Abstract
Niemann-Pick type C1 (NPC1) disease is a rare progressive neurodegenerative disorder characterized by endolysosomal cholesterol accumulation. Previous studies implicating oxidative stress in NPC1 disease pathogenesis raised the possibility that non-enzymatic formation of cholesterol oxidation products could serve as disease biomarkers. We measured these metabolites in the plasma and tissues of the Npc1−/− mouse model and found several cholesterol oxidation products that were elevated in Npc1−/− mice, were detectable prior to the onset of symptoms, and were associated with disease progression. Non-enzymatically formed cholesterol oxidation products were similarly increased in the plasma of all human NPC1 subjects studied and delineated an oxysterol profile specific for NPC1 disease. This oxysterol profile also correlated with age of disease onset and disease severity. We further show that the plasma oxysterol markers decreased in response to an established therapeutic intervention in the NPC1 feline model. These cholesterol oxidation products are robust blood-based biochemical markers for NPC1 disease that may prove transformative for diagnosis and treatment of this disorder, and as outcome measures to monitor response to therapy.
INTRODUCTION
Niemann-Pick type C1 (NPC1) disease is a rare, progressive neurodegenerative disorder with an estimated incidence in Western Europeans on the order of one in 120,000–150,000 (1). This number is almost certainly an underestimate because of the difficulty in establishing the diagnosis. Approximately 95% of Niemann-Pick type C (NPC) cases are caused by mutations of the NPC1 gene, which is located on chromosome 18q11 (2); the remaining 5% are caused by mutations in the NPC2 gene, mapped to chromosome 14q24.3 (3). In patients with NPC1 disease cholesterol and other lipids accumulate in the viscera and central nervous system (4,5). In early childhood affected individuals typically exhibit ataxia and progressive impairment of motor and intellectual function, and they usually die in adolescence. A major barrier to delivery of effective treatment for NPC disease has been the lack of a non-invasive and inexpensive diagnostic test, thus leading to diagnostic delays of >4 years (6). The time to diagnosis is critical since early intervention is likely to yield the most benefit in this disease.
Mutations in the NPC1 and NPC2 genes profoundly affect the intracellular trafficking of cholesterol and, as a consequence, lead to accumulation of free cholesterol in late endosomal/lysosomal structures (4,7). Several lines of evidence suggest that the lysosomal lipid accumulation in NPC disease is accompanied by cellular oxidative stress. In vitro studies show increased oxidative stress in cultured fibroblasts and neurons. NPC1-deficient fibroblasts show increased concentrations of reactive oxygen species (ROS) and lipid peroxidation, and exhibit a gene expression profile indicative of oxidative stress (8,9). Likewise, treatment of mouse cortical neurons with U18666A, a compound that induces an NPC cellular phenotype, leads to increased oxidative stress (10). In vivo studies similarly provide evidence of cellular and tissue oxidative stress in NPC1 disease. Murine Npc1-deficient macrophages show increased ROS and harbor signs of chronic oxidative damage (11), and in human NPC1 subjects, antioxidant capacity in serum is decreased (12). Concomitant with elevated ROS, there is a marked increase in cholesterol oxidation products, which are formed non-enzymatically after attack of cholesterol by oxygen free radicals (11). These cholesterol oxidation products, or oxysterols, are uniformly elevated in both multiple tissues and plasma of Npc mutant mice (Npc1−/−) (11,13).
We hypothesized that the elevated cholesterol oxidation products in the tissues of Npc1−/− mice are a reflection of the unique intersection of oxidative stress and excess intracellular free cholesterol that is the hallmark of NPC1 disease. In the present study, to test whether concentrations of circulating oxysterols might serve as markers for NPC1 disease, we analyzed plasma and tissue samples from Npc1−/− mice over their 11-week lifespan. Furthermore, oxysterols that associate with disease in the murine model were examined in plasma and cerebrospinal fluid (CSF) from NPC1 and age-matched control subjects. We found that several cholesterol oxidation products were markedly elevated in the plasma of NPC1 subjects, but not in other neurodegenerative or lysosomal storage diseases, and correlated with severity and age of onset of disease.
RESULTS
Cholesterol oxidation products in plasma and tissues of Npc1-deficient mice
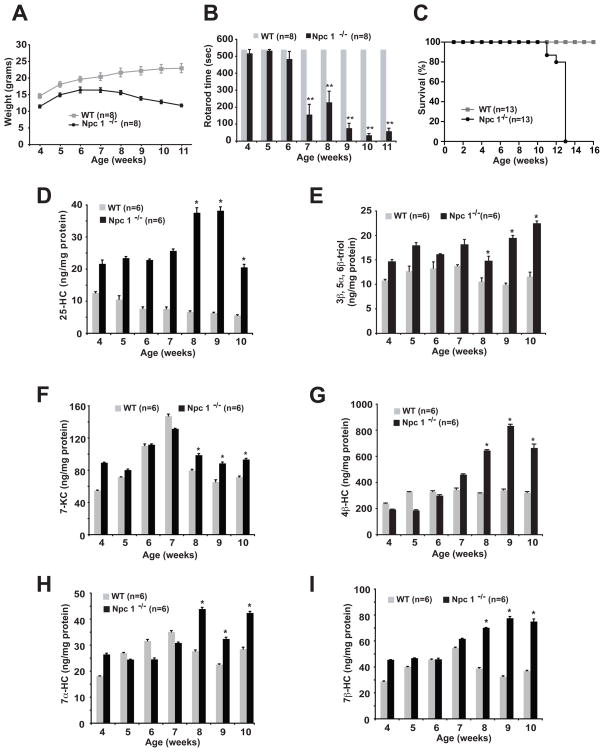
Previous demonstration of increased oxidative stress and elevated cholesterol oxidation products in the tissues of Npc1 deficient mice raised the possibility that circulating, non-enzymatically generated oxysterols might serve as markers for NPC disease. To test this possibility, we monitored plasma oxysterol concentrations over the lifespan of the BALB/c NPCnih (Npc1−/−) mouse, a naturally occurring murine model that harbors a retroposon insertion in the Npc1 gene (14). We measured oxysterol species formed exclusively by non-enzymatic reactions (cholestane-3β,5α,6β-triol (3β,5α,6β-triol), 7β-hydroxycholesterol (7β-HC), and 7-ketocholesterol (7-KC)), and species for which there is evidence for both enzymatic and non-enzymatic synthesis in vivo (4β-hydroxycholesterol (4β-HC), 7α-hydroxycholesterol (7α-HC) and 25-hydroxycholesterol (25-HC)) (Fig. 1). In contrast to control wild type (WT) littermates, Npc1−/− mice failed to appropriately gain weight and started to lose weight by 7–8 weeks of age (Fig. 2A). Npc1−/− mice exhibited decreased coordination, as evidenced by rotarod testing, beginning at seven weeks of age (Fig. 2B), and life span was markedly shortened, with a mean survival of 78 days (Fig 2C) (15). In pooled plasma samples elevations in 25-HC and 3β,5α,6β-triol were present at virtually all ages in Npc1−/− mice as compared to control mice (Figure 2, D and E). By contrast, elevations in 7-KC, 4β-HC, 7α-HC, and 7β-HC were most prominent after 7 weeks of age, when the Npc1−/− mice began losing weight and were overtly symptomatic (Fig. 2, F–I). (These oxygenated cholesterol species represent, for the most part, non-enzymatically formed oxidation products, although 4β-HC, 7α-HC and 25-HC can also be generated enzymatically). Nonetheless, a subset of cholesterol oxidation products (25-HC, 3β,5α,6β-triol, 7-KC, 7α-HC and 7β-HC) were all increased in the plasma of the 4 week Npc1−/− mice, an age that predates onset of neurological symptoms, and all of the oxysterols examined were significantly elevated in 8–10 week Npc1−/− mice.
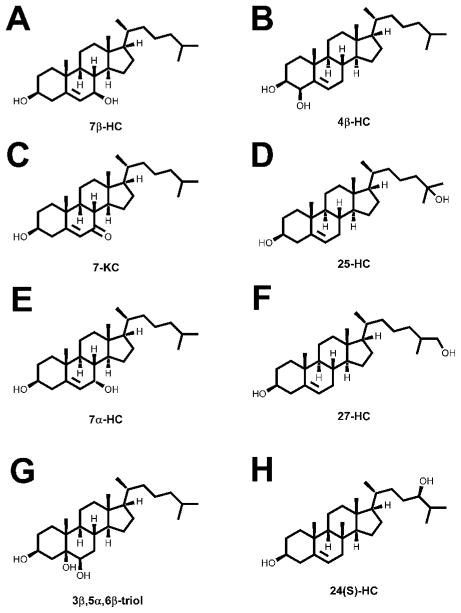
Fig. 1.
