Summary
Inflammasomes are multi-protein complexes that sense microbial molecules and endogenous danger signals in intracellular compartments. Inflammasome assembly results in caspase-1 activation, which in turn drives maturation and secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18, and induces pyroptosis to eliminate the infectious agent. The importance of inflammasomes in regulating immune responses was recognized with the discovery of polymorphisms in genes encoding inflammasome components and their linkage to aberrant production of IL-1β and IL-18 in autoimmune and hereditary periodic fevers syndromes. We review the current knowledge on the role of inflammasomes in regulating innate and adaptive immune responses with an emphasis on the role of these immune complexes in autoinflammatory disorders and autoimmune diseases such as colitis, type I diabetes, multiple sclerosis and vitiligo.
Keywords: NLR, inflammasome, NLRP3, ASC, caspase-1, autoimmunity
Introduction
The human immune system relies on two evolutionary distinct but interconnected systems to fight infections and to mount effective responses to both endogenous and exogenous insults. The evolutionarily ancient innate arm of the immune system instantly detects a wide spectrum of danger- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) by means of a limited set of germline-encoded pattern recognition receptors (PRRs) (1). Engagement of these receptors leads to mobilization of polymorphonuclear leukocytes and professional phagocytes to the site of infection or injury, where they contribute to eliminating the infectious threat and to inducing repair mechanisms in order to restore the damage elicited by the pathogen and/or the inflammatory responses of the host. In addition, the presentation of immunogenic antigens on the surface of professional antigen-presenting cells plays a pivotal role in instructing the adaptive immune system (2). As a result, dendritic cells are at the center of the communication interface that connects the innate and adaptive arms of the immune system. Whereas the innate immune system largely relies on a limited set of PRRs, the evolutionarily more recent adaptive component of the immune system is capable of generating a seemingly unlimited repertoire of antigen-specific T-cell receptors and highly specific antibodies through the random process of somatic recombination (3, 4). T and B-cell clones expressing particular antigen-specific receptors and antibodies subsequently undergo rigorous selection to prevent immune responses against autoantigens and harmless external antigens. A second aspect in which the adaptive immune system differs from the innate immune arm is that it is capable of memory responses that allow swift responses against future encounters with the same pathogen. Although the innate and adaptive components of the immune system each have particular qualities, it is the combined action of the rather broad but immediate innate immune response together with the highly specific but temporally delayed adaptive immune response that renders the immune system, as a whole, highly efficacious in clearing infections and fighting disease.
Despite their critical roles in host defense, immune processes need to be rigorously controlled at several steps to avoid tissue damage resulting from the immune system attacking the body’s own tissues. Indeed, mutations in immune genes that give rise to excessive or aberrant activation of the immune system in the absence of any apparent infection can lead to a multitude of chronic debilitating conditions. These diseases can be viewed as a continuum with autoinflammatory disorders located at one end of the spectrum and autoimmune diseases at the other. The latter are characterized by the adaptive immune system mistakenly recognizing the body’s own molecular constituents as foreign antigens of microbial origin. Alternatively, adaptive immune responses against harmless non-self antigens such as pollen may cause allergies. Both allergies and autoimmune diseases are associated with the production of antibodies against autoantigens and hyperactivation of antigen-specific T cells that target the body’s own tissues. On the other side of the spectrum, autoinflammatory diseases, such as the recently recognized periodic fever syndromes, are characterized by recurrent episodes of fever, rash, and joint inflammation in the absence of the usual hallmarks of autoimmunity such as high titers of antigen-specific T lymphocytes and autoantibodies (5). On the contrary, it is thought that unlike autoimmune diseases and allergies, deregulated innate immune responses lay at the foundation of autoinflammatory diseases, which often have a hereditary component (6). Our understanding of the molecular pathology underlying autoinflammatory diseases including familial Mediterranean fever (FMF), tumor necrosis factor-α (TNF-α) receptor-associated periodic fever syndrome (TRAPS), cryopyrin-associated periodic syndromes (CAPS), deficiency of the interleukin-1 (IL-1) receptor antagonist (DIRA), hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), and Behcet’s disease has markedly improved in recent years (7). Here, we focus on a number of autoinflammatory and autoimmune disorders that have been linked to either excessive or defective production of the pro-inflammatory cytokines IL-1β and IL-18 by inflammasome complexes.
IL-1β and IL-18: caspase-1-activated cytokines
IL-1β and IL-18 are related cytokines that were recognized early on for their ability to cause a wide variety of biological effects associated with infection, inflammation, and autoimmunity (8). IL-1β regulates systemic and local responses to infection, injury and immunological challenge by generating fever, activating lymphocytes and promoting leukocyte transmigration into sites of injury or infection (8). It can act by inducing upregulated expression of the IL-2 receptor on the surface of lymphocytes, by promoting T-cell survival, by instructing B cells to enhance antibody production, and by promoting B-cell proliferation (8–11). Importantly, IL-1β signaling was shown to promote T-helper 17 (Th17) cell differentiation (12–14). IL-18 lacks the pyrogenic activity of IL-1β, but it can promote Th1 cell polarization by inducing interferon-γ (IFNγ) production by activated T cells and natural killer cells in the presence of IL-12 (8, 9). In the absence of IL-12, IL-18 may enhance Th2 responses through the production of Th2 cytokines such as IL-4, IL-5 and IL-10 (9, 15, 16). More recently, IL-18 was shown to synergize with IL-23 to induce IL-17 production from already committed Th17 cells (17, 18).
