Abstract
Research has shown that ingestion of a single high-fat (HF) meal causes postprandial lipemia and produces a reduced brachial artery blood flow response to vascular occlusion in Caucasians. However, the forearm BF response to occlusion in Caucasian and Asian populations after a single HF meal has not been compared. Eleven healthy male Asians, mean age 26.4 (±4.2) years, height 174.2 (±7.4) cm, and weight 73.8 (±5.7) kg and eight Caucasians, mean age 26.8 (±4.6) years, height 182.9 (±5.9) cm, and weight 82.8 (±4.8) kg were studied. A randomized cross-over study design was used with a HF (50.1 g total fat) or low-fat (LF) (5.1 g total fat) test meal 1 week apart. Forearm blood flow was measured over a 2-minute period following a 4-minute occlusion (FBFO) at 2 and 4 hours following ingestion of a test meal. This study found that FBFO was significantly attenuated in Asians (19.3%; p=0.09) compared to Caucasians after the ingestion of a HF meal. When comparing LF vs HF meals in Asians, the FBFO were 336.9 ml/100 ml tissue/minute and 240.8 ml/100 ml tissue/minute, respectively (p=0.02), whereas in Caucasians, the FBFO were 344.8 ml/100 ml tissue/minute and 287.4 ml/100 ml tissue/minute, respectively. It appears Asians have a more sensitive response to a single HF meal which may be explained, in part, by genotypic variation. These findings suggest that a single HF meal may contribute to the detrimental effects on vascular health in Asian males and raises speculation regarding the cumulative impact of a chronic HF diet in this population.
Keywords: high-fat meal, forearm blood flow, ethnicity
INTRODUCTION
Obesity exponentially increases the risk for the development of cardiovascular disease (CVD), one of the leading contributors to morbidity and mortality worldwide (Wyatt et al, 2006). The prevalence of people who are either overweight or obese is exceeding 60% among American adults and is expected to reach 75% within 10 years (Wyatt et al, 2006; Zheng and Berthoud, 2007). Obesity associated diseases in non-Western countries are known to be related to the adoption of a more urbanized lifestyle in which higher dietary fat consumption is common (Chew et al, 1988; Huang et al, 1996; Jee et al, 1998; Kagawa et al, 2002; Khoo et al, 2003).
The long-term effect of a chronic HF diet on endothelial dysfunction is well described (Raitakari et al, 2000; Poppitt, 2005). However, several studies have established that even the ingestion of a single high-fat (HF) meal, (50% or more total energy from fat), elevates serum lipids in healthy subjects within 2–4 hours (Vogel et al, 1997; Bae et al, 2003; Plotnick et al, 2003) and results in impairment of endothelial function (Vogel et al, 1997; Bae et al, 2003; Plotnick et al, 2003; Jackson et al, 2007). One of the key events in the genesis of CVD is the transient postprandial hypertriglyceridemia (HTG) observed after fat-rich food ingestion. Nappo et al (2002) investigated inflammatory markers after healthy subjects ingested a HF meal. They verified significant elevations in serum triglycerides (TG) as well as cytokines such as TNF-α, TL-6, monocyte chemo-attractant protein and an increase in the expression of adhesion molecules (ICAM-1, VCAM-1) which normally are absent in the endothelium of the vascular wall. Tsai et al (2004) noted postprandial HTG induced 8-epi-glutathionc prostaglandin F2α (oxidative modification product from arachidonic acid) while decreasing glutathionc peroxidase (endogenous antioxidants) after healthy subjects ingested a HF meal. Bae et al (2001) showed that HTG after ingestion of a HF meal significantly stimulated leukocytes to produce superoxide anion radicals, which in turn results in significantly decreased endothelial function. Superoxide anion radicals reduce production of endothelium-derived nitric oxide (NO) causing cell membrane injury, and induce low-density lipoprotein (LDL) oxidation, which has cytotoxic effects on vascular endothelial cells.
There is evidence to support that even 2 hours after a single HF meal, endothelial function is impaired as assessed by vascular occlusion (Vogel et al, 1997; Bae et al, 2003; Plotnick et al, 2003; Tsai et al, 2004). These studies used ultrasonography to measure brachial artery dilation and found that elevated serum TG levels after ingestion of a HF meal were inversely related to brachial artery dilation in response to vascular occlusion and this effect was sustained for up to 4 hours. However, most of these studies were conducted on healthy Caucasians; few on Asians and none using the same meal to directly compare the response of Asians to Caucasians.
