Abstract
Background
The conical epidermal cells found on the petals of most Angiosperm species are so widespread that they have been used as markers of petal identity, but their function has only been analysed in recent years. This review brings together diverse data on the role of these cells in pollination biology.
Scope
The published effects of conical cells on petal colour, petal reflexing, scent production, petal wettability and pollinator grip on the flower surface are considered. Of these factors, pollinator grip has been shown to be of most significance in the well-studied Antirrhinum majus/bumble-bee system. Published data on the relationship between epidermal cell morphology and floral temperature were limited, so an analysis of the effects of cell shape on floral temperature in Antirrhinum is presented here. Statistically significant warming by conical cells was not detected, although insignificant trends towards faster warming at dawn were found, and it was also found that flat-celled flowers could be warmer on warm days. The warming observed is less significant than that achieved by varying pigment content. However, the possibility that the effect of conical cells on temperature might be biologically significant in certain specific instances such as marginal habitats or weather conditions cannot be ruled out.
Conclusions
Conical epidermal cells can influence a diverse set of petal properties. The fitness benefits they provide to plants are likely to vary with pollinator and habitat, and models are now required to understand how these different factors interact.
Keywords: Antirrhinum majus, conical cell, epidermis, floral scent, floral temperature, flower colour, grip, petal, pollination, wettability
INTRODUCTION
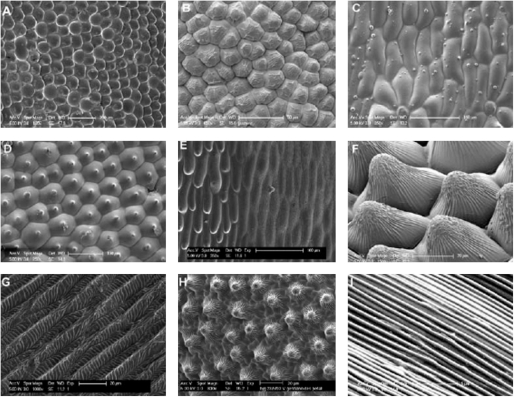
The flower epidermis is crucial to understanding petal function, as it is both the primary point of contact with the abiotic environment (influencing light capture and reflectance, evaporation, temperature and wettability) and the primary point of contact with the biotic environment (providing visual, tactile and olfactory cues to pollinating animals). The petals of most Angiosperm species have at least one of their epidermal surfaces composed of conical or papillate cells, protruding outwards from the plane of tissue. Cells that can be described as conical are found on 75–80 % of petals examined (Kay et al, 1981; Christensen and Hansen, 1998). They are usually found on the epidermis oriented towards potential pollinators (usually the adaxial epidermis), but can also be found on the other epidermal surface in some species. Conical petal epidermal cells vary greatly in overall size, with at least 10-fold differences in cell diameter readily recorded between different species (Fig. 1A, B). The steepness and height of the cone can vary dramatically, from a gentle lens shape to an almost hair-like structure in petunia (Fig. 1D–F). The base shape of the cell is most commonly hexagonal, but can be more rounded, irregular and amoeboid, or even elongated as a long rectangle, as seen on the ray florets of daisies (Fig. 1G, H). The surface structure of the cells is also variable, being anything from smooth and coated in a thin layer of wax to heavily coated in cuticular striations, which may be arranged linearly or radiate outwards from the peak of the cone (Fig. 1E, F, I).
Fig. 1.
Variation in petal epidermal cell form. (A–C) Variation in base diameter of petal epidermal cells. (A) Nerine sarniensis has cells ∼100 µm in diameter, while (B) Jasminum officinale has cells ∼20 µm in diameter. Nymphaea alba (C) lacks conical cells completely. (D–F) Variations in the steepness of the cone of conical cells. (D) Geranium procurrens; (E) Hibiscus trionum – note mixed morphology of cells; and (F) Helianthus annuus – note striations on conical cells. (G–I) The variety shown in the base shape of conical cells. Rectangular bases in (G) Gazania krebsiana and amoeboid in (H) Veronica. (I) Regular striations found on Mentzelia lindleyi.
Whatever the specifics of their morphology, conical epidermal cells are treated by many authors as a defining feature of petals. They are rarely found on leaves or any other plant surface, and are very common on petals. Within the developmental literature they are often treated as a marker for petal identity, being used to define homeotic conversions between petals and other floral organs (Irish, 2009; Ojeda et al., 2009). It is therefore surprising that a cell type used to define organ identity had no proven function until very recently. We, and other authors, have spent several years analysing the effects of conical petal cells on flower form, plant reproductive success and pollinator behaviour. In this review we set out to synthesize those studies to provide an answer to why so many Angiosperm species have conical petal epidermal cells.