Both IL-1β and IL-18 are produced as inactive cytosolic precursors that usually require caspase-1-mediated cleavage to gain biological activity (19–23). In particular, caspase-1-mediated maturation of IL-1β and IL-18 is critical for their secretion from activated monocytes and macrophages. Hence, mice and macrophages lacking caspase-1 fail to secrete mature IL-1β and IL-18 (21, 22). Caspase-1 is the founding member of an evolutionarily conserved family of cysteine proteases that specifically cleave their substrates behind aspartate residues (24). By processing substrates at targeted sites, caspases may activate or inactivate critical signaling cascades that are involved in programmed cell death, differentiation, and cell proliferation (25, 26). In particular, caspase-1 modulates inflammatory and host defense responses against microbial pathogens by processing the precursor forms of the pro-inflammatory cytokines IL-1β and IL-18 into their biologically active forms (19–22). In addition to secreting IL-1β and IL-18, caspase-1 contributes to host defense through incompletely understood mechanisms such as unconventional protein secretion and an inflammatory cell death program known as ‘pyroptosis’ that occurs in myeloid cells infected with bacterial pathogens such as Salmonella Typhimurium, Francisella tularensis and Bacillus anthracis (23, 27–30). Pyroptosis is thought to contribute to host defense by preventing pathogen replication in infected immune cells, while at the same time helping to present intracellular microbial antigens to cells of the immune system and by mediating the release of the DAMP high mobility group box 1 (HMGB1) from infected macrophages (27, 31, 32).
Inflammasomes: caspase-1-activating platforms
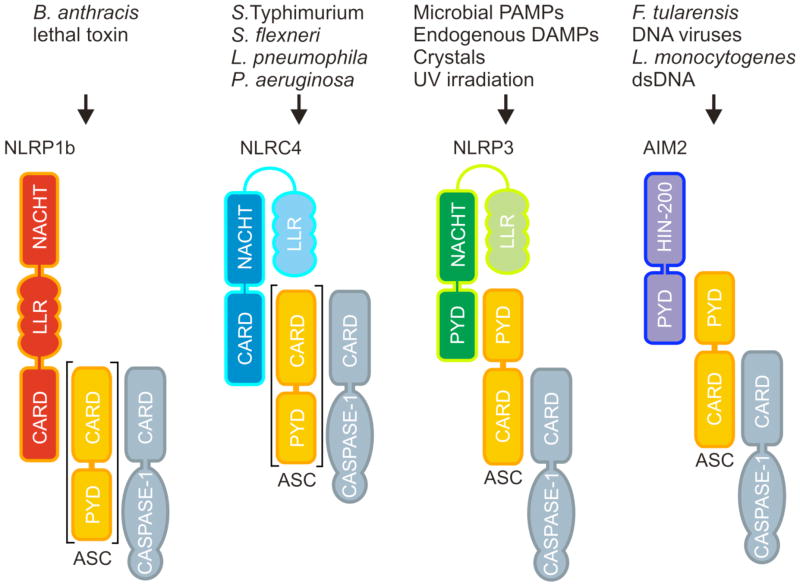
Caspase-1 is produced as an inactive zymogen in the cytosol of naive immune and epithelial cells and needs to be recruited by cytosolic multi-protein complexes known as inflammasomes to undergo proximity-induced autoactivation (33). Inflammasomes represent a group of cytosolic multi-protein complexes that are assembled around certain intracellular PRRs of the NOD-like receptor (NLR) and the HIN-200 receptor families, respectively (34). NLRs are characterized by a centrally located NACHT motif (also referred to as NBD or NOD domain) that is flanked at the N-terminus by interaction motifs of the caspase recruitment domain (CARD), pyrin, or baculovirus IAP repeat (BIR) domains (Fig. 1). These motifs are utilized for engaging NLRs in homotypic interactions with adapter proteins and effectors such as apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1. At the C-terminus, most NLRs contains an array of leucine-rich repeat (LRR) motifs that are believed to be involved in modulating NLR activity (1). Unlike NLRs, the HIN-200 family member absent in melanoma (AIM2) contains a prototypical DNA-binding HIN200 domain that is preceded by an amino-terminal pyrin motif, through which AIM2 recruits ASC and caspase-1 (Fig. 1). In addition to AIM2, the NLR proteins Nlp1b, Nlrp3, and Nlrc4 all have been shown to contribute to pathogen clearance by mediating the assembly of inflammasome complexes (35).
Fig. 1. Composition of inflammasome complexes.