Oxysterol structures.
(A–C) 3β,5α,6β-cholestane-triol (3β,5α,6β-triol), 7β-hydroxycholesterol (7β-HC) and 7-ketocholesterol (7-KC) are generated through non-enzymatic cholesterol oxidation. (D–F) 7α-hydroxycholesterol (7α-HC), 4β-hydroxycholesterol (4β-HC) and 25-hydroxycholesterol (25-HC) can be generated through both non-enzymatic and enzymatic pathways. (G–H) 24(S)-hydroxycholesterol (24(S)-HC) and 27-hydroxycholesterol (27-HC) are produced exclusively through enzymatic cholesterol oxidation.
Fig. 2.
Cholesterol oxidation products are elevated in the plasma of Npc1−/− mice.
(A) Weight gain of WT and Npc1−/− mice. (B) Rotarod performance of WT and Npc1−/− mice. Error bars for WT mice are contained within the bars. (C) Kaplan-Meier survival analysis of WT and Npc1−/− mice. (D–H) 25-HC, 3β,5α,6β-triol, 7-KC, 4β-HC, 7α-HC and 7β-HC plasma concentrations measured weekly in pooled plasma samples (n=5–8 mice/group) obtained from WT and Npc1−/− mice. For 8–10 week time points, *p≤0.05 for Npc1−/− vs. WT control. In panels D–H the error bars represent the precision of replicate testing of pooled samples.
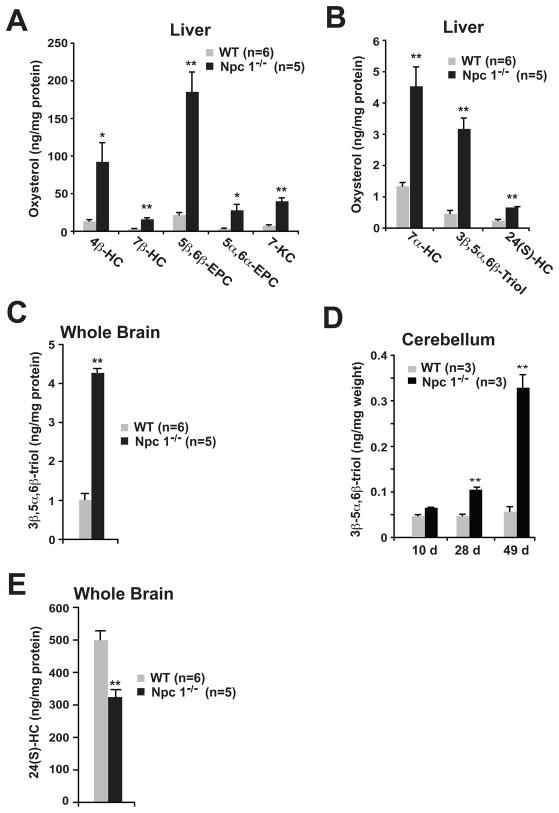
The elevated plasma oxysterols in the Npc1−/− mice were accompanied by altered tissue oxysterol levels. In livers of 9-week old Npc1−/− mice, there was marked accumulation (3.4–8.9 times increase compared to WT) of multiple non-enzymatically formed cholesterol oxidation products (4β-HC, 7β-HC, 5β,6β-epoxycholesterol, 5α,6α-epoxycholesterol, 7-KC, 7α-HC and 3β,5α,6β-triol) (Fig. 3, A and B). Elevated 3β,5α,6β-triol concentrations, in contrast to other oxidized cholesterol species, were also evident in whole brain (increased 4.3-times, p<0.001) and in the cerebellum, a region of the brain profoundly affected by the neurodegenerative disease process in the Npc1−/− mice (Fig. 3, C and D). An age dependent, significant elevation of 3β,5α,6β-triol in the cerebellum was detected beginning at 4 weeks in asymptomatic Npc1−/− mice (Fig. 3D), consistent with the known cholesterol storage and asymptomatic neuroinflammatory changes that are evident as early as 9 days in the Npc1−/− mice (16). In contrast to the non-enzymatic cholesterol oxidation products, enzymatically formed 24(S)-hydroxycholesterol (24(S)-HC) levels were attenuated 35% (p<0.001) in whole brain tissue of the 9-week old Npc1−/− mice, as compared to WT mice (Fig. 3E). Reduced formation of 24(S)-HC has been reported previously for Npc1−/− mice, as well as in human neurodegenerative diseases, and thought to be due to dysfunction and/or loss of large neurons (17,18). In spite of the reduced 24(S)-HC production, 24(S)-HC concentrations were increased 2.8-times (p<0.001) in the liver of the Npc1−/− mice (Figure 3B). These elevated steady-state concentrations of 24(S)-HC were likely a manifestation of both the hepatocellular disease in the Npc1−/− mice and the central role of the liver in clearance of plasma oxysterols (19). Taken together, these findings raised the possibility that plasma concentrations of non-enzymatically generated oxysterols might aid the in diagnosis of human NPC1 disease and be informative with respect to disease progression.
Fig. 3.
Accumulation of non-enzymatic cholesterol oxidation products in Npc1−/− mouse tissues.
(A,B) Oxysterol concentrations in livers of 9-week-old WT and Npc1−/− mice. (C) 3β,5α,6β-triol concentrations in the brain tissue of 9-week-old WT and Npc1−/− mice. (D) 3β,5α,6β-triol concentrations in cerebellar tissue of 10 day, 4-week and 7-week-old WT and Npc1−/− mice. (E) 24(S)-HC concentrations in the brain tissue of 9-week-old WT and Npc1−/− mice. Error bars represent samples from independent mice as denoted in each panel. *p<0.05 and **p<0.001 for Npc1−/− vs. WT.
Elevated cholesterol oxidation products in the plasma of human NPC1 subjects
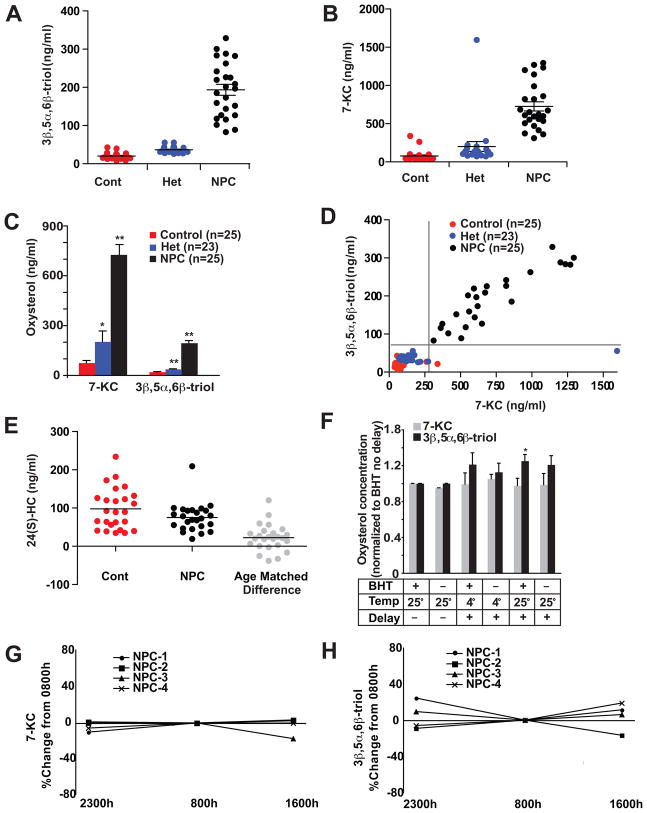
To study the association of oxysterols with human NPC1 disease, we obtained plasma samples from human NPC1 subjects enrolled in an observational study at the U.S. National Institutes of Health. We initially performed a pilot study on plasma samples from ten NPC subjects, in which we used isotope dilution GC/MS to monitor 22 distinct oxysterol species (Table S1) known to be present in human plasma (20,21). Comparison with previously reported reference values (21–23) allowed us to identify two species that were increased (3β,5α,6β-triol and 7-KC) and one that was decreased (24-HC) in the NPC1 plasma samples. On the basis of these findings, we measured these three oxysterol species in plasma samples in a validation cohort of 25 fasting NPC1 subjects (1–51 yrs; mean age 11.3 yrs), 25 controls (1–47 yrs; mean age 11.0 yrs) and 23 obligate heterozygotes (parents of NPC1 subjects) or known sibling carriers. Control subjects were matched for age, but not for gender, as there were no differences in oxysterol profiles between male and female controls. We found that the two cholesterol oxidation products, 3β,5α,6β-triol (control mean 20.1, range 7.9–42.9; NPC1 mean 193.6, range 82.9–328.8) and 7-KC (control mean 77.4, range 39.4–338.8; NPC1 mean 725.9, range 311.6–1294.1), were strikingly elevated in NPC1 subjects compared to age-matched controls (p<0.001 using Bonferroni posttest correction for multiple comparisons) (Fig. 4, A and B). Although the range of values overlapped with controls, these oxidation products were also significantly elevated in the heterozygotes. Even excluding the single heterozygote subject with marked elevation of 7-KC, mean 7-KC and 3β,5α,6β-triol levels in the heterozygotes were increased 1.8- and 1.9-times, respectively, over control subjects (7-KC, p<.05; 3β,5α,6β-triol, p<.001) (Fig. 4C). When both 7-KC and 3β,5α,6β-triol concentrations were plotted for individual subjects, these oxysterol species were sufficient to permit complete discrimination of NPC1 subjects from controls and heterozygotes (Fig. 4D). The plasma 3β,5α,6β-triol and 7-KC were highly correlated (r = 0.9), indicating that generation of these oxidation products in vivo likely involve a common process. This result is consistent with our hypothesis that increased oxysterols in NPC1 subjects reflects the unique intersection of increased intracellular free cholesterol and increased oxidative stress. Receiver-operator curve (ROC) analysis demonstrated that the area under the curve for 3β,5α,6β-triol was 1.0 and for 7-KC was 0.9984, reflecting the high sensitivity and specificity afforded by the markers.