CONICAL CELLS ENHANCE POLLINATION SUCCESS
It was impossible to establish whether conical epidermal cells enhanced pollination success until the identification of a mutant differing from a wild-type form only in their production. The mixta mutant of Antirrhinum majus was described by Noda et al. (1994), and shown to contain a null allele of the MYB-related transcription factor necessary to initiate conical cell outgrowth. The epidermis of the mutant petals was composed of flat hexagonal-based cells. This change in cell morphology had a consequence for petal colour, in that the mutant flowers appeared slightly paler and less velvety. This effect is discussed in more detail below, but was shown by Noda et al. (1994) to be due solely to cell shape. In all other respects the two lines were phenotypically identical.
To test whether conical cells enhanced pollination success, Glover and Martin (1998) grew the mixta mutant and isogenic wild-type lines (from which the mutagenic transposon had excised germinally) in mixed field plots. Four flowers per plant were emasculated prior to anther dehiscence, and tagged. Seed set by tagged flowers was scored, with the presence of seed used to indicate a pollinator visit and the absence of seed to represent no pollinator visit. The experiment was repeated in multiple plots over two summers, and in all cases the outcome was the same. Conical petal cells significantly enhanced a flower's chance of being visited by a pollinator, with the degree of difference increasing as the competition for pollinator attention between plants increased. These data confirmed that petal conical cells do enhance pollination success, but provided few clue as to why or how. Indeed, unpigmented conical- and flat-celled flowers were also used in the same experiments, produced by crossing the wild type and mixta lines to the nivea mutant, lacking the gene encoding chalcone synthase and therefore lacking all flavonoid and anthocyanin synthesis (Wienand et al., 1982). The pattern was the same in the white flowers, with conical-celled lines receiving more pollinator attention. This result indicated that the visible difference between the wild type and mixta flowers was not the primary factor in determining pollination success.
VISUAL EFFECTS OF CONICAL CELLS
Long before the isolation of a mutant line in which to investigate the effect of cell shape, Kay et al. (1981) hypothesized that conical petal cells would focus light into petals, enhancing colour. They further suggested that the cone shape might have a light-scattering effect, generating a sparkling appearance. Comparison of the mixta mutant and the wild-type form of Antirrhinum confirmed these hypotheses. Gorton and Vogelmann (1996) showed that this visual effect resulted from the ability of conical cells to act as lenses, focusing light into epidermal vacuoles that contain anthocyanin, thus enhancing petal colour saturation. At the same time conical cells scatter reflected light from the mesophyll more evenly than flat cells, resulting in a brighter sheen or velvety texture to the petal. However, the simple fact that conical cells do influence petal colour does not necessarily mean that their effect on pollination is achieved through visible effects. Indeed, the enhanced pollination of white conical-celled flowers relative to white flat-celled flowers (Glover and Martin, 1998) suggests that the observable difference in colour is a red herring. Dyer et al. (2007) used behavioural experiments to show that the colour difference between wild-type and mixta mutant flowers is detectable by bumble-bees (Bombus terrestris, one of the natural pollinators of Antirrhinum). They measured the reflectance spectra of the two flower types, and produced artificial flowers painted to reflect the same wavelengths to the same degree. Once trained to associate a food reward with one of these artificial flowers, bees presented with a choice of both colours continued to forage on the known rewarding flower type in preference to the novel colour. However, further experiments by the same authors showed that the bees had no innate preference for either colour. If both colours were presented to naïve bees, each equally rewarded, the bees foraged equally from both. Similarly, experiments to investigate the extent to which flowers of either colour were visible at defined distances indicated that neither the wild type nor the mixta mutant colour was more salient to the insect eye (Dyer et al., 2007).
Conical petal cells influence the flower visually in one other way. Baumann et al. (2007) studied the mixta mutant, various transgenic plants ectopically expressing MIXTA-like genes, and a flat-celled mutant of Petunia hybrid [phmyb1, mutant at the orthologue of MIXTA (van Houwelingen et al., 1998)]. They observed that epidermal cell morphology influenced petal reflexing, with conical-celled petals standing more upright than those with flat cells, possibly as a result of differential tension produced by the cellular outgrowth. In consequence, conical-celled petals appeared to present a larger surface area to approaching pollinators. Previous studies have shown that target size is highly significant in attracting insects, and that larger flowers are more readily detectable than smaller flowers (Spaethe et al., 2001; Spaethe and Chittka, 2003).
