Abstract
Background
Pollination is a crucial step in angiosperm (flowering plant) reproduction. Highly orchestrated pollen–pistil interactions and signalling events enable plant species to avoid inbreeding and outcrossing as a species-specific barrier. In compatible pollination, pollen tubes carrying two sperm cells grow through the pistil transmitting tract and are precisely guided to the ovules, discharging the sperm cells to the embryo sac for fertilization.
Scope
In Lilium longiflorum pollination, growing pollen tubes utilize two critical mechanisms, adhesion and chemotropism, for directional growth to the ovules. Among several molecular factors discovered in the past decade, two small, secreted cysteine-rich proteins have been shown to play major roles in pollen tube adhesion and reorientation bioassays: stigma/style cysteine-rich adhesin (SCA, approx. 9·3 kDa) and chemocyanin (approx. 9·8 kDa). SCA, a lipid transfer protein (LTP) secreted from the stylar transmitting tract epidermis, functions in lily pollen tube tip growth as well as in forming the adhesive pectin matrix at the growing pollen tube wall back from the tip. Lily chemocyanin is a plantacyanin family member and acts as a directional cue for reorienting pollen tubes. Recent consecutive studies revealed that Arabidopsis thaliana homologues for SCA and chemocyanin play pivotal roles in tip polarity and directionality of pollen tube growth, respectively. This review outlines the biological roles of various secreted proteins in angiosperm pollination, focusing on plant LTPs and chemocyanin.
Keywords: Angiosperm fertilization, Arabidopsis thaliana, chemocyanin, cysteine-rich peptides (CRPs), Lilium longiflorum, lipid transfer proteins (LTPs), plantacyanins, pollen tube tip growth, stigma/style cysteine-rich adhesin (SCA)
INTRODUCTION
Plant cells are enclosed in a rigid extracellular matrix (ECM), the cell wall, through which various cell–cell communications are accomplished in response to diverse environmental or developmental signalling cues. The haploid pollen tube cell, carrying two immotile sperm cells, is the only migrating cell in the angiosperms (Sanders and Lord, 1989). For plant sexual reproduction, pollen tube cells pass through a series of signalling events in female reproductive tissues and deliver two sperm cells into the embryo sac for double fertilization: one sperm cell fuses to the egg cell, resulting in the formation of the zygote, and the other to the central cell, resulting in the formation of the endosperm, a nutritional tissue for the developing embryo in the seed (Lord and Russell, 2002).
The pollen tube shows polar tip growth, which enables the tube cell to migrate directionally toward the ovules through the transmitting tract (TT) (Lord, 2000; Yang and Fu, 2007). When the pollen tube grows in the reproductive tract, a callose wall forms to sequester the cell cytoplasm containing the male germ unit (tube cell nucleus and two sperm cells) at the front (Lord, 2000). The tube cell is a completely separate unit from the spent pollen tube and the pollen grain (Jauh and Lord, 1995). The tube cell cytoplasm has tip-oriented, reverse fountain streaming, which is fuelled by a dynamic cytoskeleton, to convey the vesicles containing membrane and cell wall materials to the clear zone at the newly synthesized tube tip (Lord and Russell, 2002). Here, fine actin filaments and a tip-focused Ca2+ gradient are found, and a tip-localized Ca2+-dependent protein kinase functions in oscillation and reorientation of pollen tube tip growth (Holdaway-Clarke et al., 1997; Moutinho et al., 1998). In addition, a Rho-family GTPase of plant (ROP) functions in polar tip growth by controlling the dynamics of actin filament formation (Lin et al., 1996; Fu et al., 2001).
Pollen tubes grow much faster and longer in the pistil than in germination medium. The in vivo pollen tubes of Lilium longiflorum are able to travel the long stylar TT (approx. 10 cm) to reach the ovules in 3 d, while the in vitro pollen tubes grow only up to approx. 5 mm and cease growth within a day. This is probably due to a more dynamic actin organization, exocytosis/endocytosis events and tip-growth signalling occurring in the clear zone at the tip by interplay between the tube cell and the pistil TT (Lord, 2000; Cheung and Wu, 2008). The pistil tissues can provide the pollen tube with a cue for guidance as well as nourishment for tube cell growth. Polar tip growth and guidance of the pollen tube need to be further studied in the light of pollen–pistil interactions, because we have yet to understand fully the effects of the pistil on the pollen tube. Several studies have recently shown biochemical and genetic evidence that small proteins secreted from either pollen or the pistil play critical roles in pollen tube tip growth (Chae et al., 2007, 2009) and chemotropic guidance (Kim et al., 2003; Dong et al., 2005; Okuda et al., 2009). The next big question is whether these small, secreted proteins act with any receptor partner to regulate downstream signalling, as their functional counterparts do in neuronal axon guidance (Stumm and Hollt, 2007) and polarized growth of the mating yeast cell (Madden and Snyder, 1998).
ROLES OF SMALL, SECRETED PROTEINS IN VARIOUS POLLEN–PISTIL INTERACTIONS
In angiosperm fertilization, pollen tubes undertake a series of interactions with sporophytic female tissues (Franklin-Tong, 1999, 2002; Lord and Russell, 2002). The most well understood mechanism in pollen–pistil interaction is self-incompatibility (SI), which functions as a genetic gateway to prevent plant species from inbreeding. In the Brassicaceae, self-/non-self-pollen recognition is controlled by a polymorphic S-locus, where both male and female SI determinants are encoded as a set. The male determinant is the small (approx. 6 kDa), secreted pollen-coat protein, SCR/SP11 (Schopfer et al., 1999; Takayama et al., 2000) and the female determinant is the plasma membrane (PM)-localized serine/threonine receptor kinase, S-locus receptor kinase (SRK) (Stein et al., 1991). An identical S-allele ligand–receptor interaction occurring on the surface of the papillar cell triggers downstream SI signalling, consisting of some non-S-locus factors to reject the self-pollen (Ivanov et al., 2010; Tantikanjana et al., 2010). The ligand recognition autophosphorylates the receptor and recruits the M-locus protein kinase (MLPK), a PM-tethering protein (Murase et al., 2004; Kakita et al., 2007), and the Armadillo repeat-containing protein 1 (ARC1), a U-box E3 ubiquitin ligase (Gu et al., 1998; Stone et al., 1999, 2003), to the PM fraction. Subsequently, SRK, together with MLPK, phosphorylates ARC1, which targets EXO70A1, a putative component of the exocyst complex that promotes compatible pollination, to the degradation pathway (Synek et al., 2006; Samuel et al., 2009).
In the Solanaceae, the female SI determinants are S-locus RNases (S-RNases) secreted from the style (McClure et al., 1989). The stylar S-RNase is taken up through the pollen tube tip and impairs tube growth of self-pollen by degrading rRNA (Luu et al., 2000). In the non-self-pollen tubes that grow in the style, the S-RNase is degraded by an S-locus F-box protein (Sijacic et al., 2004). In Nicotiana tabacum, HT-B, a small secreted protein, participates in the SI reaction as a non-S-specific factor by controlling sequestration of the S-RNase (Goldraij et al., 2006). In the Papaveraceae, the female S-locus determinant gene encodes a small (approx. 15 kDa), soluble protein, PrsS (Papaver rhoeas stigma S) (Foote et al., 1994). It is secreted from the stigmatic papillae cells and interacts with the male determinant, PrpS (P. rhoeas pollen S), which is a small (approx. 20 kDa) protein with predicted transmembrane domains (Wheeler et al., 2009). The S-allele-specific interaction triggers Ca2+-mediated signalling, which results in actin depolymerization and programmed cell death responses in the self-pollen (Bosch and Franklin-Tong, 2008; Poulter et al., 2010).