The question that arises is whether these patterns are comparable across different race/ethnicities. For instance, there is evidence that immigrant Asians acclimatized to more energy-dense diets have an increased prevalence of obesity, CVD, and diabetes mellitus (DM) when compared to their native counterparts. Filipino women living in California, when compared with Californian Caucasian women, have a six-fold risk of DM and a nearly 4-fold risk of developing hypertension, hyperlipidemia, and insulin resistance, hallmarks of the metabolic syndrome (Araneta et al, 2002). Dhawan et al (1994) and Whincup et al (2005) showed that South Asian subjects who live in the West had higher levels of fasting insulin, fasting glucose, impaired fasting glucose, and prevalence of DM compared to Caucasians or Asians living in Asia. The increasing problem of obesity and metabolic syndrome among Asians may be compounded by the presence of a differentiated, thrifty genotype which heightens their susceptibility to vascular diseases in relation to increased body fat (Kagawa et al, 2002), There are many studies that suggest thrifty single nucleotide polymorphism (SNP) genes are involved in the individual’s sensitivity to developing CVD and DM. These genes may contribute to regulating lipids, energy metabolism and fat storage, which may be different depending on ethnicity (Kadowaki et al, 2002; Kagawa et al, 2002; Nakanishi el al, 2004; Tai et al 2004; Radha et al, 2006). Although fat is the most efficient way of storing energy, it can reduce overall health in affluent environments in which diets are abundant in saturated fat, low fiber, and highly refined carbohydrate foods (Zimmet and Thomas, 2003).
There have been no reports published comparing how Asians vs Caucasians would respond to the same HF meal. Furthermore, studies of blood flow response associated with occlusion have been accomplished using ultrasound to assess the diameter of the brachial artery. The limitation with this method is changes in brachial artery diameter are due to shear stress in the large arteries causing delay in flow, and not the response of the local vascular bed to anoxia (Vogel et al, 1997; Bae et al 2003; Plotnick et al, 2003; Pyke and Tschakovsky, 2005; Murphy et al 2007; Wu et al, 2007). A recent study (Wu et al, 2007) showed these changes in brachial artery diameter are caused by activation of transient receptor potential vanilloid-4 (TRPV4) channels mediated through an arachidonic acid intermediate. Brachial artery diameter does not directly reflect microvascular function and, as the angle of the ultrasound probe often varies during testing, there is uncertainty about the accuracy of this technique (Pyke and Tschakovsky, 2005). Therefore, the purpose of this study was to compare the effect of a HF vs LF meal in Asian vs Caucasian men on microvascular function as assessed by forearm blood flow response to a 4-minute period of vascular occlusion (FBFO) using a more direct measure of endothelial function, volume plethysmography (Whitney, 1953).
MATERIALS AND METHODS
Subjects
Healthy subjects were recruited and assigned to groups according to self reported ethnic identification. The demographics were not significantly different between the Asian and Caucasian groups (Table 1). The Asian subjects were Thai, Vietnamese, Chinese, Japanese, Indonesian, and Filipino. The inclusion criteria included being a healthy male Asian or Caucasian, a body mass index (BMI) of 18.5–24.9 kg/m2, ≤25% body fat, non-smoker, and low alcohol consumption (<3 drinks per week). Exclusion criteria included a family history of premature coronary artery disease, high blood pressure (≥140/90 mmHg), known DM, and currently taking any prescription medications.
Table 1.
Demographics of the subjectsa.
| Total (n=19) | Caucasians (n=8) | Asians (n=11) | p-valueb (95%CI) | |
|---|---|---|---|---|
| Age (yrs) | 26.6±4.3 | 26.7±4.2 | 26.4±4.2 | 0.89 (−4.68, 4.0) |
| BMI (kg/m2) | 24.5±1.6 | 24.8±1.5 | 24.3±1.5 | 0.57 (−2.1, 1.2) |
| % Body fat | 19.1±4.9 | 18.7±4.1 | 19.3±5.3 | 0.83 (−4.5, 5.5) |
Value were presented as mean±SD
Value were compared Caucasians vs Asians
All protocols and procedures of this pilot study were approved by the Institutional Review Board of Loma Linda University and all subjects signed a statement of informed consent.
Test meals
Isocaloric LF and HF meals (726 kcal) were given to the subjects at the study site in the morning after an overnight fast. The HF meal (50.1 g fat, 14 g saturated fat, 443 mg cholesterol, 22.3 g protein, 43.8 g carbohydrates) consisted of two eggs, hash browns with cheddar cheese, dry toast, margarine, and tomato ketchup. The isocaloric LF meal (5.1 g fat, 1 g saturated fat, 0 mg cholesterol, 31.3 g protein, 135.8 g carbohydrates) consisted of buttermilk pancakes, cherry topping, egg substitute, tomato ketchup, and commercial fruit juice. To assure consistency across subjects, meals were ordered from the same commercial restaurant.