EFFECTS OF CELL SHAPE ON FLORAL TEMPERATURE
A preliminary study by Comba et al. (2000) suggested that the wild type conical-celled Antirrhinum flower absorbed more direct sunlight than that of the flat-celled mixta mutant, and was therefore sometimes warmer. However, a great deal of variability in floral temperature was recorded. Increased temperature has the potential to enhance plant reproductive success in a number of ways. Maintaining their flowers at a temperature that is higher than ambient confers a fitness benefit on plants, particularly when conditions are cool, because warmer temperature speeds up the development of floral organs and of seed (Akpan and Bean, 1977; Elgersma et al., 1989). Heat might also function as a reward to pollinators, by providing a direct metabolic reward (Rands and Whitney, 2007). Dyer et al. (2006) showed that bumble-bees choose warmer feeders over cooler feeders, even when the sucrose reward is the same in both. This discrimination is based not just on the temperature of the reward, as Whitney et al. (2008) were able to train bees to choose artificial flowers that felt cooler than alternatives, by rewarding only the cooler feeder.
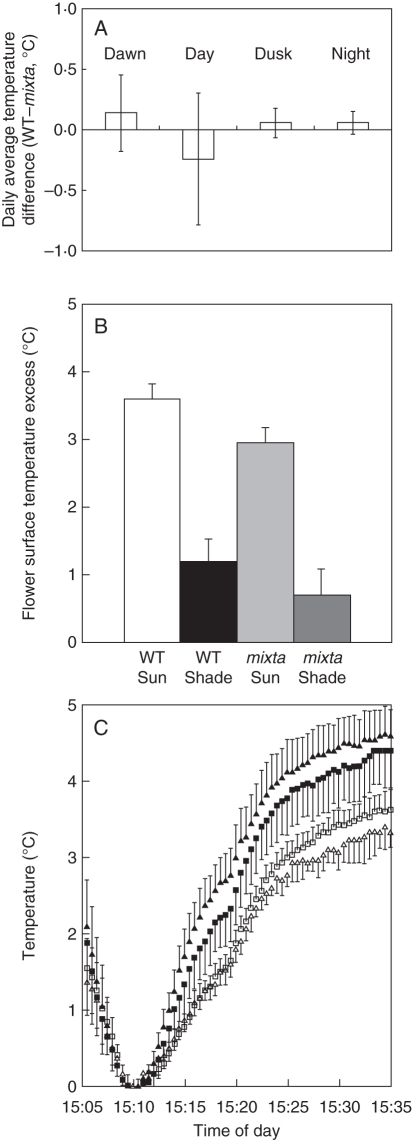
To explore further the possibility that conical cells enhance floral temperature, we planted out immature wild-type and mixta plants in the Cambridge University Botanic Garden, before flowering occurred. The temperature inside the corollas of plants of the two genotypes was monitored using thermocouples secured by a wire support taped to the stem and attached to a datalogger. Paired flowers of the two genotypes were chosen from adjacent plants matched for maturity, height and direction faced. Temperatures were recorded in such paired flowers on 16 d between 30 July and 11 September 2006, along with the ambient air temperature, all at 5 min intervals. Very little difference was found between the daily average temperatures of the two flower types, although there was a great deal of variability in the instantaneous measurements. The data were therefore divided into periods of dawn, day, dusk and night, and average differences between genotypes in these periods over the 16 d are shown in Fig. 2A. We performed an analysis of covariance (ANCOVA) with the daily average difference between paired conical- and flat-celled flowers as the response variable and, after model simplification, the significant contributory factors to the difference between genotypes were reduced to the ambient air temperature (F1,46 = 25·4; P <0·001) and period of day (F1,46 = 4·33; P <0·01). A Tukey test showed a significant difference at the 95 % confidence level between the periods ‘day’ and ‘dusk’. Both these significant results could be attributable to a tendency for mixta flowers to be warmer than the wild type at temperatures >20 °C. The apparent tendency for conical-celled flowers to be warmer around dawn was not significant in this analysis, but seemed worthy of further investigation.
Fig. 2.
Petal cell shape and temperature. (A) Difference in intrafloral temperature between paired wild-type (WT) and mixta flowers from adjacent plants matched for maturity, height and direction faced, as described in the text. Bars show means and s.d. of the daily averages of instantaneous differences in temperature between WT and mixta flowers. Dawn = 1 h either side of sunrise; day = from 2 h after sunrise to 2 h before sunset; dusk = 1 h either side of sunset; night = from 2 h after sunset to 2 h before sunrise. (B) Mean excess (± s.e.) of flower surface temperature over air temperature in sunlit and shaded flowers of each genotype, measured by hand (50 flowers of each genotype oriented towards the sun, data pooled across three dates: 7 August, 6 September and 1 November). (C) Rate of warming of flowers of different genotypes in controlled conditions: the increase in temperature for each genotype between temperature at the time shown and the minimum temperature when not illuminated (at 15:10) (means ± s.e.). Sixteen individual flowers were set out in a randomized block in a growth chamber, together with four fine thermocouples, and a light meter. Intrafloral temperatures were monitored using custom-made fine (0·05 mm) Type K thermocouples and a datalogger. Background temperature in the growth chamber was set to 12 °C. Flowers were left undisturbed under illumination for 2 h. Lights were then turned off for 5 min. The rate of increase in temperature inside each individual flower was calculated over the same 13 min time interval after lights were turned on again. Filled symbols, pigmented flowers (Nv/Nv); open symbols, white flowers (nv/nv). Triangles, conical-celled flowers (Mx/Mx); squares, flat-celled flowers (mx/mx).