Although the SI signalling pathways in some species have been well documented, most of the angiosperms are self-compatible and less is known about the mechanisms of compatible pollination. The initial step for pollen–pistil interaction is the physical adhesion of the pollen grain to the stigma. For the dry stigma of Brassicaceae, pollen coat proteins play a significant role in pollen adhesion. An Arabidopsis thaliana lipophilic molecule in the exine of the pollen coat mediates pollen grain adhesion to the dry surface of the papillar cells in a species-specific manner (Zinkl et al., 1999). Two small, secreted Brassica pollen coat proteins, SLR1-BP1 (approx. 9 kDa) and SLR1-BP2 (approx. 6 kDa), function in pollen grain adhesion by interacting with S-locus glycoprotein (SLG)-like receptor 1 (SLR1) (Luu et al., 1999; Takayama et al., 2000).
Following physical contact with the stigma, pollen becomes hydrated and produces the pollen tube. The Brassica PM-localized aquaporin-like protein, MIP-MOD (approx. 30 kDa), was shown to function in pollen hydration and germination, supposedly by regulating the water supply to pollen (Dixit et al., 2001). In tobacco, lipids are thought to be essential for pollen hydration and tube growth (Lush et al., 1998; Wolters-Arts et al., 1998). The Arabidopsis pollen coat was shown to be enriched in lipases and lipid-binding oleosins (approx. 50 kDa) including GRP17 (Mayfield et al., 2001). The T-DNA-inserted null mutant grp17-1 pollen displayed a significant delay in hydration after contact with the papillar cells (Mayfield and Preuss, 2000). In tomato, LAT52 (approx. 20 kDa), a pollen-specific small, secreted protein, is involved in pollen germination via an interaction with the pollen receptor kinase LePRK2 (Tang et al., 2002; Zhang et al., 2008). Once tomato pollen tubes germinate, LeSTIG1 (approx. 13 kDa) secreted from the stigma interacts with pollen LePRK receptors to promote tube cell growth in the stigma (Tang et al., 2004).
In the stigma and the style, pollen tubes grow in the ECM of the TT, a specialized, secretory tissue where they are guided to the female gametophyte. In lily, small, secreted proteins [stigma/style cysteine-rich adhesins (SCAs), approx. 9·3 kDa; and chemocyanin, approx. 9·8 kDa] function in adhesion-mediated and chemotropic pollen tube guidance, respectively (Park et al., 2000; Kim et al., 2003). In the Arabidopsis TT, γ-aminobutyric acid (GABA) was shown to form a gradient, contributing to precise pollen tube guidance to the micropyle (Wilhelmi and Preuss, 1996; Palanivelu et al., 2003). In tobacco, transmitting tissue-specific (TTS) glycoprotein (approx. 100 kDa) promotes pollen tube growth and its RNAi (RNA interference) plants are female sterile (Cheung et al., 1993, 1995). TTS becomes deglycosylated at the growing pollen tube tip, forming a spatio-temporal glycosylation gradient for pollen tube attraction (Wu et al., 1995). The gradient of a chemoattractant is proposed as a widespread mechanism for pollen tube guidance across angiosperm species.
Pollen tubes require cell wall-modifying activity to grow through the extracellular space in the stylar TT. A Zea mays pollen-specific extensin-like protein (Pex1, approx. 80 kDa) is a secreted, glycosylated protein with a conserved leucine-rich repeat (LRR) and a variable extensin-like domain (Stratford et al., 2001). Pex1 functions as a male factor in pollen tube growth in the TT (Rubinstein et al., 1995a, b). An expansin-like activity is also required for pollen tubes to penetrate the rigid cell walls of the TT (Cosgrove et al., 1997; Grobe et al., 1999). Pollen-specific pectin methylesterase (PME), a cell wall-modifying enzyme, was reported to enhance pollen tube tip dynamics and growth (Bosch and Hepler, 2005; Bosch et al., 2005; Jiang et al., 2005).
The female gametophyte governs pollen tube entrance to the micropyle (Shimizu and Okada, 2000; Palanivelu and Preuss, 2006). Pollen tube growth was arrested in Arabidopsis ovule-defective mutants, suggesting the presence of a signal from the female gametophyte (Hulskamp et al., 1995; Ray et al., 1997). A laser ablation study using Torenia fournieri demonstrated that the micropylar guidance signal for pollen tubes comes from the synergid cells (Higashiyama et al., 2001). Two defensin-like cysteine-rich polypeptides (TfCRP1 and TfCRP3, 8·6 and 9·8 kDa, respectively) are found in the synergid cells and act as diffusible attractants for pollen tube targeting to the egg apparatus (Okuda et al., 2009). In terms of guidance signalling, small, secreted proteins appear to be the most common factors. Their roles in successful fertilization may be accomplished through an interaction with a PM-localized partner. Z. mays egg apparatus 1 (ZmEA1), a small (approx. 10 kDa) protein with a predicted transmembrane domain, was shown to be essential in micropylar pollen tube guidance (Marton et al., 2005). Arabidopsis FERONIA is a PM-localized receptor-like kinase in the synergid cells (Escobar-Restrepo et al., 2007) and feronia pollen tubes fail to release the sperm cells following their entrance into the receptive synergid (Huck et al., 2003). Arabidopsis GENERATIVE CELL SPECIFIC 1/HAPLESS2 (GCS1/HAP2), a putative transmembrane protein (approx. 80 kDa), functions in targeting pollen tubes to the ovule as well as in fusion of the gametes at fertilization (von Besser et al., 2006; Mori et al., 2006).
DISCOVERY OF ADHESION AND CHEMOTROPIC FACTORS IN LILY POLLEN TUBE GUIDANCE
It is difficult to study in vivo pollen tube growth in the pistil so development of in vitro bioassays was necessary to increase our understanding of compatible pollination in the angiosperms. The lily flower has a large pistil (approx. 15 cm), an open hollow style and a wide stigma covered with secreted carbohydrate-rich exudates. These features allowed us to obtain sufficient amounts of tissue for in vitro assays and protein purification procedures. On the lily stigma, pollen tubes show directional growth toward the entrance to the hollow style. This guidance on the stigma was mimicked using an in vitro pollen tube reorientation assay. When pollen tubes were placed in the vicinity of an agarose well containing the purified stigma proteins with the tube tips facing away from the well, they reoriented to grow up a gradient of the chemotropic source in the well. A high resolution purification method using reverse-phase high-performance liquid chromatography (HPLC) and mass analysis identified a small, secreted plantacyanin family protein, chemocyanin (Kim et al., 2003), as the chemotropic molecule responsible for this reorientation.
In the hollow style of lily, pollen tubes grow adhering to the inner surface that consists of a layer of the specialized transmitting tract epidermis (TTE). This haptotactic (adhesion-mediated) pollen tube guidance was reconstituted in an in vitro pollen tube adhesion assay (Jauh et al., 1997). It consisted of nitrocellulose membrane-bound ECM from the style, on which lily pollen tubes grew and tightly adhered. This bioassay allowed us to isolate two adhesion molecules: SCA, a small, secreted lipid transfer protein (LTP), and low esterified pectic polysaccharides (Mollet et al., 2000; Park et al., 2000). An ionic interaction between SCA and pectins is critical in forming the functional, adhesive matrix (Mollet et al., 2000). Neither of these molecules alone was active in the adhesion assay.
