Abstract
Background
Pollen–pistil interactions are an essential prelude to fertilization in angiosperms and determine compatibility/incompatibility. Pollen–pistil interactions have been studied at a molecular and cellular level in relatively few families. Self-incompatibility (SI) is the best understood pollen–pistil interaction at a molecular level where three different molecular mechanisms have been identified in just five families. Here we review studies of pollen–pistil interactions and SI in the Asteraceae, an important family that has been relatively understudied in these areas of reproductive biology.
Scope
We begin by describing the historical literature which first identified sporophytic SI (SSI) in species of Asteraceae, the SI system later identified and characterized at a molecular level in the Brassicaceae. Early structural and cytological studies in these two families suggested that pollen–pistil interactions and SSI were similar, if not the same. Recent cellular and molecular studies in Senecio squalidus (Oxford ragwort) have challenged this belief by revealing that despite sharing the same genetic system of SSI, the Brassicaceae and Asteraceae molecular mechanisms are different. Key cellular differences have also been highlighted in pollen–stigma interactions, which may arise as a consequence of the Asteraceae possessing a ‘semi-dry’ stigma, rather than the ‘dry’ stigma typical of the Brassicaceae. The review concludes with a summary of recent transcriptomic analyses aimed at identifying proteins regulating pollen–pistil interactions and SI in S. squalidus, and by implication the Asteraceae. The Senecio pistil transcriptome contains many novel pistil-specific genes, but also pistil-specific genes previously shown to play a role in pollen–pistil interactions in other species.
Conclusions
Studies in S. squalidus have shown that stigma structure and the molecular mechanism of SSI in the Asteraceae and Brassicaceae are different. The availability of a pool of pistil-specific genes for S. squalidus offers an opportunity to elucidate the molecular mechanisms of pollen–pistil interactions and SI in the Asteraceae.
Keywords: Asteraceae, Senecio, pistil, stigma, pollen, pollen–pistil interactions, self-incompatibility, transcriptome
INTRODUCTION
The evolution of the carpel had profound consequences for the reproductive biology of angiosperms by encasing the ovule and its egg-containing female gametophyte (embryo sac) within a mass of maternal sporophytic tissue, thereby denying the sperm-dispensing male gametophyte (pollen) easy access to its mate. This physical separation of female and male gametophytes by the carpel (= pistil) gave greater powers of maternal mate discrimination to the flower and resulted in the evolution of the pollen–pistil interaction (Heslop-Harrison, 1975), a complex series of cellular and molecular interactions that effectively constitute a form of ‘courtship’ between the haploid pollen and the diploid pistil. During this molecular courtship process various recognition processes take place, often associated with active processes of discrimination and rejection of ‘incompatible’ pollen at interspecific and intraspecific levels (Hiscock and Allen, 2008). Additionally, with ovules often in limited supply, ‘compatible’ pollen tubes have to compete for ovules leading to an additional level of selection, on the male gametophyte, a consequence of the carpel that is thought to have been a major factor in the evolutionary success of angiosperms (Mulcahy, 1979; Hormazo and Herrero, 1992).
The pollen–pistil interaction is thus a fundamental process in the reproductive biology of flowering plants and has been the subject of intense research for many decades (for recent review see Hiscock and Allen, 2008). In recent years there has been much progress in identifying molecules that mediate specific events during the pollen–pistil interaction, such as pollen adhesion and hydration, pollen tube growth and navigation through the pistil, and self-incompatibility (SI) in particular (Edlund et al., 2004; Swanson et al., 2004; Takayama and Isogai, 2005; Hiscock and Allen, 2008). Nevertheless most studies have been confined to a few model species, and even among these few well-studied species there does not appear to be any general consensus among the types of molecules regulating a common programme of cellular pollen–pistil interactions necessary for compatibility (Lord, 2003; Hiscock and Allen, 2008). Such a lack of consensus is not surprising because proteins that regulate sexual reproductive processes evolve more rapidly than proteins that regulate other cellular processes (Swanson and Vacquier, 2002). This is endorsed by molecular studies of SI, the best understood pollen–pistil interaction, in which the pistil recognizes and actively rejects self-pollen or pollen from genetically closely related individuals. Here, despite identical genetic determination, different molecules have been identified regulating gametophytic SI (GSI) in the Solanaceae and Papaveraceae (see Meng et al., 2011; McClure et al., Franklin-Tong et al., 2011), whilst molecules regulating sporophytic SI (SSI) in the Brassicaceae (Takayama and Isogai, 2005) are not encoded at the S (self-incompatibility) locus of Ipomoea trifida (Convolvulaceae) (Rahmann et al., 2007).
Pollen–pistil interactions and SI have been most widely studied in species from the Brassicaceae [SSI, and self-compatible (SC)], Solanaceae (GSI and SC), Rosaceae (GSI and SC), Plantaginaceae (GSI and SC), Papaveraceae (GSI and SC), and the Liliaceae (SI and SC), where SI has been more intensively studied at a molecular level than ‘compatibility’ (self- and cross-). In the Brassicaceae SSI is regulated by a pistil-expressed S-receptor kinase (SRK), which interacts with its cognate pollen ligand SCR (S-cysteine-rich protein) to initiate recognition and subsequent rejection of self-pollen (Takayama and Isogai, 2005). In the Solanaceae, Rosaceae and Plantaginaceae, GSI is triggered by an interaction between a pistil-expressed S-RNase and a pollen-expressed F-box protein (SLF) although the details of this molecular interaction and how it triggers pollen rejection remain to be determined (Meng et al., 2011; McClure et al.). Interestingly, despite sharing identical genetic determination, GSI in poppy (Papaveraceae) is mediated by a calcium-based signalling pathway probably triggered by interaction between a pollen-expressed Ca2+-channel receptor and its cognate ligand, a stigma-expressed S-glycoprotein (Franklin-Tong et al., 2011). Compatibility has been studied most extensively in species from the Solanaceae, where in Nicotiana and Petunia it has been demonstrated that stigmatic lipids are essential for pollen development and pollen tube guidance on the stigma and once growing within the pistil other molecules such as receptor kinases and their ligands, lipid-transfer proteins (LTPs), and arabinogalactan glycoproteins variously impact on pollen tube growth and guidance. In the Brassicaceae studies of compatibility in SC Arabidopsis thaliana have identified an oleosin and LTPs as important for the initial stages of the pollen–pistil interaction (Mayfield et al., 2001), while GABA and cysteine-rich proteins have been shown to function in pollen tube guidance and attraction, respectively (reviewed in Hiscock and Allen, 2008). Interestingly, two small proteins, SCA and chemocyanin (a planticyanin), first identified in Lilium (Liliaceae; Park and Lord, 2003; Kim et al., 2003) and shown to play a role in pollen tube growth in the pistil, have now been identified in Arabidopsis, where they also appear to function in pollen–pistil interactions (reviewed in Hiscock and Allen, 2008). SCA and planticyanins thus represent the first consensus molecules potentially involved in a common compatibility pathway across diverse plant families spanning the monocot–eudicot divide.