The murine NOD-like receptor (NLR) proteins NLRP1b, NLRC4 and NLRP3, and the HIN-200 protein AIM2 assemble inflammasomes in a stimulus-specific manner. NLRP1b recognizes the cytosolic presence of the Bacillus anthracis lethal toxin. The NLRC4 inflammasome is assembled after detection of bacterial flagellin or the basal body rod component of the bacterial type III and type IV secretion systems. NLRP3 is activated when macrophages are exposed to UV irradiation, microbial PAMPs, endogenous DAMPs such as ATP, or crystals such as monosodium urate, silica, and asbestos. AIM2 directly binds dsDNA in the cytosol to induce caspase 1 activation in cells infected with Francisella tularensis, Listeria monocytogenes, or DNA viruses such as cytomegalovirus and vaccinia virus. The bipartite adapter protein ASC is required for assembly of the NLRP3 and AIM2 inflammasomes, whereas the Nlrp1b and Nlrc4 inflammasomes exist in variants that either contain or lack ASC.
Pathogens activate caspase-1 in an inflammasome-specific manner, although the different inflammasomes may have redundant roles during infection (33, 36). Similar to mammalian Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) at the cell surface and within endosomes (37), NLRs and HIN-200 proteins detect microbial components and endogenous danger signals in intracellular compartments (1, 38). Apart from AIM2, which directly binds microbial dsDNA in the cytosol of infected immune cells, the molecular mechanisms leading to activation of the Nlrp1b, Nlrc4, and Nlrp3 inflammasomes are less clear. Assembly of the latter inflammasome complexes may result from direct binding of conserved microbial proteins, polysaccharide structures and nucleic acids such as flagellin and components of the bacterial cell wall or viral envelope. Alternatively, these inflammasomes may respond indirectly to invading pathogens by monitoring changes in the concentration of cellular components such as ATP and K+, by detecting lysosomal leakage or through the production of danger signals such as reactive oxygen species and uric acid (39). The Nlrp1b inflammasome responds to Bacillus anthracis lethal toxin in the cytosol of intoxicated macrophages (40) and mutations in the Nlrp1b gene were shown to alter anthrax lethal toxin-induced macrophage cell death responses (29, 40). Notably, Nlrp1b inflammasome-induced macrophage cell death confers resistance to infection with B. anthracis spores in vivo, demonstrating the importance of inflammasome-mediated cell death (referred to as pyroptosis) for host defense (29). Activation of the NLR family member Nlrp3 comprises a two-step process that requires priming with TLR and NLR ligands to enhance NF-κB-driven transcription of Nlrp3, and the subsequent exposure to microbial toxins and ionophores such as nigericin and maitotoxin, or endogenous alarmins such as ATP and uric acid to trigger assembly of the Nlrp3 inflammasome (33, 39). Alternatively, priming and activation of the Nlrp3 inflammasome may occur simultaneously during infection of macrophages with bacterial, viral, and fungal pathogens such as Staphylococcus aureus, Streptococcus pneumonia, influenza virus, and Candida albicans, respectively (33, 39). The prominent role of the Nlrp3 inflammasome in host defense against microbial pathogens is illustrated by the observation that Nlrp3-deficient mice are hypersusceptible to candidiasis (41–43). The Nlrc4 inflammasome detects bacterial flagellin and the basal body rod component of bacterial type III and IV secretion systems of Salmonella, Pseudomonas, Legionella, and Shigella spp. (33, 44, 45). The Nlrp3 inflammasome also contributes to host defense during systemic S. Typhimurium infection, when flagellin expression is inhibited (36). In addition to the secretion of IL-1β and IL-18, the Nlrc4 inflammasome was recently shown to induce pyroptotic cell death to clear flagellin-expressing bacteria such as L. Pneumophila and B. thailandensis (28). Finally, the HIN-200 family member AIM2 responds to F. tularensis, Listeria monocytogenes, and certain DNA viruses such as cytomegalovirus and vaccinia virus by inducing caspase-1 activation through assembly of the AIM2 inflammasome (46–51). These observations illustrate the critical role of inflammasome activation in host defense to pathogens. However, inflammasome activation needs to be tightly controlled, because dysregulated activation and activity of caspase-1 was recently associated with diseases such as inflammatory bowel diseases (52–56), gouty arthritis (57), type I and II diabetes (58, 59), vitiligo (60), autoimmune Addison’s disease (59), and less common autoinflammatory disorders that are collectively referred to as cryopyrinopathies (61).