LILY SCA, A PLANT LIPID TRANSFER PROTEIN
Plant LTPs are small (7–10 kDa), basic (pI 8·8–10) proteins and are commonly found in the angiosperms as a multigene family (Vignols et al., 1994; Kader, 1996; Arondel et al., 2000). LTP protein structure has several conserved features: a 3-D globular shape composed of four α-helices, three loops and a long C-terminal tail, which are stabilized by four disulfide bridges with eight conserved cysteines (Shin et al., 1995; Gomar et al., 1996; Heinemann et al., 1996). Depending on the disulfide bond arrangement and protein size, plant LTPs can be classified into two groups: type 1 (LTP1, approx. 10 kDa) and type 2 (LTP2, approx. 7 kDa) (Kader, 1997; Douliez et al., 2000a). The most outstanding feature of this hydrophilic molecule is a hydrophobic cavity that runs through the whole molecule and, in several known cases, is capable of interacting with the acyl chain of a phospholipid molecule and fatty acids in vitro (Zachowski et al., 1998; Hamilton, 2004). Plant LTPs appear to have no specificity for binding lipids, and they even bind two monoacylated lipid monomers (Charvolin et al., 1999; Douliez et al., 2000b, 2001) or a diacylated lipid (Sodano et al., 1997). Although many LTPs were shown to be able to interact with various lipid molecules in test tubes, no in vivo LTP–lipid complex has been isolated and shown to have biological significance.
Plant LTPs contain a secretory signal peptide and are located in the extracellular space (Bernhard et al., 1991; Thoma et al., 1993). For the last two decades, diverse extracellular biological functions of plant LTPs and LTP-like proteins have been explored. Onion (Allium cepa) Ace-AMP1 and barley (Hordeum bulgare) LTP4 showed defensive roles against fungal and microbial pathogens (Phillippe et al., 1995; Molina and GarciaOlmedo, 1997). Arabidopsis defective in induced resistance 1 (DIR1) is involved in systemic acquired resistance (SAR) (Maldonado et al., 2002). Azelaic acid-induced 1 (AZI1) is involved in salicylic acid (SA)-mediated plant defence (Jung et al., 2009). Arabidopsis glycosylphosphatidylinositol-anchored LTP 1 (LTPG1), an LTP-like molecule, plays a role in cuticular wax deposition (DeBono et al., 2009). In addition, there is evidence that plant LTPs function in plant growth and development (Chae et al., 2010). Tobacco LTP2 mediates cell wall loosening in vitro (Nieuwland et al., 2005).
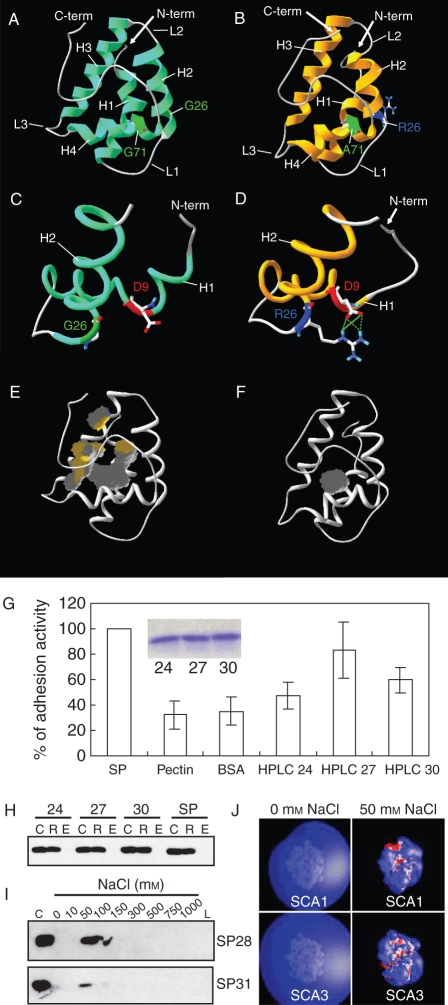
Plant LTPs are also implicated in pollen tube guidance. Lily LTP, SCA, is secreted from the pistil TTE and functions in forming an adhesive matrix with pectin that guides pollen tubes to the ovules (Mollet et al., 2000; Park et al., 2000). There are three SCA isoforms found in the lily stigma secretion with similar molecular masses (SCA1, 9370 Da; SCA2, 9384 Da; and SCA3, 9484 Da) (Chae et al., 2007). Among them, two SCAs (SCA1 and SCA3) were predicted to have a typical LTP-like structure (Fig. 1A–F). One amino acid difference (Gly26 in SCA1 and Arg26 in SCA3) between the two was predicted to result in significant structural changes, especially in the size of the internal hydrophobic cavity. Correlating with this, the two SCA isoforms showed different levels of in vitro pollen tube adhesion activity (Fig. 1G). However, they showed identical pectin binding abilities, by which SCA and pectin form an adhesive matrix via ionic interaction (Fig. 1H, I). The predicted electrostatic potentials of both SCAs show that they are not different in their charge interaction with a negative pectin moiety (Fig. 1J).
Fig. 1.
Two lily SCA isoforms that have identical pectin binding ability show different levels of pollen tube adhesion activity in vitro, with correlating structural differences. (A–F) Three-dimensional structures of SCA1 and SCA3 were generated by homology/MD modelling using the crystal structure of maize LTP (Shin et al., 1995) as the template. Both SCA1 (A) and SCA3 (B) have the typical plant LTP-like structure: a globular shape of the orthogonal four-helix bundle architecture, four disulfide bonds, an internal hydrophobic and solvent-inaccessible cavity, and a long C-terminal tail. H1–H4 indicate helices 1–4, respectively. L1–L3 represent loops 1–3. G26 in SCA1 does not interact with D9 (C). R26 in SCA3 is shown to have strong hydrogen bonding and salt bridge interactions with D9 (D). SCA1 (E) has a larger internal hydrophobic cavity (grey), compared with SCA3 (F). (G) In vitro adhesion assays using HPLC-purified SCA isoforms. SP, pectin matrix prepared with SCA-enriched size-column fractions prior to the HPLC purification as a positive control of adhesion activity; Pectin, pectin matrix alone without any protein (a negative control); BSA, pectin matrix with bovine serum albumin (BSA) replacing SCA (a negative control); HPLC 24–30, pectin matrices containing the HPLC-purified SCA proteins from size-column fractions 24 (SCA2-enriched), 27 (SCA1-enriched) and 30 (SCA3-enriched), respectively. The insert shows equal amounts of proteins (5 µg) from each fraction, applied to the bioassay. (H) In vitro pectin-binding assay. SP, positive protein control prior to HPLC purification; C, a 10 µL aliquot of protein–pectin mixtures as the loading control; R, retentate from the 100 kDa cut-off spin-column containing proteins bound to pectins; E, eluate containing proteins washed off by 1 m NaCl. (I) Ionic strengths of SCAs needed to be detached from the pectin matrix. SP28 and SP31, size-column fractions 28 (SCA1-enriched) and 31 (SCA3-enriched), respectively; C, the loading control; 0–1000 mm NaCl, eluates containing proteins that were serially washed off using a gradient of ionic strengths; L, retentate containing proteins that were left over in the spin-column after the final elution using 1 m NaCl. (J) Electrostatic potentials for homology/MD structures of SCAs. Isopotential contour plots at ±1 kBT e−1 for both SCA1 and SCA3 were generated using GRASP at 0 and 50 mm ionic strengths. Blue indicates a positive and red a negative electrostatic potential. This research was originally published in Journal of Biological Chemistry (Chae et al., 2007). Copyright the American Society for Biochemistry and Molecular Biology.
