs, or NLRs, or other receptors to increase the expression of MHC molecules, co-stimulatory ligands, and cytokines. These mature DC can then optimally activate T cells and direct their differentiation. HSPs, heat-shock proteins; MSU, monosodium urate crystal; TLRs, Toll-like receptors; CLRs, C-type lectin receptors; NLRs, NOD-like receptors.
Molecular identity of endogenous adjuvants
The molecules that are responsible for endogenous adjuvant activity have also been referred to as DAMPs (danger-associated molecular patterns). This acronym emphasized the similarities in function to the immunostimulatory molecules from microbes that were termed PAMPs (pathogen-associated molecular patterns) by Janeway (13).
While it was clear that cells contained DAMPs, the molecular identity of the bioactive molecules was unknown. To identify these molecules, cytosol was subjected to chromatographic separation, and the fractions screened for adjuvant activity in bioassays. Through this approach, one of the molecules with adjuvant activity was identified as uric acid (14).
Uric acid is produced in all cells during the catabolism of purines from DNA and RNA, and there are very high levels of this molecule constitutively present in the cytosol of normal cells (14). Moreover under conditions of injury and stress, cells may degrade their nucleic acids and this can lead to ten-fold increases in intracellular uric acid levels (14). In fact, even after cells have died, the cellular corps can continue to produce large amounts of uric acid as their DNA and RNA break down and the liberated purines continue to be metabolized by xanthine oxidase (15).
It was initially surprising that uric acid would have biological activity, because it is normally present in humans at ~60 μg/dl (in mice at about half this level), which is close to its saturating concentration (~70 μg/dl) in biological fluids. Of importance however, this molecule is completely in solution under normal physiological conditions. In contrast, it is thought that the immune system may be sensing uric acid when it undergoes a chemical phase change, nucleating into monosodium urate (MSU) microcrystals (14). This would occur when dying cells release their large intracellular pools of uric acid and create locally in the tissue interstitium a highly supersaturated solution. Moreover, the uric acid would go from the low sodium milieu of the cytosol into the high sodium-containing environment of the extracellular fluids. While nucleation of microcrystals around dead cells has not been formally proven to occur, the conditions would strongly favor this activity, and it has been shown that MSU crystals but not soluble uric acid solutions are stimulatory in vitro and in vivo (14).
There was endogenous adjuvant activity also present in fractions of cytosol-containing molecules of much higher molecular weight than uric acid (14). Therefore, uric acid is not the only endogenous adjuvant activity in cells. It is however an important one, because depletion of uric acid from cells, either by inhibiting its synthesis or hydrolyzing it through oxidative catalysis, partially but significantly reduces endogenous adjuvant activity in cells (14, 16, 17). There is also limited data potentially implicating ATP and nucleic acids as an endogenous adjuvant that promotes responses to cellular antigens (18, 19). A number of other cellular molecules have been reported to have endogenous adjuvant activity, e.g. high mobility group box 1 (HMGB1), heat shock proteins (HSPs), and granulysin, etc. (2, 20). How much these other molecules contribute to the total adjuvant activity in cells is not yet clear, because in most cases, the effect of eliminating them on this activity has not been tested.
Mechanism of action of endogenous adjuvants
Conventional microbial adjuvants are thought to work in large part by stimulating dendritic cells to mature and migrate to secondary lymphoid tissues (21). In this process, dendritic cells are stimulated to present antigen, express costimulatory molecules, and secrete cytokines in ways that are immunostimulatory for T cells and thereby initiate productive immune responses. Endogenous adjuvants may work in similar ways (Fig. 2). The injection of cell cytosol subcutaneously into mice stimulates dendritic cells in the skin to ingest particles and migrate to draining lymph nodes (22). The migrated cells are mature and express high levels of costimulatory molecules (22). Studies with MSU (14), HMGB1 (23), and granulysin (20) similarly showed that these agents would stimulate dendritic cells to mature. That this was a likely mechanism of action of endogenous adjuvants was shown in experiments where uric acid depletion inhibited the priming of T cells to antigen + dead cells but not responses stimulated by adoptively transferred antigen-bearing mature dendritic cells (16). This finding that endogenous adjuvants were needed before but not after dendritic cells had acquired the antigen and matured implied that they were acting at least in part by stimulating dendritic cells to become immunostimulatory.
Microbial adjuvants may also augment responses by stimulating the production of cytokines, such as IL-12 (24). Whether this is also the case for endogenous adjuvants has not been well studied. However, what is clear is that dying cells and MSU stimulate the production of cytokines, such as interleukin-1 (IL-1) (see sections below). IL-1 has been implicated in the adjuvant activity of alum by some (25, 26) but not other labs (27, 28, Kono and Rock unpublished result). IL-1 may also influence other events, one of which is the polarization of CD4+ T cells into T-helper 17 (Th17) cells (29, 30). Therefore, cell death and the release of endogenous adjuvants may not only influence the priming of T cells but also their differentiation into polarized effector cells.
There must be receptors on (or in) dendritic cells that are stimulated by endogenous adjuvants and activate these cells in ways that promote immune responses. However, only a few of these putative receptors have as yet been identified. One of these is the C-type lectin receptor Clec9a, which is expressed primarily on CD8-expressing dendritic cells. While its precise ligand is unknown, Clec9a is stimulated by necrotic cells and promotes CD8+ T-cell responses to antigens associated with the dead cells (31). Anti-Clec9a antibodies have also been shown target antigens into dendritic cells and promote immune responses (32). There is also evidence that dendritic cells are stimulated by granulysin (20) and HMGB1 (33-36) through Toll-like receptor 4 (TLR4) and/or RAGE (receptor for advanced glycation end products) and that cellular nucleic acids can stimulate TLR7 and TLR9 on B cells to promote antibody responses (19, 37, 38). Similarly, the P2X7 ATP receptor has been implicated in augmenting responses to cells (18). NLRP3 senses MSU, but it has not yet been shown that this receptor is the one through which this endogenous molecule stimulates dendritic cells and provides adjuvant activity (39). An important area of future research will be the molecular identification of endogenous adjuvants and their receptors as well as elucidation of their mechanisms of action and contributions to immune responses.