Nlrp3 polymorphisms and cryopyrinopathies
Nlrp3 shares the presence of a centrally located NACHT motif with all other NLR family members (1). This NACHT motif is flanked at the N-terminus by a pyrin domain to allow homotypic interactions with the bipartite adapter protein ASC in the Nlrp3 inflammasome. At the C-terminus, Nlrp3 contains an array of 12 LRR motifs believed to be involved in modulating Nlrp3 activity and in sensing (indirectly) microbial ligands and endogenous alarmins (39, 62). Notably, three auto-inflammatory conditions of which the primary symptoms are urticarial skin rashes and prolonged episodes of fever have each been linked to gain-of-function mutations in Nlrp3 (6, 63). The three Nlrp3-associated autosomal-dominant periodic fever syndromes are known as familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and chronic infantile neurological cutaneous and articular syndrome/neonatal onset multisystem inflammatory disease (CINCA/NOMID) (64, 65). Rather than being separate diseases, they represent a disease continuum, with FCAS the most mild and CINCA/NOMID the most severe. Patients suffering from one of these disorders, which are collectively referred to as the cryopyrin/Nlrp3-associated periodic syndromes (CAPS), can further present with arthralgia, headaches, elevated spinal fluid pressure, cognitive deficits, sensorineural hearing loss and renal amyloidosis (64, 65). More than 70 inherited and de novo disease-associated mutations have been identified so far, a large majority of which are situated within and around the Nlrp3 NACHT domain (7). The CAPS-associated mutations are believed to induce conformational changes that render Nlrp3 constitutively active, which results in continuous caspase-1 activation (66). As a consequence, mononuclear cells from CAPS patients secrete significantly more spontaneous and induced IL-1β and IL-18 (67). Excessive production of the latter cytokines may explain most of the clinical manifestations of these syndromes apart from bony overgrowth in CINCA/NOMID patients (68–70). Indeed, analysis of mice expressing CAPS-associated Nlrp3 mutations confirmed the role of the inflammasome and showed that CAPS symptoms are partially dependent on IL-1β (71, 72).
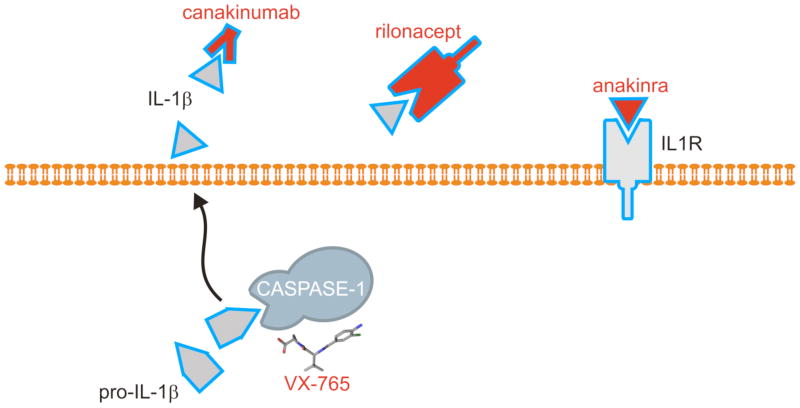
Until recently, treatment options for patients with CAPS disorders were limited. Our increased understanding of the genetic basis and the molecular mechanisms underlying these debilitating disorders allowed for the translation of basic research findings into highly efficacious new therapies targeting IL-1β or its receptor on effector cells (Fig. 2). Daily administration of the IL-1 receptor antagonist anakinra (Kineret, Amgen) provided marked amelioration of disease symptoms in CAPS patients (68, 73). Similarly, the safety and efficacy of the human IL-1β neutralizing monoclonal antibody canakinumab (Ilaris, Novartis) and rilonacept (Arcalyst, Regeneron), a fusion protein of the ligand-binding domains of human IL-1 receptor (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) with the Fc portion of a human immunoglobulin G1 (IgG1), in the treatment of CAPS have recently been demonstrated (74, 75). Chemical caspase-1 inhibitors represent a fourth mechanism to prevent overproduction of IL-1β in CAPS patients. Vertex Pharmaceuticals developed an orally active caspase-1 inhibitor (VX-765) that is currently under study for the treatment of epilepsy. Interestingly, VX-765 inhibited IL-1β secretion from LPS-stimulated peripheral blood mononuclear cells of FCAS patients (76), but clinical trials analyzing its efficacy in CAPS patients have not been registered. It thus remains to be determined whether this drug and analogues thereof can be deployed as a fourth option for treating CAPS.
Fig. 2. Mechanisms of therapeutic molecules interfering with inflammasome effector pathways.
Anakinra (Kineret, Amgen) corresponds to a recombinant form of the IL-1 receptor and thus targets the IL-1 receptor to displace endogenous IL-1 from the receptor. In contrast, canakinumab (Ilaris, Novartis) is a human IL-1β neutralizing monoclonal antibody raised against human IL-1β, whereas rilonacept (Arcalyst, Regeneron) represents the ligand-binding domains of human IL-1 receptor (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) that are fused with the Fc portion of a human immunoglobulin G1 (IgG1). The latter two molecules target IL-1β in circulation, thus scavenging it from the IL-1 receptor. Finally, VX-765 (Vertex Pharmaceuticals) is a small-molecule inhibitor that specifically inhibits caspase-1 in activated immune cells.