This structure–function study suggests dual roles for SCA in pollen tube adhesion and tip growth. SCA may act as a lectin-like molecule on the pollen tube cell wall back from the tip, where adhesive pectins are mainly found, providing an ionic ‘glue’ for the link to the pectins on the surface of the stylar TTE (Lord, 2000; Mollet et al., 2007). However, stylar SCA was also shown to bind the tip region of in vitro growing pollen tubes and then internalize to the cytoplasm of the tube cell through an endocytotic pathway (Kim et al., 2006). Specific correlation of the internal hydrophobic cavity volume to adhesion activity implies that an as yet unknown SCA-binding partner may exist at the pollen tube tip to influence tube growth and thereby adhesion rates. SCA may function in pollen tube tip growth signalling.
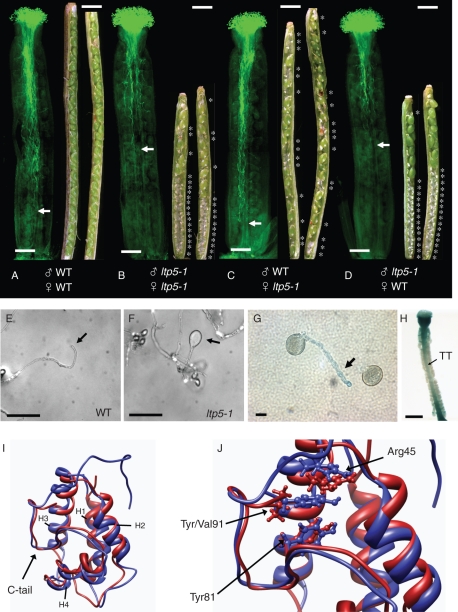
The proposed role of SCA in pollen tube tip growth was further evidenced by a genetic study using A. thaliana. A genome-wide screening of SCA-like Arabidopsis LTP proteins and the phenotypic examination of T-DNA insertional mutants revealed one SCA-like Arabidopsis LTP mutant (ltp5-1, SALK104674) displaying disturbed pollen tube growth in the pistil and decreased seed numbers in the mature siliques (Chae et al., 2009). This was initially thought to be caused solely by a pistil defect, since SCA was known to be secreted from the stylar TTE and to act as a female factor to guide lily pollen tube growth. However, the reciprocal cross-pollination study showed that the defect in ltp5-1 in pollen tube growth was mainly dependent on pollen itself (Fig. 2A–D). The ltp5-1 pollen tubes grew only up to the middle of the wild-type pistil in 12 h (Fig. 2D), by which time the wild type has arrived at the base of the pistil (Fig. 2A). Therefore, no fertilized ovules were found in the bottom half of the pistil in this cross (Fig. 2D), similar to the mutant pollination (Fig. 2B). When in vitro grown, the ltp5-1 pollen tubes displayed abnormally swollen tips and growth cessation in 6 h (Fig. 2F). The LTP5 gene expression was found in pollen tubes at a low level (Fig. 2G). To date, several Arabidopsis LTP genes and lily SCA have been shown to be expressed in various tissues, but not in pollen (Thoma et al., 1994; Clark and Bohnert, 1999; Arondel et al., 2000; Park and Lord, 2003). The LTP5 gene was also weakly expressed in the pistil TT (Fig. 2H). The ltp5-1 pistil seed sets were decreased when wild-type pollen was used in a cross (Fig. 2C), suggesting that the ltp5-1 mutation might interfere with pollen tube guidance to the female gametophyte. Further study revealed that ltp5-1 was a gain-of-function mutant (Chae et al., 2009).
Fig. 2.
A gain-of-function mutant for Arabidopsis LTP5, an SCA-like LTP, shows defects in polar pollen tube tip growth and pistil function for seed formation. (A–D) In vivo reciprocal cross-pollination of ltp5-1 to wild-type plants. Flowers at stage 12 (Smyth et al., 1990) were emasculated a day before each cross-pollination (n = 15 per cross). At 12 h after pollination, 6–7 pistils were fixed, and pollen tube growth was examined by aniline blue staining. The remaining pollinated pistils ripened into mature siliques in 8 d. Siliques were then dissected for examination of fertilized ovules. Scale bars = 200 mm. Arrows indicate the pollen tube front in the pistil. Asterisks designate unfertilized ovules in the silique. Scale bars = 1 mm. (E, F) Pollen from mature flowers was grown on solid germination medium in vitro for 6 h at room temperature. Arrows indicate pollen tube tips. Scale bars = 100 mm. (G, H) GUS (β-glucuronidase) assay of an LTP5pro:GUS flower. (G) A weak level of gene expression (arrow) was identified in pollen tubes grown on the solid medium in vitro for 6 h. Scale bars = 10 mm. (H) A dissected pistil showed a low level of gene expression in the pistil TT (arrow). Scale bar = 400 mm. (I) Superposition of ribbon representations of the structures of LTP5 and ltp5-1. The structures were generated using homology modelling and 1 ns molecular dynamics simulations. The additional, predominantly hydrophobic, C-terminal tail of ltp5-1 is shown to cap one side of the protein, which is known to be an entrance for a putative ligand to the internal hydrophobic cavity in maize LTP (Han et al., 2001). Red, LTP5; blue, ltp5-1; H1–H4, helix 1–4. (J) A focused view of the superposition of (I) is shown, with residues of interest (Arg45, Tyr81, Val91 and Tyr91) depicted in ball and stick representations. Replacement of Val91 in LTP5 with Tyr91 in ltp5-1 results in stabilizing π-cation interactions with Arg45 and π-stacking interactions with Tyr81. The colouring scheme is the same as in (I). This research was originally published in The Plant Cell (www.plantcell.org) (Chae et al., 2009). Copyright American Society of Plant Biologists.
As for lily SCAs, both Arabidopsis LTP5 and the aberrant ltp5-1 proteins were predicted to have a typical plant LTP structure (Fig. 2I). However, ltp5-1 was shown to have an additional C-terminal tail (Fig. 2I, blue). Interestingly, Tyr91 in the ltp5-1 tail sequence was predicted to localize in close proximity to Arg45 and Tyr81, which are crucial residues in maize LTP that interact with a lipid molecule (Han et al., 2001). Although there is no evidence that SCAs or Arabidopsis LTP5 have any ligand in their hydrophobic cavities, the structural studies suggest that these LTPs may function in pollen tube tip growth by interacting with a putative binding partner. The ballooned pollen tube tip of ltp5-1 is highly similar to those of ROP signalling mutants (Li et al., 1999; Fu et al., 2001; Gu et al., 2006) and its putative upstream receptor kinase (Zhang and McCormick, 2007), suggesting that Arabidopsis LTP5 may act as a cue for pollen tube tip growth signaling.
DO ARABIDOPSIS SCA-LIKE LTPS PLAY DIVERSIFIED ROLES IN PLANT REPRODUCTION?