To extend our understanding of the molecular regulation of pollen–pistil interactions involved in compatibility and SI it is important to extend our studies of these key reproductive processes to species in other families from the monocots and eudicots. With this in mind a study of pollen–pistil interactions and SI was initiated in Senecio squalidus as a model species in the Asteraceae, a family that had hitherto received relatively limited study in these areas of reproductive biology. Here we review our current understanding of these processes in the Asteraceae generally, and S. squalidus specifically.
POLLEN–PISTIL INTERACTIONS AND SI IN THE ASTERACEAE
The Asteraceae is the second largest family of flowering plants, containing approximately 1620 genera and 22 750 species (APG III, 2003). The family includes many important crop plants (e.g. sunflower, lettuce and chicory), ornamental plants (e.g. ‘daisies’, gerberas and chrysanthemums), as well as some invasive weedy species (e.g. centaurea and star thistle). Early studies of pollen–pistil interactions in the Asteraceae were directed exclusively at SI species. Indeed, the first genetic accounts of SSI were made from studies in Crepis foetida (Hughes and Babcock, 1950) and Parthenium argentium (Gerstel, 1950) and then extended through a subsequent study of Cosmos bipinnatus (Crowe, 1954). These pioneering studies of SSI showed the system to be controlled by a single S locus with multiple S alleles that could display dominance-recessive relationships in both pollen and pistil – the latter property being one of the confounding factors in the ‘delayed’ discovery of this system relative to the simpler GSI system, first described by East and Mangelsdorf (1925). Extensive confirmation of SSI in the Brassicaceae then followed with the classic studies of Brassica by Bateman (1952). From this point on genetic and, later, molecular genetic studies of SSI have tended to focus on species in the Brassicaceae whilst similar studies in the Asteraceae did not occur until fairly recently (Hiscock, 2000b; Allen et al., 2010b). A recent review of the phylogenetic distribution of SSI within the Asteraceae (Ferrer and Good-Avila, 2007) estimated 63 % of species to be SI (presumably all SSI), with the remaining species a mixture of pseudo-self-incompatibility (PSI, 10 %) and SC (27 %). This high percentage of SI species in the Asteraceae suggests that SI is the ancestral breeding system within the family although the phylogenetic support for this assumption is inconclusive (Ferrer and Good-Avila, 2007).
Structural and cytological studies of compatible and incompatible pollen–pistil interactions in species from the Asteraceae (Cosmos, Ambrosia and Helianthus) and the Brassicaceae (Brassica and Raphanus) identified similarities between their shared SSI systems at a cellular level (Knox, 1973; Howlett et al., 1975; Dickinson and Lewis, 1975; Vithanage and Knox, 1977). These similarities included: the release of exine-held pollen coat soon after contact was made between pollen and stigma – this being a consequence of both compatible and incompatible pollinations; the arrest of incompatible pollen at the stigma surface soon after germination; and the deposition of callose in incompatible pollen tubes and in stigma cells adjacent to the arrested pollen tubes.
Electron microscopy studies of the stigma surface of Helianthus (Vithanage and Knox, 1977) and various other Asteraceae species (Heslop-Harrison and Shivanna, 1977) reported it to be of the ‘dry’ type – another commonality with the Brassicaceae (Heslop-Harrison and Shivanna, 1977). Angiosperm stigmas have been classified into two broad categories, ‘wet’ and ‘dry’, depending on whether or not they possess a surface secretion (Heslop-Harrison and Shivanna, 1977; Heslop-Harrison, 1981). Wet stigma species include members of the Solanaceae, Rosaceae and Liliaceae, while dry stigmas, as well as being typical of the Brassicaceae, are also found in the grasses (Poaceae) and Papaveraceae. This fundamental difference in stigma type has been found to correlate with broad differences between the pollen–pistil interaction in species with wet vs. dry stigmas (Heslop-Harrison, 2000; Johnson and Preuss, 2003; Lord, 2003; Edlund et al., 2004). For instance, in species with wet stigmas pollen capture is non-specific and pollen hydration within the secretion is passive and unregulated, whereas in species with dry stigmas (e.g. Arabidopsis) pollen capture and adhesion show a degree of species specificity (Zinkl et al., 1999; Zinkl and Preuss, 2000) and pollen hydration on the stigma is a highly regulated process (Dickinson, 1995). Epidermal cells of wet stigmas tend to lack a continuous cuticle, so penetration of the stigma by the pollen tube is fairly easy, whereas species with dry stigmas usually possess a continuous cuticle which presents a major barrier to pollen tube penetration that must be overcome by pollen secreting hydrolytic enzymes, such as cutinase (Hiscock et al., 1994, 2002a).
STUDIES OF REPRODUCTION, POLLEN–PISTIL INTERACTIONS AND SI IN S. SQUALIDUS
Senecio squalidus has an intriguing evolutionary history and population biology that make it a unique ‘model’ offering unconventional opportunities for studies of plant reproductive biology, particularly SI (see Hiscock, 2000a; Hiscock et al., 2003). S. squalidus is a recent diploid hybrid species derived from a cross between S. aethnensis and S. chrysanthemifolius that occur on Mt Etna, Sicily, where hybrids still flourish today (James and Abbott, 2005; Abbott et al., 2009). Material from this hybrid zone was introduced to Britain around 1690, where it was cultivated in the Oxford Botanic Gardens (Harris, 2002). UK S. squalidus evolved from this founder population after its escape and subsequent spread from Oxford during the late eighteenth century. Genetic studies confirmed that, like other Asteraceae species, SI in S. squalidus is controlled sporophytically by a single S locus (Hiscock et al., 2000b), and population genetic studies predict that between seven and eleven S-alleles are present in UK populations, with two to six per local population (Brennan et al., 2006). One aspect of studying SI in S. squalidus is to discern how the species has been able to maintain a strong system of SI and yet colonize the UK so rapidly with such a small reserve of S-alleles (Brennan et al., 2005). Unusually high levels of dominance interactions between S-alleles and pseudo-self-compatibility are together predicted to facilitate maintenance of SI whilst allowing effective mating and seed production for colonization (Brennan et al., 2005, 2006, 2010). In addition to its intriguing population biology, which offers unique opportunities for studies of the population genetics and evolution of SSI, S. squalidus has many other attributes that make it a good model for studies of pollen–pistil interactions and plant reproductive biology more generally: (1) it is easily grown in a glasshouse, taking approximately 6 months to reach flowering from seed; (2) under glasshouse conditions it flowers continuously producing large numbers of flower heads (capitula); and (3) it can be propagated clonally by cuttings, facilitating maintenance of defined S genotypes.