On three separate sunny mornings we measured the surface temperature excess of conical- and flat-celled flowers, choosing flowers facing directly towards the sun, and recording the sun/shade status of the flower at the time. Temperatures were recorded using a custom-made fine (0·05 mm) Type K (chrome/alumel) thermocouple touching the petal surface, and then of the air 2 cm to the right. Figure 2B shows the mean floral surface temperature excess (floral surface temperature minus local air temperature) of 50 flowers of each of the conical-celled wild-type Antirrhinum and flat-celled mixta mutant Antirrhinum lines. There was a small difference in temperature excess between conical- and flat-celled flowers (ANCOVA, blocked by date of reading: F1,98 = 3·4: P = 0·067), but the sunlit/shaded status of the flower was clearly the major factor (F1,98 = 31·6; P <0·001). We conclude that conical petal cells may have an effect on floral surface temperature in some conditions, but that the effect is small.
To avoid field variability, we attempted to simulate dawn under controlled conditions and compare intrafloral temperatures. Sixteen individual flowers, four each from plants of the four genotypes Mx/Nx (magenta, conical-celled), mx/Nv (magenta, flat-celled), mx/Nv (white, conical-celled) and mx/nv (white, flat-celled) were set out in a randomized block in a growth chamber (all lines as described in Glover and Martin, 1998). Figure 2C shows the mean temperature within the corolla of flowers of each of the four lines when the lights were switched on after a period of dark. The only statistically significant difference was between the warming rate of pigmented (Nv) and white (nv) flowers, with pigmentation significantly increasing the rate of floral warming [one-way analysis of variance (ANOVA): d.f. = 3; F = 31·05; P <0·001]. A Tukey test on all pairwise comparisons showed significant differences at the 95 % confidence level between genotypes differing at the NIVEA locus, but not between genotypes differing at the MIXTA locus.
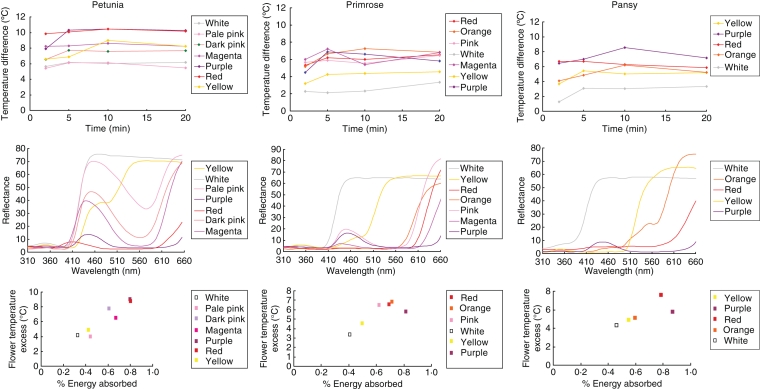
To relate the observed small effect of cell shape on temperature to the potential warming effects of pigment (Kevan, 1975; Molgaard, 1989; Jewell et al., 1994; McKee and Richards, 1998) reflectance spectra were obtained for each of six Viola × wittrockiania, seven Petunia grandiflora and seven Primula flower colour morphs (Supplementary Data Table S1, available online) with an Ocean Optics (Dunedin, FL, USA) spectrophotometer (S2000) relative to a white reflection standard (Fig. 3A). The reflectance spectra were subsequently analysed to estimate the amount of energy absorbed by each flower. This was done by calculating the percentage reflection at each wavelength for each flower, using the spectrophotometer light source as the white standard. Light reflected is not available to be absorbed and so provides a relative indicator of energy absorption. Four flowers of each colour morph were placed in random positions in a block in a controlled environment room. Boxes of flowers were refrigerated for 10 min and then returned to the bench. A K type thermocouple was used to measure the surface temperature at two independent positions on one petal over the following 20 min. Air temperature was also recorded. All measurements were repeated three times.
Fig. 3.
Comparison of recorded flower temperature excess (temperature difference) and reflectance data (reflectance) gathered for each colour morph of pansy (Viola × wittrockiania), petunia (Petunia grandiflora) and primrose (Primula). (A) The ‘flower temperature excess’ (the positive difference between flower temperature and air temperature) for each line of each of the three species tested. (B) The reflectance spectra for each line. (C) Estimate of energy absorbed (made by calculating the percentage reflection at each wavelength) plotted against flower temperature excess for each line. For all three species, floral temperature increased with the amount of energy absorbed (Pearson correlation coefficients: petunia, r = +0·947, t5 = 6·56, P = 0·001; primrose, r = +0·815, t4 = 2·81, P = 0·048; pansy, r =+ 0·759, t3 = 2·02, P = 0·137).