The Nlrp3 inflammasome in inflammatory bowel disease
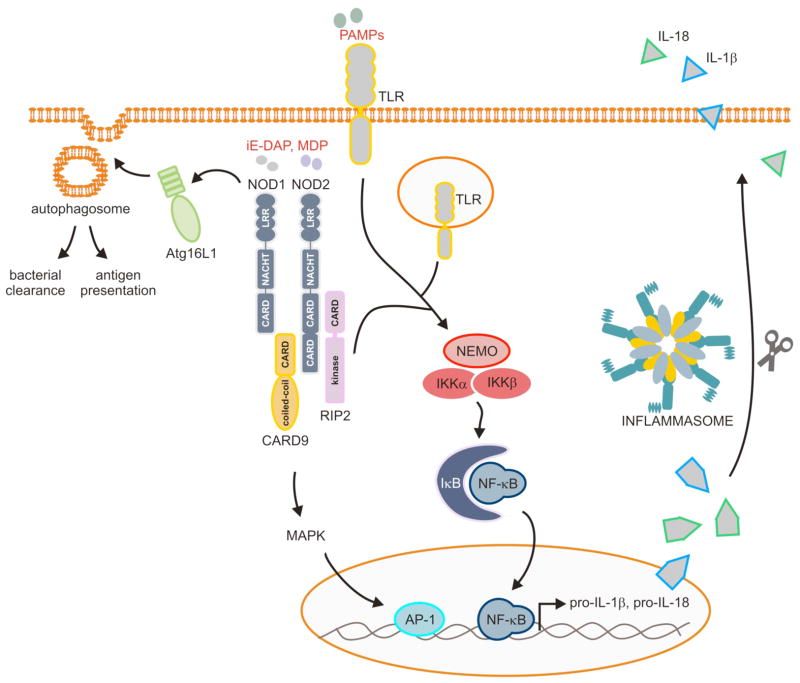
In addition to the CAPS-associated polymorphisms in the coding sequence of Nlrp3, mutations in the Nlrp3 promoter that result in decreased Nlrp3 expression and reduced IL-1β production have recently been linked with increased susceptibility to Crohn’s disease in humans (52). This finding was in line with previous studies showing that polymorphisms in the genes encoding the inflammasome effector IL-18 and the IL-18 receptor accessory protein correlated with increased susceptibility to Crohn’s disease (77, 78). Moreover, decreased secretion of the inflammasome cytokine IL-1β was noted in monocytes of Crohn’s disease patients that have been stimulated with the bacterial peptidoglycan fragment muramyl dipeptide (MDP) (79–81). MDP and a second peptidoglycan fragment called iE-DAP activate the Nlrp3-related NLRs Nod1 and Nod2, respectively (82–85). The importance of the latter NLRs in immune signaling was realized with the discovery that they represent key susceptibility genes for the development inflammatory bowel disease (IBD). Indeed, the genes encoding Nod1 and Nod2 are mutated in 15–20% of IBD patients (85–87). Ligation of Nod1 and Nod2 by respectively iE-DAP and MDP triggers recruitment of the adapter proteins CARD9 and RIP2, which subsequently leads to activation of the transcription factors NF-κB and AP-1 in Paneth cells, epithelial cells, and professional antigen-presenting cells (Fig. 3). This results in the production of cytokines, chemokines and other inflammatory mediators that induce immune cell activation. Nod1 and Nod2 may also contribute to immune responses by regulating autophagosome formation in cooperation with autophagy-related protein Atg16L1 (88). Interestingly, Atg16L1 has also been identified as key susceptibility gene for the development of Crohn’s disease (89, 90). In this respect, it is interesting to note that Nod2 variants containing Crohn’s disease-associated mutations failed to recruit Atg16L1 to the plasma membrane and to induce autophagosome formation (88). This observation suggests that in addition to its role in regulating NF-κB activation and cytokine secretion, Crohn’s disease-linked mutations in Nod2 might also contribute to disease by hampering the induction of autophagic responses.
Fig. 3. NLR- and TLR-mediated regulation of cytokine production and autophagosome formation.
Members of the Toll-like receptor (TLR) and NOD-like receptor (NLR) families recognize conserved microbial components called pathogen-associated molecular patterns (PAMPs) that correspond with molecules vital for microbial survival such as flagellin, nucleic acid structures unique to bacteria and viruses and bacterial cell wall components such as lipopolysaccharide (LPS). TLRs are located at the cell surface and in endosomes of immune cells, whereas NOD-like receptors (NLRs) and HIN-200 proteins detect pathogens located in intracellular compartments. PAMP recognition by these receptors triggers a number of protective responses, including the production of pro-inflammatory cytokines and chemokines through activation of the transcription factors NF-κB and AP-1. In addition, the NLR proteins Nod1 and Nod2 may regulate antigen presentation and bacterial clearance by promoting autophagosome formation through the recruitment of Atg16L1 to the plasma membrane. Finally, the NLRs Nlrp1b, Nlrp3 and Nlrc4; and the HIN-200 protein AIM2 induce assembly of inflammasome complexes, which are responsible for caspase-1 activation and the subsequent secretion of mature IL-1β and IL-18.