Sterile cell death triggers an inflammatory response
The concept that cell injury can help to trigger adaptive immune responses is relatively recent. In contrast, it has been known since ancient times that injury induces inflammation (40), and inflammation is now recognized to be an innate immune response. In response to cell injury, the immune system rushes its defenses to the affected site. There is rapid vasodilatation, which increases delivery of blood-borne defenses to the affected area, protein-rich fluid then leaks from vessels into the tissue space, which delivers soluble immune defenses, and leukocytes migrate from post-capillary venules into the interstitial space, which brings cellular defense to site of injury (5). These events were first described in the mid-19th century before there was any clear concept that innate immunity even existed (41-43). Such inflammation occurs whenever there is significant necrosis occurring in vivo, and its choreography is so stereotypical that its presence and progression is used by pathologists to date the time of tissue injury, e.g. in a myocardial infarct. While, it has long been known that cell death stimulates inflammation, the mechanisms through which this occurred were largely unknown until recently.
What in dying cells triggers an inflammatory response?
The innate immune system somehow senses cell death in ways that lead to inflammation. Since unperturbed living cells do not cause inflammation, it follows that upon death, there are changes in cells that promote inflammation. Similar to what was discussed in the section on endogenous adjuvants, it is thought that after death cells expose components (DAMPs) that are not normally seen on living cells (1, 2) (Fig 2). In support of this concept, simple rupture of the plasma membrane makes cells proinflammatory, and injection of their intracellular contents is proinflammatory (44). In recent years a number of candidate proinflammatory DAMPs have been identified.
The endogenous adjuvant uric acid was an obvious candidate for a proinflammatory DAMP, because, as discussed above, it is released from dying cells and recognized by the innate immune system. Moreover, MSU was known to be highly proinflammatory, as it is the etiological agent for the inflammatory disease gout, a condition that occurs in hyperuricemic patients (45), and it causes robust inflammation when injected into normal animals (46). To investigate whether uric acid is important to the death-induced sterile inflammatory response, it was depleted from animals by expressing a transgenic uricase construct or by blocking its synthesis with xanthine oxidase inhibitors. In such uric acid-depleted mice, the inflammatory responses to cell death were significantly reduced (15). In contrast, in the same uric acid-depleted animals, inflammatory responses to microbial stimuli [zymosan or lipopolysaccharide (LPS)] or irritant particles (silica) were not reduced (15). These findings indicated that uric acid depletion was not generally anti-inflammatory but selectively affected inflammation to cell death. Similarly, uric acid depletion decreases inflammation and damage in the lung induced by bleomycin or elastase damage (47, 48). Therefore, uric acid is a proinflammatory DAMP that helps stimulate the death-induced inflammatory response.
The inhibition of inflammation to cell death in uric acid-depleted mice while significant was only partial; this finding suggested that other proinflammatory DAMPs must exist and be contributing to these responses, and indeed, there are many candidates for this function. One of these candidates is ATP. It can function as a ‘find me’ signal for dead cells (49, 50) and also stimulate the production of proinflammatory cytokines, like IL-1, from macrophages (51, 52). It was reported recently that pharmacological depletion of ATP or elimination of its receptor, P2X7, markedly inhibited inflammation in vivo to thermal injury of the liver (53), indicating that ATP can function as a significant proinflammatory DAMP. Another such DAMP is SAP130 that binds the C-type lectin receptor Mincle that is expressed on leukocytes (54). Antibodies to Mincle inhibited inflammation to cell death in the thymus (54). A role for non-muscle myosin has been shown in cardiac ischemia reperfusion (55). In this case, the released molecule does not have proinflammatory activity by itself but upon release becomes bound by natural antibodies to form immune complexes that then activate complement (55). Yet other potential DAMPs that have been implicated in some models of cell death-induced inflammation are HMGB1 (56, 57), nucleic acids (RNA and DNA) (58-61), mitochondrial components (53, 62, 63), lactoferrin (64), and heat shock proteins (65, 66), among other molecules.
It may be that the contribution of these various DAMPs will vary in different situations. There could be cell type differences, because some DAMPs may be expressed in a tissue-restricted manner. One example is a class of DAMPs called Alarmins (67, 68). These are molecules such as β-defensins that are expressed primarily in leukocytes. In addition to cell-type differences, there could be differences in the contribution of different DAMPs, depending on the anatomical location or type of injury. This might explain why DAMPs that have been reported to be required for some responses do not seem to be necessary for others. Although there is evidence for a role of ATP, HMGB1, and Mincle (53, 54), in our assay systems, we have not observed reductions in cell-death induced inflammation when ATP is depleted or in animals that genetically lack the P2X7 ATP receptor, Mincle (Kono, Patel and Rock unpublished result), or HMGB1 (69). In the case of ATP, this perhaps is not surprising because in many situations, e.g. ischemia, dying cells will have depleted their intracellular stores of this nucleotide (70) yet are highly inflammatory.
Role of TLRs, TIR-adapters, and IL-1
While the mechanism by which dying cells stimulated inflammation was unknown, there has been considerable progress over the last decade in elucidating how microbes stimulate inflammation. One of the important mechanisms that triggers inflammation to infection is the recognition of microbial molecules by pattern recognition receptors, such as TLRs (71). While TLRs were first shown to recognize unique microbial chemical structures that were not made by the host, there were subsequently a number of reports suggesting that these receptors could also recognize some of the host’s own molecules (e.g. HMGB1, DNA, and HSPs) (72); although a caveat to these findings was the possibility that the stimulatory activity of some of these putative autologous TLR ligands was actually due to contamination with microbial molecules (and this was probably an artifact in some cases) (73, 74). Nevertheless these findings raised the possibility that TLRs might be involved in sensing cell death, and in response, triggering inflammation.