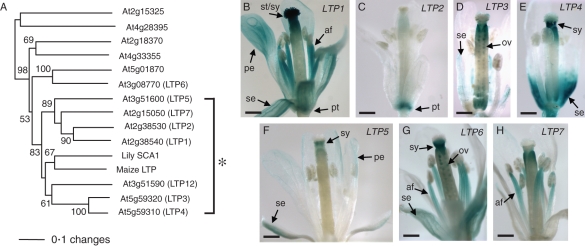
In the A. thaliana genome, about a hundred LTP or LTP-like molecules are found, and 13 conventional plant LTPs are highly homologous to lily SCA (>40 % amino acid identity) (Fig. 3A). These Arabidopsis LTP genes are also known as pathogenesis-related (PR)-14 genes (Sels et al., 2008). Although functional redundancy appears to occur in Arabidopsis SCA-like LTPs, a recent study proposes that they may be highly diversified in their roles in plant growth and fertilization (Chae et al., 2010). Arabidopsis LTP1/2, LTP3/4 and LTP5/12 genes are located right next to each other in tandem orientation, appearing as duplicated pairs (Arondel et al., 2000). The LTP1/2 and LTP3/4 pairs showed >80 % cDNA sequence identity, but their gene expression patterns are highly varied in Arabidopsis reproductive tissues. LTP1 is present most abundantly in the stigma and the style (Fig. 3B), where pollen tubes initiate their growth, while LTP2 was found only in the pedicel (Fig. 3C). LTP3 showed its specific expression in the ovules (Fig. 3D), while LTP4 was expressed in the style (Fig. 3E). LTP5 displayed the weakest level of gene expression among the SCA-like LTP genes (Fig. 3F); however, it has specific gene expression in pollen tubes and the pistil TT (Fig. 2G, H). LTP6 also showed gene expression in the style and the ovule (Fig. 3G), but LTP7 does not show any significant gene expression in reproductive tissues (Fig. 3H). These diversified gene expression patterns of SCA-like LTP genes suggest that each gene plays its own role in the pistil for pollen tube growth and guidance.
Fig. 3.
The SCA-like LTP group in Arabidopsis thaliana. (A) Phylogenetic relationships of SCA and SCA-like LTPs in Arabidopsis. The asterisk indicates lily SCA, maize LTP and seven closely related Arabidopsis SCA-like LTPs. The values on the branches indicate the number of bootstrap replicates supporting the branch. Only bootstrap replication values >50 are shown. This research was originally published in The Plant Cell (www.plantcell.org) (Chae et al., 2009). Copyright American Society of Plant Biologists. (B–H) GUS analysis for gene expression patterns of SCA-like Arabidopsis LTP genes in mature floral tissues. This research was originally published in Journal of Experimental Botany (Chae et al., 2010). Copyright the Society of Experimental Biology.
LILY CHEMOCYANIN, A PLANTACYANIN FAMILY MEMBER
Plantacyanins are small ECM proteins that belong to the ancient, plant-specific phytocyanins, which are classified as a subfamily of blue copper proteins (Ryden and Hunt, 1993). The blue copper proteins have a conserved copper-binding site, formed by two histidines, one cysteine, and one methionine, glutamine or leucine. Unlike other blue copper proteins, two histidines in the copper-binding site of plantacyanins were shown to be exposed to the surface (Einsle et al., 2000), which may facilitate an interaction with ligands. Plantacyanins from Arabidopsis, spinach and cucumber harbour one methionine at the fourth copper-binding site, which is thought to provide a high redox potential (Nersissian et al., 1998), but lily plantacyanin contains leucine at this site instead (Kim et al., 2003). A ragweed plantacyanin, Ra3, does not contain histidines in the binding site and does not display copper-binding activity (Hunt et al., 1985). Their copper-binding abilities and reactive oxygen species (ROS) production with respect to structural changes need to be further evaluated to understand their biological roles in plants.
No functionality of plantacyanins had been identified until chemocyanin, the lily plantacyanin secreted from the pistil, was shown to act as an external signal to regulate in vitro pollen tube reorientation (Kim et al., 2003). Localization of external chemocyanin at the tip of in vitro growing pollen tubes (Kim et al., 2004) may be related to ROS accumulation at the dynamic tip membrane. ROS are known to influence intracellular signalling by activating calcium channels in the plasma membrane (Pei et al., 2000; Foreman et al., 2003). Activated calcium channels trigger calcium influx through the plasma membrane of the growing pollen tube tip (Hepler et al., 2001), which results in a tip-focused intracellular calcium gradient for directional pollen tube growth (Malho et al., 2000). A reorientation of the tip-focused calcium gradient occurs during pollen tube tip reorientation (Hepler et al., 2001).
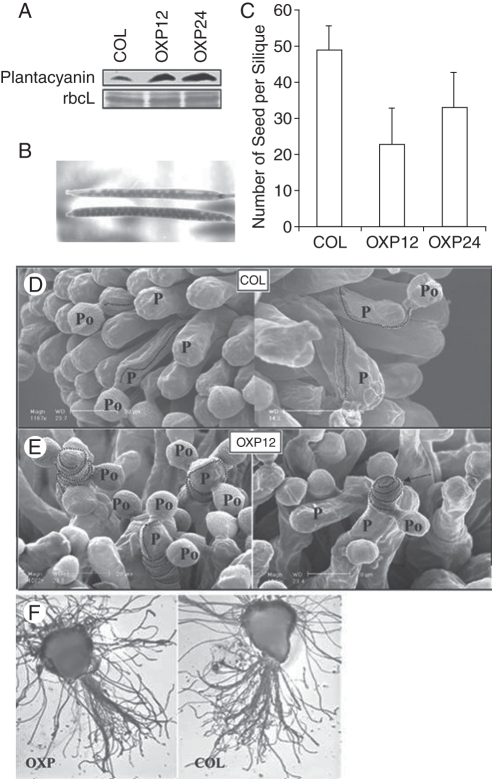
There is a single plantacyanin gene (At2g02850) found in the Arabidopsis genome. The amino acid identity with lily chemocyanin is 51·9 %. Unfortunately, the knock-down did not display any phenotype. The gene expression was most abundant in the inflorescence, especially in the stigma and the style (Dong et al., 2005). Immunolocalization showed that plantacyanin is present in the surface of the stigmatic papillar cell and in the TT from the style to the ovary, where pollen tubes germinate and are guided to the ovule (Dong et al., 2005). On the papillar cells of the Arabidopsis plant over-expressing plantacyanin, many wild-type pollen tubes could not grow toward the style/ovary, making many turns around the papillar cell surface and ending their growth at the papillar cell tip (Fig. 4E). This loss of directionality might be due to a disturbed gradient of guidance cues with over-expression of plantacyanin. This failure in pollen tube guidance resulted in smaller seed sets in plantacyanin over-expression lines, compared with the wild type (Fig. 4B and C).
Fig. 4.
Plantacyanin over-expression lines were used to examine the effect of increased levels of Arabidopsis plantacyanin in the stigma on pollination with wild-type (COL) pollen. Plantacyanin protein levels in the pistils (A) at flower stage 12–13 of OXPs (homozygous T2 generation) are much higher than those of the wild type (COL), as revealed by protein blots. The protein loading control used was Ponceau S staining of the Rubisco large subunit. (B, C) Over-expression pistils pollinated with wild-type pollen produce siliques with fewer seeds than the wild type (COL). The numbers of T2 homozygous pistils hand pollinated after emasculation of flowers are COL (n = 5), OXP12 (n = 21) and OXP24 (n = 23). Values are means ±s.d. (D) Scanning electron microscope images of wild-type pollen on wild-type stigma (two images). Dotted lines trace the path of the pollen tube after it penetrates the papilla cell wall. P, papilla cell, Po, pollen grain. (E) Wild-type pollen on over-expression stigmas showed aberrant tube growth after penetration of the papilla cell wall. Pollen tubes make many turns around the papilla cell in the over-expression stigmas (OXP12; left). One pollen tube shown (OXP12; right) grew away from the style and ended up at the papilla cell tip (arrow). In a semi-in vivo analysis (F), the over-expression stigma (left) and the wild-type stigma (right) were pollinated with wild-type pollen and cultured on an Arabidopsis pollen growth medium. Pollen tubes that penetrate the stigma/style were quantified. No significant difference in number was found between the transgenic and control samples. Scale bars = 20 µm. This research was originally published in Plant Physiology (www.plantphysiol.org) (Dong et al., 2005). Copyright American Society of Plant Biologists.
