Abstract
Background and Aims
During sexual reproduction in higher angiosperms, the pollen tubes are directed to the ovules in the pistil to deliver sperm cells. This pollen tube attraction is highly species specific, and a group of small secreted proteins, TfCRPs, are necessary for this process in Torenia fournieri.
Methods
A candidate pollen tube attractant protein in Torenia concolor, a related species of T. fournieri, was isolated and the attractant abilities between them were compared.
Key Results
TcCRP1, an orthologous gene of TfCRP1 from T. concolor, is expressed predominantly in the synergid cell. The gene product attracted pollen tubes in a concentration-dependent manner, but attracted fewer pollen tubes from the other species.
Conclusions
The results indicated that this class of CRP proteins is a common pollen tube attractant in Torenia species. The sequence diversity of these proteins is important for species-specific pollen tube attraction.
Keywords: Torenia fournieri, T. concolor, sexual reproduction, TcCRP1, fertilization, pollen tube guidance, synergid cell, defensin, cysteine-rich polypeptide, CRP, speciation
INTRODUCTION
Higher angiosperms have evolved a unique fertilization system. Unlike gymnosperms, the sperm cells of angiosperms do not have the ability to move long distances. Instead, the pollen tube carries the sperm cells to the female embryo sac for fertilization. When pollen grains are deposited on the female stigma, they hydrate and pollen tubes grow to penetrate into the female tissue. Pollen tubes continue to grow inside the style and, once they reach to the ovary, they change their growth direction toward the ovule. The pollen tube enters into the embryo sac from the micropyle of the ovule, and bursts to release sperm cells. The growth direction of pollen tubes must be tightly and precisely regulated throughout this long journey (Higashiyama, 2010).
After the finding of the pollen tube, attempts have been made to identify the chemoattractants that determine pollen tube growth orientation in the female pistil. In classic studies, calcium ions were first thought to be a candidate for the attraction molecule, because a calcium concentration gradient could be formed to attract the pollen tube through the female tissue (Mascarenhas and Machlis, 1962). However, as external calcium ions are required for pollen tube tip growth, and the growth stimulation and the change in growth orientation of pollen tubes were not distinguished in this system, whether attractant molecules really exist was not determined (Higashiyama and Hamamura, 2008).
Based on the genetic analysis of Arabidopsis thaliana, several mutants defective in the tube attraction were identified. In an analysis of plants lacking embryo sacs, pollen tubes were found to be able to growth inside the ovary but could not direct themselves toward the ovule (Hulskamp et al., 1995; Ray et al., 1997). Pollen tubes could reach to the funiculus of the ovule, but could not find the micropyle and failed to fertilize in magatama mutants defective in the development of female gametophytic cells, suggesting the involvement of the embryo sac for guidance (Shimizu and Okada, 2000).
The importance of the embryo sac for pollen tube attraction has also been shown during the in vitro fertilization of Torenia fournieri (Higashiyama et al., 1998). When synergid cells were ablated by UV laser, pollen tubes were not attracted to the ovule, suggesting that synergid cells secrete attractants (Higashiyama et al., 2001; Higashiyama, 2002). The necessity of synergid cells for attraction was also shown by the identification of an arabidopsis transcription factor, MYB98, which is predominantly expressed in synergid cells and whose mutation caused a defect in micropylar pollen tube attraction (Kasahara et al., 2005). The synergid cell is also required for pollen tube rupture and sperm cell release into the embryo sac (Huck et al., 2003; Rotman et al., 2003; Escobar-Restrepo et al., 2007).
A key feature of pollen tube attraction is that it is species-specific. When ovules from two related species are placed on a medium close enough to cause interference of signals from each ovule, pollen tubes are attracted to the ovules from the same species. Adding excess calcium ions to the medium does not perturb the attraction, suggesting that the attractant is not a simple universal molecule such as calcium, but rather a more complex molecule that can vary even in closely related species (Higashiyama et al., 2006).
Recently, a family of secreted proteins that have the ability to attract pollen tubes was discovered (Okuda et al., 2009). Using an expressed sequence tag analysis of cDNAs from the isolated synergid cells of T. fournieri, it was found that genes encoding cysteine-rich polypeptides (CRPs) are abundantly expressed in the synergid cells. Among them, TfCRP1 (LURE1) and TfCRP3 (LURE2) proteins attracted pollen tubes in vitro. Down-regulation of TfCRP1 and TfCRP3 caused less attraction of the pollen tubes to the ovule in vitro. Based on these results, it was concluded that TfCRP1 and TfCRP3 are pollen tube attractants responsible for micropylar attraction in Torenia (Okuda et al., 2009; Goto et al., 2011).
These pollen tube attractants possess six cysteine residues, a cysteine-stabilized α-helix β-sheet (CSαβ) motif and a γ-core motif, which are typical molecular features of antimicrobial defensin proteins (Cornet et al., 1995; Thomma et al., 2002; Yount and Yeaman, 2004; Yount et al., 2007). Another defensin-like protein from maize, ZmES4, is involved in pollen tube rupture, suggesting the importance of this family of protein not only for defence response but also for reproduction (Amien et al., 2010). Rapid sequence divergence is also a feature of defensin proteins (Maxwell et al., 2003; Patil et al., 2004), thus these pollen tube attractants were thought to be good candidates for species-specific pollen tube guidance.