Indeed some studies have found a potential role for TLRs in the inflammatory response to cell death. TLR2/TLR4 double knockout mice injected with sterile dead cells generate less inflammation compared to wildtype animals, although the reduction in this response is modest (69); in contrast, in the same system responses were normal in TLR1, TLR3, TLR6, TLR7, TLR9, and TLR11 single knockout mice. Recent studies have shown that TLR2, TLR4, and/or TLR9 can also play a role in the ischemia-reperfusion (I/R) injury in heart, brain, liver, intestine, and kidney (75, 76). In these cases, DAMPs (e.g. HMGB1, HSPs, and DNA, etc.) are thought to be released from the initial ischemic tissue, and the tissue damage is exacerbated by the intense inflammatory responses after reperfusion occurs. In addition, in a drug-induced liver injury model, a reduction in inflammation was observed in TLR9-deficient mice (59). Overall the contribution of individual TLRs in the response to cell death, although varied, has been in general partial and in our hands relatively minor. A caveat to these loss-of-function studies is that if multiple TLRs participate in these responses (so the loss of any one might have a minimal effect), then a larger role for these receptors could have been missed.
When stimulated TLRs initiate a signaling cascade by recruiting intracellular TLR-IL-1 receptor (TIR) adapter molecules, three of four TIR adapters were completely dispensable for death-induced inflammation in vivo. However one TIR adapter, myeloid differentiation factor 88 (MyD88), was essential for the inflammatory response to injected dead cells (69). This was surprising, given the apparent limited role of TLRs in these responses, and suggested that other MyD88-dependent receptors might play a key role. Examination of mutant mice lacking such receptors revealed that the MyD88-dependent IL-1 receptor (IL-1R) was essential for the death-induced sterile inflammatory response (69). Reciprocal experiments showed that neutralization of IL-1α or genetic deficiency of IL-1 inhibited these responses (44, 69) (Fig. 3). In contrast, inflammatory responses to a microbial stimulus such as zymosan were intact in mice lacking the IL-1 pathway. These data pointed to a key role of IL-1 in the death-induced sterile inflammatory response.
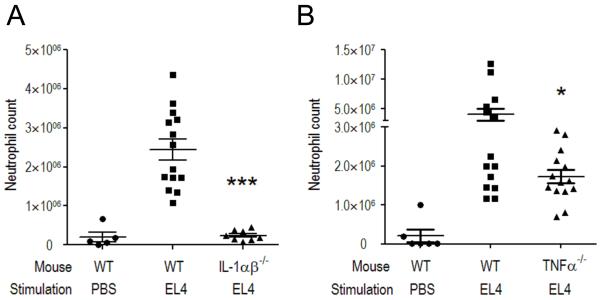
Fig. 3. Role of IL-1 and TNFα in cell death-induced inflammatory responses.
Wildtype (WT) C57BL/6 and (A) IL-1αβ double-deficient or (B) TNFα-deficient mice were injected intraperitoneally with 30 million heat-shocked necrotic EL4 cells. Total neutrophil number in the peritoneal cavity was determined 14 h after stimulation. The data are combined results of three experiments with values from individual mice displayed along with means ± SEM. *p<0.05, ***p<0.0001 versus WT group. The data shown that TNFα can make some contribution to the cell death-induced inflammatory responses; notably this contribution is less than from IL-1.
The finding that IL-1 played a dominant role in cell death-induced inflammation was surprising, because there are other proinflammatory cytokines produced in inflammatory responses that have redundant effects with IL-1. Moreover, tumor necrosis factor-α (TNFα) appears to be a more important cytokine in driving inflammation in a number of other conditions, such as in autoimmune diseases. However, in the sterile inflammatory response to cell death, the contribution of TNFα appears to be more modest than IL-1 (Fig. 3). Indeed, it may be generally true that IL-1 is a dominant cytokine driving sterile inflammatory responses in non-autoimmune situations (see below).
The sources of IL-1 in the death-induced sterile inflammatory response
Since IL-1 was required for the death-induced sterile inflammatory response, it was important to understand where it was coming from and how it was generated. One possibility was that the IL-1 was being released from the dying cells themselves (77). This could occur because cells that are making IL-1 contain intracellular pools of the cytokine. Remarkably it is poorly understood how IL-1 is normally released from cells other than the fact that it does not follow a typical secretion pathway (78). It has long been known that IL-1 can be released experimentally by breaking cells open, and therefore necrotic death, in which cells swell and lose integrity of their plasma membrane, is one way the cytokine could be released.
Support that IL-1 from dying cells could stimulate inflammation came when it was observed that dead IL-1α -deficient dendritic cells did not trigger responses while IL-1-sufficient ones did (77). However, dendritic cells can make a lot of IL-1, and this IL-1 is sufficient to trigger inflammation even when live dendritic cells are injected in vivo (44). In contrast, injection into mice of a variety of other dead cell types that genetically lack both IL-1α and IL-1β stimulated an inflammatory response that was equivalent to that of wildtype necrotic cells (44). Therefore, the IL-1 that is driving the sterile inflammatory response in many cases is not coming directly from the dead cell. In these situations, the IL-1 must be produced by cells in the host upon recognition of cell death. That this is the case was formally shown by the loss or inflammatory response to dead cells in mice that genetically lack either IL-1α or IL-1β (44).
What is the cellular sensor of cell death for the sterile inflammatory response?