A gradient of Arabidopsis plantacyanin was found in the embryo sac (Dong et al., 2005) with as yet unknown functionality. When travelling through the pistil TT, Arabidopsis pollen tubes emerge through breaks in the septum epidermis and adhere to the surface of this secretory epidermis until precisely targeted to the ovules (Lord, 2000). Further study is necessary to determine whether plantacyanin acts as a signalling cue in pollen tube guidance to the ovule. Some Arabidopsis SCA-like LTPs are also present in the female gametophyte (Fig. 3) and may be involved in adhesion-assisted guidance. The activity of lily chemocyanin is synergistically enhanced by the presence of SCA in the pollen tube reorientation assay (Kim et al., 2003), so both may interact in guidance in the pistil.
OUTLOOK
A series of biochemical and genetic studies on plantacyanin (Kim et al., 2003; Dong et al., 2005) and LTPs (Park et al., 2000; Chae et al., 2007, 2009), and defensin-like proteins (Okuda et al., 2009) revealed their pivotal roles in pollen tube guidance during compatible angiosperm fertilization. By analogy, they may be proposed to function through a putative interacting partner such as a membrane receptor. In Arabidopsis, small, secreted proteins function in diverse receptor-mediated signalling events. Secreted CLV3 interacts with a membrane-bound receptor–protein complex (CLV1 and CLV2) to regulate Arabidopsis shoot apical meristem cell proliferation and differentiation (Clark, 2001). EPIDERMAL PATTERNING FACTOR1 (EPF1) functions in stomata cell differentiation and its role is dependent on TOO MANY MOUTHS (TMM) receptor-like protein and ERECTA (ER) family receptor kinases (Hara et al., 2007). In neuronal axon guidance, the CXC motif chemokine stromal cell-derived factor 1 (SDF1) is a small, secreted protein that interplays with the CXC chemokine receptor 4 (CXCR4) (Stumm and Hollt, 2007). Yeast mating pair cells utilize small, secreted a/α factors and PM-localized Ste2p receptors to trigger downstream signalling for polarized cell growth toward each partner (Madden and Snyder, 1998).
The fact that Arabidopsis SCA-like LTPs appear to be multifunctional in plant vegetative growth and sexual reproduction (Chae et al., 2010) suggests possible interactions with diverse putative receptors. A mammalian β-defensin, a small, secreted protein, was proposed to participate in diverse cell signalling events by interacting with different receptors for immunity and pigmentation (Candille et al., 2007; Dorin and Jackson, 2007). One plant guidance molecule may trigger either attractive or repulsive signalling, depending on the combination of its putative receptors, as in the neuron. Netrin, a secreted chemoattractant for neuronal outgrowth, plays dual-opposite roles via different combinations of its interacting receptors, UNC-5 and UNC-40 (Hong et al., 1999). The field of pollination/fertilization remains an exciting frontier in plant signalling, and small proteins play a leading role.
ACKNOWLEDGEMENTS
Research in the laboratory of E.M.L was supported by the National Science Foundation, USA.
LITERATURE CITED
- Arondel V, Vergnolle C, Cantrel C, Kader JC. Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Science. 2000;157:1–12. doi: 10.1016/s0168-9452(00)00232-6. [DOI] [PubMed] [Google Scholar]
- Bernhard WR, Thoma S, Botella J, Somerville CR. Isolation of a cDNA clone for spinach lipid transfer protein and evidence that the protein is synthesized by the secretory pathway. Plant Physiology. 1991;95:164–170. doi: 10.1104/pp.95.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development. 2006;133:4761–4769. doi: 10.1242/dev.02683. [DOI] [PubMed] [Google Scholar]
- Bosch M, Franklin-Tong VE. Self-incompatibility in Papaver: signalling to trigger PCD in incompatible pollen. Journal of Experimental Botany. 2008;59:481–490. doi: 10.1093/jxb/erm195. [DOI] [PubMed] [Google Scholar]
- Bosch M, Hepler PK. Pectin methylesterases and pectin dynamics in pollen tubes. The Plant Cell. 2005;17:3219–3226. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Cheung AY, Hepler PK. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiology. 2005;138:1334–1346. doi: 10.1104/pp.105.059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, et al. A defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Li Z, Li K, et al. Two SCA (stigma/style cysteine-rich adhesin) isoforms show structural differences that correlate with their levels of in vitro pollen tube adhesion activity. Journal of Biological Chemistry. 2007;282:33845–33858. doi: 10.1074/jbc.M703997200. [DOI] [PubMed] [Google Scholar]
- Chae K, Kieslich C, Morikis D, Kim S, Lord EM. A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization. The Plant Cell. 2009;21:3902–3914. doi: 10.1105/tpc.109.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Gonong BJ, Kim S-C, et al. A multifaceted study of stigma/style cysteine-rich adhesin (SCA)-like Arabidopsis lipid transfer proteins (LTPs) suggests diversified roles for these LTPs in plant growth and reproduction. Journal of Experimental Botany. 2010;61:4277–4290. doi: 10.1093/jxb/erq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvolin D, Douliez JP, Marion D, Cohen-Addad C, Pebay-Peyroula E. The crystal structure of a wheat non-specific lipid transfer protein (nsLTP1) complexed with two molecules of phospholipid at 2·1 angstrom resolution. European Journal of Biochemistry. 1999;264:562–568. doi: 10.1046/j.1432-1327.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- Cheung AY, May B, Kawata EE, Gu Q, Wu HM. Characterization of cDNAs for stylar transmitting tissue-specific proline-rich proteins in tobacco. The Plant Journal. 1993;3:151–160. [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82:383–393. doi: 10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Clark AM, Bohnert HJ. Cell-specific expression of genes of the lipid transfer protein family from Arabidopsis thaliana. Plant and Cell Physiology. 1999;40:69–76. doi: 10.1093/oxfordjournals.pcp.a029476. [DOI] [PubMed] [Google Scholar]
- Clark SE. Cell signalling at the shoot meristem. Nature Reviews Molecular Cell Biology. 2001;2:276–284. doi: 10.1038/35067079. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Bedinger P, Durachko DM. Group I allergens of grass pollen as cell wall-loosening agents. Proceedings of the National Academy of Sciences, USA. 1997;94:6559–6564. doi: 10.1073/pnas.94.12.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBono A, Yeats TH, Rose JKC, et al. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. The Plant Cell. 2009;21:1230–1238. doi: 10.1105/tpc.108.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Rizzo C, Nasrallah M, Nasrallah J. The Brassica MIP-MOD gene encodes a functional water channel that is expressed in the stigma epidermis. Plant Molecular Biology. 2001;45:51–62. doi: 10.1023/a:1006428007826. [DOI] [PubMed] [Google Scholar]
- Dong J, Kim ST, Lord EM. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiology. 2005;138:778–789. doi: 10.1104/pp.105.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin JR, Jackson IJ. GENETICS: β-defensin repertoire expands. Science. 2007;318:1395. doi: 10.1126/science.1151370. [DOI] [PubMed] [Google Scholar]
- Douliez JP, Michon T, Elmorjani K, Marion D. Structure, biological and technological functions of lipid transfer proteins and indolines, the major lipid binding proteins from cereal kernels. Journal of Cereal Science. 2000a;32:1–20. [Google Scholar]
- Douliez JP, Michon T, Marion D. Steady-state tyrosine fluorescence to study the lipid-binding properties of a wheat non-specific lipid-transfer protein (nsLTP1) Biochimica et Biophysica Acta. 2000b;1467:65–72. [PubMed] [Google Scholar]
- Douliez JP, Jegou S, Pato C, Molle D, Tran V, Marion D. Binding of two mono-acylated lipid monomers by the barley lipid transfer protein, LTP1, as viewed by fluorescence, isothermal titration calorimetry and molecular modelling. European Journal of Biochemistry. 2001;268:384–388. doi: 10.1046/j.1432-1033.2001.01889.x. [DOI] [PubMed] [Google Scholar]