Reproductive development in S. squalidus
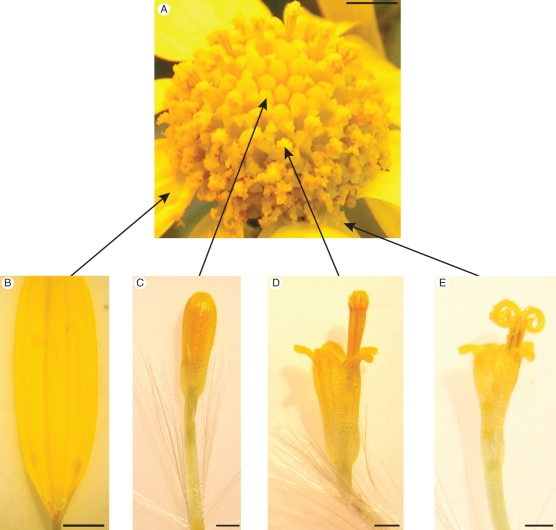
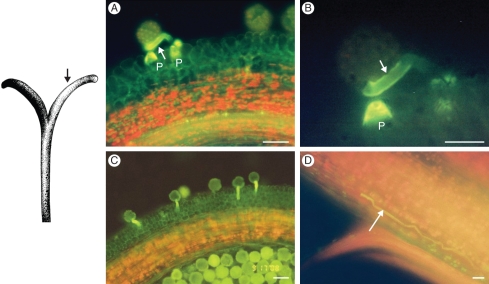
Senecio squalidus possesses a capitulum-type inflorescence, typical of the Asteraceae. The inflorescence consists of an outer whorl of carpellate ray florets and inner whorls of cosexual disc florets (Fig. 1). The individual disc florets develop sequentially, with florets from the outer whorls maturing before those in the centre of the inflorescence (Fig. 1). The pistil possesses a bi-lobed semi-dry stigma, a style and a single ovary. The receptive surface of the stigma, the papillae cells, is protected in immature pistils where the two stigmatic lobes are pressed tightly together (Fig. 2A). As the pistil matures and grows past the anthers, sterile pseudo-papillae at the ends of the stigmatic lobes collect pollen from the anthers and present this to pollinators (Figs 2B and 3). At maturity the two lobes of the stigma come apart to reveal the receptive papillae cells (Fig. 3).
Fig. 1.
Stages of floret development in S. squalidus. (A) Developing captiulum; arrows indicate florets of different developmental stages; scale bar = 2 mm. (B) Ray floret; scale bar = 1 mm. (C) Immature disc floret. (D) Disc floret stage 1 (anther mature). (E) Disc floret stage 2 (pistil mature). Scale bars in (C–E) = 0·5 mm.
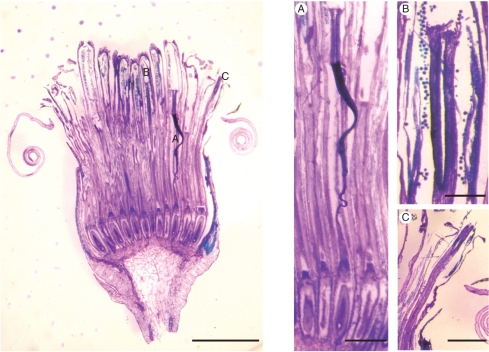
Fig. 2.
Section through a Senecio squalidus capitulum, showing individual disc florets in different stages of development. Inset pictures show detail of pistils: (A) entire pistil; (B) developing pistil; (C) emerging pistil. Section stained with Toluidine blue. Scale bars = 5 mm (main image), and 1 mm (insets).
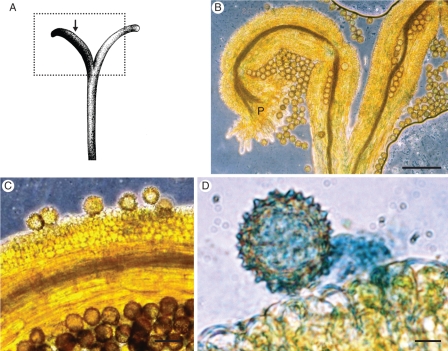
Fig. 3.
(A) Illustration of Senecio squalidus pistil. (B) Squash preparation of S. squalidus stigma, stained with aniline blue (section of pistil indicated by hatched line in A). P, pseudo-papillae cells; scale bar = 0·1 mm. (C) Detail of stigma papillae cells with pollen grains attached (indicated by black arrow in A); scale bar = 25 µm. (D) Detail of pollen tube penetrating papillae cells; scale bar = 5 µm.
S. squalidus possesses a semi-dry stigma
Because early studies of stigma surfaces in the Asteraceae reported the dry type it was always assumed that this was the case and it was not until Elleman et al. (1992) carried out a comparative study of pollen–stigma interactions in Brassica, poppy, Cosmos and Helianthus, using novel anhydrous fixation conditions, from which it emerged that stigmas of Asteraceae species were not entirely dry, but rather appeared to produce a small amount of surface secretion. This finding was subsequently confirmed by Hiscock et al. (2002b) in an extensive structural and cytological study of the pollen–stigma interaction in S. squalidus, and other Asteraceae, which led to a reclassification of the Asteraceae stigma as ‘semi-dry’, reflecting the fact that small amounts of a lipid-rich secretion were always present in the basal regions of stigmatic papillae where the cuticle was absent (Hiscock et al., 2002b; Fig. 4). This study revealed that the stigma of S. squalidus shows characteristics of both dry- and wet-stigma surfaces. The stigmatic papillae possess a cuticle on their surface, but unlike in dry stigma species (e.g Brassica sp.) this is not continuous, and does not extend to the base of the cells (Hiscock et al., 2002b). The mature semi-dry surface of the S. squalidus stigma produces small amounts of an extracellular secretion, on the surface of the papillae cells and secretion of this lipid-rich material is enhanced when pollen makes contact with the stigma, irrespective of whether the pollen is compatible or incompatible (Hiscock et al., 2002b).
Fig. 4.