In this study an orthologous gene of TfCRP1 was isolated from Torenia concolor and named TcCRP1. TcCRP1 was expressed exclusively in the synergid cells. Purified TcCRP1 protein attracted T. concolor pollen tubes in vitro. It attracted T. fournieri pollen tubes less frequently, suggesting that the diversification of CRP1 proteins among species may contribute to species-specific pollen tube attraction.
MATERIALS AND METHODS
Plant materials, crosses and pollen tube observations
Torenia fournieri ‘Blue and White’ and T. concolor were grown in a plant growth room or in a chamber under the ambient conditions. Because both T. fournieri and T. concolor are self-fertilizing and do not auto-pollinate, emasculation was not needed before the crossing experiment. Fully opened flowers were hand-pollinated with pollen grains from the same species or other species. The pistils were collected 24 h later and fixed in the fixation solution (ethanol : acetic acid = 9 : 1) overnight. Subsequently they were rehydrated with 90 % ethanol and 70 % ethanol, respectively, for 20 min. After rehydration, they were kept in 1 n NaOH solution overnight, then stained with 1 % aniline blue solution dissolved in 0·1 m K3PO4 (pH 12·4) for >1 h. They were mounted in 50 % glycerol and observed under an IX71 fluorescence microscope (Olympus, Tokyo, Japan).
For the pollen tube elongation experiment, hand-pollinated pistils were cut at the junction between the style and ovary and placed on the pollen tube growth medium. Pollen tubes emerged from the cut end of the style approx. 8 h after pollination. The pistils were dissected 24 h after pollination to pick up the ovules and to monitor fertilization in vivo. Pistils were mounted on 0·12 m sorbitol and observed under a BX51 microscopy (Olympus) to determine whether the pollen tube entered into the embryo sac.
Isolation of synergid cells and egg cells
Flowers 2 d after maturation were used as material to isolate synergid cells. Pistils were dissected and carpel walls were removed to take ovaries. The ovaries were sunk in the 300 µL of enzyme solution [1 % (w/v) cellulase (Worthington), 0·3 % macerozyme RS (Yakult), 0·05 % pectlyase Y-23 (Yakult), 5 mm CaNO3, 0·4 m mannitol] for 1 h at 28 °C. Synergid and egg cells separated from ovule tissues were distinguished by their morphology, captured in the pico pipette (Altair, Japan), pooled separately by cell type, and washed with 100 µL of wash solution (CaNO3, 0·4 m mannitol) three times. Cells were kept frozen until being used.
RNA isolation, the cloning of TcCRP1 and RT-PCR
The mRNA from isolated synergid cells and egg cells was extracted using RNAquous-Micro (Ambion, Austin, TX, USA) with Plant RNA Isolation Aid (Ambion). The RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) was used for the remaining tissues. cDNA was synthesized using SuperScript® reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The expression of cell-type specific marker genes in these mRNAs (Kawano et al., 2011) was confirmed to clarify that no contamination of other cell types in the pool of synergid cell mRNA and egg cell mRNA. TcCRP1 was first isolated from ovule cDNA by PCR using gene-specific degenerate primers (Supplementary Data Table S1, available online). Full-length cDNA was isolated by 5′- and 3′- RACE-PCR (GENE RACER Kit; Invitrogen) and the sequence was confirmed. The intron sequence was determined from the genomic sequence. Thirty-five-cycles of RT-PCR were conducted using cDNA from sporophytic tissues as templates, and 50 cycles of RT-PCR were performed using cDNA from synergid cells and egg cells as templates. The full-length TcCRP1 sequence including the intron was deposited to the GenBank under the Accession No. AB594190.
Alignment and phylogenetic analyses of TfCRPs and TcCRP1
Signal sequences were predicted using SignalP 3·0 software (Bendtsen et al., 2004). Mature CRP proteins were aligned using the ClustalX version 2·0 algorithms (Larkin et al., 2007) and modified manually. A phylogenetic tree was generated using the neighbor–joining method (Saitou and Nei, 1987) in Clustal X.
Construction
His-tagged TfCRP1 was constructed as previously reported (Okuda et al., 2009). TcCRP1 cDNA corresponding to its mature peptide encoding region was amplified using the following primer set and subcloned into the pBluescriptII SK (+) vector (Toyobo, Osaka, Japan):
forward primer: 5′-CACCGGATCCGGTCAAATTCCACCT GAG-3′
reverse primer: 5′-CTCGAGTTATTTCTTTTTCTTTTCA GAAC-3′
The underlines represent the BamHI recognition sequence in the forward primer and the XhoI recognition sequence in the reverse primer, respectively. After subcloning, The BamHI and XhoI recognition sequences were cleaved using BamHI and XhoI restriction enzymes and then ligated into the pET28a vector in Escherichia coli BL21 cells to generate His-tagged TcCRP1.
Protein purification and refolding
One and a half litres of BL21 E. coli culture cells expressing His-tagged TfCRP1 or TcCRP1 proteins were collected 24 h after the induction of protein expression. Protein purification and refolding was done as described previously (Okuda et al., 2009). Refolding reagents were removed from the protein solution [50 mm Tris–HCl (pH 8·0) buffer] by dialysis.