What is the host cell that is sensing cell death and in response producing the IL-1 that drives the sterile inflammatory response? There could be many candidates because a large number of cell types can produce IL-1α, and there are several that can also make IL-1β. To narrow the field of potential cell types, experiments were performed with chimeric mice in which either their bone marrow or parenchymal elements (radioresistant cells) genetically lacked IL-1α and IL-1β. These experiments showed that the sterile inflammatory response to injected dead cells required IL-1 genes (both IL-1α and IL-1β) in the bone marrow cells (44). In addition, there was a reduction, albeit partial, in mice whose radioresistant elements lacked IL-1β (44).
The next question was which kind(s) of bone marrow-derived cell(s) was the source of IL-1 in these responses. The fact that the cell was of bone marrow origin pointed to a leukocyte, but this did not narrow the field of candidates very far, as many of these cells can make IL-1. To determine the identity of the relevant cell(s), experiments were performed to assess the impact of eliminating various leukocytes. The conditional depletion of cells expressing high levels of CD11b in CD11b-promoter-driven diphtheria toxin receptor transgenic mice markedly inhibited the inflammatory response to dead cells injected intraperitoneally (44). Under these same conditions, the CD11b cell-depleted mice generated normal inflammatory responses to another proinflammatory stimuli injected intraperitoneally, such as MIP-2 (44). Therefore, in the absence of the CD11bhigh cells, animals still have the necessary components to successfully mount inflammatory responses, but they are markedly impaired in doing so in response to cell death.
One of the principal cell types eliminated in the CD11b-DTR transgenic mouse is the macrophage. To determine whether this was the relevant cell type, macrophages were transferred back into CD11b-depleted mice. The adoptive transfer of these cells could reconstitute the response to dead cells (44) (Fig. 4). Even though highly purified macrophages supported the responses, a limitation of using primary cells is the possibility that a contaminating cell type was contributing to the reconstitution. To address this question, a cloned macrophage cell line was transferred, and it too was able to reconstitute responses (44). Therefore, these studies indicated that macrophages are a key cell participating in the death-induced inflammatory response.
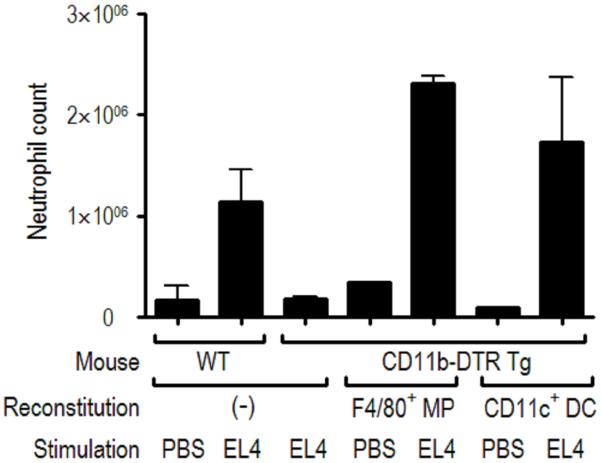
Fig. 4. Analysis of the cells required for the generation of cell death-induced inflammation.
Wildtype (WT, FVB/N) or CD11b-DTR-Tg mice were injected intravenously with 500 ng of diphtheria toxin (500 ng); this procedure depletes macrophages in the transgenic mice. The treated animals were then reconstituted with F4/80+ macrophages (MPs) from thioglycollate-elicited peritoneal cells or CD11c+ dendritic cells (DCs) from spleens of Flt3L-treated mice (C57BL/6 mice injected with Flt3L-transduced cells). Neutrophil number in the peritoneum was determined 14 h after intraperitoneal injection of heat-shocked necrotic EL4 cells or PBS and displayed as the mean ± SEM. The data show that loss of CD11b+ cells in the host markedly inhibits inflammation to dead cells and that these responses can be reconstituted by the adoptive transfer of MPs or DCs.
The role of dendritic cells in these responses was also investigated. This was of particular interest, because these cells do respond to dead cells (14, 22) and a subset of them can express CD11b. However, conditional depletion of CD11c-expressing cells in a CD11c-promoter-driven-DTR transgenic mouse did not reduce the death-induced inflammatory responses in the peritoneal model, even though there was good depletion of dendritic cells (44). Interestingly, adoptive transfer of dendritic cells into CD11b-depleted mice can also reconstitute the inflammatory to dead cells (Fig. 4). Dendritic cells therefore have some capacity to participate in this response, and it is possible that in tissues where dendritic cells are more abundant than in the peritoneum, these cells might play a role in addition to macrophages.
With the identification of a key role for macrophages in the death-induced sterile inflammatory response, the obvious question was whether these cells were the source of IL-1 that was needed for these responses. To address this question, IL-1-sufficient versus IL-1-deficient macrophages were transferred into CD11b-depleted mice. It was found that IL-1-deficient macrophages did not reconstitute inflammation, and they were indeed therefore a source of IL-1 in these responses (44). While IL-1α -deficient macrophages failed to reconstitute responses, IL-1β-deficient ones could (44). Since the radiation chimera studies discussed above indicated IL-1β -sufficient bone marrow was required for death-induced inflammation, the finding that IL-1β-deficient macrophages reconstituted inflammation in CD11b-depleted mice suggests that there may be an additional bone marrow-derived cell that contributes IL-1β to these responses.
