Abstract
Background and Aims
Plants are sessile organisms that face selection by both herbivores and pollinators. Herbivores and pollinators may select on the same traits and/or mediate each others' effects. Erysimum capitatum (Brassicaceae) is a widespread and variable plant species with generalized pollination that is attacked by a number of herbivores. The following questions were addressed. (a) Are pollinators and herbivores attracted by similar plant traits? (b) Does herbivory affect pollinator preferences? (c) Do pollinators and/or herbivores affect fitness and select on plant traits? (d) Do plant compensatory responses affect the outcome of interactions among plants, pollinators and herbivores? (e) Do interactions among E. capitatum and its pollinators and herbivores differ among sites and years?
Methods
In 2005 and 2006, observational and experimental studies were combined in four populations at different elevations to examine selection by pollinators and herbivores on floral traits of E. capitatum.
Key Results
Pollinator and herbivore assemblages varied spatially and temporally, as did their effects on plant fitness and selection. Both pollinators and herbivores preferred plants with more flowers, and herbivory sometimes reduced pollinator visitation. Pollinators did not select on plant traits in any year or population and E. capitatum was not pollen limited; however, supplemental pollen resulted in altered plant resource allocation. Herbivores reduced fitness and selected for plant traits in some populations, and these effects were mediated by plant compensatory responses.
Conclusions
Individuals of Erysimum capitatum are visited by diverse groups of pollinators and herbivores that shift in abundance and importance in time and space. Compensatory reproductive mechanisms mediate interactions with both pollinators and herbivores and may allow E. capitatum to succeed in this complex selective environment.
Keywords: Compensation, Erysimum capitatum, Brassicaceae, evolutionary trade-off, floral traits, flower production, geographic mosaic, herbivory, multi-species interactions, natural selection, pollen limitation, pollination, resource limitation
INTRODUCTION
The evolution of reproductive traits in animal-pollinated plants has been increasingly viewed in light of natural selection by both pollinators and herbivores (Louda and Potvin, 1995; e.g. Armbruster, 1997; Herrera et al., 2002; Irwin et al., 2004; Armbruster et al., 2009; Gomez et al., 2009; Whittall and Carlson, 2009). Pollinators and herbivores can interact in several ways to simultaneously influence the evolution of plant traits. First, selection by herbivores can be so strong that it precludes the possibility of selection by pollinators (e.g. Herrera, 2000). For instance, selection by ungulates feeding on flowering stalks overrides selection by pollinators in both Erysimum mediohispanicum and Hormathophyllum spinosum (Gomez and Zamora, 2000; Herrera et al., 2002; Gomez et al., 2009). Secondly, the same or correlated traits can be under selection by both pollinators and herbivores, producing adaptive compromises and exaptations (Galen and Cuba, 2001; Ehrlen, 2002; Irwin et al., 2003; Adler and Bronstein, 2004; Armbruster et al., 2009). For example, ants which reduced fitness and bumblebees which increased fitness in Polemonium viscosum preferred similar floral characteristics, producing opposing selective effects (Galen and Cuba, 2001; Galen and Butchart, 2003). Thirdly, herbivory can alter plant attractiveness to pollinators directly, by damaging attractive structures, or indirectly, through plant responses to damage (e.g. Juenger and Bergelson, 1997; Krupnick et al., 1999; McCall and Irwin, 2006; McCall, 2008, 2010). Pollinators often prefer flowers that are undamaged, and in many cases any form of floral damage may reduce pollinator visitation (Adler, 2000; McCall, 2010).
Plant responses to herbivory may either limit or exacerbate the effects of damage. Herbivory may result in compensatory responses such as increased flower production (Paige and Whitham, 1987; Agrawal, 2000; Juenger et al., 2000), reducing any negative effects of damage on plant–pollinator interactions. However, plants also may reduce flower production when damaged (Krupnick et al., 1999; Sharaf and Price, 2004). Krupnick et al. (1999), for example, found that floral herbivory caused plants to produce fewer flowers and receive fewer pollinator visits. Such developmental responses are critical components of the complex interplay among plants, pollinators and herbivores, but rarely have been addressed in multiple populations in the context of both pollinator visitation and herbivory.
Pollinator and herbivore assemblages often vary among plant populations (Herrera, 1988; Kearns, 1992; Dilley et al., 2000; Rand, 2002; Bradley et al., 2003; Aigner, 2005; Gomez et al., 2010) and can create geographically distinct selection regimes that result in phenotypic differentiation among plant populations (Thompson, 1988; Rey et al., 2006; Gomez et al., 2009). This may be particularly true for plant species that occupy montane regions, which are very heterogeneous over short distances in both micro-climate and co-occurring species (reviewed in Lomolino, 2001). In addition, elevational gradients often have been associated with population differentiation in plants (Linhart and Grant, 1996). For this reason, mountainous regions provide excellent opportunities to examine the effects of different pollinator and herbivore communities on plant traits.