Pollen tube attraction assay
Purified proteins ware mixed with Alexa488-conjugated dextran or Alexa546-conjugated dextran and vortexed with 5 % (w/v) gelatin to generate gelatin beads containing these proteins. Pollen tube culture and the procedure of the in vitro pollen tube attraction assay were as described previously (Okuda et al., 2009).
Estimation of the Ka/Ks rate
The nucleotide sequences encoding the signal peptide and mature polypeptide in TfCRP1 and TcCRP1 were subjected to counting the ratio of nonsynonymous substitutions/ nonsynonymous sites (Ka) and synonymous substitutions/synonymous sites (Ks) using DnaSP version 5·0 (Librado and Rozas, 2009).
RESULTS
Flower structure and pollen tube elongation in the style of Torenia species
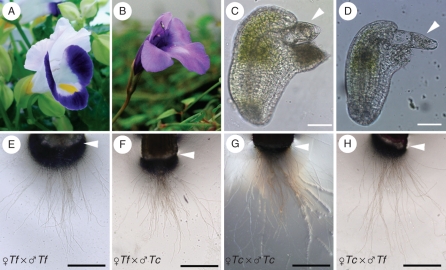
Initially pollen tube elongation in the style and attraction to the ovules were compared in Torenia species to investigate pollen tube guidance. Torenia concolor was used as a partner for the crossing experiment, because it has a similar floral structure to T. fournieri (Fig. 1A, B). Similar to T. fournieri, the embryo sac of T. concolor was protruded from the ovule (Fig. 1C, D), and the synergid cells, the egg cell and a part of the central cell could be easily observed under the microscope.
Fig. 1.
Flower morphology and pollen tube attraction in Torenia species: (A, B) flowers of (A) T. fournieri and (B) T. concolor; (C, D) protruding embryo sac of (C) T. fournieri and (D) T. concolor; (E–H) pollen tubes emerging from the cut end of the style (arrowheads). The cross combinations (female × male) are indicated: Tf = Torenia fournieri; Tc = T.concolor. Scale bars: (C, D) = 50 µm; (E–H) = 200 µm.
It has been previously observed that pollen tubes grow through the style, enter the ovary and fertilize the ovule within 24 h after intra- and interspecific pollination in T. fournieri and T. concolor (Higashiyama et al., 2006). However, the time needed for pollen tubes to grow through the style was not clear. Some secreted molecules, such as chemocyanins, function in chemotropic attraction of pollen tubes elongating in the style of lilies (Kim et al., 2003). Self- and interspecies crossing experiments between T. fournieri and T. concolor were performed because it was necessary to adjust the culture periods for the in vitro pollen tube attraction assay and we wanted to clarify whether pollen tubes could grow through the style of the other species at the same time. After hand pollination, the pistils were cut at the junction of the style and the ovary, and the styles were placed in a pollen tube germination medium to observe pollen tube emergence from the cut end of the style (Fig. 1E–H). When the stigma of T. fournieri was pollinated with T. fournieri pollen grains, pollen tubes did not emerge from the cut end of the style at the time point of 6 h after pollination, but instead emerged from the other end of the style 8 h after pollination, and the tubes continued growing on the medium (Fig. 1E). When the style of T. concolor was pollinated with T. fournieri pollen grains, the pollen tubes emerged from the cut end of the style 8 h after pollination, the same timing as the self-pollination of T. fournieri (Fig. 1F). Similarly, both T. concolor and T. fournieri pollen tubes emerged from the cut end of T. concolor style 8 h after pollination (Fig. 1G, H). These results suggest that the elongation of the pollen tube was not affected by the plant species, and that the speed of growth of the pollen tubes and the timing to enter the ovary were the same in T. fournieri and T.concolor.
Interspecies cross between T. fournieri and T. concolor revealed less pollen tube attraction to ovules from different species
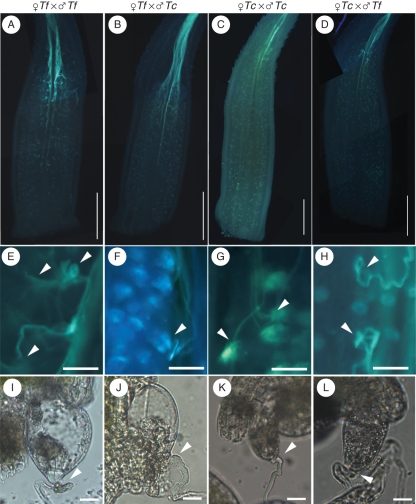
It has previously been observed that reduced penetration of pollen tubes into the embryo sac occurs during cross pollination (Higashiyama et al., 2006). When T. fournieri pistils were self-pollinated, all the ovules accepted pollen tubes, while only 4·5 % of the ovules accepted pollen tubes when T. concolor pollen grains were used. When T. concolor pistils were self-pollinated, 80·3 % of the ovules accepted pollen tubes, while only 18·9 % of the ovules accepted pollen tubes when T. fournieri pollen grains were used (Higashiyama et al., 2006). To determine how these pollen tubes behaved in vivo and when defects in tube guidance were occurred, pollen tube elongation was observed in vivo in the ovary (Fig. 2). Stigmas of T. fournieri and T. concolor were pollinated with either self- or cross-pollen grains and observed 24 h after pollination. As the result, pollen tubes grew normally in the style in all the self- and cross-pollination experiments. During self-crossing of T. fournieri, the pollen tubes changed their growth orientation toward the ovules upon arriving at the top part of the ovary (Fig. 2A, E). All of the pollen tubes that came close to the ovule were trapped at the entrance of the protruding embryo sac, suggesting that they entered and fertilized the ovule (Fig. 2I). When T. concolor pollen grains were crossed with the T. fournieri stigma, the pollen tubes grew normally and entered the ovary (Fig. 2B). However, most of the pollen tubes did not change their direction to the ovule (Fig. 2F), and only a few ovules with pollen tubes at the micropylar end were observed (Fig. 2J), suggesting that they did not precisely attract the pollen tubes. In the cross of T. fournieri pollen grains with T. concolor, fewer pollen tubes were directed toward the ovule compared with the T. concolor self-cross (Fig. 2K, L). These results suggest that short-range pollen tube guidance, from the ovary to the ovule, is important for precise fertilization among Torenia species.