Fig. 4.
Elevated plasma concentrations of cholesterol oxidation products in human NPC1 subjects.
(A) 3β,5α,6β-triol and (B) 7-KC concentrations in plasma samples in age-matched control (n=25), NPC1 subjects (n=25), and heterozygotes (n=25). (C) Mean plasma 7-KC and 3β,5α,6β-triol levels for control, NPC1 and heterozygote subjects. *p<0.05 for heterozygotes vs. controls; **p<0.001 for heterozygotes vs. controls, and NPC1 vs. controls. (D) 7-KC concentrations as a function of 3β,5α,6β-triol concentrations for individual control, NPC1 and heterozygote subjects. (E) 24(S)-HC concentrations in fasting plasma samples in age-matched control and NPC1 subjects, and difference between age-matched subjects. *p<0.05 for difference between controls and NPC1 subjects. (F) Stability tests of 7-KC and 3β,5α,6β-triol in plasma samples processed in the presence and absence of BHT, or after a 24-hour delay after storage at 4°C or at room temperature. *p<0.05 for BHT/delay/room temperature vs. BHT/no delay (G,H) Diurnal variation of 7-KC and 3β,5α,6β-triol in plasma of NPC1 subjects.
The striking elevation in circulating cholesterol oxidation products is consistent with known increases in the free cholesterol precursor and oxidant tone in NPC1-deficient tissues (8,9,11–13). The cholesterol oxidation products were unlikely to have been formed in the circulation or subsequent to the blood collection, because no significant association was found between plasma cholesterol and oxidation products (Fig. S1, A and B). As compared to controls, plasma 3β,5α,6β-triol/cholesterol and 7-KC/cholesterol ratios in NPC1 subjects were elevated 9.9 and 10.6-times, respectively, thus demonstrating that enhanced oxidant stress, rather than an increase in oxidation product resulting from an increase in precursor, was responsible for the elevated plasma oxysterols (Fig. S1, C and D). Even among Familial Hypercholesterolemia subjects with total plasma cholesterol > 300 mg/dl, the plasma 3β,5α,6β-triol and 7-KC levels were within the normal range (Fig. S1E). Furthermore, the lack of association between plasma cholesterol oxidation products and serum Trolox Equivalent antioxidant capacity (TEAC), a measure of total antioxidant capacity, demonstrate that elevated serum levels of 3β,5α,6β-triol and 7-KC are independent of the oxidative environment in the plasma (Fig. S1F).
In contrast to the elevated non-enzymatic oxidation products, mean plasma 24(S)-HC, an enzymatically formed oxysterol, was lower in NPC1 subjects than in controls (control mean 97.7, range 34.8–234.1; NPC1 mean 75.1, range 19.3–209.3) (Fig. 4E). In light of the age-dependent decline in circulating 24(S)-HC levels (22), plasma 24(S)-HC values were further analyzed. We found that 24(S)-HC plasma concentrations were significantly reduced in the NPC1 subjects, as compared to age-matched controls (Fig. 4E. p<0.005 using Bonferroni posttest correction for multiple comparisons). In humans, 24(S)-HC is formed almost exclusively in the central nervous system and its concentrations in plasma reflect the balance between cerebral production and hepatic clearance (17). Therefore, neurodegenerative changes in the NPC1 subjects would be expected to yield lower plasma 24(S)-HC concentrations, similar to those reported for subjects with Alzheimer disease or neuroinflammation (17). The considerable overlap in plasma 24(S)-HC concentrations between the groups, however, suggests that by itself plasma 24(S)-HC is not a sufficiently robust marker to permit discrimination between NPC1 and unaffected individuals.
To examine the stability of the oxysterol measurements in the plasma samples, we measured the oxysterols under different processing conditions. Routine samples were collected in EDTA and butylated hydroxytoluene (BHT)-containing tubes, immediately centrifuged, and the plasma was removed and stored at −80°C. All processing of archived samples was performed under argon, which prevented formation of adventitious cholesterol oxidation products. Stability of the oxysterols was determined by collecting the plasma samples in the presence or absence of BHT and by processing after overnight storage at either 4°C or at room temperature. 7-KC demonstrated remarkable stability and was unaffected by the absence of BHT, processing delay or storage temperature (Fig. 4F). Detection of the 3β,5α,6β-triol species was similarly unaffected by altered processing of the plasma samples. We observed, however, up to a 25% increase in 3β,5α,6β-triol concentrations after a processing delay and storage at room temperature (p<0.05), possibly reflecting conversion of precursor oxidation products, 5α,6α-epoxycholesterol and 5β,6β-epoxycholesterol, to the tri-hydroxylated 3β,5α,6β-triol species. On the other hand, oxysterol concentrations in plasma samples appear to be stable during storage at −80°C. Repeat 3β,5α,6β-triol and 7-KC determinations on samples stored at −80°C for up to six weeks showed a coefficent of variance of <10%. Furthermore, we can detect elevated 3β,5α,6β-triol and 7-KC concentrations in plasma samples from NPC1 subjects that have been archived for up to ten years. We also investigated whether 7-KC or 3β,5α,6β-triol demonstrated diurnal variation. We found no evidence of a diurnal pattern for either 7-KC or 3β,5α,6β-triol, although the non-fasting oxysterol determinations deviated from fasting levels by up to 16% and 23% for 7-KC and 3β,5α,6β-triol, respectively (Fig. 4, G and H). Nonetheless, the observed intra-subject variation for the oxysterol measurements was small compared to the differences between NPC1 and control values.
Because the plasma cholesterol oxidation products are manifestations of tissue oxidative stress, we explored whether the clinical presentation of disease in the NPC1 subjects might reflect the degree of cellular oxidative stress and, ultimately, the severity of the intracellular cholesterol trafficking defect. Using skin fibroblasts obtained from study subjects, we measured free cholesterol levels, as well as low-density lipoprotein (LDL)-stimulated cholesterol esterification in the fibroblasts. Impaired delivery of LDL-derived cholesterol to the endoplasmic reticulum (ER) for re-esterification is a hallmark of the NPC1 cellular lesion. Free cholesterol levels were increased 3.6–10.0-times in the NPC1 fibroblasts, as compared to WT fibroblasts. Consistent with this finding, LDL-stimulated cholesterol esterification was markedly reduced in the fibroblasts of the majority of the NPC1 subjects, and, not unexpectedly, was strongly associated with the cellular free cholesterol (r=-0.64; p<0.01) (Fig. S2). However, the association of these cellular phenotypes with the oxysterol biomarkers was relatively modest (r=0.22 for 7-KC and r=0.25 for 3β,5α,6β-triol), indicating that the amount of circulating oxysterol appears to be determined only in part by the severity of the NPC1 cellular lesion.