Figure 3A shows the ‘flower temperature excess’ (the positive difference between flower temperature and air temperature) for each line of each of the three species, with the reflectance spectra shown below (Fig. 3B). A two-way ANOVA with repeated measures demonstrated that there was a significant effect of flower colour on flower temperature excess in P. grandiflora (d.f. = 24, F.pr < 0·01), Viola × wittrockiania (d.f. = 27, F.pr < 0·01) and Primula (d.f. = 21, F.pr = 0·013).
When the reflectance spectra obtained were analysed to estimate the amount of energy absorbed by calculating the percentage reflection at each wavelength for each flower line, it was found in all three species that there was a correlation between our estimate of the amount of energy available to be absorbed and the subsequent floral temperature (Fig. 3C). There was a clear trend in all three species for petals with lighter colours to be cooler than those with darker colours. Red, orange and purple flowers were generally the warmest. The difference in floral warming conferred by petal colour was as much as 6 °C, considerably more than the differences observed between conical-celled and flat-celled flowers. Overall, we conclude that the possession of pigments which capture light results in the capture of heat as well, substantially warming flowers relative to air temperature. This warming effect is considerably greater than the limited effect achieved by epidermal cell shape. This suggests that moderation of floral temperature is unlikely to be a significant factor in the production of conical petal epidermal cells by most plant species.
SCENT PRODUCTION IS NOT DIRECTLY AFFECTED BY CELL SHAPE
The idea that scent production might be influenced by petal epidermal morphology stems from two main sources. First, analysis of the site and mechanisms of volatile production in the petals of Antirrhinum and petunia has demonstrated that the adaxial epidermis, i.e. the one consisting of conical cells, is the main site of activity of the enzymes that synthesize floral scent (Dudareva and Pichersky, 2000; Kolosova et al., 2001). It is therefore plausible that change to the shape or fate of those cells would influence scent production, although we note that in other species, such as rose, both conical adaxial and flat abaxial petal epidermal cells produce scent (Bergougnoux et al., 2007). Secondly, our observations that epidermal morphology could influence floral temperature might also suggest an effect on scent, since temperature will affect volatilization of key scent components.
Whitney et al. (2009a) used a gas chromatography–mass spectrometry (GC-MS) analysis to demonstrate that there was no difference between the wild type and the mixta mutant line in the types, ratios or amounts of the different components of Antirrhinum floral scent. Changing epidermal shape does not appear to influence the synthesis of scent compounds. However, it remains possible that small temperature differences do influence the release of volatiles under particular conditions, and it is also possible that the change in cell shape affects the shape of the dispersal plumes. These effects are very difficult to test experimentally, and are also unlikely to be significant in the maintenance of the conical form by the great majority of Angiosperm species.
FLOWER WETTABILITY IS REDUCED BY CONICAL CELLS
The extent to which a tissue is hydrophobic, causing water to bead and roll off, can have enormous consequences for its function. In a series of classic papers, Barthlott and Neinhaus (1997) showed that the leaves of the Sacred Lotus, Nelumbo nucifera, were superhydrophobic, resulting in the removal not only of any water that contacted the leaf, but also of dirt particles that were removed by the water. This self-cleaning effect was named the Lotus effect, and was shown to be caused by the papillate shape of the leaf epidermal cells of this aquatic plant (Neinhaus and Barthlott, 1997).
Perhaps unsurprisingly, then, analysis of wettability of the wild type and mixta mutant lines of Antirrhinum also generated a significant difference. Flat-celled petals were generally more wettable than conical-celled petals, with the wild-type petals showing some evidence of patchy superhydrophobicity (Whitney et al., 2011). This phenomenon has the potential to influence reproductive success in a number of ways. First, wet tissues do not hold their shape well, and may be unattractive to pollinators. Secondly, reduced wettability may limit the extent to which water films interfere with gas exchange in those petals with stomata (Smith and McClean, 1989; Brewer and Smith, 1995). Thirdly, if superhydrophobicity is associated with a self-cleaning effect it is possible that conical cells aid in the removal of display-reducing dirt particles or potentially infectious fungal spores or virus particles. Fourthly, it is also possible that self-cleaning is important in removing insect scent marks. Many pollinators leave short-lived scent marks on flowers; these scent marks indicate to pollinators that the flowers have been recently visited and therefore emptied of nectar (Goulson et al., 2000; Saleh et al., 2006, 2007). The ability to remove such marks might be significant in increasing the pollinator visitation rate, although such an effect is only likely to be in play in the rain or at dawn, times when foraging by most pollinators is much reduced anyway. Finally, it is also possible that superhydrophobic properties ensure that water drops roll off the petal before dew or rain can dilute the nectar. Dilute nectar would significantly decrease the attractiveness of a flower to foraging pollinators. While any of these effects might be biologically significant in specific instances, their general reliance on pollinator foraging during or immediately after substantial rainfall makes them unlikely to be very important in the majority of habitats.