As described above, Nlrp3 was recently identified as a third NLR family member that is associated with IBD (52). Nevertheless, our understanding of the precise role the Nlrp3 inflammasome plays in intestinal homeostasis was less clear. To resolve this issue, several groups made use of experimental colitis models in mice to examine how the Nlrp3 inflammasome might provide protection against gut inflammation (53, 55, 56, 91, 92). The dextran sodium sulfate- (DSS) and 2,4,6-trinitrobenzene sulfonate (TNBS)-induced colitis models represent two of the most commonly used experimental colitis models for investigating innate immune mechanisms of colitis, although the models are less suitable for studying clinical features of human IBD that are associated with adaptive immune responses (93). Oral administration of DSS or TNBS is directly toxic to the colonic epithelium and triggers inflammation by disrupting the compartmentalization of commensal bacteria in the gut (94, 95). Early studies using the DSS-induced colitis model suggested IL-1β and IL-18 production contribute to intestinal inflammation (96–99), but the concept of inflammasome signaling being detrimental in IBD is being reevaluated based on recent reports suggesting that the Nlrp3 inflammasome confers protection against colitis and colitis-associated tumorigenesis (53, 55, 56, 91, 92). Indeed, Nlrp3−/− mice suffered from increased sensitivity to body weight loss, rectal bleeding, diarrhea, and mortality, thus suggesting that Nlrp3 inflammasome signaling provides protection against DSS-induced colitis (53, 56, 91). Similar responses were observed when Nlrp3−/− mice were subjected to the acute TNBS-induced colitis model (53, 91). Moreover, mice lacking the inflammasome proteins ASC also suffered from more severe histopathological changes during both the acute and chronic phases of DSS-induced colitis, which resulted in increased morbidity and mortality in DSS-fed caspase-1-deficient mice (53, 55, 56). Given that gut inflammation is an important predisposing factor for the development of colorectal cancer (100), mice lacking components of the Nlrp3 inflammasome were also subjected to the azoxymethane (AOM)/DSS tumorigenesis model (56, 92). Nlrp3−/− mice as well as those lacking ASC or caspase-1 suffered from increased dysplasia and tumor formation (56, 92). This observation suggests that elevated inflammatory responses and increased destruction of the epithelial barrier in the absence of a functional Nlrp3 inflammasome drives colitis-associated tumorigenesis. Notably, caspase-1-deficient mice consistently showed increased susceptibility to colitis relative to mice lacking Nlrp3, suggesting that additional inflammasomes may regulate caspase-1 activation in the gastrointestinal tract. In this respect, one study showed caspase-1 activation by the Nlrc4 inflammasome to provide protection against colitis-associated colon tumorigenesis, although it failed to confirm the induction of increased tumor loads in Nlrp3-deficient mice (101). The discrepant results in Nlrp3−/− mice may stem from variability in a number of parameters influencing disease outcome, including the protocol used to induce gut inflammation, the genetic background of the mice, and the composition of their gut microbiota. This may also explain why one recent study proposed Nlrp3−/− mice to be protected from DSS-induced colitis (102), while four other studies showed hypersusceptible responses in these mice (53, 55, 56, 91). Although further analysis is required to resolve this issue, a protective role for Nlrp3 appears likely based on genetic evidence from IBD patients and experimental colitis studies in mice lacking inflammasome effector molecules. Indeed, polymorphisms leading to decreased Nlrp3 expression are associated with increased risk for developing CD (52). Moreover, mice lacking the inflammasome substrates IL-1β and IL-18 (103) or their cognate receptors (103, 104) were also reported to be more susceptible to DSS-induced colitis. In agreement, mice lacking myeloid differentiation factor 88 (MyD88), an adapter protein required for both TLR-mediated upregulation of IL-1β and IL-18 transcripts as well as for signaling downstream of their cognate receptors, are hyper-susceptible to DSS-induced colitis (95, 105, 106).
In light of the important roles inflammatory cytokines such as IL-1β, IL-6, and TNF-α play in promoting colitis and inflammation-associated tumorigenesis (100), the roles of IL-1β and IL-18 in protection against colitis and colon tumorigenesis downstream of inflammasome assembly was analyzed in additional depth. Unlike IL-1β, the production of IL-18 was markedly increased in serum and colon tissue of DSS-fed mice (53, 55). Moreover, il-18−/− and il-18r1−/− mice were protected against tissue damage in the DSS-induced colitis and the AOM/DSS tumorigenesis models (107). Finally, morbidity was markedly reduced by administering exogenous IL-18 to DSS-fed caspase-1-deficient mice (53). Thus, IL-18 appears to play a major role downstream of the inflammasome in providing protection against colitis-associated inflammation, tissue damage, and tumor formation. This may be linked to its role in repair of the gut epithelium by promoting increased division of stem cells at the base of crypts to replace damaged enterocytes (53, 55, 108). In addition, IL-1β and/or IL-18 may regulate the production of short peptides with bactericidal activity called ‘defensins’ by cells of the colonic crypt, because crypts of Nlrp3−/−mice contained altered defensin levels (91). The importance of these bactericidal peptides is illustrated by the observation that their expression is diminished in colons of Crohn’s disease patients (109, 110). Thus, recent findings point to a key role for inflammasomes in regulating homeostasis of the gut epithelium and suggest that controlled delivery of exogenous IL-18 in the gut might prove beneficial in treating ulcerative colitis and/or Crohn’s disease.