Specificity of plasma oxysterols for NPC disease
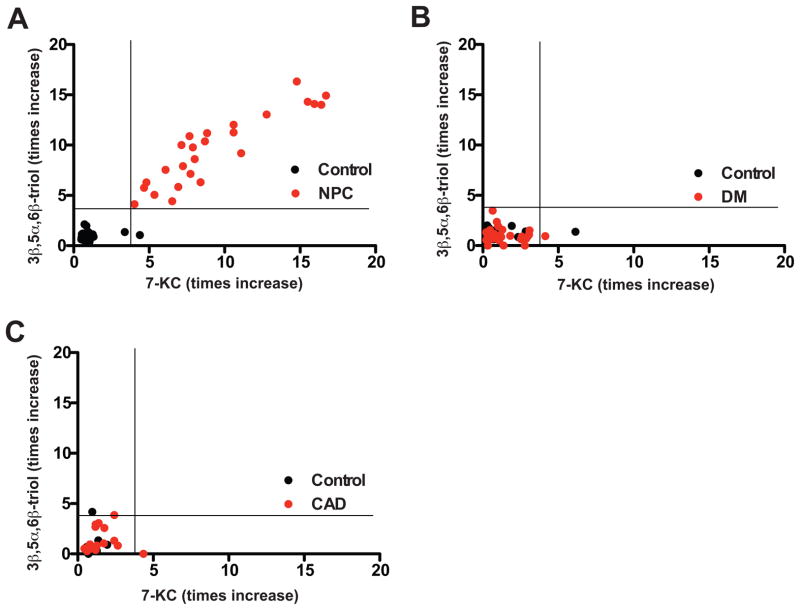
To evaluate the specificity of the plasma oxysterol profile for NPC1 disease, we measured oxysterols in subjects with type 2 diabetes and in subjects with coronary artery disease (CAD), two common conditions that are associated with oxidative stress. In diabetics, plasma 3β,5α,6β-triol and 7-KC concentrations were increased only 1.4- and 1.2-times, respectively compared with controls matched for age, gender, smoking status and statin usage (Fig. 5). In subjects with angiographically proven CAD, plasma 3β,5α,6β-triol or 7-KC concentrations were increased 1.4- and 1.5-times, respectively compared with matched controls. By contrast, these biomarkers were elevated 9.6- and 9.3-times, respectively, in the NPC1 subjects. Moreover, none of the diabetic or CAD subjects exhibited oxysterol levels that overlapped with the range of values for the NPC1 subjects.
Fig. 5.
Plasma levels of cholesterol oxidation products in human subjects with diabetes and coronary artery disease.
(A) Plasma 7-KC and 3β,5α,6β-triol levels re-plotted for individual control and NPC1 subjects (heterozygote subjects have been omitted), (B) Plasma 7-KC and 3β,5α,6β-triol levels plotted for individual control and diabetic (DM) subjects (n=34), which have been matched for age, gender, smoking status and statin usage. (C) Plasma 7-KC and 3β,5α,6β-triol levels plotted for individual control subjects and subjects with angiographically-proven coronary artery disease (CAD) (n=18), which have been matched for age, gender, smoking status and statin usage.
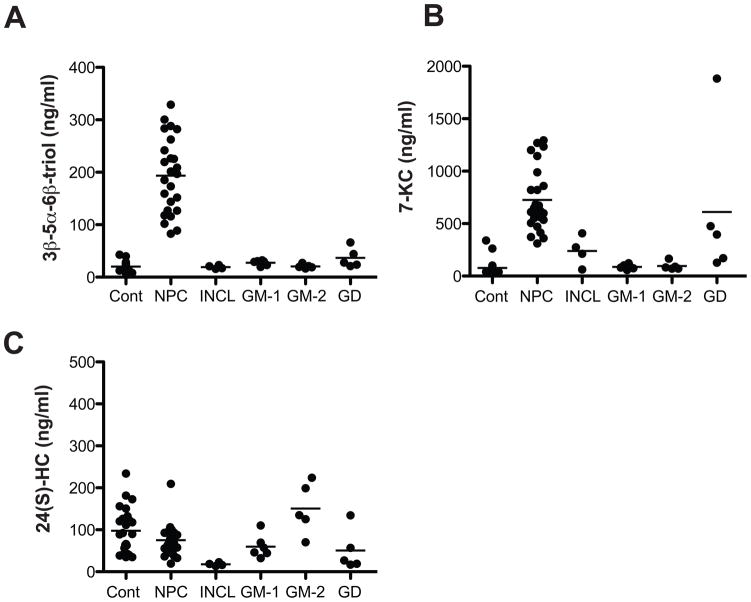
We further examined the circulating oxysterol concentrations in several other lysosomal storage diseases (infantile neuronal ceroid lipofuscinosis (INCL), GM-1 gangliosidosis, GM-2 gangliosidosis, and Gaucher disease) that have similar clinical presentations (e.g., hepatosplenomegaly and cognitive impairment) to NPC1 disease and for which a diagnostic blood-based test that differentiated NPC1 disease from other lysosomal storage diseases would be extremely useful. Plasma 3β,5α,6β-triol and 7-KC concentrations were significantly elevated in NPC1 subjects (Fig. 6, A and B). 3β,5α,6β-triol concentrations, and to a lesser extent 7-KC, were able to differentiate NPC1 subjects from subjects with other lysosomal storage diseases. Plasma 24(S)-HC concentrations were similar in subjects with NPC1 and other lysosomal storage diseases, with the exception of INCL subjects in which the 24(S)-HC was profoundly reduced (Figure 6C), consistent with the widespread cortical atrophy that typifies this disorder.
Fig. 6.
Comparison of plasma oxysterol concentrations in NPC1 disease and other lysosomal storage diseases.
(A) 3β,5α,6β-triol, (B) 7-KC and (C) 24(S)-HC concentrations in fasting plasma samples from control, NPC1, infantile neuronal ceroid lipofuscinosis (INCL), GM-1 gangliosidosis (GM-1), GM-2 gangliosidosis (GM-2) and Gaucher disease (GD) subjects. For 3β,5α,6β-triol, p<0.001 for NPC1 vs. INCL, GM-1, GM-2 and GD; for 7-KC, p<0.001 for NPC1 vs. GM-1 and GM-2 and p<0.01 for NPC1 vs. INCL
Cholesterol oxidation products in the CSF of human NPC1 subjects
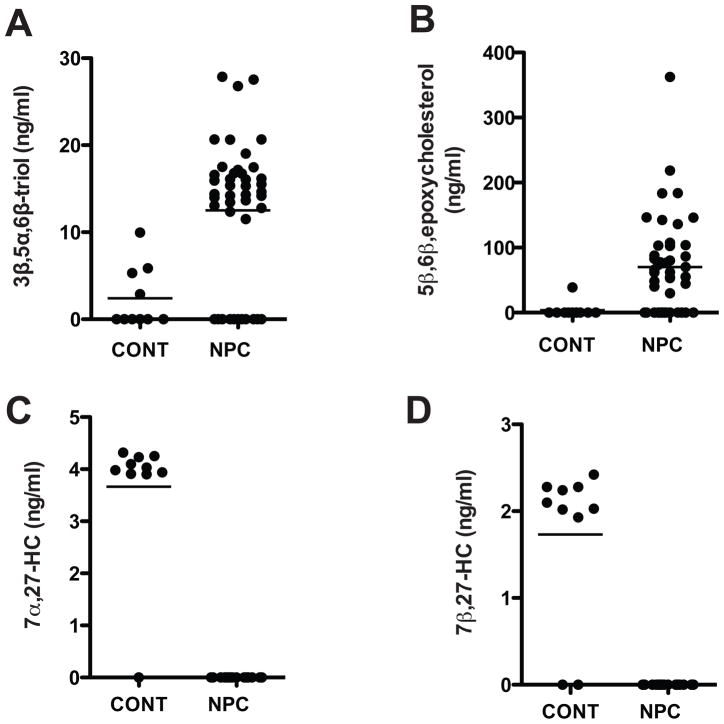
In NPC1 subjects, the elevated plasma cholesterol oxidation products were likely formed in multiple peripheral tissues prior to release into the circulation, similar to situation in Npc1−/− mice (11,13). To identify specific biomarkers for neurodegeneration in NPC1 disease, the CSF in NPC1 study subjects and pediatric controls was monitored for possible enrichment of CNS-derived cholesterol oxidation products. CSF oxysterol concentrations, however, were only ~10% that of corresponding plasma concentrations and below limits of detection in approximately a quarter of the subjects. Significant elevations were observed in the NPC1 subjects for 3β,5α,6β-triol (p<0.001) (Fig. 7A), which is increased in brain tissue of Npc1−/− mice (Fig. 3, C and D), and 5β,6β-epoxycholesterol (p<0.01) (Fig. 7B), a precursor of the 3β,5α,6β-triol. By contrast, 7α,27-HC and 7β,27-HC (Fig. 7, C and D), which were undetectable in the all of the NPC1 subjects, were present in the CSF of the control subjects. Surprisingly, we could not detect 24(S)-HC, a measure of CNS cholesterol turnover, in the CSF of either the NPC1 or control subjects. This could reflect reduced 24(S)-HC synthesis, or more likely, conversion to oxidation products not detectable by the selective ion monitoring employed in our study. Thus, NPC subjects demonstrate a distinct CSF oxysterol profile in which there is alteration of specific cholesterol oxidation products, consistent with the cholesterol oxidation signature found in the brain tissue of the Npc1−/− mice.