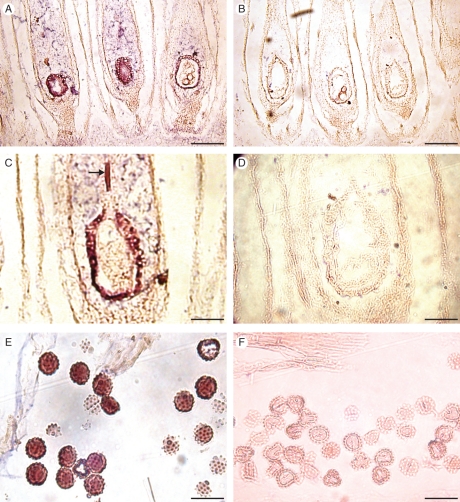
The semi-dry stigma of Senecio squalidus stained for the presence of lipids, peroxidase and reactive oxygen species (ROS). (A) Stigma stained with Sudan black b to visualize the presence of lipids, indicated by black staining. (B) Control stigma stripped of lipids by placing in 10 % SDS prior to staining. (C) Stigma stained with 0·1 m guaiacol, 0·1 m H2O2, in 20 mm phosphate buffer, pH 4·5, to visualize peroxidase activity. (D) Control of C. (E) Stigma stained with TMB-HCl (3,39,5,59-tetramethylbenzidine-HCl, 0·1 mg ml−1 in TRIS acetate, pH 5·0) showing the localized activity of ROS in papillae cells, indicated by blue staining. (F) Control of (E). Scale bars = 50 µm.
The stigma surface stains positive for the presence of lipids, carbohydrate, protein, reactive oxygen species (ROS) and peroxidases, components that have been identified as important for stigma function (McInnis et al., 2006; Fig. 4). Since the discovery of high levels of ROS, mainly in the form of hydrogen peroxide, in Senecio stigmas, it has been shown that accumulation of high levels of ROS is a general feature of angiosperm stigmas when they reach maturity and are optimally receptive to compatible pollen (McInnis et al., 2006). Nevertheless, the biological function of these ROS remains a mystery. Two possible functions of stigmatic ROS might be: (1) protecting stigmas from pathogen attack in a similar way to that proposed for ROS in protecting nectar from infection by bacteria and fungi (Carter and Thornburg, 2004), and (2) as a signal to germinating pollen (McInnis et al., 2006).
Pollen–pistil interactions in S. squalidus
Upon landing on the stigma, the pollen grain rapidly releases pollenkitt (pollen coat) from the exine onto the stigma surface, leading to the formation of an ‘attachment foot’ beneath the pollen grain (Hiscock, 2000a; Hiscock et al., 2002b). The attachment foot is structurally complex, and is formed of a mixture of pollen-wall material and stigmatic extracellular secretion. The cytoplasm of the papillae cells directly below pollen grains has been observed to contain large numbers of vesicles, suggesting that these cells are actively producing secretion (Hiscock, 2000a; Hiscock et al., 2002b). During a compatible pollination, after the pollen grain hydrates (within 15–30 min of landing on the stigma surface), a pollen tube emerges and grows between the papillae cells (Fig. 5). The pollen tube penetrates the stigma and grows intercellularly through the cells of the stigmatic cortex, before turning 90 ° and growing parallel with the transmitting cells of the stigmatic lobe and style, towards the ovary (Hiscock et al., 2002b).
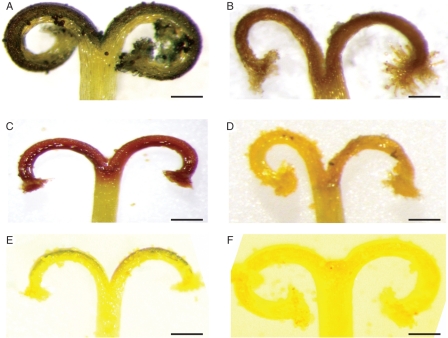
Fig. 5.
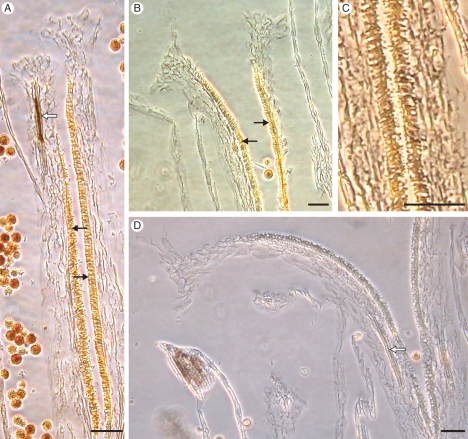
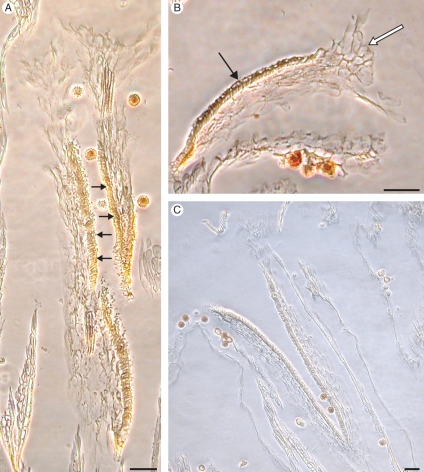
Incompatible and compatible pollinations in Senecio squalidus. Squash preparations of stigmas stained with aniline blue and viewed under UV light. (A,B) Incompatible pollination; pollen tube (arrow) blocked from entering papillae (P). (C) Compatible pollination; pollen tubes penetrating stigma tissue. (D) Compatible pollen tube growing through transmitting tissue (arrow). Scale bars = 0·25 µm.
The SI response in S. squalidus
The site of the SI response in S. squalidus is variable, but typically occurs at the stigma surface, where incompatible pollen tubes are often arrested prior to germination. However, some incompatible pollen grains do produce pollen tubes; these are arrested on the stigma surface or, on rare occasions, after penetration of the stigma (Hiscock et al., 2002b). Pollen tube arrest is accompanied by the accumulation of vesicles, and deposition of callose plugs in papillae cells directly below incompatible pollen tubes (Hiscock, 2000a; Hiscock et al., 2002b; Fig. 5).
Despite the extensive research of SSI in population genetic studies of S. squalidus, the genetic S-locus controlling SSI remains unidentified. Previous studies of S. squalidus have shown that orthologues of the Brassica S-gene, SRK, are not expressed exclusively in the stigma, or linked to the S-locus (Hiscock et al., 2003; Tabah et al., 2004). From this evidence it has been concluded that SSI in Senecio species operates through a different molecular mechanism from that found in Brassica (Hiscock et al., 2003). Subsequent studies using isoelectric focusing identified stigma-specific peroxidases (SSPs), which were associated with defined S-alleles and expressed exclusively in the pistil (Hiscock et al., 2003). Expression of these was developmentally regulated with maximal expression occurring at anthesis when SI is functioning. In situ hybridizations revealed that expression of SSP was specific to the stigmatic papillae cells. However, the SSP gene did not segregate with defined S-alleles and so tight linkage cannot be established between the SSP genes and the S-locus (McInnis et al., 2005).