Fig. 2.
In vivo pollen tube growth and attraction to the ovule: aniline blue staining of (A–D) pollen tubes in the ovary and (E–H) pollen tubes attracted to the ovules (arrowheads). (I–L) DIC images of ovules attracting pollen tubes. The cross combinations (female × male) are indicated above each column of images: Tf = Torenia fournieri; Tc = T.concolor. Scale bars: (A–D) = 1 mm; (E–H) = 100 µm; (I–L) = 20 µm.
Isolation of TcCRP1, an orthologue of TfCRP1, and its synergid-specific expression
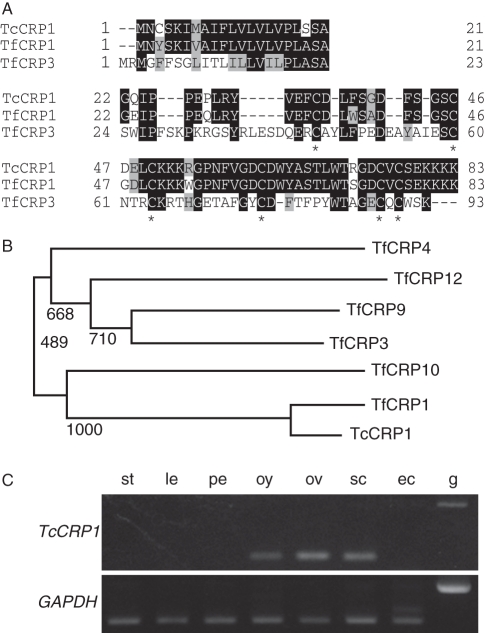
In vivo crossing experiments suggested that short-range guidance is critical for species-specific pollen tube attraction in T. fournieri and T. concolor. Previously TfCRP1 (LURE1) and TfCRP3 (LURE2) proteins had been isolated as pollen tube guidance molecules secreted from the synergid cells (Okuda et al., 2009). Because TfCRP1 and TfCRP3 function as relatively short-range guidance cues within a 200 µm diameter, they were considered as good candidates for species-specific pollen tube guidance molecules. To investigate whether pollen tube attraction in T. concolor is regulated by these proteins or not, the orthologous genes of TfCRP1 and TfCRP3 were isolated from the cDNA of T. concolor ovules. Using gene-specific and degenerated primers, genes with similarity to TfCRP1 were amplified; however, it was not possible to amplify that of TfCRP3. RACE-PCR and PCR with genomic DNA revealed total sequence similarity and gene structure between TfCRP1 and the amplified fragment, therefore it was named TcCRP1 (Supplementary Data Fig. S1, available online). The predicted TcCRP1 protein had a signal peptide at its N-terminus, and the mature TcCRP1 protein had high sequence similarity with TfCRP1, rather than TfCRP3 (Fig. 3A). A phylogenetic analysis of group1 DEFL CRPs (Okuda et al., 2009) and TcCRP1 showed that TcCRP1 was closest to TfCRP1 within the group (Fig. 3B). All cysteine residues, necessary for intramolecular disulfide-bond formation and tertial structure of CRP molecules (Thomma et al., 2002), are conserved among TfCRP1 and TcCRP1, although eight of 62 amino acid residues in the mature protein were different between them (Fig. 3A). RT-PCR analysis revealed that TcCRP1 was expressed in the ovule-containing tissues and strongly expressed in synergid cells but not egg cells (Fig. 3C). These results suggest that TcCRP1 has similar molecular features to TfCRP1 so we thought it was a good pollen tube attractant candidate in T. concolor.
Fig. 3.
Amino acid sequence of TcCRP1 and TcCRP1 expression. (A) Multiple alignments of TcCRP1, TfCRP1 and TfCRP3 proteins. Sequences in the top row represent signal sequences, and those in the middle and bottom rows represent mature proteins. Conserved amino acids in all three sequences are shown in black boxes, and in two of the three are shaded. Conserved cysteine residues have asterisks. (B) Phylogenetic analysis of group 1 (LURE-like) CRPs. Bootstrap values are shown on each branch. (C) RT-PCR of TcCRP1. GAPDH served as a control. Abbreviations: st, style; le, leaf; pe, petal; oy, ovary; ov, ovules; sc, synergid cells; ec, egg cells; g, genomic DNA.