Forearm blood flow
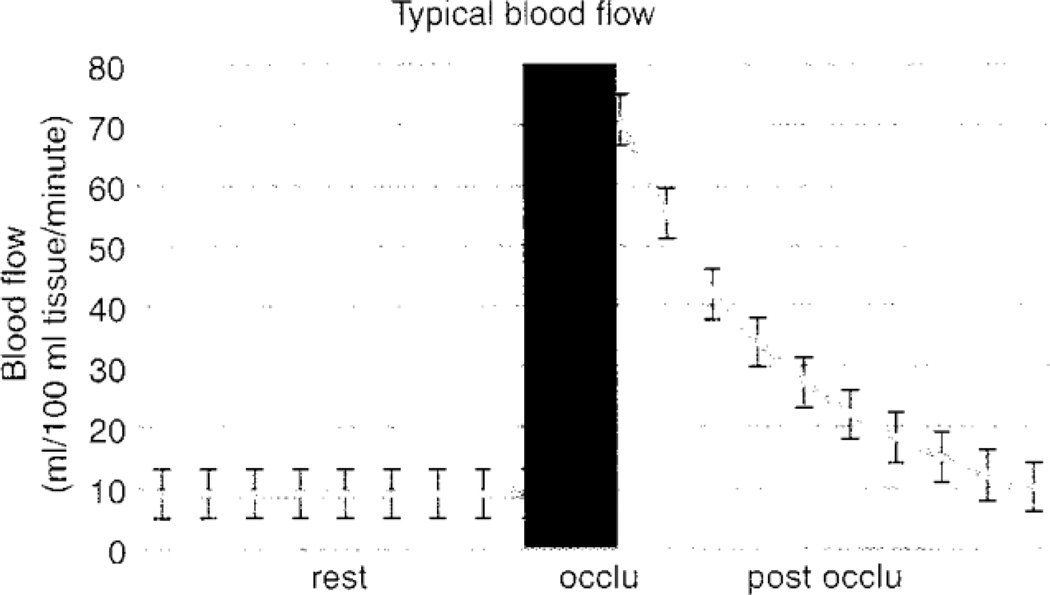
Relative volume changes in forearm blood flow were measured by automatic venous occlusion strain gauge plethysmography (EC6, D.E. Hokanson, Bellevue, WA). Data were transduced by a data acquisition system (MP150, BIOPAC Systems, Goleta, CA) and the resulting slopes digitalized at a rate of 2,000 samples per second with a 24 bit analog to digital converter using AcqKnowledge version 3.9.1 (BIOPAC Systems, Goleta, CA). An arterial occlusion cuff was placed around the wrist and the upper arm. The Whitney strain gauge was placed on the widest part of the proximal forearm. Thirty seconds prior to flow measurement, the wrist occlusion cuff was inflated to 200 mmHg to exclude hand blood flow. The upper arm cuff was inflated to a sub-systolic pressure (50 mmHg) for 5 seconds and deflated for 7 seconds to intermittently occlude the venous circulation. Baseline values were obtained during the first two minutes. The upper arm cuff was then inflated and clamped to a pressure of 200 mmHg for 4 minutes. Post-occlusion measurements were obtained for 2 minutes after deflation. Data from atypical forearm blood flow trace are shown in Fig 1.
Fig 1.
Blood flow at rest and after 4-minutes of vascular occlusion. This was recorded every 12 seconds for 2 minutes following the release of the arterial occlusion cuff on the brachial artery. Peak blood flow after occlusion was measured at 3 seconds post-occlusion. Blood flow is expressed in ml/100 ml forearm tissue/minute.
Blood pressure and heart rate
Continuous radial arterial blood pressure (BP) and heart rates (HR) were measured using a non-invasive device (Fusion TM, BIOPAC Systems, Goleta, CA). The Fusion unit uses a sensor strapped to the wrist over the anterior distal radius. Appropriate calibration procedures were used for each subject. Radial BP was measured continuously in 15 second cycles, and digitalized on line at a rate of 2,000 samples per second with a 24 bit analogue to digital convertor using an MP150 (BIOPAC Systems, Goleta, CA) and graphed on AcqKnowledge version 3.9.1 by BIOPAC.
Procedures
Subjects fasted 12 hours prior to participating in the study. The order of HF/LF breakfast meals was randomly assigned and separated with a one week wash out period. Subjects were asked to abstain from dietary supplements for 1 week prior to starring the study and avoid engaging in physical exercise 48 hours prior to the experimental meal. Measurements were taken over a 4-day period, 2 days for obtaining baseline data (no test meal) and 2 days during the experiment (test meals). Baseline measurement reliability was established using the data from the two days (no test meal) prior to the experiment day. Prior to the measurements, subjects lay comfortably on a plinth in a thermally neutral room for 20 minutes (23.8°C). Before ingestion of the test breakfast meal, resting FBFO, HR and BP were measured to establish an average baseline. Measurements of FBFO, HR and BP were taken at 2-hours and 4-hours postprandial. Subjects were allowed to sit or walk slowly, and only consumption of water was allowed during the assessment period.