Other sterile triggers of inflammation also trigger IL-1-dependent inflammation
As discussed above, urate is a proinflammatory DAMP that helps stimulate part of the inflammation to dying cells. Since this response is IL-1 dependent, it is perhaps not surprising that the inflammatory response to urate crystals was also found to require this cytokine (69, 79). In addition to urate crystals, there is a diverse set of particles that can induce inflammation in vivo. These irritant particles include silica crystals, calcium pyrophosphate crystals, alum (precipitated aluminum salt), and asbestos particles. The finding that inflammation to urate was IL-1-dependent led to examination of the role of this cytokine in inflammatory responses to these stimuli and remarkably responses to all these particles were dependent on IL-1 (25, 80-84). Subsequent studies found that particles that were not traditionally thought of inducers of inflammation, such as cholesterol crystals, would similarly stimulate IL-1-dependent inflammation when they are injected or form in vivo (85) (see below). All of these particles were also shown to stimulate macrophages to produce bioactive IL-1β in vitro (25, 80-82, 85)
The mechanisms by which bioactive IL-1 is produced are best studied for IL-1β. This cytokine is initially synthesized, as is a long precursor that is biologically inactive (pro-IL-1β) (30). When macrophages producing pro-IL-1β are stimulated with ATP or irritant particles, the pro-caspase 1 zymogen (inactive protease) assembles into a molecular complex called the inflammasome and is cleaved into active form (86, 87). The catalytically active caspase 1 then cleave pro-IL-1β to its mature and biologically active form (86).
Inflammasomes consist not only of caspase 1 but also of a scaffolding protein called ASC and an NLR protein, the latter of which is thought to be the activating component of the complex (87). The NLR protein that was necessary for MSU crystals to stimulate macrophages to produce IL-1β was found to be NLRP3 (79). Subsequently, it was found that NLRP3 was also required for macrophages to produce IL-1 when stimulated by many irritant particles, including silica, asbestos, alum, calcium pyrophosphate, and cholesterol crystals (25, 80, 82, 83, 85). The IL-1-dependent inflammatory response to cell death in vivo is significantly reduced in NLRP3-deficient mice (59, 63, Lai and Rock, unpublished result).
Priming of macrophages to make IL-1
For macrophages to be stimulated by sterile particles to make IL-1 in vitro, they must first be ‘primed’ with a stimulus that induces the synthesis of pro-IL-1β and also upregulates the expression of NLRP3 (88, 89). The stimuli that can prime macrophages include TLR agonists and cytokines like TNF. These priming stimuli may work by activating NFκB, an important transcription factor for IL-1β and presumably also for NLRP3 (88). What is surprising and not understood is that the while priming is essential for responses to particles in vitro, the same particles stimulate IL-1-dependent responses in vivo without a co-injected priming stimulus. This finding implies that something is providing the priming stimulus in vivo. Conceivably the particles are stimulating the production of cytokine in vivo that prime macrophages. Indeed, it is clear that particles like urate crystals can stimulate cells through other pathways than the NLRP3 inflammasome (90-92). Alternatively, it is possible that some of the IL-1-producing cells in vivo reside in a state where priming is not required, either due to their differentiation state or signals in the host environment (e.g. microbial products). In fact, in some of the examples discussed above where TLRs contribute to IL-1-dependent sterile inflammatory responses, it is conceivable that they are doing so by helping to prime macrophages.
How do dead cells and irritant particles trigger inflammasomes?
The NLR polypeptides are leucine-rich repeat proteins and are thought to function as intracellular sensors that control the activation of inflammasomes. In support of this concept mutations in NLRP3 lead to spontaneously active inflammasomes (or ones that have a lower threshold of activation) and as a consequence cause IL-1-dependent autoinflammatory diseases (93). Exactly what NLRP3 senses and how it activates the inflammasome is still not well understood. A number of microbial molecules can stimulate the activation of NLRP3 inflammasomes and have been considered as putative ligands for this NLR protein (93), although direct binding has not yet been documented.
Since NLRP3 is a cytosolic and nuclear protein, it was a mystery as to how it could be sensing dead cells or irritant particles that are extracellular and excluded from the cell interior by the plasma membrane. When macrophages encounter these particles, they rapidly ingest them by phagocytosis, and this process was found to be essential for particle triggering of NLRP3-dependent IL-1 production (80). In contrast, soluble activators of NLRP3, such as ATP, did not require phagocytosis. While internalization may bring the particles one step closer to NLRP3, the particles and NLRP3 are still in anatomically distinct compartments. As the next step in the pathway, there is evidence for two distinct mechanisms by which particles in phagosomes may stimulate NLRP3 (Fig. 5).
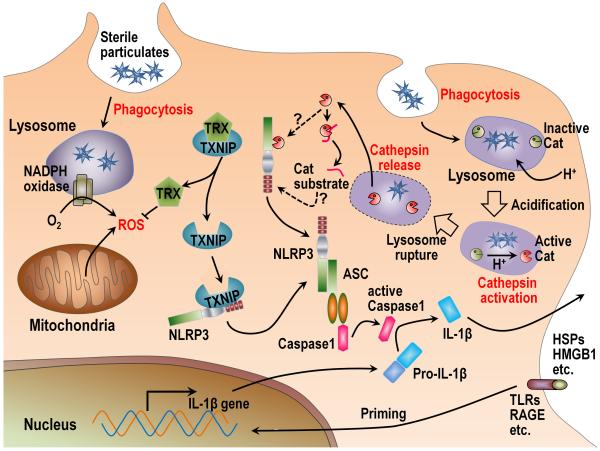
Fig. 5. Mechanisms of NLRP3 inflammasome activation by sterile stimuli.