CONICAL CELLS AID POLLINATOR GRIP ON THE FLOWER
The possibility that petal epidermal cell morphology might provide tactile cues for pollinators was first proposed by Kevan and Lane (1985), who showed that bees could be trained to recognize epidermal surfaces of different species or different plant organs by touch alone. Tactile cues might operate through the antennae, or through the feet of insects after landing. Kevan and Lane (1985) proposed that this ability might allow pollinators to orient themselves on the petal and might therefore function as a tactile nectar guide.
Our recent work has shown that bumble-bees can discriminate conical- and flat-celled petals by touch alone, that conical epidermal cells provide grip on the otherwise slippery petal surface and that bumble-bee preference for conical epidermal cells increases as the flower becomes more difficult to manipulate (Whitney et al., 2009a, b). Using epoxy replicas of conical- and flat-celled petals, to remove any confounding effects of temperature, scent or colour, we used differential conditioning (Dyer and Chittka, 2004) to teach bees to associate a reward with a flat-celled surface but a punishment (bitter-tasting quinine) with a conical-celled surface. Bees learned very quickly not to drink from the cup associated with the conical-celled surface, showing that they could distinguish these visually identical surfaces through touch alone. We found that bees had no preference for either epoxy surface when both contained a sucrose reward and were offered at a horizontal angle, but if we made the disks harder to handle by presenting them vertically, then bees showed a highly significant preference for the conical-celled disks. Colour coding the two disk types with different pigments enabled the bees to learn that one colour was easier to handle than the other, and they then used the colour as a cue to visit the conical-celled flowers preferentially. When we explored the same effect with Antirrhinum flowers we found a similar result – presenting the flowers in an easy to handle fashion, with the mouth held open, removed the preference for the conical-celled line. These observations provide an explanation for the data of Comba et al. (2000), who observed in field trials that bees rejected flat-celled flowers both before landing (presumably having learned to associate the visible colour difference of the two Antirrhinum lines with the difference in grip) and after landing (when the slippery surface of the flat-celled flower proved difficult to manipulate). While an effect on grip might be expected only to influence pollinators handling flowers whose morphology makes them harder to manipulate, such as the zygomorphic Antirrhinum and other personate flowers, it might also be significant in improving grip on structurally simpler flowers growing in windy or wet habitats.
OPTIMIZING PETAL MICROMORPHOLOGY FOR POLLINATOR AND HABITAT
The studies detailed in this review have shown that conical petal epidermal cells do significantly affect flower colour, flower wettability and the tactile handling of the flower by pollinators, have a limited effect on floral temperature and might have an indirect effect on scent dispersal. This range of effects is itself very broad, but becomes broader still when we consider the variation in epidermal cell morphology described in the Introduction and shown in Fig. 1. However, the number of possible combinations of plant species, pollinator species and abiotic habitats in which they interact is greater still, and it is against this diversity of ecological interaction that petal cell function must be considered.
For an Antirrhinum plant growing in a warm, dry temperate habitat and pollinated by nectar-gathering bumble-bees, providing pollinator grip may be the primary role of conical petal cells. However, many other plants experience pollination in very different situations. For a plant flowering in low light conditions the ability to enhance light capture by petal cells may be of more significance, generating a stronger colour signal. For a plant flowering in a tropical rainforest, the ability to shed water before petals become waterlogged might be the most important role of conical cells. Even the limited effects on floral temperature that we report in this study might be of significance in key habitats. Invertebrate pollinators experience significant difficulty in warming their bodies to a sufficient temperature to allow use of their flight muscles (Bishop and Armbruster, 1999). Previous studies have shown that flowers that are warmer than the surrounding air, or that warm up quickly at dawn, can provide a significant metabolic reward to insects, allowing them to begin foraging more quickly than if they had not sheltered in such flowers (Herrara, 1995; Seymour et al., 2003; Sapir et al., 2006; Norgate et al., 2010). In key marginal habitats the small warming effect of conical cells at dawn may be sufficient to explain their maintenance. In these habitats, the altered primary role of the conical cells could lead to an equal selective pressure to maintain their presence but perhaps in a different shape that maximizes their most important function.