Inflammasome signaling during experimental autoimmune encephalomyelitis
As discussed above, IL-1β and IL-18 play major roles in the induction of adaptive immune responses by regulating the differentiation and activation of Th1, Th2, and Th17 cells in a context-dependent manner (8–16). As such, IL-1β signaling was recently demonstrated to contribute to Th17-driven exacerbation of experimental autoimmune encephalomyelitis (EAE) (12, 13). This T-cell-mediated autoimmune disease results from autoreactive T cells targeting oligodendrocytes and is characterized by neuroinflammation featuring infiltration of lymphocytes and microglia demyelination and axonal loss (111). In agreement with its central role in the production of mature IL-1β and IL-18, mice lacking caspase-1 were shown to be markedly protected from EAE incidence and severity (112, 113). More recently, this protective phenotype was attributed to the lack of mature IL-18 production in these mice, because il-18−/− mice were protected to a similar extent against EAE (114). Upstream of caspase-1, an important role for Nlrp3 and ASC was provided by studies showing that mice lacking these inflammasome components were protected from EAE development because of reduced Th1 and Th17 responses (113, 114). In agreement with results obtained in this mouse model of multiple sclerosis, caspase-1 expression was found to be augmented in clinical brain samples of multiple sclerosis patients (115, 116). This suggests that (bio)chemically interfering with caspase-1 activity and/or neutralizing bioactive IL-18 in the brain might provide clinical benefit to patients.
Nlrp1 mutations associate with vitiligo, Addison's disease, and type I diabetes
In addition to the disease-linkage studies described above connecting the Nlrp3 inflammasome to autoinflammatory and autoimmune diseases, recent reports identified single nucleotide polymorphisms (SNPs) in the promoter and coding regions of Nlrp1 that associated with vitilgo, vitiligo-associated autoimmune diseases, Addison’s disease, and type I diabetes (59, 60, 117, 118). One study identified a strong and independent association of SNPs located within the promoter region and the coding sequence of the Nlrp1 gene, respectively, with vitiligo and vitiligo-associated autoimmune diseases (60). The results of this study were independently confirmed in a cohort of Romanian vitiligo patients (117). Vitiligo is a rare and chronic autoimmune disease that affects less than 1% of the population and that is characterized by the T-cell-mediated destruction of melanocytes. Nlrp1 is highly expressed in T cells and Langerhans cells in the skin (119), explaining the potential role of mutant Nlrp1 in debilitating autoimmune diseases of the skin and other organs. This ultimately results in depigmentation in irregular patches of the skin and hair (120).
As with most autoimmune disorders, vitiligo patients often suffer from concurrent autoimmune diseases. Notably, specific SNPs in and around NALP1 were found to associate with an extended autoimmune and autoinflammatory disease phenotype in vitiligo patients suffering from autoimmune Addison’s disease as well (60). The latter disease is characterized by immune-mediated destruction of the adrenal cortex, which leads to defective production of mineralocorticoid, glucocorticoid, and adrenal androgen. Notably, two separate studies identified a coding SNP in Nlrp1 (SNP rs12150220) that demonstrated strong linkage to Addison’s disease independently of vitiligo (59, 118). This SNP also showed linkage to type I diabetes, although it failed to reach genome-wide significance (59). Similar to the CAPS-associated SNPs in Nlrp3 (7), most of the 177 identified SNPs in the Nlrp1 gene (including rs12150220) localized to the Nlrp1 NACHT domain (60). However, the functional effects of disease-associated Nlrp1b SNPs have not (yet) been validated experimentally. Nevertheless, the observations mentioned above suggest a model in which they may induce conformational changes in Nlrp1 that reduce the threshold for assembly of the Nlrp1 inflammasome, thus triggering unwarranted activation of caspase-1 and excessive production of IL-1β and IL-18 in immune cells of patients suffering from vitiligo, Addison’s disease, and/or type I diabetes. Future efforts aimed at analyzing the effect of caspase-1 inhibitors and IL-1β neutralizing therapies in patients carrying disease-linked SNPs in Nlrp1 may facilitate the development of new approaches for therapeutic intervention in these debilitating diseases.
Concluding remarks
A wealth of information has emerged in recent years linking deregulated inflammasome signaling to human autoinflammatory and autoimmune diseases. However, our understanding of the precise role(s) inflammasomes play in modulating disease outcome is still in its infancy, and many important questions regarding the composition of the molecular machinery regulating inflammasome activation and activity remain to be addressed. Further elucidation of inflammasome pathways combined with an in-depth understanding of how they contribute to rare and common autoimmune and autoinflammatory disorders alike may open up the horizon for the development of new therapies for a breath of human afflictions.
Acknowledgments
This work is supported by National Institute of Health Grants AR056296 and AI088177, a NIAMS Centers of Excellence for Influenza Research and Surveillance (CEIRS) grant and the American Lebanese Syrian Associated Charities (ALSAC) to T-D. K. and by grants from the Fund for Scientific Research-Flanders and the European Union Framework Program 7 Marie-Curie grant 256432 to M.L.
Footnotes
The author declares no competing financial interests.