There are two distinct mechanisms proposed as to how NLRP3 inflammasomes are activated by sterile particles. The first model suggests that after phagocytosis, the particles stimulate ROS from NADPH oxidase and/or mitochondria. The increased level of ROS in the cytosol is then sensed by thioredoxin (TRX) and causes its dissociation from thioredoxin-binding protein (TXNIP). The released TXNIP then binds and activates NLRP3 through interaction with LRR and NATCH domains of NLRP3. The second model also requires phagocytosis of the sterile particles. In this pathway phagosomes acidify and the drop in pH causes Cathepsin (Cat) activation. Through some unknown mechanism, some of the particulate-containing phagosomes rupture and release their contents into the cytosol. This vacuolar rupture is somehow sensed by NLRP3, possibly by binding a cleavage product of activated cathepsins or by the cathepsins cleaving NLRP3 in a way that activates it. NLRP3 associates with ASC and pro-Caspase 1 to form the inflammasome and cleaves the Caspase 1 zymogen into its active form. Active Caspase 1 then cleaves pro-IL-1β into active IL-1β. In most cases, the synthesis of pro-IL-1β is induced by “priming”, which requires the stimulation of pattern recognition receptors or cytokines to stimulate transcription of pro-IL-1β.
In one pathway, internalized particles stimulate the production of reactive oxygen species (ROS), and this process has been linked to the NLRP3 pathway because elimination of ROS with chemical scavengers sometimes inhibits the production of IL-1 (82). Phagosomes contain an NADPH-oxidase complex that can be triggered by internalized particles to produce large amounts of ROS, which normally functions as part of the intracellular anti-microbial response. This oxidative burst was initially thought to be the source of the reactive products driving the IL-1 response (82). However, the particle-stimulated IL-1 response was subsequently found to be intact in cells that genetically lack NADPH oxidase, so this enzyme cannot be the only source of ROS that triggers this response (80, 94-96). More recently, it has been suggested that the ROS stimulating the NLRP3 inflammasome is instead coming from mitochondria (96), although it is not yet clear how the ingestion of particles stimulates these organelles to make these reactive species. More recent data have suggested that once ROS are generated they cause thioredoxin-binding protein (TXNIP) to dissociate from thioredoxin and bind to the leucine-rich repeat and NATCH domains of NLRP3, causing its activation (97).
In contrast to the above results, some other experiments have not observed any inhibition of particle-stimulated IL-1 responses by ROS scavengers and SOD1 mutations that lead to increased ROS levels decreased caspase 1 activation and IL-1 production (96, 98-100). Similarly, in TXNIP-deficient macrophages, others could not observe a reduction in IL-1β secretion upon stimulation with several NLRP3 inflammasome activators, such as IAPP, MSU, or ATP (101). While the reasons for these differences are not clear, they might be due to specific cell types or conditions used in different experimental systems. It also suggests that there are additional pathways that can operate, and indeed there is evidence that this is the case.
A second pathway also initiates when particles are internalized into phagosomes and starts with events that are part of normal phagosomal physiology (Fig. 5). As part of the process that coverts the phagosome into a catabolic vacuole, it fuses with lysosomes and a proton antiporter in the membrane acidifies the vesicle (80). This drop in pH causes the lysosomal acid optimal proteases (Cathepsins) to become catalytically active, a process that is important for hydrolyzing the proteins that have been ingested by the phagocyte. In addition, it turns out the activation of at least two of these proteases, Cathepsin B and L, also contributes to the triggering of the NLRP3 inflammasome, as discussed below. What happens next is that for reasons that are not yet understood, a fraction of the particle-containing phagosome rupture and release their contents into the cytosol (80). While this event places the particles into the same subcellular compartment as the NLRP3 inflammasome, the particles themselves are not directly triggering this pathway. Instead, it is the event of phagosomal rupture and specifically the release of Cathepsin B and L (or the cleavage products from their substrates) that seems to be somehow be sensed by NLRP3. In support of this concept, simply rupturing vesicles by osmotic lysis (without particles) activates the NLRP3 inflammasome in macrophages, and this process requires activated Cathepsins (80). Exactly how the activated Cathepsins are sensed by NLRP3 is not presently known. It may be that after cleavage, one of substrates becomes a stimulatory ligand for NLRP3 or the Cathepsins might cleave NLRP3 in a way that leads to its activation (Fig. 5).
While most work has focused on how DAMPs stimulate cytokine production and inflammation, there may also be regulatory mechanisms that serve to limit these responses. Thus, there are emerging data that at the same time that DAMPs stimulate inflammation, they also engage some regulatory receptors (e.g. CD24, Siglec-10/G), which then serve to limit the magnitude of the inflammatory responses (102).
Innate sensing of host damage
The finding that NLRP3 senses vesicular rupture has led to a new concept that this innate sensor is monitoring the health of cells and responds to internal cell damage. This may not be the only thing that NLRP3 senses, as there is evidence that it is stimulated by other molecules including products of microbes and ATP. However, at least some of these molecules are ones that can disrupt membranes (e.g. listeriolysin O, nigericin, maitotoxin, and hemolysins, etc.) (103-105), so it is possible that these agents might also stimulate NLRP3 by causing vesicular rupture.
NLRP3 is not the only example where the immune system is monitoring the health of cells. γδ T cells in the skin monitor keratinocytes for damage and in response help stimulate tissue repair (106, 107). Natural killer (NK) cell receptors monitor cells for MIC proteins, which are expressed under certain conditions of cell stress (108, 109). It has been suggested that the release of heat shock proteins (many of which are induced upon cell stress) may stimulate innate cells (110, 111); the release of HMGB1 upon cell damage can stimulate TLRs and RAGE (56, 112). Mincle senses SAP130 from necrotic cells to stimulate inflammation (54); Clec9a recognizes unidentified ligands from necrotic cells and enhances cross priming of CD8+ T cells (31). Thus, an emerging theme is that beyond surveillance for infections the innate immune system may employ sensors to monitor the health of host cells and respond in ways to repair tissues or eliminate abnormal cells.