This potential trade-off between functions is further emphasized by the variety of surface structures that can be found on individual petals. Here, conical cells are present, potentially to enhance pollinator grip, but surface structures with other functional roles, such as the generation of structural colour, can also be present, often in distinct patterns on the flower (Whitney et al., 2009c, d). The proportion of conical cells to other surface morphologies could depend on the complex selective biotic and abiotic pressures occurring in each habitat.
Over the last 12 years, our studies, and those of other authors, have provided a range of insights into how conical petal epidermal cells might provide selective advantage to the plants that possess them. The challenge for the next decade is to explore the diversity of conical cell form in diverse habitats, developing models and rules to determine how petal epidermal morphology can best fit plants to their individual environments.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Sean Rands for help and advice with statistics, Adrian Dyer for guidance in establishing bee experiments, and the Cambridge University Botanic Garden for access to many plant species. This work was supported by Natural Environment Research Council grant NE/C000552/1. D.P. was supported by a University Research Opportunities Programme studentship.
LITERATURE CITED
- Akpan EEJ, Bean EW. The effects of temperature upon seed development in three species of forage grasses. Annals of Botany. 1977;41:689–695. [Google Scholar]
- Barthlott W, Neinhuis C. The purity of sacred lotus or escape from contamination in biological surfaces. Planta. 1997;202:1–8. [Google Scholar]
- Baumann K, Perez-Rodriguez M, Bradley D, et al. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development. 2007;134:1691–1701. doi: 10.1242/dev.02836. [DOI] [PubMed] [Google Scholar]
- Bergougnoux V, Caissard JC, Jullien F, et al. Both the adaxial and abaxial epidermal layers of the rose petal emit volatile scent compounds. Planta. 2007;226:853–866. doi: 10.1007/s00425-007-0531-1. [DOI] [PubMed] [Google Scholar]
- Bishop JA, Armbruster WS. Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Functional Ecology. 1999;13:711–724. [Google Scholar]
- Brewer CA, Smith WK. Leaf surface wetness and gas exchange in the pond lily Nuphar polysepalum (Nymphaeaceae) American Journal of Botany. 1995;82:1271–1277. [Google Scholar]
- Christensen K, Hansen H. SEM-studies of epidermal patterns of petals in the angiosperms. Opera Botanica. 1998;135:1–91. [Google Scholar]
- Comba L, Corbet SA, Hunt H, Outram S, Parker JS, Glover BJ. The role of genes influencing the corolla in pollination of Antirrhinum majus. Plant, Cell and Environment. 2000;23:639–647. [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiology. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer AG, Chittka L. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften. 2004;91:224–227. doi: 10.1007/s00114-004-0508-x. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Bees associate warmth with floral colour. Nature. 2006;442:525. doi: 10.1038/442525a. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Mutations peturbing petal cell shape and anthocyanin synthesis influence bumblee perception of Antirrhinum majus flower colour. Arthropod-Plant Interactions. 2007;1:45–55. [Google Scholar]
- Elgersma A, Stephenson AG, den Nijs APM. Effects of genotype and temperature on pollen tube growth in perennial ryegrass (Lolium perenne L.) Sexual Plant Reproduction. 1989;2:225–230. [Google Scholar]
- Glover BJ, Martin C. The role of petal cell shape and pigmentation in pollination success in Antirrhinum majus. Heredity. 1998;80:778–784. [Google Scholar]
- Gorton HL, Vogelmann TC. Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiology. 1996;112:879–888. doi: 10.1104/pp.112.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D, Stout JC, Langley J, Hughes WHO. Identity and function of scent marks deposited by foraging bumblebees. Journal of Chemical Ecology. 2000;26:2897–2912. [Google Scholar]
- Herrera CM. Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology. 1995;76:218–228. [Google Scholar]
- van Houwelingen A, Souer E, Spelt K, Kloos D, Mol J, Koes R. Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida. Plant Journal. 1998;13:39–50. doi: 10.1046/j.1365-313x.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- Irish V. Evolution of petal identity. Journal of Experimental Botany. 2009;60:2517–2527. doi: 10.1093/jxb/erp159. [DOI] [PubMed] [Google Scholar]
- Jewell J, McKee J, Richards AJ. The keel colour polymorphism in Lotus corniculatus L.: differences in internal flower temperatures. New Phytologist. 1994;128:363–368. doi: 10.1111/j.1469-8137.1994.tb04020.x. [DOI] [PubMed] [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society. 1981;83:57–84. [Google Scholar]
- Kevan PG. Sun-tracking solar furnace in high arctic flowers: significance for pollination and insects. Science. 1975;189:723–726. doi: 10.1126/science.189.4204.723. [DOI] [PubMed] [Google Scholar]