Concentration-dependent pollen tube attraction activity of TcCRP1 against the T. concolor pollen tube
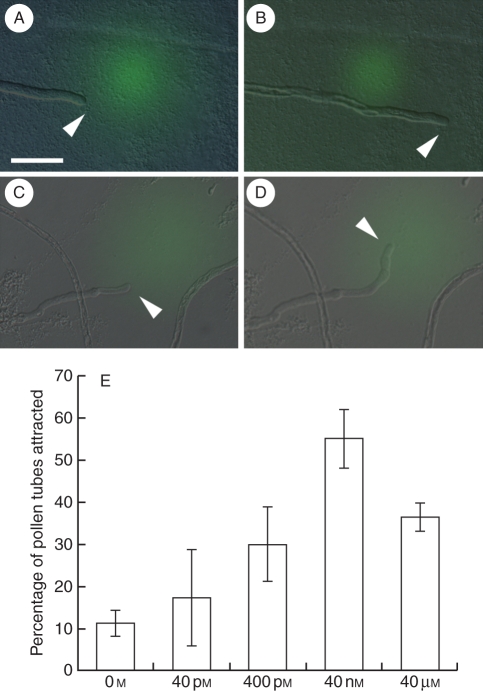
Next tests were done to find out if TcCRP1 could attract T. concolor pollen tubes. Recombinant His-tagged TcCRP1 protein was expressed in E. coli and purified in an Ni-column. After refolding, the protein was embedded in the gelatin beads and placed in front of pollen tubes grown through the style. Generally, pollen tubes on solid agarose medium grow slightly wavy. In fact, when the beads that contain buffer only were used, most of the pollen tubes did not change their direction of growth, but 11·1 % of the pollen tubes did due to this random waving growth (Fig. 4A, B). When the beads that contained 40 nm of TcCRP1 protein were used, 55·1 % of the pollen tubes turned to the beads (Fig. 3C, D). This pollen tube attraction by TcCRP1 decreased when a lower protein concentration was tested, suggesting that the attraction was concentration-dependent (Fig. 3E). When 40 µm of TcCRP1 protein was tested, pollen tube attraction decreased to 36·4 %, suggesting that a high concentration of attractant molecules affects recognition of the attractant concentration gradient from the signal source. These results suggest that TcCRP1 functions as a pollen tube attractant in T. concolor in a concentration-dependent manner.
Fig. 4.
Pollen tube attraction by TcCRP1: (A, B) T. concolor pollen tube just after (A) and 20 min after (B) applying assay buffer only (green); (C, D) T. concolor pollen tube just after (C) and 20 min after (D) applying TcCRP1 protein (green). Arrowheads indicate the pollen tube tip. (E) Concentration-dependent pollen tube attraction by TcCRP1. Data are means ± s.d. (n > 3 with >10 pollen tubes per replicate). Scale bar = 50 µm.
Species-specific pollen tube attraction by CRPs
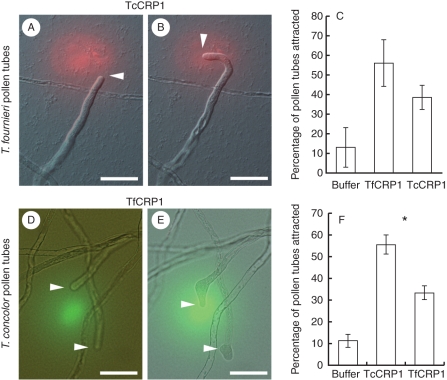
Defensin-like CRP proteins often have divergent amino acid sequences between orthologues (Thomma et al., 2002). Because eight amino acid substitutions occur between TfCRP1 and TcCRP1 (Fig. 3A), and fewer pollen tubes were attracted to the embryo sac from different species (Fig. 2), these different amino acids may contribute to pollen tube attraction by the same species. Next tests were done to find out if these orthologous CRP1s could attract pollen tubes from different species (Fig. 5). The attraction activity of TfCRP1 and TcCRP1 were compared against T. fournieri and T. concolor pollen tubes. When beads containing 40 nM of TfCRP1 protein, which is the most efficient concentration for pollen tube attraction against T. fournieri pollen tubes in vitro (Okuda et al., 2009) were applied, 56·3 % of the pollen tubes were attracted to the protein beads in T. fournieri pollen tubes (Fig. 5C). In contrast, when 40 nm of TcCRP1, which is the most effective concentration for pollen tube attraction against T. concolor, were added (Fig. 4), only 38·7 % of T. fournieri pollen tubes were attracted (Fig. 5A–C) and the remaining 61·3 % of the pollen tubes continued growing straight. Although it was not significantly different (Fischer's exact test, P = 0·12), the attraction of T. fournieri pollen tubes by TfCRP1 was 1·45-fold higher than that of TcCRP1. When 40 nm TfCRP1 was applied to T. concolor pollen tubes, 33·3 % were attracted and the remaining 66·7 % ignored the protein-containing beads and kept growing straight. The attraction rate of T. concolor pollen tubes by TcCRP1 was 1·65-fold higher than that of TfCRP1, and this attraction was significantly higher than that of TfCRP1 (Fig. 5D–F) (Fischer's exact test, P < 0·05). These results suggest that CRP1s most effectively attracted pollen tubes from the same species.
Fig. 5.