Statistical and data analysis
Because this was a pilot study, there was not enough information regarding variances to calculate sample size for comparing blood flow between the two ethnic groups. As an alternative, the sample size was determined using range of means and standard deviations of blood flow after consuming a HF meal in each ethnic group from previous studies. The sample size necessary for the study of comparing blood flow between the two ethnic groups was determined by a power analysis. The range of means and standard deviations for blood flow after consuming a HF meal in Caucasians and Asians were from 584±211 to 628±249 ml/minute and 424±89 to 711±109 ml/minute, respectively. The criterion for significance of this pilot study was set at 0.1 because of a high variation among subjects and the small sample size. A sample size of 10 subjects in each group was deemed necessary to achieve an adequate power of 80%.
Data was entered using the statistical software SAS© System version 9.10. Values are expressed as mean ±SD. The distribution of FBFO was positively skewed, therefore, a log transformation was applied before further analyses. Confidence intervals (CI) of 95% with low and high ranges were calculated. Arterial resistance (AR) was calculated from mean BP divided by forearm BF. Mean BP was calculated using the formula systolic BP− diastolic BP/3+ diastolic BP. The cardiac work index (CWI) was calculated with: HR × Mean BP. Mixed model analyses were used to evaluate the mean of the FBFO, HR, BP, AR, and CWI in subjects before and after ingestion of the test meals.
RESULTS
HF meal and forearm blood flow
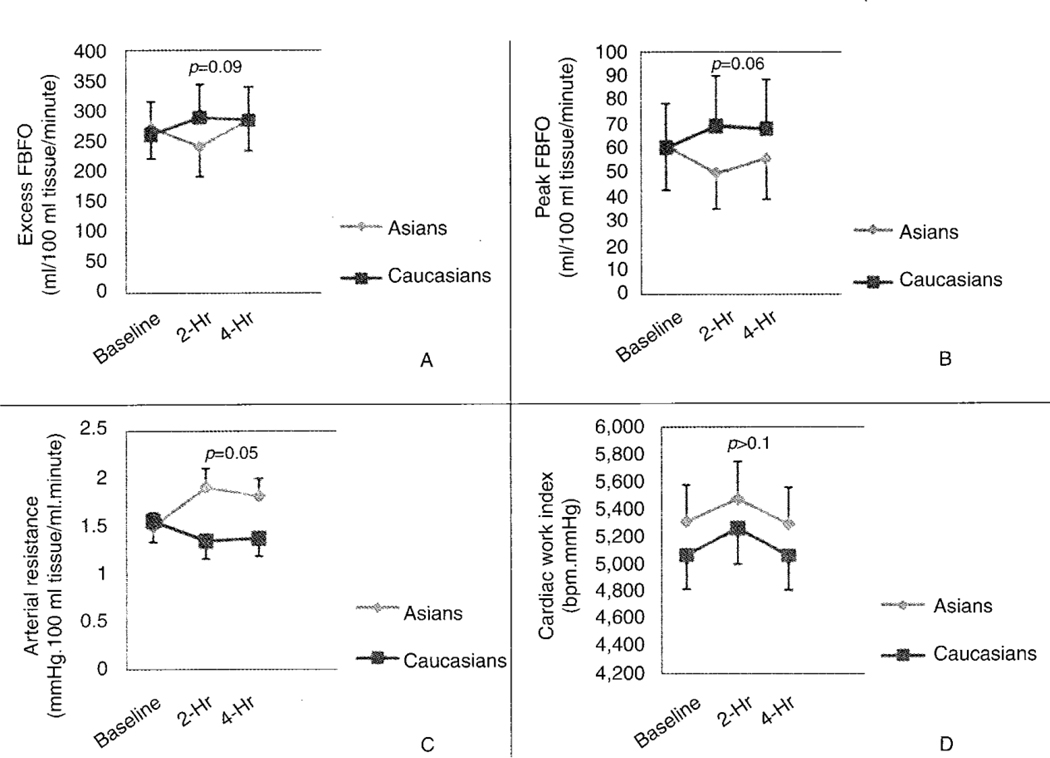
When the baseline blood flow response to anoxia in the Asian group was compared to that at 2 hours after the ingestion of a HF meal, the total blood flow over 2 minutes decreased from 271.3 ml/100 ml of forearm tissue/minute (95%CI 201.9–364.4) at baseline to 240.8 ml/100 ml of forearm tissue/minute (95%CI 183.9–315.4) after ingestion of a HF meal (p=0.12; Fig 2A). Furthermore, in Asians the peak blood flow after occlusion was significantly decreased from 62.2 ml/100 ml forearm tissue/minute. (95%CI 47.1–78.6) at baseline to 49.0 ml/100 ml forearm tissue/minute (95%CI 38.3–62.7) after the ingestion of a HF meal (p=0.01; Fig 2B). Arterial resistance significantly increased after ingestion of a HF meal in Asians (p=0.01; Fig 2C). In Asians, heart rate, blood pressure, and cardiac work index did not change significantly after ingestion of a HF meal (Fig 2D). In Caucasians, there was no significant change in total blood flow response to anoxia over 2 minutes, peak blood flow after occlusion, heart rate, blood pressure, arterial resistance or cardiac work index observed 2 hours post- ingestion of a HF meal compared to baseline.