Fig. 7.
CSF oxysterol profile in NPC1 and control subjects.
(A) 3β,5α,6β-triol, (B) 5β,6β-epoxycholesterol, (c) 7α-27-HC and (d) 7β-27-HC concentrations in CSF from control and NPC1 subjects with established disease.
Correlation of oxysterol biomarkers with severity of human NPC1 disease
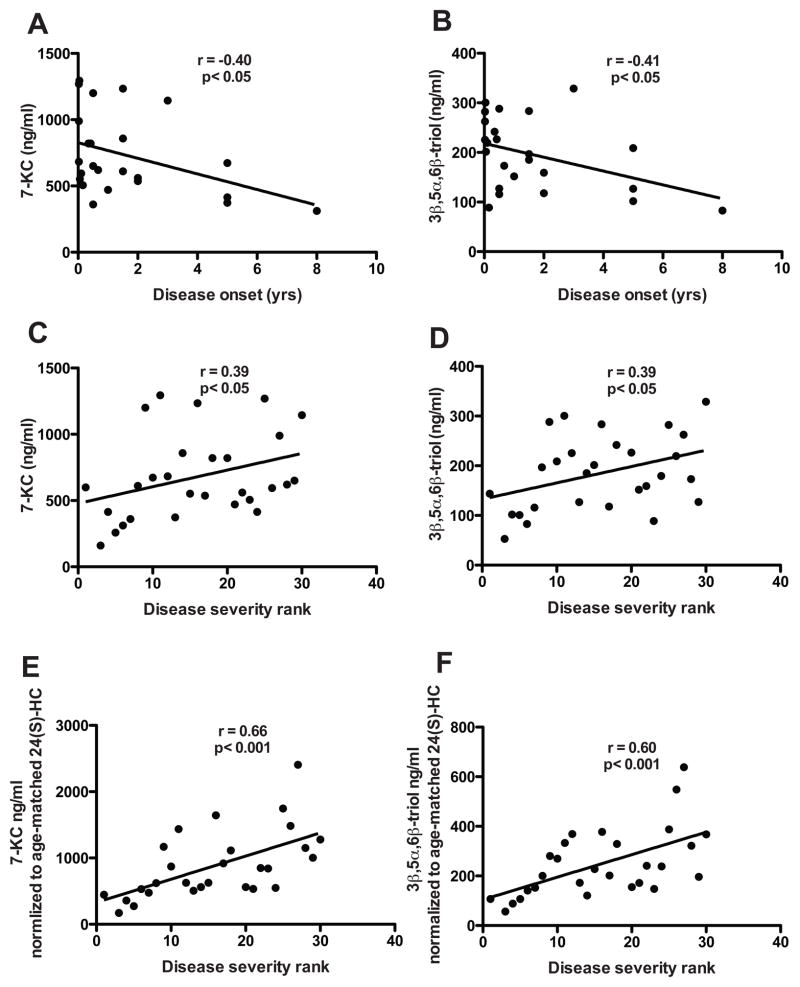
The wide range in 7-KC (311–1294 ng/ml) and 3β,5α,6β-triol (89–328 ng/ml) plasma concentrations within this phenotypically heterogeneous cohort of patients with NPC1 disease raised the possibility that the magnitude of the oxysterol biomarkers might be informative regarding disease severity. We found that the age of initial presentation of disease in the NPC1 subjects, which ranged from 1 week to 8 years, was significantly associated with plasma concentrations of both 7-KC (r = −0.41, p<0.05) and 3β,5α,6β-triol (r = −0.40, p<0.05) (Fig. 8, A and B). Furthermore, NPC1 disease severity, based on severity scale rank within the cohort (6), correlated significantly with concentrations of both 7-KC (r = 0.39, p<0.05) and 3β,5α,6β-triol (r = 0.39, p<0.05) (Fig. 8, C and D). An even more robust association emerged between disease severity and an oxysterol index that incorporates either 7-KC or 3β,5α,6β-triol concentrations, which vary directly with severity, and age-corrected 24(S)-HC levels, which vary inversely with disease severity (for 7-KC, r = 0.66, p<0.001; for 3β,5α,6β-triol, r = 0.60, p<0.001) (Fig. 8, E and F). Together, these findings indicate the oxysterol biomarkers may be informative with respect to the clinical course of disease.
Fig. 8.
Correlation of plasma oxysterol concentrations with age of NPC1 disease onset and disease severity.
(A) Plasma 7-KC and (B) 3β,5α,6β-triol concentrations correlated with age of disease onset in NPC1 subjects. For 7-KC: r = −0.40, p<0.05; For 3β,5α,6β-triol, r = −0.41, p<0.05. (C) Plasma 7-KC and (D) 3β,5α,6β-triol concentrations correlated with disease severity rank in NPC1 subjects. For panels C–E, the severity rank increases with clinical severity of disease. For 7-KC: r = 0.39, p<0.05; For 3β,5α,6β-triol: r = 0.39, p<0.05. (E,F) Correlation of ratio of (E) plasma 7-KC and (F) 3β,5α,6β-triol to age-corrected 24(S)-HC values with disease severity rank in NPC1 subjects. For 7-KC: r = 0.66, p<0.001; For 3β,5α,6β-triol, r = 0.60, p<0.001.
Circulating oxysterol biomarkers are decreased in response to therapy
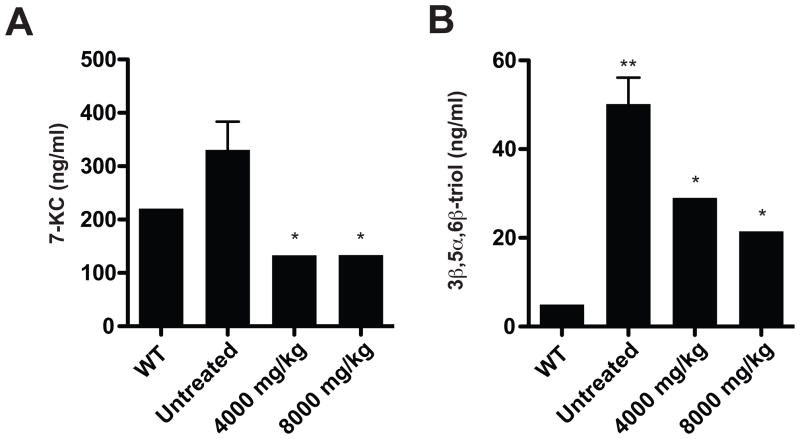
To assess whether 7-KC and 3β,5α,6β-triol might serve as markers of therapeutic efficacy in NPC1 disease, we measured the oxysterols in a feline model of NPC1 disease that is well documented and known to resemble the juvenile-onset form of the disease in children (5,24). In the NPC1 cats serum 7-KC and 3β,5α,6β-triol concentrations were increased 1.3- and 9.4-times, as compared to WT littermates (Fig. 9) Notably, the relative increase in 3β,5α,6β-triol concentration in the affected cats was identical to that observed in human NPC1 subjects. Serum samples were analyzed in 16–18-week NPC1 cats that were untreated or treated with a single dose of 2-hydroxypropyl-β-cyclodextrin (cyclodextrin; 4000 mg/kg or 8000 mg/kg subcutaneously), which has been shown to prolong the lifespan in both the NPC1 mouse and feline models (25–28). Initiation of cyclodextrin treatment at three weeks led to amelioration of clinical neurological disease and significant reduction of 3β,5α,6β-triol and 7-KC concentrations in the serum of the cyclodextrin-treated cats (Fig. 9). These findings raise the possibility that the oxysterol biomarkers may provide metrics to follow efficacy of therapy and/or disease progression in human NPC1 subjects.
Fig. 9.
Circulating oxysterol biomarkers are decreased in response to cyclodextrin therapy.
(A) Serum 7-KC and (B) 3β,5α,6β-triol concentrations were measured in untreated WT (4–16 weeks) and NPC1 (16 weeks) cats, and in NPC1 cats (16–18 weeks) treated with a single subcutaneous injection of 4000 or 8000 mg/kg at 3 weeks (n=2–4/group). *p≤0.05 for cyclodextrin-treated vs. untreated animals, and **p<0.01 for untreated NPC1 vs. WT animals.