TRANSCRIPTOMIC ANALYSIS OF THE S. SQUALIDUS PISTIL
During its colonization of the UK, S. squalidus hybridized with native S. vulgaris (groundsel) to form the allohexaploid hybrid species S. cambrensis (Welsh ragwort) within the last 60 years (Abbott and Lowe, 2004). Despite their close evolutionary relationships these three Senecio species are highly divergent in flower head morphology and mating system. Senecio squalidus has large outer ray flowers in its capitulum and is SI while S. vulgaris has no ray flowers and is SC, and S. cambrensis has short or variable ray flowers and is usually SC, although functional SI has been found in synthetic hybrids (Brennan et al., 2010). These three Senecio taxa therefore offer unique opportunities to study the regulation of ray flower development and SI/SC. To further study these developmental processes anonymous cDNA microarrays were created using floral tissue from the three taxa and the primary triploid hybrid of S. vulgaris × S. squalidus, S. × baxteri (Hegarty et al., 2005).
Great variation in gene expression was detected between the three floral transcriptomes (Hegarty et al., 2005, 2006) and sequencing identified potential candidate genes for pollen–pistil interactions and SI (Allen et al., 2010a). ‘Virtual subtraction’ analyses identified genes up-regulated in SI S. squalidus compared with SC S. vulgaris and S. cambrensis. Northern blot analysis confirmed tissue-specific expression in pollen or pistil (or both) and directed identified genes of interest for more detailed study. One such gene showed a particularly interesting expression profile, being developmentally regulated with transcripts present in both pollen and pistil (Allen et al., 2010a). This gene showed significant orthology to SF21, a gene of unknown function originally identified in Helianthus annuus (sunflower) (Kräuter-Canham et al., 1997). The SF21 gene family is widespread in plants (Okuda and Kondoh, 1999), and there is evidence of specialization of SF21 genes to function in reproductive processes, particularly in the Asteraceae, where Helianthus and Senecio SF21 genes show pistil- and pollen-specific expression. S. squalidus SF21 has multiple copies with one gene copy expressed in pollen, and the other in pistil tissues (Fig. 6; Allen et al., 2010a). In Senecio SF21 has been proposed to act in compatible pollen tube guidance from the stigma to the ovary (Allen et al., 2010a). If SF21 does function in pollen tube guidance, this important role may explain the high degree of conservation of gene sequence across the angiosperms. Certainly, the SF21 gene family represents a rare example of a family of highly conserved pistil- and pollen-expressed genes.
Fig. 6.
In situ hybridizations on longitudinal sections of Senecio squalidus pistils. Pistils at ovule developmental stages 2 and 3 hybridized with SF21 antisense probe (A,C,E) and hybridized with sense probe (B,D,F). (A) Base of pistil with staining in ovules. (C) Expression is localized to the integument cells surrounding the embryo sac and transmitting tissue immediately above ovule (black arrow). (E) Expression was also detected in mature pollen grains. Scale bars: (A,B) = 50 µm; (C–F) = 25 µm.
To identify additional genes potentially involved in pollen–pistil interactions and SI in S. squalidus we recently created a pistil-enriched cDNA library using suppression subtractive hybridization (SSH) (Allen et al., 2010b). The resulting data set contained both novel genes to S. squalidus and genes previously identified in similar studies of other unrelated species from the Brassicaceae, Poaceae, Solanaceae and Liliaceae. The Senecio pistil data set was expected to contain genes potentially involved in mediating the female side of SI, including primary S-recognition genes. Thus, any novel pistil genes identified could be considered as potential candidate SI genes. Novel genes identified in this study included a WNK (with no K/lysine) kinase with a putative calcium-binding domain, a kinase-interacting protein, a membrane-associated protein, a nematode resistance protein and several hypothetical proteins of unknown function (Allen et al., 2010b; Table 1). These novel pistil-specific genes may be candidate SI genes as the pistil data set is expected to contain genes potentially involved in mediating the female side of SI, including primary S-recognition genes.
Table 1.
Putative function and expression characteristics of candidate SI/pollen–pistil genes in Senecio squalidus
| Accession number | Gene description | Gene annotation | Putative function | Site of expression |
|---|---|---|---|---|
| GO255172 | Fbox | Fbox | Protein fate | Pollen |
| GO255101 | ABC transporter | ABC | Transport | Pistil |
| GO255139 | NECIV | NECIV | Defence | Pistil |
| GO255232 | Integral membrane protein | IMP | Cell wall | Pistil |
| GO255201 | CBS-domain | CBS | Unknown | Pistil |
| GO255154 | Calcium-kinase | CaKin | Signalling | Pollen |
| GO255182 | Nodulin/mtn3 | Nod | Signalling/transport | Pistil |
| GO255107 | Membrane-associated protein | MAP | Signalling/transport | Pistil |
| GO255153 | Kinase interacting protein | KIP | Signalling | Pollen |
| GQ227732 | Sunflower 21a | SF21a | Signalling | Pollen/pistil |
| GQ227733 | Sunflower 21b | SF21b | Signalling | Pollen |
| GO255242 | Sunflower 3 | SF3 | Transcription | Pollen |
| GO255239 | Sunflower 16 | SF16 | Transcription | Pollen |
| GO255140 | Stigma specific peroxidase | SSP | Defence | Pistil |
| GO255135 | Putative binding protein | Bind | Transport | Pistil |
| GO255123 | myo-Inositol oxygenase | Myo | Metabolism | Pistil |
| GO255100 | UDP glycosyltransferase | UDP | Defence | Pistil |
| GO255085 | Nematode resistance protein | NR | Defence | Pistil |
| GO255235 | Unknown protein | Unk | Unknown | Pistil |
| GO255177 | Dehydration sensitive | Dehyd | Stress | Pistil |
Two pistil-expressed genes were of particular interest as candidates for pollen–pistil interactions and SI. First, the Senecio pistil-specific membrane associated protein (MAP) was found to be expressed in the papillar cells and transmitting tissue of the stigma (Fig. 7). The MAP protein is predicted to have three transmembrane helices, with most of the protein lying extracellularly. In S. squalidus the nucleotide sequence of MAP exhibits relatively high S-genotypic polymorphism, which is elevated in the extracellular region (Allen et al., 2010b). The closest homologue in Arabidopsis, AtMAMI (Arabidopsis thaliana membrane-associated mannitol induced) is related to vesicle-associated membrane proteins (VAMPs), which were also returned on a BLAST-X search using MAP (Galaud et al., 1997). VAMPs are predicted to function in membrane trafficking of molecules from vesicles to the cell membrane. Mannitol is a potent ROS quencher and a chemical commonly produced by fungi to suppress ROS-mediated plant defence systems (Jennings et al., 1998). ROS are present at high levels in the receptive cells of the stigma, offering a potential obstacle to pollen tube development; it is possible that the production of mannitol suppresses ROS, to allow pollen tube penetration and growth. If the Senecio MAP was also induced by mannitol, the presence of this chemical would potentially signal the presence of a pollen grain and stimulate membrane trafficking in the papillar cells of the stigma during the pollen–stigma interaction. The cytoplasm of S. squalidus papillae directly below pollen grains has been observed to contain large numbers of vesicles, suggesting that these cells are actively producing secretion (Hiscock et al., 2002b), and pollen tube arrest during the SI response is characterized by pronounced ‘swellings’ in cell walls of papillae cells in direct contact with incompatible pollen tubes (Hiscock, 2000a; Hiscock et al., 2002b). The Senecio AtMAMI might well be involved in these secretion activities in papillae.