- Einsle O, Mehrabian Z, Nalbandyan R, Messerschmidt A. Crystal structure of plantacyanin, a basic blue cupredoxin from spinach. Journal of Biological Inorganic Chemistry. 2000;5:666–672. doi: 10.1007/s007750000154. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo J-M, Huck N, Kessler S, et al. The FERONIA receptor-like kinase mediates male–female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Foote HC, Ride JP, Franklin-Tong VE, Walker EA, Lawrence MJ, Franklin FC. Cloning and expression of a distinctive class of self-incompatibility (S) gene from Papaver rhoeas L. Proceedings of the National Academy of Sciences, USA. 1994;91:2265–2269. doi: 10.1073/pnas.91.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE. Signaling and the modulation of pollen tube growth. The Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE. The difficult question of sex: the mating game. Current Opinion in Plant Biology. 2002;5:14–18. doi: 10.1016/s1369-5266(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang ZB. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. Journal of Cell Biology. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldraij A, Kondo K, Lee CB, et al. Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature. 2006;439:805–810. doi: 10.1038/nature04491. [DOI] [PubMed] [Google Scholar]
- Gomar J, Petit MC, Sodano P, et al. Solution structure and lipid binding of a nonspecific lipid transfer protein extracted from maize seeds. Protein Science. 1996;5:565–577. doi: 10.1002/pro.5560050402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe K, Becker WM, Schlaak M, Petersen A. Grass group I allergens (beta-expansins) are novel, papain-related proteinases. European Journal of Biochemistry. 1999;263:33–40. doi: 10.1046/j.1432-1327.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Gu TS, Mazzurco M, Sulaman W, Matias DD, Goring DR. Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proceedings of the National Academy of Sciences, USA. 1998;95:382–387. doi: 10.1073/pnas.95.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li SD, Lord EM, Yang ZB. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. The Plant Cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Progress in Lipid Research. 2004;43:177–199. doi: 10.1016/j.plipres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Han GW, Lee JY, Song HK, et al. Structural basis of non-specific lipid binding in maize lipid transfer protein complexes revealed by high-resolution X-ray crystallography. Journal of Molecular Biology. 2001;308:263–278. doi: 10.1006/jmbi.2001.4559. [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes and Development. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann B, Andersen KV, Nielsen PR, Bech LM, Poulsen FM. Structure in solution of a four-helix lipid binding protein. Protein Science. 1996;5:13–23. doi: 10.1002/pro.5560050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annual Review of Cell and Developmental Biology. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, et al. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. The Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- Hulskamp M, Schneitz K, Pruitt RE. Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. The Plant Cell. 1995;7:57–64. doi: 10.1105/tpc.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, George D, Yeh L. Ragweed allergen Ra3: relationship to some type 1 copper-binding proteins. Journal of Molecular Evolution. 1985;21:126–132. doi: 10.1007/BF02100086. [DOI] [PubMed] [Google Scholar]
- Ivanov R, Fobis-Loisy I, Gaude T. When no means no: guide to Brassicaceae self-incompatibility. Trends in Plant Science. 2010;15:387–394. doi: 10.1016/j.tplants.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Jauh GY, Lord EM. Movement of the tube cell in the lily style in the absence of the pollen grain and the spent pollen tube. Sexual Plant Reproduction. 1995;8:168–172. [Google Scholar]
- Jauh GY, Eckard KJ, Nothnagel EA, Lord EM. Adhesion of lily pollen tubes on an artificial matrix. Sexual Plant Reproduction. 1997;10:173–180. [Google Scholar]
- Jiang LX, Yang SL, Xie LF, et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell. 2005;17:584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- Kader JC. Lipid transfer proteins in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- Kader JC. Lipid transfer proteins: a puzzling family of plant proteins. Trends in Plant Science. 1997;2:66–70. [Google Scholar]
- Kakita M, Murase K, Iwano M, et al. Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. The Plant Cell. 2007;19:3961–3973. doi: 10.1105/tpc.106.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang KL, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences, USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dong J, Lord EM. Pollen tube guidance: the role of adhesion and chemotropic molecules. Current Topics in Developmental Biology. 2004;61:61–79. doi: 10.1016/S0070-2153(04)61003-9. [DOI] [PubMed] [Google Scholar]
- Kim ST, Zhang KL, Dong J, Lord EM. Exogenous free ubiquitin enhances lily pollen tube adhesion to an in vitro stylar matrix and may facilitate endocytosis of SCA. Plant Physiology. 2006;142:1397–1411. doi: 10.1104/pp.106.086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin YK, Heath RM, Zhu MX, Yang ZB. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. The Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YK, Wang YL, Zhu JK, Yang ZB. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. The Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord E. Adhesion and cell movement during pollination: cherchez la femme. Trends in Plant Science. 2000;5:368–373. doi: 10.1016/s1360-1385(00)01744-1. [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD. The mechanisms of pollination and fertilization in plants. Annual Review of Cell and Developmental Biology. 2002;18:81–105. doi: 10.1146/annurev.cellbio.18.012502.083438. [DOI] [PubMed] [Google Scholar]
- Lush WM, Grieser F, Wolters-Arts M. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiology. 1998;118:733–741. doi: 10.1104/pp.118.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu DT, Marty-Mazars D, Trick M, Dumas C, Heizmann P. Pollen–stigma adhesion in Brassica spp involves SLG and SLR1 glycoproteins. The Plant Cell. 1999;11:251–262. doi: 10.1105/tpc.11.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu DT, Qin XK, Morse D, Cappadocia M. S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature. 2000;407:649–651. doi: 10.1038/35036623. [DOI] [PubMed] [Google Scholar]
- Madden K, Snyder M. Cell polarity and morphogenesis in budding yeast. Annual Review of Microbiology. 1998;52:687–744. doi: 10.1146/annurev.micro.52.1.687. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- Malho R, Camacho L, Moutinho A. Signaling pathways in pollen tube growth and re-orientation. Annals of Botany. 2000;85:59–68. [Google Scholar]
- Marton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science. 2005;307:573–576. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- Mayfield JA, Preuss D. Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nature Cell Biology. 2000;2:128–130. doi: 10.1038/35000084. [DOI] [PubMed] [Google Scholar]
- Mayfield JA, Fiebig A, Johnstone SE, Preuss D. Gene families from the Arabidopsis thaliana pollen coat proteome. Science. 2001;292:2482–2485. doi: 10.1126/science.1060972. [DOI] [PubMed] [Google Scholar]
- McClure BA, Haring V, Ebert PR, et al. Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature. 1989;342:955–957. doi: 10.1038/342955a0. [DOI] [PubMed] [Google Scholar]
- Molina A, GarciaOlmedo F. Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. The Plant Journal. 1997;12:669–675. doi: 10.1046/j.1365-313x.1997.00669.x. [DOI] [PubMed] [Google Scholar]
- Mollet JC, Park SY, Nothnagel EA, Lord EM. A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. The Plant Cell. 2000;12:1737–1749. doi: 10.1105/tpc.12.9.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Faugeron C, Morvan H. Cell adhesion, separation and guidance in compatible plant reproduction. Annual Plant Reviews. 2007;25:69–90. [Google Scholar]
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nature Cell Biology. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- Moutinho A, Trewavas AJ, Malho R. Relocation of a Ca2+-dependent protein kinase activity during pollen tube reorientation. The Plant Cell. 1998;10:1499–1509. doi: 10.1105/tpc.10.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K, Shiba H, Iwano M, Che FS, Watanabe M, Isogai A, Takayama S. A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science. 2004;303:1516–1519. doi: 10.1126/science.1093586. [DOI] [PubMed] [Google Scholar]