DISCUSSION
A major impediment to the development of effective treatments for Niemann-Pick C disease has been the lack of outcome measures to evaluate therapeutic efficacy in this cholesterol storage disorder. A number of recent in vitro and in vivo studies indicate the presence of increased oxidative stress in NPC1 disease (8–11,13). On the basis of these studies, we explored the possibility that oxidative attack of the excess free cholesterol in these patients might lead to increased formation of cholesterol oxidation products, which could serve as disease biomarkers. Using the Npc1−/− mouse model, we identified cholesterol oxidation products that were elevated, both in tissues and plasma, and were associated with disease progression. Non-enzymatically formed cholesterol oxidation products were similarly increased in the plasma of human NPC1 subjects. The specific oxysterol species differed to some degree between humans and mice, and this may reflect inherent differences in sterol metabolism between the species. Measurement of plasma oxysterols allowed for sensitive and specific detection of NPC1 disease in mice and humans. Furthermore, plasma oxysterol concentrations were correlated with the severity and age of disease onset. In mice lacking the Npc1 gene, alterations in plasma oxysterols could be detected prior to onset of clinical disease, and in NPC1 patients were evident when there were minimal to no neurological symptoms. These cholesterol oxidation products can serve as NPC1 disease-specific biochemical markers and may prove useful for early detection of NPC1 disease and for evaluation of therapeutics in clinical trials.
In vivo conversion of lipoprotein cholesterol to oxygenated cholesterol metabolites (oxysterols) occurs in all tissues through both enzymatic and non-enzymatic pathways (Fig. 1). Side-chain oxysterols, which are generated by oxidation of the isooctyl side-chain of cholesterol, are biologically active and regulate acute sterol homeostatic responses via transcriptional and posttranslational mechanisms. These oxysterols, which include 24-, 25- and 27-HC, are predominantly synthesized by cytochrome P450 enzymes that reside in the ER (e.g., CYP46A1) and mitochondria (e.g., CYP27A1). In contrast to side-chain oxysterols, steroid ring-modified oxysterols are principally generated non-enzymatically as a result of the susceptibility of the C-5,6 double bond and the C-7 vinylic methylene group of cholesterol to radical and nonradical oxidation reactions (29,30). The major products of free-radical based oxidation of cholesterol include 7α-HC, 7β-HC, 7-KC and the epimeric 5,6-epoxides (30). The latter species are abundant in oxidized LDL and in macrophages, and serve as substrates for the ubiquitous cholesterol epoxide hydrolase to yield 3β,5α,6β-triol (29,31).
In vivo, multiple mechanisms are likely responsible for generation of the free radicals responsible for cholesterol oxidation, including iron-catalyzed reduction of H2O2 by superoxide by iron or peroxynitrite (30). Superoxide anions interact with polyunsaturated fatty acyl chains in membranes to produce hydroperoxy lipid radicals that are required for formation of both 5,6-epoxycholesterols, the precursor to 3β,5α,6β-triol, and 7-KC. In cells the enzyme complex primarily responsible for generation of superoxide anions is the NADPH oxidase complex (32). This complex is activated by lactosylceramide-enrichment of cell surface glycosphingolipid signaling domains (33,34). In light of the marked accumulation of lactosylceramide in multiple tissues in NPC1 subjects (35), it seems plausible that the well-characterized cholesterol-induced perturbations of glycosphingolipid metabolism in NPC disease, may be contribute to ROS production through activation of NADPH oxidase.
Non-enzymatically formed oxysterols have been reported to be elevated in human diseases associated with oxidative stress. The major circulating cholesterol oxidation products–7α-HC, 7β-HC, 7-KC and the epimeric 5,6-epoxides–have been shown to be elevated in the plasma of diabetic (36,37) and obese subjects (38). Increased 7α-HC and 7β-HC concentrations were also found in subjects with normal plasma cholesterol and atherosclerotic disease (39). In diabetes and atherosclerosis, however, the relatively modest increase in circulating oxysterol concentrations (increased ~1.3-times) and considerable overlap between subject groups suggests that these metabolites may have limited utility as disease markers in these disorders. By contrast, mean plasma 7-KC and 3β,5α,6β-triol concentrations were increased 9.4- and 9.6-times, respectively, in the NPC1 subjects, and, when used in combination, these species allowed discrimination of NPC1 subjects from age-matched controls with 100% sensitivity and specificity. These plasma oxysterols are stable, can be reproducibly measured, and exhibit only modest within subject variation, which auger well for their use as clinical biomarkers for NPC1 disease. In addition to their utility in monitoring disease, non-enzymatically formed oxysterols may also contribute to disease pathogenesis through their ability to promote apoptosis or to stimulate inflammation (29,40,41). In particular, the 3β,5α,6β-triol species, which is elevated in the cerebellar tissue of Npc1−/− mice and in the CSF of human NPC1 subjects, has been reported to be cytotoxic and promote mitochondrial dysfunction (42).
In contrast to the marked elevation of cholesterol oxidation products, NPC1 disease was associated with reduced enzymatic formation of 24(S)-HC in the brain tissue of Npc1−/− mice. Because the rate of 24(S)-HC flux into the plasma reflects cholesterol turnover in metabolically-active neurons in the CNS, reduced plasma 24(S)-HC concentrations in NPC1 may result from the loss of sterol 24-hydroxylase-expressing neuronal populations (e.g., Purkinje cells). Reduced plasma 24(S)-HC concentrations have previously been reported in Alzheimer disease and in the setting of neuroinflammation (17). Moreover, the profound reduction in 24(S)-HC concentrations in the subjects with infantile neuronal lipofuscinosis (Figure 6C), a lysosomal storage disease characterized by rapidly progressive brain atrophy, provides further support for 24(S)-HC as a general marker of neurodegeneration. Concomitant with reduced 24(S)-HC, we detected increased formation of 3β,5α,6β-triol in the brain tissue of the Npc1−/− mice. Thus, non-enzymatic cholesterol oxidation may explain the augmented CYP46A1-independent sterol excretion in the Npc1−/− mice (18).
Barriers to the development of more effective treatments for NPC disease have been its rare disease status and the lack of outcome measures to evaluate efficacy of therapy in clinical trials. The finding that 7-KC and 3β,5α,6β-triol markers were significantly lowered by treatment with cyclodextrin, a compound that prolongs survival in NPC1 animal models (25–28) and is being administered to human NPC1 subjects through an FDA-approved single patient investigational new drug application, provides a biochemical-based, surrogate end-point that could facilitate clinical evaluation of cyclodextrin and other emerging therapeutics.
Circulating oxysterols may additionally serve as metrics for monitoring the clinical course of NPC1 disease. Although correlation of the oxysterol markers with disease progression is clearly limited by the pleiotropic nature of the disease (e.g., multiple disease genotypes) and small sample size in our study, we were able to demonstrate significant correlations between plasma concentrations of 7-KC and 3β,5α,6β-triol and age of disease onset and severity. Moreover, plasma oxysterol concentrations expressed as the ratio of cholesterol oxidation products to age-normalized 24(S)-HC was even more informative than individual oxysterols in predicting disease severity. The higher predictive power of this oxysterol index may reflect inclusion in the index of markers of both oxidative stress and neuronal damage/loss, processes that are central to the pathogenesis of NPC1 disease. Given the variability in disease progression, even among siblings with a common genotype, these oxysterol biomarkers may prove most useful when applied longitudinally in individual NPC1 subjects.
An unexpected finding was the near doubling in plasma 7-KC and 3β,5α,6β-triol among the NPC1 heterozygotes, since humans with NPC1 haploinsufficiency have not been reported to develop clinical symptoms of NPC1 disease. On the other hand, Purkinje cell loss and enhanced phosphorylation of tau is present in aged Npc1+/− mice, and in a feline NPC1 model heterozygotic cats exhibit intermediate biochemical phenotypes for cholesterol esterification and liver lipid accumulation (43,44). Moreover, abnormal filipin staining patterns have been reported in skin fibroblasts from obligate human heterozygotes, indicating accumulation of lysosomal free cholesterol, which may serve as substrate for non-enzymatic formation of cholesterol oxidation products (45). Because of the wide phenotypic spectrum of NPC1 and the fact that a skin biopsy is necessary for biochemical diagnosis, NPC1 disease is likely underdiagnosed. Thus, the carrier frequency for NPC1 mutations is likely higher than the 0.6% predicted by a disease incidence of 1:120,000. If the increased circulating oxysterols are indicative of tissue oxidative stress, then human NPC1 heterozygotes, in whom the magnitude of the oxysterol elevation is comparable to those of diabetic and atherosclerotic subjects (36,37,39), may be at risk for neurodegeneration or other oxidative stress-related diseases. Thus, it is possible that therapeutic strategies under development for NPC1 subjects, such as antioxidant therapy to reduce ROS or small molecule chaperones to increase NPC1 protein stability, may also reduce disease risk in heterozygotic subjects.