Interspecies pollen tube attraction by CRP1: (A, B) T. fournieri pollen tube (A) just after and (B) 20 min after applying the TcCRP1 protein (red); (C) comparison of the T. fournieri pollen tube attraction rate using buffer, 40 nm of TfCRP1 and 40 nm of TcCRP1; (D, E) T. concolor pollen tube (D) just after and (E) 20 min after applying the TfCRP1 protein (green); (F) comparison of the T. concolor pollen tube attraction rate using buffer, 40 nm of TcCRP1 and 40 nm of TfCRP1. (C, F) Data are means ± s.d. (n > 3 with >10 pollen tubes per replicate). The asterisk in (F) indicates a significant difference between TcCRP1 and TfCRP1 (Fisher's exact test, P < 0·05). Scale bars = 50 µm.
CRP1 may be diverse under the positive selection
In some classes of CRP proteins, typically defensin-like proteins, the amino acid sequences of the orthologous proteins are less conserved between species (Maxwell et al., 2003; Patil et al., 2004). This rapid evolution is thought to contribute to their defensive functions against diverse pathogens. Because pollen tubes from different species were less attracted to the CRP1 protein compared with its own pollen tubes (Fig. 5), we thought that CRP1 might selectively change the amino acid sequence. To demonstrate this point, the rate of nonsynonymous substitutions versus synonymous substitutions (Ka/Ks) was calculated in the CRP proteins. If Ka/Ks is smaller than 1, most of the nucleotide substitutions are silent and do not affect amino acid substitution. If Ka/Ks is larger than 1, the protein might change the sequence selectively (Fiebig et al., 2004).
Because the signal peptide was cleaved out and the mature polypeptide functions in pollen tube attraction, the Ka/Ks ratios was calculated separately for the signal peptide and the mature protein region. The Ka/Ks ratio in the signal peptide region was smaller than 1 (0·801) and that of the mature peptide region was larger than 1 (1·153), suggesting that the mature CRP1 protein may change to alter the amino acid sequence selectively.
DISCUSSION
Micropylar pollen tube guidance may be a key step for species recognition in Torenia
Multiple steps are believed to maintain successful fertilization within the species and prevent undesired crosses with different species (Johnson and Preuss, 2002; Shimizu, 2002; Swanson et al., 2004; Chapman and Goring, 2010). The first reproductive barrier to prevent undesired crosses is pollen–stigma recognition. When distantly related plant species are cross-pollinated, pollen grains placed on the stigma do not adhere to the stigma surface (Zinkl et al., 1999). In some combinations, although pollen grains attach to the stigma, they fail to hydrate and pollen tubes do not germinate. Several prevention steps have been observed when crossing more related species, including the arrest of pollen tube growth in the style, a lack of funiculus guidance, and a lack of micropylar guidance (Shimizu, 2002; Kikuchi et al., 2007).
When T. fournieri and T. concolor were crossed, pollen tubes grew normally through the style (Figs 1E–H and 2A–D), indicating that the mechanism to support pollen tube growth through the style is maintained between these species. In contrast, fewer ovules guided the pollen tubes from crossing partner species at least to the filliform apparatus of the protruding embryo sac (Fig. 2), suggesting this rather short-range guidance is a key step for species recognition in Torenia.
Identification of TcCRP1 as a pollen tube attractant in T. concolor
A CRP1-like gene, TcCRP1, was found in the T. concolor ovule cDNA based on nucleotide sequence similarity (Fig. 3). The entire nucleotide sequence similarity, including the intron, synergid cell-abundant expression, and protein sequence similarity suggested that TcCRP1 is related to TfCRP1. Indeed, the recombinant TcCRP1 protein attracted T. concolor pollen tubes in vitro, suggesting that TcCRP1 is a TfCRP1 orthologue and a pollen tube attractant in T. concolor (Fig. 4). TcCRP1 attracted pollen tubes in a concentration-dependent manner, and it attracted pollen tubes most effectively at the concentration of 40 nm, which is similar to the attraction by TfCRP1 (Okuda et al., 2009). These results suggest conservation of CRP1-like molecules as a micropylar pollen tube attractant within Torenia.
Functional similarities and differences in CRP1 and CRP3
Although TcCRP1 showed strong attractant activity to T. concolor pollen tubes, the attraction activity against T. fournieri pollen tubes was weak (Fig. 5). When 40 nm TcCRP1 was used in the assay, the attraction rate was 55·1 % and 30·3 % for T. concolor pollen tubes and T. fournieri pollen tubes, respectively (Fig. 5). This same tendency was seen when TfCRP1 was applied to T. concolor pollen tubes. Because eight of 62 amino acid residues were different between mature TfCRP1 and TcCRP1, key residues may influence the attractant activities in these amino acids.
A mutational analysis of radish defensin Rs-AFP2 has been performed to identify the antifungal activity site (De Samblanx et al., 1997). Two adjacent sites affecting its activity were found: one was located between the second and third β-strand, and the other was in the loop region connecting the first β-strand and the α-helix. The loop region sequence of some β-defensins is positively selected (Gao and Zhu, 2010). Most of the amino acid substitutions between TfCRP1 and TcCRP1 are located in similar positions, suggesting the importance of these regions for defensin-like protein function.