- Nersissian AM, Immoos C, Hill MG, Hart PJ, Williams G, Herrmann RG. Uclacyanins, stellacyanins, and plantacyanins are distinct subfamilies of phytocyanins: plant-specific mononuclear blue copper proteins. Protein Science. 1998;7:1915–1929. doi: 10.1002/pro.5560070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland J, Feron R, Huisman BAH, et al. Lipid transfer proteins enhance cell wall extension in tobacco. The Plant Cell. 2005;17:2009–2019. doi: 10.1105/tpc.105.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biology. 2006;6:7. doi: 10.1186/1471-2229-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Park SY, Lord EM. Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Molecular Biology. 2003;51:183–189. doi: 10.1023/a:1021139502947. [DOI] [PubMed] [Google Scholar]
- Park SY, Jauh GY, Mollet JC, et al. A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. The Plant Cell. 2000;12:151–163. doi: 10.1105/tpc.12.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Phillippe B, Cammue BPA, Thevissen K, et al. A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiology. 1995;109:445–455. doi: 10.1104/pp.109.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter NS, Wheeler MJ, Bosch M, Franklin-Tong VE. Self-incompatibility in Papaver: identification of the pollen S-determinant PrpS. Biochemical Society Transactions. 2010;38:588–592. doi: 10.1042/BST0380588. [DOI] [PubMed] [Google Scholar]
- Ray S, Park SS, Ray A. Pollen tube guidance by the female gametophyte. Development. 1997;124:2489–2498. doi: 10.1242/dev.124.12.2489. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL, Broadwater AH, Lowrey KB, Bedinger PA. Pex1, a pollen-specific gene with an extensin-like domain. Proceedings of the National Academy of Sciences, USA. 1995a;92:3086–3090. doi: 10.1073/pnas.92.8.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL, Marquez J, SuarezCervera M, Bedinger PA. Extensin-like glycoproteins in the maize pollen tube wall. The Plant Cell. 1995b;7:2211–2225. doi: 10.1105/tpc.7.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden L, Hunt L. Evolution of protein complexity: the blue copper-containing oxidases and related proteins. Journal of Molecular Evolution. 1993;36:41–66. doi: 10.1007/BF02407305. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR. Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. The Plant Cell. 2009;21:2655–2671. doi: 10.1105/tpc.109.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Lord EM. Directed movement of latex particles in the gynoecia of three species of flowering plants. Science. 1989;243:1606–1608. doi: 10.1126/science.243.4898.1606. [DOI] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self-incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. [DOI] [PubMed] [Google Scholar]
- Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiology and Biochemistry. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development. 2000;127:4511–4518. doi: 10.1242/dev.127.20.4511. [DOI] [PubMed] [Google Scholar]
- Shin DH, Lee JY, Hwang KY, Kim KK, Suh SW. High-resolution crystal structure of the nonspecific lipid transfer protein from maize seedlings. Structure. 1995;3:189–199. doi: 10.1016/s0969-2126(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Sijacic P, Wang X, Skirpan AL, et al. Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature. 2004;429:302–305. doi: 10.1038/nature02523. [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodano P, Caille A, Sy D, dePerson G, Marion D, Ptak M. 1H NMR and fluorescence studies of the complexation of DMPG by wheat non-specific lipid transfer protein. Global fold of the complex. FEBS Letters. 1997;416:130–134. doi: 10.1016/s0014-5793(97)01185-x. [DOI] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proceedings of the National Academy of Sciences, USA. 1991;88:8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Arnoldo M, Goring DR. A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science. 1999;286:1729–1731. doi: 10.1126/science.286.5445.1729. [DOI] [PubMed] [Google Scholar]
- Stone SL, Anderson EM, Mullen RT, Goring DR. ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. The Plant Cell. 2003;15:885–898. doi: 10.1105/tpc.009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford S, Barnes W, Hohorst DL, et al. A leucine-rich repeat region is conserved in pollen extensin-like (Pex) proteins in monocots and dicots. Plant Molecular Biology. 2001;46:43–56. doi: 10.1023/a:1010659425399. [DOI] [PubMed] [Google Scholar]
- Stumm R, Hollt V. CXC chemokine regulator receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. Journal of Molecular Endocrinology. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Synek L, Schlager N, Eliáš M, Quentin M, Hauser M-T, Žárský V. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. The Plant Journal. 2006;48:54–72. doi: 10.1111/j.1365-313X.2006.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Shiba H, Iwano M, et al. Isolation and characterization of pollen coat proteins of Brassica campestris that interact with S locus-related glycoprotein 1 involved in pollen–stigma adhesion. Proceedings of the National Academy of Sciences, USA. 2000;97:3765–3770. doi: 10.1073/pnas.040580797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S. A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. The Plant Cell. 2002;14:2277–2287. doi: 10.1105/tpc.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. The Plant Journal. 2004;39:343–353. doi: 10.1111/j.1365-313X.2004.02139.x. [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Nasrallah JB. Complex networks of self-incompatibility signaling in the Brassicaceae. Current Opinion In Plant Biology. 2010;13:520–526. doi: 10.1016/j.pbi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Thoma S, Hecht U, Kippers A, Botella J, Devries S, Somerville C. Tissue specific expression of a gene encoding a cell wall localized lipid transfer protein from Arabidopsis. Plant Physiology. 1994;105:35–45. doi: 10.1104/pp.105.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma S, Kaneko Y, Somerville C. A nonspecific lipid transfer protein from Arabidopsis is a cell wall protein. The Plant Journal. 1993;3:427–436. doi: 10.1046/j.1365-313x.1993.t01-25-00999.x. [DOI] [PubMed] [Google Scholar]
- Vignols F, Lund G, Pammi S, et al. Characterization of a rice gene coding for a lipid transfer protein. Gene. 1994;142:265–270. doi: 10.1016/0378-1119(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Wheeler MJ, de Graaf BHJ, Hadjiosif N, et al. Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature. 2009;459:992–995. doi: 10.1038/nature08027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi LK, Preuss D. Self-sterility in Arabidopsis due to defective pollen tube guidance. Science. 1996;274:1535–1537. doi: 10.1126/science.274.5292.1535. [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. Lipids are required for directional pollen tube growth. Nature. 1998;392:818–821. doi: 10.1038/33929. [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang H, Cheung AY. A pollen tube growth-stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82:395–403. doi: 10.1016/0092-8674(95)90428-x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Fu Y. ROP/RAC GTPase signaling. Current Opinion in Plant Biology. 2007;10:490–494. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski A, Guerbette F, Grosbois M, Jolliot-Croquin A, Kader JC. Characterisation of acyl binding by a plant lipid transfer protein. European Journal of Biochemistry. 1998;257:443–448. doi: 10.1046/j.1432-1327.1998.2570443.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wengier D, Shuai B, Gui C-P, Muschietti J, McCormick S, Tang W-H. The pollen receptor kinase LePRK2 mediates growth-promoting signals and positively regulates pollen germination and tube growth. Plant Physiology. 2008;148:1368–1379. doi: 10.1104/pp.108.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl GM, Zwiebel BI, Grier DG, Preuss D. Pollen–stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development. 1999;126:5431–5440. doi: 10.1242/dev.126.23.5431. [DOI] [PubMed] [Google Scholar]