Fig 2.
Comparison of Asians to Caucasians before, 2-hours and 4-hours following ingestion of a single HF meal in total blood flow response to anoxia over 2 minutes (excess FBFO) (A); peak blood flow after occlusion (peak FBFO) (B); arterial resistance (C); and cardiac work index (D). The excess FBFO was calculated from the area under the exponential curve (2-minutes post-occlusion) subtracted by the resting blood flow.
Comparison of a HF to a LF meal
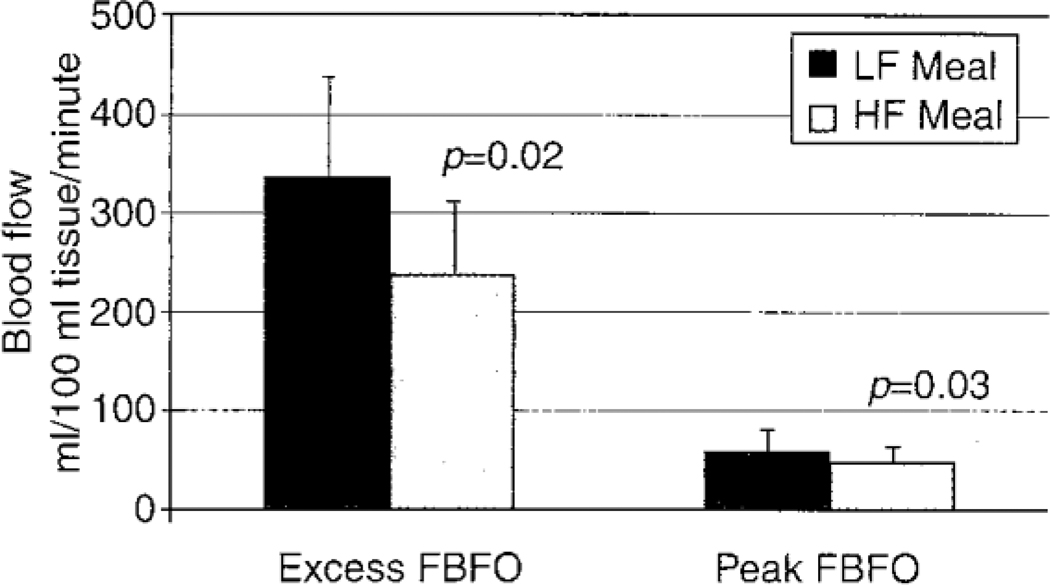
When comparing a HF to a LF meal within ethnic groups, there was a significant difference in total blood flow response to anoxia over 2 minutes in Asians (240.8 vs 336.9 ml/100 ml forearm tissue/minute, respectively; p=0.018) (Fig 3), The difference in peak blood flow following occlusion after ingestion of a HF meal (49.9 ml forearm tissue/minute) compared to a LF meal (62.2 ml forearm tissue/minute) was also significant (p=0.03) (Fig 3), as was the difference in arterial resistance after ingestion of a HF compared to a LF meal (1.89±0.16 and 1.52±0.16 mmHg.100 ml tissue/ml.minute, respectively) (p=0.04). The difference in cardiac work index after ingestion of a HF compared to a LF meal in Asians (5,472.9±250.4 vs 5,928.52±285.78 bpm.mmHg) was statistically significant (p=0.07). In Caucasians, the changes in total blood flow response to anoxia over 2 minutes, peak blood flow after occlusion, heart rate, blood pressure, arterial resistance, and cardiac work index post-ingestion of a HF compared to a LF meal were not statistically significant (p>0.1).
Fig 3.