In contrast, although entire sequence similarity between TfCRP1 and TfCRP3 is low (35 % identical), both proteins show almost the same activities (about 55 % attraction) for T. fournieri pollen tubes (Okuda et al., 2009). Two possibilities have been proposed for the function of CRP-related pollen tube attraction. First, although their sequences are diverse, both TfCRP1 and TfCRP3 possess a CSαβ motif and a γ-core motif, typical molecular features of antimicrobial defensin proteins (Cornet et al., 1995; Thomma et al., 2002; Yount and Yeaman, 2004; Yount et al., 2007). These factors may result in a similarity of their tertial structures and in the interaction with the pollen tubes. Second, because TfCRP1 and TfCRP3 act solely in pollen tube attraction (Okuda et al., 2009), they may be received by different machinery, possibly by different receptors on the pollen tube surface. Future investigations on the key amino acid residues that yield species-specificity, as well as the identification of their tertial structures and receptors, will shed light on how these small secreted polypeptides function in pollen tube attraction.
Rapid evolution and diversification of reproductive proteins
Many genes that mediate in sexual reproduction, such as the sperm–egg interaction in sea urchin and the pollen–stigma interaction in Arabidopsis thaliana, have diverged rapidly, which is often a result of adaptive evolution (Swanson and Vacquier, 2002). Although when these Torenia species diverged from a common ancestor is unclear, the sequence diversities of the CRP genes expressed in the synergid cells suggests their importance in pollen tube attraction and they may be a driving force in speciation.
Although CRP genes evolve rapidly, entire regions in a gene do not change in the same manner. The nucleotide sequence encoding a signal peptide is more conserved than the sequence encoding a mature peptide, and the sequences encoding mature peptides are often under positive selection (Morrison et al., 2003; Semple et al., 2006). A comparison of the Ka/Ks value between nucleotide sequences encoding signal sequences and mature proteins in TfCRP1 and TcCRP1 revealed that the protein-encoding region was less conserved between them. This result supports the assumption that the LURE-class of pollen tube attractants (CRP1 and CRP3) has diversity in their sequences.
Diversification of micropylar pollen tube attractants in a wide range of plant species
Several mutants defective in micropylar pollen tube guidance have been reported in A. thaliana. A mutation in the MAGATAMA3 gene, which encodes a yeast Sen1-like RNA helicase, causes pollen tube misguidance near the micropyle (Shimizu et al., 2008). CENTRAL CELL GUIDANCE1, a transcription regulator expressed in the central cell, and MYB98, a transcription factor expressed in synergid cells, also affect micropylar guidance (Kasahara et al., 2005; Chen et al., 2007). These genes are thought to function as the upstream regulator for producing attractant molecules or regulating guidance signalling, so their precise contribution to guidance is still unclear (Higashiyama, 2010). GABA, a candidate guidance molecule that is produced in the sporophytic cells of the pistil, can create a concentration gradient through the pistil (Palanivelu et al., 2003). However, as adding excess external GABA does not affect pollen tube guidance by the ovule, it may be a general stimulation factor for tube attraction functioning together with other attractants (Higashiyama et al., 2006).
The maize gene ZmEA1 is expressed predominantly in the egg apparatus (egg and synergid cells) (Marton et al., 2005). Knockdown of ZmEA1 causes defects in pollen tube penetration into the intercellular space of micropylar nucellus cells. Recently, an N-terminal cleaved, predicted mature ZmEA1 protein of 49 amino acids was shown to attract maize pollen tubes in vitro (Dresselhaus and Marton, 2009). As ZmEA1 possesses only two cysteine residues, its secondary structure is not related to CRP proteins such as TfCRP1 and TfCRP3. Whether these different classes of small secreted proteins can function as attractants in both species, or whether they are species-specific attractants is unknown. Because ZmEA1-like genes are limited to the Poaceae species (Marton et al., 2005), it may be a monocotyledon-specific attractant. Further discovery of pollen tube attractants, particularly in dicotyledons including A. thaliana, will reveal the evolution, conservation and diversification of pollen tube attractants in plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank S. Yamashita and G. Kokubugata for plant material, N. Iwata for supporting experiments, and S. Nishida for discussions and comments. This work was supported by research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (nos 20870020 and 21770041 to M.M.K; nos 18075004 and 19370017 to T.H.; no. 18GS0314-01 to N.S.), the Japan Science and Technology Agency (PRESTO project to T.H.), the Yamada Science Foundation (to T.H.) and The Mitsubishi Foundation (to T.H.), Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists to D.S. and S.O., and the Japan Society for the Promotion of Science Bilateral Programs.