The availability of a simple, quantitative blood test for diagnosis of NPC disease would provide an unprecedented opportunity for early disease detection and the possibility of intervention in neurologically asymptomatic subjects. Specifically, a blood-based screening or diagnostic test would be applicable to infants presenting with cholestatic jaundice, in which the prevalence of NPC is as high as 8% (46); any infant or child with hepatomegaly or splenomegaly, in which there is suspected storage disease; children with mild neurological deficits or learning disorders; and adolescents or adults with psychiatric disease and neurological symptoms (47). Although not yet FDA-approved for NPC, clinical evidence is accumulating that miglustat, an iminosugar that inhibits the synthesis of glycosphingolipids, may slow neurological progression (48), and miglustat has been approved for use in Europe for treatment of NPC (49). Given that the length of the neurologically asymptomatic phase may be a major factor in determining the age of neurological onset and that disease progression after neurological involvement is linear (6), identification of affected patients prior to the onset of neurological symptoms is critical. The current diagnostic standard for NPC1 is an invasive skin biopsy and filipin staining in one of several specialized clinical laboratories world-wide (50). The lack of a non-invasive screening or diagnostic test contributes to a significant diagnostic delay, which has been shown to be 4.3 years in a predominately pediatric cohort and 6.2 years in adults with NPC1 disease (6,51). Recent advances in mass spectrometric detection of oxysterols raise the possibility that these cholesterol-derived biomarkers could be quantified with sufficient sensitivity to enable implementation of newborn screening (52,53). A similar approach to detection of sterol compounds is being pursued for Smith-Lemli-Opitz syndrome, another inborn error of sterol metabolism (54). The finding that circulating oxysterols are informative with respect to NPC1 disease severity suggests that oxysterol biomarkers may also be useful for following disease progression or response to therapy by providing outcome measures in clinical trials. Validation of these oxysterol biomarkers is currently underway in an NIH-sponsored clinical trial (61).
MATERIALS AND METHODS
Animals
BALB/c NPCnih mice were obtained from Jackson Laboratories and maintained on a standard chow diet. Rotarod performance was monitored as described (15). Experimental procedures were approved by the Washington University Animal Studies Committee and were conducted in accordance with the USDA Animal Welfare Act and the Public Health Service Policy for the Humane Care and Use of Laboratory Animals. NPC1 cats were raised in the animal colony of the School of Veterinary Medicine at the University of Pennsylvania under NIH and USDA guidelines for the care and use of animals in research (55). Treatment with 2-hydroxypropyl-β-cyclodextrin was performed as described previously (55). The experimental protocol was approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Human Subjects
NPC1 fibroblasts and plasma were obtained from individuals enrolled in NIH protocol 06-CH-0186 (Evaluation of Biochemical Markers and Clinical Investigation of Niemann-Pick Disease, type C; PI: F.D. Porter). This clinical protocol was approved by the NICHD Institutional Review Board and the analysis of coded human samples was approved by the Human Studies Committee at Washington University. Plasma samples for diabetes and coronary artery disease subjects were obtained from a prospective registry (PI: S. Marso) for patients who have been admitted to St. Luke’s Hospital for in-hospital coronary angiography.
Cells
NPC1 mutant fibroblasts were obtained from skin biopsy specimens and control human fibroblasts were obtained from ATCC (CRL-1474). Fibroblasts cell lines were cultured as described (56).
Oxysterol determinations
Plasma samples were collected in tubes containing K3-EDTA and butylated hydroxytoluene (BHT) and stored at −80°C. CSF samples were collected using standard procedures in tubes containing BHT and stored at −80°C. Samples were analyzed in a blinded fashion. Prior to analysis, BHT (50 μg/ml) was added to plasma and CSF samples. For total oxysterol measurements, 200 pmol deuterated 27-HC (d5-27-HC) as an internal standard (IS) were added under a continuous argon stream, samples saponified (RT for 2 hrs) and chlorofom:methanol extraction performed twice as described (11,57). All sample processing was performed under argon to prevent adventitious cholesterol oxidation. Oxysterol purification was accomplished using aminopropyl (Waters Sep-Pak Vac RC 500mg NH2 Cartridges) and silica columns (Isolute 100mg SI 10mL XL cartridges) (58). Following solid phase separation from other lipid species, oxysterols were derivitized using pyridine:hexamethyldisilazane:trimethylchlorosilane (3:2:1) (21), and analyzed by gas chromatography/mass spectrometry (GC/MS) using an Agilent Technologies 5975B inert XL MSD with an Agilent Technologies 6890N Network GC System. Oxysterols were monitored with ions at m/z 472 (7-KC); m/z 456 (3β,5α,6β-triol, 7α-HC, 7β-HC, 4β-HC, and 27-HC); m/z 413 (24(S)-HC); m/z 131 (25-HC); and m/z 461 (d5-27-HC). Quantitative GC/MS determinations were calculated from triplicate injections and from the linear response range of standard curves established for each oxysterol/IS pair. Recovery of oxysterol species in plasma samples was typically in the 60–70% range.
Cholesterol esterification
LDL-stimulated cholesterol esterification in fibroblasts was performed as described (59).
Cholesterol determinations
For fibroblasts, lipids were isolated from cells by extraction in hexane: isopropanol (3:2), and cellular protein determined by BCA. Free cholesterol was quantified by GC/MS as previously described (60) and normalized to total cellular protein.
Statistical analyses
Results are expressed as mean ± SEM. For group comparisons, the statistical significance of differences in mean values was determined by a two-tailed single-factor ANOVA or Student’s t test. To perform correlations, data was analyzed using Pearson and Spearman correlations, as appropriate. A p value of 0.05 or less was considered significant. A Bonferroni posttest correction was used to adjust for multiple comparisons. For correlations with NPC1 disease severity, study subjects were ranked according to a disease severity scale from highest (most affected) to lowest (least affected) (6). For calculation of oxysterol ratios, 24(S)-HC values were first normalized to age-matched control values, which were interpolated from an exponential decay curve modeling the age-dependent decline in 24(S)-HC levels in control subjects (22).
Supplementary Material
Acknowledgments
We are grateful to the Hadley Hope Fund and Ed Cutler (Phlebotomy Services International) for their assistance in obtaining samples from control subjects. We also thank Drs. Anil Mukherjee, Cynthia Tifft, Bob Shamburek, and Ellen Sidransky for non-NPC1 patient samples. This study was also supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (FDP) and a Bench to Bedside award from the Office of Rare Diseases (FDP). The authors express their appreciation to the families and patients who participated in this study.
Funding: This work was performed in the Metabolomics Core at Washington University. The authors received support from the Washington University Specialized Centers of Clinically Oriented Research grant P50 HL083762 (D.S.O), RR02512 (C.V.), Dana’s Angels Research Trust (D.S.O, and N.Y.), and Ara Parseghian Medical Research Foundation (D.S.O., N.Y. and C.V.) Support was also provided by P20 RR020643 and P60 DK020579. Human samples were obtained under NIH protocol 06-CH-0186.
Abbreviations used
- 3β,5α,6β-triol
cholestane-3β,5α,6β-triol
- 4β-HC
4β-hydroxycholesterol
- 7α-HC
7α-hydroxycholesterol
- 7β-HC
7β-hydroxycholesterol
- 7-KC
7-ketocholesterol
- 24(S)-HC
24(S)-hydroxycholesterol
- 25-HC
25-hydroxycholesterol
- BHT
butylated hydroxytoluene
- CAD
coronary artery disease
- DM
diabetes mellitus
- GC/MS
gas chromatography/mass spectrometry
- NPC1
Niemann-Pick type C1
- ROS
reactive oxygen species
Footnotes
Author Contributions: F.D.P., J.E.S. and D.S.O. planned the studies. M.H.L., D.E.S. and S.J.L. performed the experiments. M.H.L., V.M. and R.S. carried out GC-MS analyses and analysis of data. S.E.G. performed analysis cholesterol esterification and data analysis. D.O. assisted with animal studies. D.D. provided reagents. R.F. performed the TEAC assays. J.H. and S.M. designed studies and provided human samples for analysis. C.A.W. carried out cholesterol measurements. C.V. performed the NPC1 cat treatment studies. N.Y. was responsible for sample procurement from study subjects. F.D.P., J.E.S. and D.S.O. wrote the manuscript.