Comparing the change in total blood flow response to anoxia over 2 minutes (excess FBFO) and peak blood flow after occlusion (peak FBFO) at 2 hours following the ingestion of a HF vs LF meal in healthy Asians.
Comparison of Asians to Caucasians
There was a statistically significant difference (p=0.09) in total blood flow response to anoxia over 2 minutes after ingestion of a HF meal at 2 hours (Asians had a 12.6% decrease compared to Caucasians who had a 10.6% increase) (Fig 2A). Similarly, there was a significant difference (p=0.06) in the peak blood flow after occlusion, between Asians (a 21.9% decrease) and Caucasians (a 14.3% increase) 2 hours after the ingestion of a HF meal (Fig 2B). Asians had a significantly greater arterial resistance (a 27.3% increase) (p=0.054) compared to Caucasians (a 15.5% increase) 2 hours following the ingestion of a HF meal (Fig 2C). Percent changes in heart rate, blood pressure, and cardiac work index (Fig 2D) were similar between the two groups.
DISCUSSION
This was a pilot study with the aim of comparing the acute effects of the same HF meal on forearm blood flow response to occlusion between Asians and Caucasians using venous occlusion plethysmography. Since alteration in thrifty genes found in Asians contributes to the increasing prevalence of obesity and CVD in Asian (Kadowaki et al, 2002; Kagawa et al, 2002; Nakanishi et al, 2004; Tai et al, 2004; Radha et al, 2006), it was felt that Asians would be more susceptible to a single HF meal.
This study found forearm blood flow response to vascular occlusion was significantly attenuated in Asians after ingestion of a single HF meal, but not in Caucasians. In Asians, forearm arterial resistance significantly increased postprandially without any measurable increases in heart rate or blood pressure, showing the increase in arterial resistance was not primarily influenced by central sympathetic outflow but the increased vascular resistance was probably mediated by a local endothelial response. One possible mechanism of diminished blood flow in response to anoxia after the ingestion of a HF meal may be related to increased free fatty acid (FFA) which affects nitric oxide (NO) production and reduces NO bioavailability. An increase in plasma FFA concentration after ingestion of a HF meal is associated with the induction of proinflammatory cytokines (Nappo et al, 2002) and reactive oxygen species (ROS) within the vascular wall (Tripathy et al, 2003; Tsai et al, 2004). Elevated plasma FFA can cause an increase in intranuclear nuclear factor kappa B (NF-κB) binding activity and ROS generation by mononuclear cells (MNCs) and poly-morphonuclear leukocytes (PMNs) (Tripathy et al, 2003). The NF-κB translocates to the nucleus and activates transcription of genes that are involved in the inflammatory response, such as proinflammatory cytokines (IL-6, TNF-α), adhesion molecule (VCAM-1, ICAM-1) (Nappo et al, 2002), and enzyme generating ROS (Tripathy et al, 2003). Superoxides react and can inactivate NO by producing peroxynitrite (Sears, 2002). Superoxides also rapidly degrade tetrahydrobiopterin (BH4) which also reduces NO production (Sears, 2002). These factors taken together can reduce NO bioavailability leading to vasoconstriction. If this mechanism, which has been seen with chronic HF diets, works after a single HF meal, it would explain the results seen in the present investigation.
This finding of decreased forearm blood flow in response to vascular occlusion after ingestion of a HF meal in Asians, but not in Caucasians, may be due to the influence of thrifty genes. The thrifty gene, which encodes the production of peroxisome proliferator activated receptors (PPAR), a nuclear sub-transmitter that regulates carbohydrate metabolism is a Pro12Ala of the PPARγ gene (Kagawa et al, 2002). This single nucleotide polymorphism (SNP) has been reported to have a preventative role in DM (Hara et al, 2002; Tai et al, 2004) by decreasing insulin resistance in Caucasians, but not in Asians (Radha et al, 2006). The frequency of PPARγ Pro12Ala polymorphism has been shown to be considerably lower in Asians than in Caucasians (Kagawa et al, 2002; Tai et al, 2004). Other possible thrifty SNP genes that may contribute to the difference in fat and energy metabolism between Asians and Caucasians are the uncoupling protein-3 (UCP3) gene and the intestinal fatty acid binding protein-2 (FABP2) gene. The t/t homozygote recessive c55tSNP of UCP3 gene is associated with a higher BMI (48% in Asians and 22% in Caucasians) (Kagawa et al, 2002). The Ala54TheSNP of the FABP2 gene is associated with a higher FABP2 (55% in Asians and 27% in Caucasians), which can enhance intestinal FFA absorption and has been linked with obesity (Kagawa et al, 2002; Nakanishi et al, 2004). These thrifty SNP genes may heighten the susceptibility to insulin insensitivity and vascular disease related to increase visceral adiposity in Asians.