LITERATURE CITED
- Amien S, Kliwer I, Marton ML, et al. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biology. 2010;8:e1000388. doi: 10.1371/journal.pbio.1000388. doi:10.1371/journal.pbio.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3·0. Journal of Molecular Biology. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Goring DR. Pollen–pistil interactions regulating successful fertilization in the Brassicaceae. Journal of Experimental Botany. 2010;61:1987–1999. doi: 10.1093/jxb/erq021. [DOI] [PubMed] [Google Scholar]
- Chen YH, Li HJ, Shi DQ, et al. The central cell plays a critical role in pollen tube guidance in Arabidopsis. The Plant Cell. 2007;19:3563–3577. doi: 10.1105/tpc.107.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet B, Bonmatin JM, Ptak M, Vovelle F. Refined 3-dimensional structure of insect defensin A in water from NMR data. Journal of Trace and Microprobe Techniques. 1995;13:335–336. [Google Scholar]
- De Samblanx GW, Goderis IJ, Thevissen K, et al. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. Journal of Biological Chemistry. 1997;272:1171–1179. doi: 10.1074/jbc.272.2.1171. [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Marton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Current Opinion in Plant Biology. 2009;12:773–780. doi: 10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, et al. The FERONIA receptor-like kinase mediates male–female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Fiebig A, Kimport R, Preuss D. Comparisons of pollen coat genes across Brassicaceae species reveal rapid evolution by repeat expansion and diversification. Proceedings of the National Academy of Sciences of the USA. 2004;101:3286–3291. doi: 10.1073/pnas.0305448101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Zhu S. Identification and characterization of the parasitic wasp Nasonia defensins: positive selection targeting the functional region? Developmental and Comparative Immunology. 2010;34:659–668. doi: 10.1016/j.dci.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Goto H, Okuda S, Mizukami A, et al. Chemical visualization of an attractant peptide, LURE. Plant Cell Physiology. 2011;52:49–58. doi: 10.1093/pcp/pcq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T. The synergid cell: attractor and acceptor of the pollen tube for double fertilization. Journal of Plant Research. 2002;115:149–160. doi: 10.1007/s102650200020. [DOI] [PubMed] [Google Scholar]
- Higashiyama T. Peptide signaling in pollen–pistil interactions. Plant Cell Physiology. 2010;51:177–189. doi: 10.1093/pcp/pcq008. [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Hamamura Y. Gametophytic pollen tube guidance. Sexual Plant Reproduction. 2008;21:17–26. [Google Scholar]
- Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T. Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. The Plant Cell. 1998;10:2019–2032. doi: 10.1105/tpc.10.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, et al. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Inatsugi R, Sakamoto S, et al. Species preferentiality of the pollen tube attractant derived from the synergid cell of Torenia fournieri. Plant Physiology. 2006;142:481–491. doi: 10.1104/pp.106.083832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- Hulskamp M, Schneitz K, Pruitt RE. Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. The Plant Cell. 1995;7:57–64. doi: 10.1105/tpc.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Preuss D. Plotting a course: multiple signals guide pollen tubes to their targets. Developmental Cell. 2002;2:273–81. doi: 10.1016/s1534-5807(02)00130-2. [DOI] [PubMed] [Google Scholar]
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. The Plant Cell. 2005;17:2981–2992. doi: 10.1105/tpc.105.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano N, Susaki D, Sasaki N, Higashiyama T, Kanaoka MM. Isolation of gametophytic cells and identification of their cell-specific markers in Torenia fournieri, T. concolor and Lindernia micrantha. Cytologia. 2011 in press. [Google Scholar]
- Kikuchi S, Kino H, Tanaka H, Tsujimoto H. Pollen tube growth in cross combinations between Torenia fournieri and fourteen related species. Breeding Science. 2007;57:117–122. [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences of the USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2·0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Marton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by Egg Apparatus 1 of maize. Science. 2005;307:573–576. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP, Machlis L. Chemotropic response of Antirrhinum majus pollen to calcium. Nature. 1962;196:292–293. doi: 10.1104/pp.39.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AI, Morrison GM, Dorin JR. Rapid sequence divergence in mammalian beta-defensins by adaptive evolution. Molecular Immunology. 2003;40:413–421. doi: 10.1016/s0161-5890(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Morrison GM, Semple CA, Kilanowski FM, Hill RE, Dorin JR. Signal sequence conservation and mature peptide divergence within subgroups of the murine beta-defensin gene family. Molecular Biology and Evolution. 2003;20:460–470. doi: 10.1093/molbev/msg060. [DOI] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Patil A, Hughes AL, Zhang G. Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiological Genomics. 2004;20:1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- Ray SM, Park SS, Ray A. Pollen tube guidance by the female gametophyte. Development. 1997;124:2489–2498. doi: 10.1242/dev.124.12.2489. [DOI] [PubMed] [Google Scholar]
- Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Current Biology. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Semple CA, Taylor K, Eastwood H, Barran PE, Dorin JR. Beta-defensin evolution: selection complexity and clues for residues of functional importance. Biochememical Society Transactions. 2006;34:257–262. doi: 10.1042/BST20060257. [DOI] [PubMed] [Google Scholar]
- Shimizu KK. Ecology meets molecular genetics in Arabidopsis. Population Ecology. 2002;44:221–233. [Google Scholar]
- Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development. 2000;127:4511–4518. doi: 10.1242/dev.127.20.4511. [DOI] [PubMed] [Google Scholar]
- Shimizu KK, Ito T, Ishiguro S, Okada K. MAA3 (MAGATAMA3) helicase gene is required for female gametophyte development and pollen tube guidance in Arabidopsis thaliana. Plant Cell Physiology. 2008;49:1478–1483. doi: 10.1093/pcp/pcn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Edlund AF, Preuss D. Species specificity in pollen–pistil interactions. Annual Review of Genetics. 2004;38:793–818. doi: 10.1146/annurev.genet.38.072902.092356. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nature Reviews Genetics. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proceedings of the National Academy of Sciences of the USA. 2004;101:7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount NY, Andres MT, Fierro JF, Yeaman MR. The gamma-core motif correlates with antimicrobial activity in cysteine-containing kaliocin-1 originating from transferrins. Biochimica et Biophysica Acta. 2007;1768:2862–2872. doi: 10.1016/j.bbamem.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Zinkl GM, Zwiebel BI, Grier DG, Preuss D. Pollen–stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development. 1999;126:5431–5440. doi: 10.1242/dev.126.23.5431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.