Fig. 7.
In situ hybridizations on longitudinal sections of mature Senecio squalidus pistils hybridized with MAP antisense probe (A–C) and hybridized with sense probe (D). (A) Upper section of emerging pistil showing expression in papillar cells of stigma (black arrows) and transmitting tissue (white arrow). (B) Fully mature and reflexed pistil exhibiting expression in papillar cells (black arrows). No expression was detected in pseudopapillae cells at the tips of the stigma. (C) Close-up of papillar cells. (D) Corresponding sense control showing no expression in papillar cells (black arrow) or transmitting tissue (white arrow). Scale bars = 50 µm.
The second candidate gene, a pistil-specific nodulin/mtn3 gene (Nod), is expressed exclusively in the papillar cells of the S. squalidus stigma, where it appears to be developmentally regulated, reaching maximal expression as the stigmatic lobes reflex to expose the papillar cells (Fig. 8). Originally identified in Medicago trunculata root nodule tissue, mtn3 is predicted to be involved in a hypersensitive defence response (Gamas et al., 1996). The nodulin/mtn3 gene family includes a number of genes that play specialized roles in reproductive tissues (Yang et al., 2006; Guan et al., 2008). The role of pollen-expressed members of this gene family has been investigated in Arabidopsis and rice. In rice, OS8NG (a gene expressed primarily in pollen but also in leaves) appears to have a dual function; it has been implicated in blight resistance in leaves and also functions in pollen development (Yang et al., 2006). In Arabidopsis, RPG1 has been shown to be essential for pollen wall development (Guan et al., 2008). Despite the large number of pistil-specific nodulin/mtn3 genes in Arabidopsis and other plant species, the role of these genes in pistils has not been studied. Nod possesses seven predicted transmembrane regions, with one variable and two highly conserved intracellular domains. The conserved domains are essential for function in RPG1, which is predicted to act by regulating membrane traffic in the pollen tapetum (Guan et al., 2008). A similar role for Nod in S. squalidus is proposed; as putative regulators of membrane traffic in papillae cells these proteins could be important mediators of pollen–stigma interactions. The presence of pollen- and pistil-specific nodulin/mtn3 genes in the pollen tapetum and stigmatic papillae cells has intriguing implications, raising the possibility that these proteins could play similar or complementary roles in the primary receptive tissue of pollen grains and stigmas.
Fig. 8.
In situ hybridizations on longitudinal sections of mature Senecio squalidus pistils hybridized with Nodulin antisense probe (A,B) and hybridized with sense probe (C). (A) Upper section of emerging pistil showing expression in papillar cells of stigma (black arrows). (B) Fully mature and reflexed pistil exhibiting increased expression in papillar cells (black arrow). No expression was detected in pseudopapillae cells (unfilled arrow). Staining of pollen grains occurred due to high levels of intrinsic pollen cytosolic alkaline phosphatase activity (Knox and Heslop-Harrison, 1969); no expression of Nod was detected in pollen via northern blot analysis (Fig. 7B). (C) Corresponding sense control showing no expression in papillar cells. Scale bars = 50 µm.
Comparative analysis of the Senecio pistil transcriptome with the four other available pistil transcriptomes – Oryza sativa (rice), Crocus sativus (crocus), Arabidopsis thaliana and Nicotiana tabacum (tobacco) – allowed us to identify key similarities and differences between these diverse species which represent the three major clades of the angiosperms (Asterids, Rosids and Monocots) and three stigma states (wet, dry and semi-dry). Our data showed that the pistil transcriptome of S. squalidus is generally more similar to the dry stigma transcriptome of Arabidopsis but also contains genes expressed in wet stigmas (Allen et al., 2010b). Additionally, our analysis indicated that certain classes of genes were common to the pistil transcriptomes of the five angiosperm species sampled to date, suggesting they are important for pistil function and hence are conserved between diverse angiosperm groups (Allen et al., 2010b). This suggests that some of the complex interactions underlying pistil function in diverse species with wet, dry and semi-dry stigmas are shared and have been inherited from the common ancestor of monocots and eudicots (Hiscock and Allen, 2008).
CONCLUSIONS AND PROSPECTS
Despite previously perceived similarities in stigma structure and SI between the Brassicaceae and Asteraceae, recent studies in Senecio have shown that both are fundamentally different: Senecio, and other Asteraceae species sampled, possess a semi-dry stigma (Hiscock et al., 2002b) rather than a completely dry (Brassica-type) stigma, and the sporophytic SI system of Senecio (and by implication other Asteraceae) does not operate through the Brassica-type SRK/SCR(SP11) molecular machinery (Hiscock et al., 2003). To understand evolutionary homologies between Asteraceae pollen–pistil interactions and SSI and those of other families it is therefore important to identify and characterize molecules regulating these critical reproductive processes in this large and important family.
Our recent transcriptomic studies in Senecio have identified numerous genes potentially involved in pollen–pistil interactions and SI in the Asteraceae. Some pistil-specific and pollen-specific genes are novel to Senecio, whereas others have been identified previously in pollen or pistils of unrelated species (Allen et al., 2010a, b). Novel Senecio pollen and pistil genes are particularly interesting given that the Senecio SSI system has yet to be characterized at a molecular level. Indeed, research on another elusive SSI system in Ipomoea (Convolvulaceae) has recently identified putative S-genes, which have no homologous matches in the databases (Rahmann et al., 2007). Similarly the recently identified Papaver pollen determinant is also encoded by a novel gene (Franklin-Tong, 2008). Of the pistil-specific candidates in S. squalidus, two genes, MAP and Nod, expressed in stigmatic papillae may play important roles in the recognition of pollen grains, so are the focus of ongoing studies. Both MAP and Nod are localized to the cell membrane, where they could potentially regulate membrane traffic during pollen hydration and germination. Comparative transcriptome analysis has also highlighted potentially important conserved pistil genes, such as SF21, and phylogenetic analysis of this gene family indicates these genes are ancient and show a high degree of sequence conservation across different angiosperm taxa (Allen et al., 2010a); this suggests a fundamental role for SF21 in reproduction. Determining the precise function of SF21 and other candidate genes will be a vital step in assessing their importance in controlling pollination in Senecio and other angiosperm taxa.