There were some limitations in the present investigation. The findings of this study can only be applied to young healthy men in the same age range. The Asians, who were used in this study, had been living in the US, rather than in Asia; therefore, the results may be different than those who had not been consuming a US diet, which is particularly high in fat. In addition, there is a need for a larger sample size as this study data showed a strong relationship between ethnic groups but the difference did not reach conventional levels of statistical significance (p≤0.05).
Despite the limitations described above, this is the first study to demonstrate a significant reduction in endothelial function using volume plethysmography after ingestion of the same HF meal in Asians compared to Caucasians. The 50 g of fat in the HF meal in the present study was lower than that used in previous studies; the effect may have been more pronounced if a higher fat meal had been used. Even though there was high cholesterol content in this HF test meal, it is unknown if there was an acute effect due to cholesterol after ingestion of the HF test meal on vascular function. The total fat content of a HF meal (50–80 g of fat) can cause transient vascular dysfunction (Poppitt, 2005). After ingestion of a HF meal, postprandial triglyceride levels significantly increase, whereas other lipids values, such as cholesterol, LDL-c, and HDL-c, will remain the same (Vogel et al, 1997; Nappo et al, 2002; Sejda et al, 2002; Plotnick et al, 2003; Giannattasio et al, 2005). The absorption of dietary triglyceride is extremely efficient; up to 90% is absorbed, but only about 40% of dietary cholesterol is absorbed directly in the small intestine (Cunnane and Griffin, 2002; Whitney et al, 2002). Vegetables and fruits were excluded from the present study. Commercial fruit juices, which contain mainly sugar and artificial flavors, were used instead of fresh-squeezed juices. Therefore, vitamins and antioxidants usually found in fresh-squeezed juice, which could alter the results of the study, were not seen as a significant concern.
Even though the blood chemistry and inflammatory markers were not measured in this study there is sufficient evidence to support there is a biomarker response after ingestion of a HF meal (Vogel et al, 1997; Nappo et al, 2002; Sejda et al, 2002; Plotnick et al, 2003; Bae et al, 2003; Tsai et al, 2004). This was a pilot study to investigate whether or not there was a significant difference in endothelial function after ingesting the same HF meal between Asian and Caucasians before further studies are carried out to investigate blood chemistry and inflammatory cytokines related to vascular function. Future studies investigating thrifty genes, blood chemistry and inflammatory cytokines related to vascular function will be important to determine the underlying mechanisms involved in the response to a HF meal, and will improve our understanding of the physiological responses which may lead to higher rates of CVD currently seen in Asian populations.
REFERENCES
- Araneta MRG, Wingard DL, Baratt-Conner E. Type 2 diabetes and metabolic syndrome in Filipina-American women: a high-risk non-obese population. Diabetic Care. 2002;25:494–499. doi: 10.2337/diacare.25.3.494. [DOI] [PubMed] [Google Scholar]
- Bae J, Schwemmer M, Lee I, et al. Postprandial hypertriglyceridemia-induced endothelial dysfunction in healthy subjects in independent of lipid oxidation. Int J Cardiol. 2003;87:259–267. doi: 10.1016/s0167-5273(02)00347-9. [DOI] [PubMed] [Google Scholar]
- Bae J, Bassenge E, Kim KB, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155:517–523. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- Chew WLC, Mak PK, Tan YT, Cheong CK. A comparison of the levels of total serum cholesterol (1974–1984) and its relations to ischemic heart disease in Singapore and the United Stated of America. Singapore Med J. 1988;29:6–10. [PubMed] [Google Scholar]
- Cunnane SC, Griffin BA. Nutrition and metabolism of lipids. In: Gibney MJ, Vorster HH, Kok FJ, editors. Introduction to human nutrition malden. MA: Blackwell Science; 2002. pp. 81–115. [Google Scholar]
- Dhawan J, Bray CL, Warburton R, Ghambhir DS, Morris J. Insulin resistance, high prevalence of diabetes, and cardiovascular risk in immigrant Asians. Genetic or environment effect? Br Heart J. 1994;72:413–421. doi: 10.1136/hrt.72.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio C, Zoppo A, Gentile G, et al. Acute effect of high-fat meal on endothelial function in moderately dyslipidemia subjects. Arterioscler Thromb Vasc Biol. 2005;25:406–410. doi: 10.1161/01.ATV.0000152231.93590.17. [DOI] [PubMed] [Google Scholar]