References
- 1.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 3.Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–125. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- 4.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 5.Dickie LJ, Savic S, Aziz A, Sprakes M, McDermott MF. Periodic fever syndrome and autoinflammatory diseases. F1000 Med Rep. 2010:2. doi: 10.3410/M2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aksentijevich I, et al. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–1285. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maliszewski CR, et al. Cytokine receptors and B cell functions. I. Recombinant soluble receptors specifically inhibit IL-1- and IL-4-induced B cell activities in vitro. J Immunol. 1990;144:3028–3033. [PubMed] [Google Scholar]
- 12.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino T, et al. Cutting edge: IL-18-transgenic mice: in vivo evidence of a broad role for IL-18 in modulating immune function. J Immunol. 2001;166:7014–7018. doi: 10.4049/jimmunol.166.12.7014. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 17.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 20.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 21.Ghayur T, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 22.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 23.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- 25.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 26.Lamkanfi M, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases leave the beaten track: caspase-mediated activation of NF-kappaB. J Cell Biol. 2006;173:165–171. doi: 10.1083/jcb.200509092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terra JK, et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of rancisella tularensis. Proc Natl Acad Sci USA. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamkanfi M, et al. Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia. J Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- 35.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 40.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 41.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–80. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren SE, et al. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol. 2010;185:818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchiya K, et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol. 2010;185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 49.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villani AC, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 58.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 59.Magitta NF, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 2009;10:120–124. doi: 10.1038/gene.2008.85. [DOI] [PubMed] [Google Scholar]
- 60.Jin Y, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 61.Lamkanfi M, Kanneganti TD. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol. 2010;42:792–795. doi: 10.1016/j.biocel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 63.Shinkai K, McCalmont TH, Leslie KS. Cryopyrin-associated periodic syndromes and autoinflammation. Clin Exp Dermatol. 2008;33:1–9. doi: 10.1111/j.1365-2230.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 67.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman HM, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neven B, Prieur AM, Quartier dit Maire P. Cryopyrinopathies: update on pathogenesis and treatment. Nat Clin Pract Rheumatol. 2008;4:481–489. doi: 10.1038/ncprheum0874. [DOI] [PubMed] [Google Scholar]
- 70.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 71.Brydges SD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N Engl J Med. 2003;348:2583–2584. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 74.Hoffman HM, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 75.Lachmann HJ, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 76.Stack JH, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175:2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 77.Tamura K, et al. IL18 polymorphism is associated with an increased risk of Crohn’s disease. J Gastroenterol. 2002;37(Suppl):111–116. doi: 10.1007/BF03326428. [DOI] [PubMed] [Google Scholar]
- 78.Zhernakova A, et al. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82:1202–1210. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Heel DA, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2- associated Crohn’s disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 80.Li J, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 81.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Inohara N, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 83.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 84.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 85.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 86.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–589. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]
- 87.McGovern DP, et al. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14:1245–1250. doi: 10.1093/hmg/ddi135. [DOI] [PubMed] [Google Scholar]
- 88.Travassos LH, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 89.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 91.Hirota SA, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn’s disease. Trends Mol Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 94.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 95.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Sivakumar PV, et al. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siegmund B, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 98.Siegmund B. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64:1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- 99.Bauer C, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-alpha mRNA expression. Dig Dis Sci. 2007;52:1642–1652. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 100.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 101.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 103.Takagi H, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 104.Lebeis SL, Powell KR, Merlin D, Sherman MA, Kalman D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2009;77:604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Araki A, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 106.Fukata M, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 107.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 109.Simms LA, Doecke JD, Walsh MD, Huang N, Fowler EV, Radford-Smith GL. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 2008;57:903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 110.Wehkamp J, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pittock SJ, Lucchinetti CF. The pathology of MS: new insights and potential clinical applications. Neurologist. 2007;13:45–56. doi: 10.1097/01.nrl.0000253065.31662.37. [DOI] [PubMed] [Google Scholar]
- 112.Furlan R, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- 113.Shaw PJ, Lukens JR, Burns S, Chi H, McGargill MA, Kanneganti TD. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gris D, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ming X, et al. Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J Neurol Sci. 2002;197:9–18. doi: 10.1016/s0022-510x(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 116.Huang WX, Huang P, Hillert J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult Scler. 2004;10:482–487. doi: 10.1191/1352458504ms1071oa. [DOI] [PubMed] [Google Scholar]
- 117.Jin Y, Birlea SA, Fain PR, Spritz RA. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol. 2007;127:2558–2562. doi: 10.1038/sj.jid.5700953. [DOI] [PubMed] [Google Scholar]
- 118.Zurawek M, Fichna M, Januszkiewicz-Lewandowska D, Gryczynska M, Fichna P, Nowak J. A coding variant in NLRP1 is associated with autoimmune Addison’s disease. Hum Immunol. 2010;71:530–534. doi: 10.1016/j.humimm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Kummer JA, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 120.Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res. 2007;20:271–278. doi: 10.1111/j.1600-0749.2007.00384.x. [DOI] [PubMed] [Google Scholar]