Despite many years of research, relatively little is known about general mechanisms regulating pollen–pistil interactions. Current research indicates a high diversity of molecules recruited for the same processes in different species, but there are fewer examples of conserved pollen–pistil genes (Hiscock and Allen, 2008). More comparative studies of pollen and pistil transcriptomes are therefore needed, especially in basal angiosperms, to identify further conserved and unique genes potentially involved in angiosperm reproductive processes.
ACKNOWLEDGEMENTS
We thank Andrew Hughes for technical support. Work described in this review was supported by the Natural Environment Research Council (NERC), the Biotechnology and Biological Sciences Research Council (BBSRC) and the Lady Emily Smyth Research Station, University of Bristol.
LITERATURE CITED
- Abbott RJ, Lowe AJ. Origins, establishment and evolution of new polyploid species: Senecio cambrensis and S. eboracensis in the British Isles. Biological Journal of the Linnean Society. 2004;82:467–474. [Google Scholar]
- Abbott RJ, Brennan AC, James JK, Forbes DG, Hegarty MJ, Hiscock SJ. Recent hybrid origin and invasion of the British Isles by a self-incompatible species, Oxford ragwort (Senecio squalidus L., Asteraceae) Biological Invasions. 2009;11:1145–1158. [Google Scholar]
- Allen AM, Lexer C, Hiscock SJ. Characterisation of sunflower-21 (SF21) genes expressed in pollen and pistil of Senecio squalidus (Asteraceae) and their relationship with other members of the SF21 gene family. Sexual Plant Reproduction. 2010a;23:173–186. doi: 10.1007/s00497-010-0137-9. [DOI] [PubMed] [Google Scholar]
- Allen AM, Lexer C, Hiscock SJ. Comparative analysis of pistil transcriptomes reveals conserved and novel genes expressed in dry, wet, and semidry stigmas. Plant Physiology. 2010b;154:1347–1360. doi: 10.1104/pp.110.162172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APG III, The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Bateman AJ. Self-incompatibility in angiosperms: I. Theory. Heredity. 1952;6:285–310. [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. Modes and rates of selfing and associated inbreeding depression in the self-incompatible plant Senecio squalidus (Asteraceae): a successful colonizing species in the British Isles. New Phytologist. 2005;168:475–486. doi: 10.1111/j.1469-8137.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. The population genetics of sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae): S allele diversity across the British range. Evolution. 2006;60:213–224. [PubMed] [Google Scholar]
- Brennan AC, Tabah DA, Harris SA, Hiscock SJ. Sporophytic self-incompatibility in Senecio squalidus (Asteraceae): S allele dominance interactions and modifiers of cross-compatibility and selfing rates. Heredity. 2010;106:113–123. doi: 10.1038/hdy.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. Is the nectar redox cycle a floral defense against microbial attack? Trends in Plant Sciences. 2004;9:320–324. doi: 10.1016/j.tplants.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Crowe L. Incompatibility in Cosmos bipinnatus. Heredity. 1954;8:1–11. [Google Scholar]
- Dickinson HG. Dry stigmas, water and self-incompatibility in Brassica. Sexual Plant Reproduction. 1995;8:1–10. [Google Scholar]
- Dickinson HG, Lewis D. Duckett JC, Racey PA, editors. Interaction between the pollen grain coating and the stigmatic surface during compatible and incompatible interspecific pollinations in Raphanus. The Biology of the Male Gamete. Biological Journal of the Linnean Society. 1975;7(Suppl. 1):165–175. [Google Scholar]
- East EM, Mangelsdorf AJ. A new interpretation of the hereditary behaviour of self-sterile plants. Proceedings of the National Academy of Sciences USA. 1925;11:166–171. doi: 10.1073/pnas.11.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell. 2004;16:S84–S97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman CJ, Franklin-Tong VE, Dickinson HG. Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytologist. 1992;121:413–424. doi: 10.1111/j.1469-8137.1992.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Ferrer MM, Good-Avila SV. Macrophylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytologist. 2007;173:401–414. doi: 10.1111/j.1469-8137.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE. Self-incompatibility in Papaver rhoeas: progress in understanding mechanisms involved in regulating self-incompatibility in Papaver. In: Frankloin-Tong VE, editor. Self-incompatibility in flowering plants. Berlin: Springer-Verlag; 2008. pp. 237–258. [Google Scholar]
- Gamas P, Niebel Fde C, Lescure N, Cullimore J. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Molecular Plant and Microbe Interactions. 1996;9:233–242. doi: 10.1094/mpmi-9-0233. [DOI] [PubMed] [Google Scholar]
- Galaud JP, Laval V, Carriere M, et al. Osmotic stress activated expression of an Arabidopsis plasma membrane-associated protein: sequence and predicted secondary structure. Biochimica et Biophysica Acta. 1997;1341:79–86. doi: 10.1016/s0167-4838(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Gerstel DU. Self-incompatibility studies in Guayule. Genetics. 1950;35:482–506. doi: 10.1093/genetics/35.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN. RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiology. 2008;147:852–863. doi: 10.1104/pp.108.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SA. Introduction of Oxford ragwort, Senecio squalidus L. (Asteraceae), to the United Kingdom. Watsonia. 2002;24:31–43. [Google Scholar]
- Hegarty M, Jones J, Wilson I, et al. Development of anonymous cDNA microarrays to study changes to the Senecio floral transcriptome during hybrid speciation. Molecular Ecology. 2005;14:2493–2510. doi: 10.1111/j.1365-294x.2005.02608.x. [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Barker GL, Wilson ID, Abbott RJ, Edwards KJ, Hiscock SJ. Transcriptome shock after interspecific hybridization in Senecio is ameliorated by genome duplication. Current Biology. 2006;16:1652–1659. doi: 10.1016/j.cub.2006.06.071. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Incompatibility and the pollen–stigma interaction. Annual Review of Plant Physiology. 1975;26:403–425. [Google Scholar]
- Heslop-Harrison Y. Stigma characteristics and angiosperm taxonomy. Nordic Journal of Botany. 1981;1:401–420. [Google Scholar]
- Heslop-Harrison Y. Control gates and micro-ecology: the pollen-stigma interaction in perspective. Annals of Botany. 2000;85:5–13. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. The receptive surface of the angiosperm stigma. Annals of Botany. 1977;41:1233–1258. [Google Scholar]