- Hara K, Kubota N, Tobe K, et al. The role of PPARγ as a thrifty gene both in mice and humans. Br J Nutr. 2002;84:S235–S239. doi: 10.1079/096582197388608. [DOI] [PubMed] [Google Scholar]
- Huang B, Rodriguez BL, Burchfiel CM, Chuou PH, Curb JD, Yano K. Acculturation and prevalence of diabetes among Japanese-American men in Hawaii. Am J Epidemiol. 1996;177:674–681. doi: 10.1093/oxfordjournals.aje.a008980. [DOI] [PubMed] [Google Scholar]
- Jackson KG, Armah CK, Minihane AM. Meal fatty acids and postprandial vascular reactivity. Biochem Soc Trans. 2007;35:451–453. doi: 10.1042/BST0350451. [DOI] [PubMed] [Google Scholar]
- Jee SH, Appel LJ, Suh I, Whelton PK, Kim IS. Prevalence of cardiovascular disease risk factors in South Korean adults: results from the Korean Medical Insurance Corporation (KMIC) study. Ann Epidemiol. 1998;8:14–21. doi: 10.1016/s1047-2797(97)00131-2. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Hara K, Kubota N, Tobe K, Terauchi Y, Yamauchi T. The role of PPAR gamma in high-fat diet-induced obesity and insulin resistance. J Diabetes Complications. 2002;16:41–45. doi: 10.1016/s1056-8727(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Kagawa Y, Yanagisawa Y, Hasegawa K, et al. Single nucleotide polymorphisms of thrifty genes for energy metabolism: evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochem Biophys Res Comm. 2002;295:207–222. doi: 10.1016/s0006-291x(02)00680-0. [DOI] [PubMed] [Google Scholar]
- Khoo KL, Tan H, Liew JP, Deslypere E, Janus E. Lipids and coronary heart disease in Asia. Atherosclerosis. 2003;169:1–10. doi: 10.1016/s0021-9150(03)00009-1. [DOI] [PubMed] [Google Scholar]
- Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27:936–942. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- Nappo F, Esposito K, Cioffi M, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. The effect of polymorphism in the intestinal fatty acid-binding protein 2 genes on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6:45–49. doi: 10.1111/j.1463-1326.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- Plotnick GD, Corretti MC, Vogel RA, Hesslink R, Wise JA. Effect of supplemental phytonutrients on impairment of the flow-mediated brachial artery vasoactivity after a single high-fat meal. J Am Coll Cardiol. 2003;41:1744–1749. doi: 10.1016/s0735-1097(03)00302-4. [DOI] [PubMed] [Google Scholar]
- Poppitt SD. Postprandial lipemia, hemostasis, inflammatory response and other emerging risk factors for cardiovascular disease: the influence of fatty meals. Curr Nutr Food Sci. 2005;1:23–34. [Google Scholar]
- Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radha V, Vimaleswaran KS, Babu HNS, et al. Role of genetic polymorphism peroxisome proliferators-activated receptor-γ2 Prol2Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects. Diabetes Care. 2006;29:1046–1051. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- Raitakari O, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol. 2000;36:417–422. doi: 10.1016/s0735-1097(00)00758-0. [DOI] [PubMed] [Google Scholar]
- Sears CE, Casadei B. Mechanisms controlling blood flow and arterial pressure. Surgery. 2002;20:i–v. [Google Scholar]
- Sejda T, Kovar J, Pitha J, Cifkova R, Svandova E, Poledne The effect of an acute fat load on endothelial function after different dietary regimens in young health volunteers. Physiol Res. 2002;51:99–105. [PubMed] [Google Scholar]
- Tai SE, Corella D, Deurenberg-Yap M, Adiconis X, Chew SK, Tan CE. Differential effects of the C1431T and Prol2Ala PPARγ gene variants on plasma lipids and diabetes risk in an Asian population. J Lipid Res. 2004;45:674–685. doi: 10.1194/jlr.M300363-JLR200. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- Tsai W, Li Y, Lin C, Chao T, Chen J. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci. 2004;106:315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Gilg JA, Owen CG, Odoki K, Alberti KG, Cook CG. British South Asians aged 13–16 years have higher fasting glucose and insulin levels than Europeans. Diabetes Med. 2005;22:1275–1277. doi: 10.1111/j.1464-5491.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Whitney RJ. The measurement of volume changes in human limbs. J Physiol. 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney EN, Cataldo CB, Rolfes SR. The lipids: triglyceride, phospholipids, and sterols. In: Howe E, editor. Understanding normal and clinical nutrition. 6th ed. Belmont, CA: Thomson Learning; 2002. pp. 129–159. [Google Scholar]
- Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology. 2007;23:75–83. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Thomas CR. Genotype, obesity and cardiovascular disease-has technical and social advancement outstripped evolution? J Intern Med. 2003;254:114–125. doi: 10.1046/j.1365-2796.2003.01170.x. [DOI] [PubMed] [Google Scholar]