REFERENCES AND NOTES
- 1.Vanier MT, Millat G. Clin Genet. 2003;64:269–281. doi: 10.1034/j.1399-0004.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 2.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Science. 1997;277:228–231. [Google Scholar]
- 3.Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 4.Ory DS. Biochim Biophys Acta. 2000;1529:331–339. doi: 10.1016/s1388-1981(00)00158-x. [DOI] [PubMed] [Google Scholar]
- 5.Walkley SU, Suzuki K. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Yanjanin NM, Velez JI, Gropman A, King K, Bianconi SE, Conley SK, Brewer CC, Solomon B, Pavan WJ, Arcos-Burgos M, Patterson MC, Porter FD. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturley SL, Patterson MC, Pentchev P. Proc Natl Acad Sci U S A. 2009;106:2093–2094. doi: 10.1073/pnas.0812934106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy JV, Ganley IG, Pfeffer SR. PLoS ONE. 2006;1:e19. doi: 10.1371/journal.pone.0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampieri S, Mellon SH, Butters TD, Nevyjel M, Covey DF, Bembi B, Dardis A. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh CH, Whiteman M, Li QX, Halliwell B, Jenner AM, Wong BS, Laughton KM, Wenk M, Masters CL, Beart PM, Bernard O, Cheung NS. J Neurochem. 2006;98:1278–1289. doi: 10.1111/j.1471-4159.2006.03958.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JR, Coleman T, Langmade SJ, Scherrer DE, Lane L, Lanier MH, Feng C, Sands MS, Schaffer JE, Semenkovich CF, Ory DS. J Clin Invest. 2008;118:2281–2290. doi: 10.1172/JCI32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu R, Yanjanin NM, Bianconi S, Pavan WJ, Porter FD. Mol Genet Metab. 2010 doi: 10.1016/j.ymgme.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tint G, Pentchev P, Xu G, Batta A, Shiefer S, Salen G, Honda A. J Inher Metab Dis. 1998;21:853–863. doi: 10.1023/a:1005474803278. [DOI] [PubMed] [Google Scholar]
- 14.Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 15.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Proc Natl Acad Sci U S A. 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid PC, Sakashita N, Sugii S, Ohno-Iwashita Y, Shimada Y, Hickey WF, Chang TY. J Lipid Res. 2004;45:582–591. doi: 10.1194/jlr.D300032-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Bretillon L, Siden A, Wahlund LO, Lutjohann D, Minthon L, Crisby M, Hillert J, Groth CG, Diczfalusy U, Bjorkhem I. Neurosci Lett. 2000;293:87–90. doi: 10.1016/s0304-3940(00)01466-x. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Lund EG, Turley SD, Russell DW, Dietschy JM. J Lipid Res. 2003;44:1780–1789. doi: 10.1194/jlr.M300164-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 20.Axelson M, Larsson O. J Biol Chem. 1996;271:12724–12736. doi: 10.1074/jbc.271.22.12724. [DOI] [PubMed] [Google Scholar]
- 21.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 22.Lutjohann D, Breuer O, Ahlborg G, Nennesmo I, Siden A, Diczfalusy U, Bjorkhem I. Proc Natl Acad Sci U S A. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iuliano L, Micheletta F, Natoli S, Ginanni Corradini S, Iappelli M, Elisei W, Giovannelli L, Violi F, Diczfalusy U. Anal Biochem. 2003;312:217–223. doi: 10.1016/s0003-2697(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 24.Somers KL, Royals MA, Carstea ED, Rafi MA, Wenger DA, Thrall MA. Mol Genet Metab. 2003;79:99–103. doi: 10.1016/s1096-7192(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 25.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Li H, Repa JJ, Turley SD, Dietschy JM. J Lipid Res. 2008;49:663–669. doi: 10.1194/jlr.M700525-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Proc Natl Acad Sci U S A. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorkhem I, Diczfalusy U. Arterioscler Thromb Vasc Biol. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 30.Murphy RC, Johnson KM. J Biol Chem. 2008;283:15521–15525. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman JW, Morisseau C, Hammock BD. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Cathcart MK. Arterioscler Thromb Vasc Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee S. Arterioscler Thromb Vasc Biol. 1998;18:1523–1533. doi: 10.1161/01.atv.18.10.1523. [DOI] [PubMed] [Google Scholar]
- 34.Iwabuchi K, Nagaoka I. Blood. 2002;100:1454–1464. [PubMed] [Google Scholar]
- 35.Vanier MT. Biochim Biophys Acta. 1983;750:178–184. doi: 10.1016/0005-2760(83)90218-7. [DOI] [PubMed] [Google Scholar]
- 36.Mol MJ, de Rijke YB, Demacker PN, Stalenhoef AF. Atherosclerosis. 1997;129:169–176. doi: 10.1016/s0021-9150(96)06022-4. [DOI] [PubMed] [Google Scholar]
- 37.Ferderbar S, Pereira EC, Apolinario E, Bertolami MC, Faludi A, Monte O, Calliari LE, Sales JE, Gagliardi AR, Xavier HT, Abdalla DS. Diabetes Metab Res Rev. 2007;23:35–42. doi: 10.1002/dmrr.645. [DOI] [PubMed] [Google Scholar]
- 38.Alkazemi D, Egeland G, Vaya J, Meltzer S, Kubow S. J Clin Endocrinol Metab. 2008;93:4282–4289. doi: 10.1210/jc.2008-0586. [DOI] [PubMed] [Google Scholar]
- 39.Prunet C, Petit JM, Ecarnot-Laubriet A, Athias A, Miguet-Alfonsi C, Rohmer JF, Steinmetz E, Neel D, Gambert P, Lizard G. Pathol Biol (Paris) 2006;54:22–32. doi: 10.1016/j.patbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Diestel A, Aktas O, Hackel D, Hake I, Meier S, Raine CS, Nitsch R, Zipp F, Ullrich O. J Exp Med. 2003;198:1729–1740. doi: 10.1084/jem.20030975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vejux A, Lizard G. Mol Aspects Med. 2009;30:153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Wang T, Huang K. Chem Biol Interact. 2009;179:81–87. doi: 10.1016/j.cbi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Brown DE, Thrall MA, Walkley SU, Wurzelmann S, Wenger DA, Allison RW, Just CA. J Inherit Metab Dis. 1996;19:319–330. doi: 10.1007/BF01799262. [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Ko M, Yanagisawa K, Michikawa M. J Biol Chem. 2005;280:27296–27302. doi: 10.1074/jbc.M503922200. [DOI] [PubMed] [Google Scholar]
- 45.Vanier MT. Wien Klin Wochenschr. 1997;109:68–73. [PubMed] [Google Scholar]
- 46.Yerushalmi B, Sokol RJ, Narkewicz MR, Smith D, Ashmead JW, Wenger DA. J Pediatr Gastroenterol Nutr. 2002;35:44–50. doi: 10.1097/00005176-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Meyer P, Martus S, Weiss R, Oheim K, Heinze M, Wittstock, Rolfs A. Am Soc Hum Genetics. 2009 [Google Scholar]
- 48.Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 49.Wraith JE, Imrie J. Ther Clin Risk Manag. 2009;5:877–887. doi: 10.2147/tcrm.s5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wraith JE, Baumgartner MR, Bembi B, Covanis A, Levade T, Mengel E, Pineda M, Sedel F, Topcu M, Vanier MT, Widner H, Wijburg FA, Patterson MC. Mol Genet Metab. 2009;98:152–165. doi: 10.1016/j.ymgme.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Sevin M, Lesca G, Baumann N, Millat G, Lyon-Caen O, Vanier MT, Sedel F. Brain. 2007;130:120–133. doi: 10.1093/brain/awl260. [DOI] [PubMed] [Google Scholar]
- 52.Honda A, Yamashita K, Hara T, Ikegami T, Miyazaki T, Shirai M, Xu G, Numazawa M, Matsuzaki Y. J Lipid Res. 2009;50:350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Jiang X, Ory DS, Han X. Rapid Commun Mass Spectrom. 2007;21:141–152. doi: 10.1002/rcm.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffiths WJ, Wang Y, Karu K, Samuel E, McDonnell S, Hornshaw M, Shackleton C. Clin Chem. 2008;54:1317–1324. doi: 10.1373/clinchem.2007.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward S, O’Donnell P, Fernandez S, Vite CH. Pediatr Res. 2010;68:52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frolov A, Srivastava K, Daphna-Iken D, Traub LM, Schaffer JE, Ory DS. J Biol Chem. 2001;276:46414–46421. doi: 10.1074/jbc.M108099200. [DOI] [PubMed] [Google Scholar]
- 57.Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS. J Biol Chem. 2003;278:25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- 58.Kaluzny MA, Duncan LA, Merritt MV, Epps DE. J Lipid Res. 1985;26:135–140. [PubMed] [Google Scholar]
- 59.Millard EE, Srivastava K, Traub L, Schaffer JE, Ory DS. J Biol Chem. 2000;275:38445–38451. doi: 10.1074/jbc.M003180200. [DOI] [PubMed] [Google Scholar]
- 60.Krakowiak PA, Wassif CA, Kratz L, Cozma D, Kovarova M, Harris G, Grinberg A, Yang Y, Hunter AG, Tsokos M, Kelley RI, Porter FD. Hum Mol Genet. 2003;12:1631–1641. doi: 10.1093/hmg/ddg172. [DOI] [PubMed] [Google Scholar]
- 61.NIH protocol 09-CH-0185: Biomarker Validation for Niemann-Pick, type C: Safety and Efficacy of N-Acetyl Cycteine.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.