Inflammasome/caspase 1-independent pathways
The inflammasome plays an essential role in generating mature IL-1β in vitro. Macrophages lacking any of the inflammasome components (NLRP3, ASC, or caspase 1) make essentially no mature IL-1 when stimulated in culture with sterile particles (80, 81). However, the situation is more complicated in vivo. We and others have found that a variable but sometimes substantial sterile inflammatory response can be seen in caspase 1-deficient mice (69, 113-117). This contrasts with the much more marked reduction of these responses that is consistently observed in IL-1β-deficient mice. These data imply that there must be a caspase 1-independent pathway for generating mature IL-1β in vivo. Indeed, there are other proteases that can cleave IL-1β (30) and some evidence that these may operate in vivo (113, 118).
Downstream targets of IL-1
Thus far we have focused on how IL-1 is produced. The next question is once IL-1 is produced, where is it acting to cause inflammation? This has been investigated by examining where the IL-1R needs to be expressed. This receptor is broadly expressed on both leukocytes and tissue parenchymal elements (119, 120). However, when IL-1R-deficient bone marrow is transplanted into wildtype mice, the chimeric animals mount perfectly normal sterile inflammatory responses, at least to crystals or dead cells (46, 69). This indicates that leukocytes or other bone marrow-derived elements do not need to be stimulated by IL-1 to generate the sterile inflammatory responses. This was somewhat surprising, because IL-1 can induce more IL-1 (30). It might have been expected that this autoamplification would have been important for optimal production of IL-1 from leukocytes, and this obviously cannot occur in the absence of the IL-1R. In contrast, if IL-1R-sufficient bone marrow is transferred into irradiated IL-1R-deficient mice, then the inflammatory responses are markedly attenuated. These results indicate that the key target of IL-1 is a radio-resistant parenchymal element. The identity of this element(s) is presently under investigation.
Medical importance
The host pays a price for mobilizing an inflammatory response. It causes symptoms such as fever, malaise, and pain, which by themselves can compromise normal function. Moreover, the leukocytes that infiltrate the tissues are ‘trigger-happy’ and relatively imprecise in their aim; as a result, proteases, ROS, and other toxic products are released from these cells when they are alive and also leak from them when they are dead (5). These products, while useful in the killing of microbes, can and do inflict damage in the tissue. Moreover, the bioactive mediators that are produced may act on tissue elements in ways that can lead to pathological changes (e.g. stimulating abnormal proliferation or collagen deposition).
These ‘costs’ of the inflammatory response may be a small price to pay in response to an infection, where the alternative can be death or significant morbidity. However, in situations where the stimulus is sterile, the cost-benefit ratio may be less favorable. The host response may contribute relatively little to defense, and the net effect may be damage. The host can deal quite well with small amounts of damage. However, when the damage is substantial, chronic, and/or repetitive, then there can be pathological consequences, such as loss of function and fibrosis, and this underlies the pathogenesis of a number of diseases.
There are a number of diseases that are caused by sterile inflammation and several of these are thought to be directly related to the NLRP3-IL-1 pathways described above. Among these are a collection of diseases that are caused when particles, such as the ones discussed above, are deposited in tissues. When urate crystals form in the joints of hyperuricemic patients, the IL-1-dependent inflammatory response causes the arthritis the disease of gout (46, 79, 121). A similar problem occurs when crystals of calcium pyrophosphate deposit in joints, and when this happens, the particles cause the condition of pseudogout (79, 122). Another set of examples is when individuals inhale silica crystals or certain forms of asbestos that cause an inflammatory response in the lung that ultimately leads to pulmonary fibrosis in the diseases of silicosis and asbestosis (123). Given these findings, it is possible that this response to inhaled particles may similarly contribute diseases caused too many other inhaled particulates such as those in cigarette smoke or air pollution.
The pathology caused by the innate response to particles may also contribute to a number of other conditions that are not traditionally thought of as particle-based diseases. One intriguing possibility is the disease of atherosclerosis. In this condition, plaques form in the walls of arteries when lipids and cholesterol deposit and the underlying smooth muscle cells proliferate (124). When this process advances to a point where it interferes with blood flow then it causes ischemic disease. It is clear that what is driving this disease is a sterile inflammatory response in the vessel wall (124). However, what is causing the sterile inflammation is unclear, other than it is related somehow to modified low density lipoproteins. While it has long been recognized that cholesterol crystals are present in advanced atherosclerotic lesions, it has more recently been shown that microscopic cholesterol crystals can be found inside of macrophages as early as plaques can be detected (85). Moreover, when such crystals are ingested or form in macrophages, they trigger the NLRP-3 inflammasome-dependent production of IL-1 in a cathepsin-dependent pathway (85). Remarkably, blocking this inflammatory pathway in vivo reduces the inflammatory response to cholesterol crystals and markedly reduces plaque formation in experimental models of atherosclerosis. Beyond atherosclerosis, there is some intriguing data that the deposition of insoluble proteins may trigger the same pathways to cause conditions like Alzheimer’s disease (81) and type II diabetes (101). There is also emerging data that IL-1 (and the NLRP3) may be involved in metabolic syndrome in which obese individuals develop among other things insulin resistance and diabetes (6, 125, 126). At its core metabolic syndrome may be a sterile inflammatory disease of adipose tissue. What incites this inflammation is unclear, but in this condition, there is death of adipose tissue with subsequent phagocytosis of the fat cells by macrophages (127-129). This process is something that would be expected to trigger the NLRP3-IL-1 pathway.
There are also diseases that can be exacerbated by sterile inflammation. An example of this is disease processes that result in cell death. In this situation the inflammatory response to dying cells can compound and extend the tissue damage. This is thought to occur e.g. in ischemic diseases, such as a myocardial infarct or stroke or toxic damage, e.g. in drug-induced liver injury (130). In fact, it is likely that this process may to contribute to the pathogenesis of a number of diseases wherein pathological processes lead to cell death.