- Kevan PG, Lane MA. Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Sciences, USA. 1985;82:4750–4752. doi: 10.1073/pnas.82.14.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Sherman D, Karlson D, Dudareva N. Cellular and subcellular localization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiology. 2001;126:956–964. doi: 10.1104/pp.126.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee J, Richards AJ. Effect of flower structure and flower colour on intrafloral warming and pollen germination and pollen-tube growth in winter flowering Crocus L. (Iridaceae) Botanical Journal of the Linnean Society. 1998;128:369–384. [Google Scholar]
- Mølgaard P. Temperature relations of yellow and white flowered Papaver radicatum in North Greenland. Arctic and Alpine Research. 1989;21:83–90. [Google Scholar]
- Neinhuis C, Barthlott W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Annals of Botany. 1997;79:667–677. [Google Scholar]
- Noda KI, Glover BJ, Linstead P, Martin C. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature. 1994;369:661–664. doi: 10.1038/369661a0. [DOI] [PubMed] [Google Scholar]
- Norgate M, Boyd-Gerny S, Simonov V, Rosa MGP, Heard TA, Dyer AG. Ambient temperature influences Australian native stingless bee (Trigona carbonaria) preference for warm nectar. PLoS ONE. 2010;5:e12000. doi: 10.1371/journal.pone.0012000. doi:10.1371/journal.pone.0012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda I, Francisco-Ortega J, Cronk QC. Evolution of petal epidermal micromorphology in Leguminosae and its use as a marker of petal identity. Annals of Botany. 2009;104:1099–1110. doi: 10.1093/aob/mcp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands SA, Whitney HM. Floral temperature ad optimal foraging: is heat a feasible floral reward for pollinators? PLos ONE. 2008;3:e2007. doi: 10.1371/journal.pone.0002007. doi:10.1371/journal.pone.0002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh N, Ohashi K, Thomson JD, Chittka L. Facultative use of the repellent scent mark in foraging bumblebees: complex versus simple flowers. Animal Behaviour. 2006;71:847–854. [Google Scholar]
- Saleh N, Scott AG, Bryning GP, Chittka L. Distinguishing signals and cues: bumblebees use general footprints to generate adaptive behavior at flowers and nest. Arthropod-Plant Interactions. 2007;1:119–127. [Google Scholar]
- Sapir Y, Shimida A, Néeman G. Morning floral heat as a reward to the pollinators of the Oncocyclus irises. Oecologia. 2006;147:53–59. doi: 10.1007/s00442-005-0246-6. [DOI] [PubMed] [Google Scholar]
- Seymour RS, White CR, Gibernau M. Heat reward for insect pollinators. Nature. 2003;426:243–244. doi: 10.1038/426243a. [DOI] [PubMed] [Google Scholar]
- Smith WK, McClean TM. Adaptive relationship between leaf water repellency, stomatal distribution and gas exchange. American Journal of Botany. 1989;76:465–469. [Google Scholar]
- Spaethe J, Chittka L. Interindividual variation of eye optics and single object resolution in bumblebees. Journal of Experimental Biology. 2003;206:3447–3453. doi: 10.1242/jeb.00570. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumble bees: flower size and colour affect search time and flight behavior. Proceedings of the National Academy of Sciences, USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney HM, Dyer AG, Chittka L, Rands SA, Glover BJ. Flower temperature can act as both cue and reward for bumblebees. Naturwissenschaften. 2008;95:845–850. doi: 10.1007/s00114-008-0393-9. [DOI] [PubMed] [Google Scholar]
- Whitney H, Chittka L, Bruce T, Glover BJ. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Current Biology. 2009a;19:1–6. doi: 10.1016/j.cub.2009.04.051. [DOI] [PubMed] [Google Scholar]
- Whitney H, Federle W, Glover BJ. Grip and slip: mechanical interactions between insects and the epidermis of flowers and flower stalks. Communicative and Integrative Biology. 2009b;2:505–508. doi: 10.4161/cib.2.6.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney H, Kolle M, Andrew P, Chittka L, Steiner U, Glover BJ. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science. 2009c;323:130–133. doi: 10.1126/science.1166256. [DOI] [PubMed] [Google Scholar]
- Whitney H, Kolle M, Alvarez-Fernandez R, Steiner U, Glover BJ. Contributions of iridescence to floral patterning. Communicative and Integrative Biology. 2009d;2:230–232. doi: 10.4161/cib.2.3.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney H, Poetes R, Steiner U, Chittka L, Glover BJ. Determining the contribution of epidermal cell shape to petal wettability using isogenic Antirrhinum lines. PLOS ONE. 2011 doi: 10.1371/journal.pone.0017576. 6(3): e17576. doi:10.1371/journal.pone.0017576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienand U, Sommer H, Schwarz-Sommer Z, et al. A general method to identify plant structural genes among genomic DNA clones using transposable element induced mutations. Molecular and General Genetics. 1982;187:195–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.