- Hiscock SJ. Self-incompatibility in Senecio squalidus L. (Asteraceae) Annals of Botany. 2000a;85(Suppl. A):181–190. doi: 10.1093/aob/mcr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ. Genetic control of self-incompatibility in Senecio squalidus L. (Asteracae): a successful colonizing species. Heredity. 2000b;85:10–19. doi: 10.1046/j.1365-2540.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytologist. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Coleman J, Dewey FM, Dickinson HG. Identification and localization of an active cutinase in the pollen of Brassica napus L. Planta. 1994;193:377–384. [Google Scholar]
- Hiscock SJ, Bown D, Gurr SJ, Dickinson HG. Serine esterases are required for pollen tube penetration of the stigma in Brassica. Sexual Plant Reproduction. 2002a;15:65–74. [Google Scholar]
- Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG. The stigma surface and pollen–stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. International Journal of Plant Sciences. 2002b;163:1–16. [Google Scholar]
- Hiscock SJ, McInnis SM, Tabah DA, Henderson CA, Brennan ACE. Sporophytic self-incompatibility in Senecio squalidus (Asteraceae) – the search for S. Journal of Experimental Botany. 2003;54:169–174. doi: 10.1093/jxb/erg005. [DOI] [PubMed] [Google Scholar]
- Hormazo JI, Herrero M. Pollen selection. Theoretical and Applied Genetics. 1992;83:663–672. doi: 10.1007/BF00226682. [DOI] [PubMed] [Google Scholar]
- Howlett BJ, Knox RB, Paxton JD, Heslop-Harrison J. Pollen wall proteins: physicochemical characterization and role in self-incompatibility in Cosmos bipinnatus. Proceedings of the Royal Society of London B. 1975;188:167–182. [Google Scholar]
- Hughes MB, Babcock EB. Self-incompatibility in Crepis foetida L. subsp. Rhoeadifolia Bieb. Schinz et Keller. Genetics. 1950;35:570–588. [PMC free article] [PubMed] [Google Scholar]
- James JK, Abbott RJ. Recent, allopatric, homoploid hybrid speciation: the origin of Oxford ragwort, Senecio squalidus (Asteraceae), in the British Isles. Evolution. 2005;59:2533–2547. [PubMed] [Google Scholar]
- Jennings DB, Ehrenshaft M, Pharr DM, Williamson JD. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proceedings of the National Academy of Sciences USA. 1998;95:15129–15133. doi: 10.1073/pnas.95.25.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Preuss D. On your mark, get set, GROW! LePRK2–LAT52 interactions regulate pollen tube growth. Trends in Plant Science. 2003;8:97–99. doi: 10.1016/S1360-1385(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Kim S, Mollet J-C, Dong J, Zhang K, Park S-Y, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RB. Pollen-wall proteins: pollen–stigma interactions in ragweed and Cosmos (Compositae) Journal of Cell Science. 1973;12:421–443. doi: 10.1242/jcs.12.2.421. [DOI] [PubMed] [Google Scholar]
- Kräuter-Canham R, Bronner R, Evrad JL, Hahne G, Friedt W, Steinmetz A. A transmitting tissue- and pollen-expressed protein from sunflower with sequence similarity to the human RTP protein. Plant Science. 1997;129:191–202. [Google Scholar]
- Lord EM. Adhesion and guidance in compatible pollination. Journal of Experimental Botany. 2003;54:47–54. doi: 10.1093/jxb/erg015. [DOI] [PubMed] [Google Scholar]
- Mayfield JA, Fiebig A, Johnstone SE, Preuss D. Gene families from the Arabidopsis thaliana pollen coat proteome. Science. 2001;292:2482–2485. doi: 10.1126/science.1060972. [DOI] [PubMed] [Google Scholar]
- McClure, et al. Compatibility and incompatibility in S-RNase-based systems. Manuscript #10622R of Special Edition. doi: 10.1093/aob/mcr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis SM, Costa LM, Gutiérrez-Marcos JF, Henderson CA, Hiscock SJ. Isolation and characterization of a polymorphic stigma-specific class III peroxidase gene from Senecio squalidus L. (Asteraceae) Plant Molecular Biology. 2005;57:659–677. doi: 10.1007/s11103-005-1426-9. [DOI] [PubMed] [Google Scholar]
- McInnis SM, Desikan R, Hancock JT, Hiscock SJ. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk. New Phytologist. 2006;172:221–228. doi: 10.1111/j.1469-8137.2006.01875.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Sun P, Kao T-h. S-RNase-based self-incompatibility in Petunia inflata. Annals of Botany. 2011;108 doi: 10.1093/aob/mcq253. 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy DL. The rise of angiosperms: a genecological factor. Science. 1979;206:20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Okuda T, Kondoh H. Identification of new genes Ndr2 and Ndr3 which are related to Ndr1/RTP/Drg1 but show distinct tissue specificity and response to N-myc. Biochemical and Biophysical Research Communications. 1999;266:208–215. doi: 10.1006/bbrc.1999.1780. [DOI] [PubMed] [Google Scholar]
- Park SY, Lord EM. Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Molecular Biology. 2003;51:183–189. doi: 10.1023/a:1021139502947. [DOI] [PubMed] [Google Scholar]
- Poulter NS, Bosch M, Franklin-Tong VE. Proteins implicated in mediating self-incompatibility-induced alterations to the actin cytoskeleton of Papaver pollen. Annals of Botany. 2011;108 doi: 10.1093/aob/mcr022. 659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmann MH, Uchiyama M, Kuno M, et al. Expression of stigma- and anther-specific genes located in the S locus region of Ipomoea trifida. Sexual Plant Reproduction. 2007;20:73–85. [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nature Reviews Genetics. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Tabah DA, McInnis SM, Hiscock SJ. Members of the S-receptor kinase multigene family in Senecio squalidus L. (Asteraceae), a species with sporophytic self-incompatibility. Sexual Plant Reproduction. 2004;17:131–140. [Google Scholar]
- Takayama S, Isogai A. Self-incompatibility in plants. Annual Review of Plant Biology. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- Vithanage HIMV, Knox RB. Development and cytochemistry of stigma surface and response to self and foreign pollination in Helianthus annuus. Phytomorphology. 1977;27:168–179. [Google Scholar]
- Yang B, Sugio A, White FF. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proceedings of the National Academy of Sciences USA. 2006;103:10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl GM, Preuss D. Dissecting Arabidopsis pollen–stigma interactions reveals novel mechanisms that confer mating specificity. Annals of Botany. 2000;85:15–21. [Google Scholar]
- Zinkl GM, Zweibel BI, Grier DG, Preuss D. Pollen–stigma adhesion in Arabidopsis: a species-specific interaction mediated by hydrophobic molecules in the pollen exine. Development. 1999;126:5431–5440. doi: 10.1242/dev.126.23.5431. [DOI] [PubMed] [Google Scholar]