Conclusions
As we have reviewed, it is becoming increasingly clear that the innate immune system not only monitors the host for microbes but also the health of the host’s own cells. In response to cell death, the innate immune system alerts the adaptive immune system to a potential problem in ways that promote the generation of responses. In parallel, it also rapidly mobilizes innate defenses to the site of injury through the generation of an inflammatory response.
Similar sterile inflammatory responses are generated to other kinds of non-microbial macroscopic particles. One of the important insights into these seemingly unrelated processes is that there is a common underlying pathway in which IL-1 is produced and is one of the key cytokines driving the ensuing inflammation. There are increasing insights into the identity of the cells that detect the sterile stimuli, how they sense such stimuli at the molecular level, and then respond to make IL-1. This is important, because there is also increasing recognition that these processes contribute to the pathogenesis of a large number of diseases.
Acknowledgements
This publication was made possible by grants from the NIH and AAF to K.L.R and a core support Grant (5 P30 DK32520) from the National Institute of Diabetes and Digestive and Kidney Diseases. H.K. was supported in part by funds from Sanofi Aventis. The authors thank Dipti Karmarkar and Hiroshi Kataoka for critical reading of the manuscript.
Footnotes
There are no other potential conflicts of interest.
References
- 1.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 5.Majno G, Joris I. Cells, tissues, and disease: principles of general pathology. 2nd ed. Oxford University Press; New York; Oxford: 2004. [Google Scholar]
- 6.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 8.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes. XVII-XXIV. The Journal of Pathology and Bacteriology. 1926;29:31–40. [Google Scholar]
- 10.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 11.den Haan JM, Bevan MJ. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr Opin Immunol. 2001;13:437–441. doi: 10.1016/s0952-7915(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 15.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol. 2006;176:3905–3908. doi: 10.4049/jimmunol.176.7.3905. [DOI] [PubMed] [Google Scholar]
- 17.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 18.Weber FC, et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207:2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tewary P, et al. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–3474. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Rock KL. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur J Immunol. 2002;32:155–162. doi: 10.1002/1521-4141(200201)32:1<155::AID-IMMU155>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Rovere-Querini P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 25.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 31.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idoyaga J, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 34.Apetoh L, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 36.Dumitriu IE, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 37.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 38.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaemi-Oskouie F, Shi Y. The Role of Uric Acid as an Endogenous Danger Signal in Immunity and Inflammation. Curr Rheumatol Rep. 2011 doi: 10.1007/s11926-011-0162-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majno G, Commonwealth Fund . The healing hand: man and wound in the ancient world. Harvard University Press; Cambridge, Mass.; London: 1975. [Google Scholar]
- 41.Jarcho S. William Addison on blood vessels and inflammation (1841-43) Am J Cardiol. 1971;28:223–225. doi: 10.1016/0002-9149(71)90373-0. [DOI] [PubMed] [Google Scholar]
- 42.Waller A. Microscopical observation on the perforation of capillaries by the corpuscles of the blood and on the origin of mucus- and pus globules. Philosophical Magazine (3rd Series) 1846;29:397–405. [Google Scholar]
- 43.Cohnheim J. Ueber Entzündung und Eiterung. Virchows Arch (Path Anat) 1867;40:1–79. [Google Scholar]
- 44.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- 46.Chen CJ, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasse P, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 48.Couillin I, et al. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J Immunol. 2009;183:8195–8202. doi: 10.4049/jimmunol.0803154. [DOI] [PubMed] [Google Scholar]
- 49.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari D, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 52.Ferrari D, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 53.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 57.Andersson U, Tracey KJ. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annual Review of Immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180:6868–6876. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- 66.Galloway E, et al. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 70.Jennings RB, Hawkins HK, Lowe JE, Hill ML, Klotman S, Reimer KA. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978;92:187–214. [PMC free article] [PubMed] [Google Scholar]
- 71.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. Article ID 672395, 21 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 74.Tsan MF, Baochong G. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 75.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 76.Arslan F, Keogh B, McGuirk P, Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/704202. Article ID 704202, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eder C. Mechanisms of interleukin-1beta release. Immunobiology. 2009;214:543–553. doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 79.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 80.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cassel SL, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 88.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 92.Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–6378. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- 93.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 94.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauernfeind F, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 97.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 98.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 99.Latz E. NOX-free inflammasome activation. Blood. 2010;116:1393–1394. doi: 10.1182/blood-2010-06-287342. [DOI] [PubMed] [Google Scholar]
- 100.van de Veerdonk FL, et al. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 104.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 105.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 109.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 110.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 111.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 112.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 113.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 115.Joosten LA, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fantuzzi G, et al. Response to local inflammation of IL-1beta-converting enzyme-deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 117.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1 beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 118.Stehlik C. Multiple interleukin-1beta-converting enzymes contribute to inflammatory arthritis. Arthritis Rheum. 2009;60:3524–3530. doi: 10.1002/art.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deyerle KL, Sims JE, Dower SK, Bothwell MA. Pattern of IL-1 receptor gene expression suggests role in noninflammatory processes. J Immunol. 1992;149:1657–1665. [PubMed] [Google Scholar]
- 120.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- 121.Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther. 2006;8(Suppl 1):S1. doi: 10.1186/ar1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beck C, Morbach H, Richl P, Stenzel M, Girschick HJ. How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol Int. 2009;29:229–238. doi: 10.1007/s00296-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 123.Fujimura N. Pathology and pathophysiology of pneumoconiosis. Curr Opin Pulm Med. 2000;6:140–144. doi: 10.1097/00063198-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mandrup-Poulsen T. IAPP boosts islet macrophage IL-1 in type 2 diabetes. Nat Immunol. 2010;11:881–883. doi: 10.1038/ni1010-881. [DOI] [PubMed] [Google Scholar]
- 126.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 127.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 128.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 129.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 130.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]