Observational and experimental studies across an elevational gradient in the Colorado Rocky Mountains were combined to examine natural selection by pollinators and herbivores on traits of Erysimum capitatum. Erysimum capitatum is a widespread native species that varies in flower colour, flower number, size, and other characteristics throughout its range (Price, 1984). Preliminary observations of this species suggested it was visited by many different pollinators and herbivores, and that the composition of pollinator and herbivore communities varied among sites. The following questions were addressed. (a) Are pollinators and herbivores attracted by similar plant traits? (b) Does herbivory affect pollinator preferences? (c) Do pollinators and/or herbivores affect fitness and select on plant traits? (d) Do plant compensatory responses affect the outcome of interactions among plants, pollinators and herbivores? (e) Do interactions among E. capitatum and its pollinators and herbivores differ among sites and years?
MATERIALS AND METHODS
Study species
Erysimum capitatum (Brassicaceae) is native throughout most of the United States (USDA, 2004) and northern Mexico in lowlands and montane areas (Turner, 2006). It is often a biennial, although life history varies among individuals and populations, and requires insect visitation for full fruit set (Price, 1984). Plants flower from early spring to mid-summer (USDA, 2004), and populations of E. capitatum are characterized by variation in flower colour and other traits across the species range (Price, 1984; Weber, 1990). Flowers may be white, lavender, or range from yellow to red (Price, 1984). All populations and individuals studied are recognized as a single species. The taxonomy and identification of E. capitatum is currently under debate, however, and the great phenotypic variation within this species may be the result of hybridization events between subspecies (Turner, 2006).
Populations
Four study populations were located in the Front Range of the eastern slope of the Rocky Mountains of Colorado. The Elk Meadow population (ELK) is in a subalpine dry meadow at 2900 m. The population at Nederland (NED) is in open lower montane Ponderosa pine forest at 2590 m. The Walker Ranch population (WAK) is in open lower montane Ponderosa pine forest at 2209 m. The Greenbelt Plateau population (GBT) is in a short-grass prairie at 1980 m. Complete descriptions of these vegetation types are in Marr (1967). In the lowest-elevation population, GBT, flowering begins as early as March, and continues through early June. In the highest-elevation population, flowering begins in late June and continues through mid-July, though some plants have flowers as late as August. At each location, a combination of observational and experimental studies was performed.
Observational study and selection analysis
To examine the nature of pollinator and herbivore preferences, and their relationship with fitness, plant traits, pollinator and herbivore visitation, plant fitness in the four study populations was measured on 50–200 haphazardly selected flowering plants in 2005 and 2006. Sample sizes differed among populations and years for several reasons. First population size and density differed both among populations and between years. In addition, an attempt was made to choose plants before they had bolted in 2006 in NED and WAK – some of these plants did not bolt.
Phenotypic characters
Corolla width, corolla tube depth, petal colour, number of open flowers, stem diameter and the total number of flowers that had opened by harvest were measured. The count variables (open and total flowers) were square-root transformed to improve normality.
Flower colour was quantified by comparing flowers to colour chips in the field, and the accuracy of this method confirmed using digital photographs and standardized RGB colour values. Study populations had no individuals with white or lavendar flowers, so yellower colour was quantified as a higher number and redder colour as a lower number. Flower width and flower depth were measured in the field with digital calipers. Width was the diameter of the flower from the tip of one corolla lobe to the tip of the opposite lobe, and depth was the distance from the base of the calyx to the angle between the claw and the limb of the corolla. Flowers were considered to be ‘open’ if they retained at least three petals (flowers with damaged limbs were counted as ‘open’ if they retained at least three attached claws). Inflorescence height was measured from the ground to the apex of the tallest inflorescence. Heights were measured at harvest so that all plants would be at the same developmental stage. Stem diameter was measured 30 mm above the ground on the tallest inflorescence.
Measurements of plant characteristics were repeated on up to 4 d, or on multiple flowers per day, though it was not possible to obtain equal repetitions of all measurements on all plants due to weather and flower availability. In 2005, corolla width and depth were measured for the oldest and newest open flowers, and one haphazardly chosen flower in between. When plants had three or fewer open flowers, all were measured. Preliminary analyses of 2005 data suggested that these multiple measures were unnecessary, as the difference in floral characters among plants was much greater than differences in floral measurements on the same plant, so in 2006, corolla width and depth were measured on one flower per plant.
Multiple measurements for all phenotypic characters were collapsed into a single measurement per character per plant. For characters such as corolla width, where differences among measurements due to intraplant variation or measurement error were likely to be the biggest contributors to within-plant measurement differences, the averages of all measurements per plant were used as the character values. For characters such as inflorescence height, for which the greatest contributor to differences among measurements was likely to be plant growth, the maximum recorded values for each plant were used as the character values.
Sampling schemes differed slightly between years. In 2005, an attempt was made to choose plants that would have open flowers throughout the planned period of pollinator observations. In 2006, sample sizes were increased in many sites. Because of this change, plants were larger, on average, in 2005 than in 2006. This may have changed estimated effects of plant size variables if there were non-linear effects of size characters, which were not tested for.
Insect visitation
To quantify pollinator preferences and the relationships between visitation and fitness, individual observers watched groups of one to ten plants for 10-min periods between 0900 h and 1500 h, which covered peak visitation times. Observations of each plant were repeated twice per day for as many days as possible given weather, and availability of flowering plants (total number of minutes per population are in Supplementary Data Table S1, available online). Visitors were identified to functional groups (described below) to reduce training time for observers, and insects were not collected until all observations had been completed to avoid influencing visitation on subsequent days. Ants were included regardless of where on the plant they were observed because tracked individual ants always collected nectar but rarely contacted reproductive parts.
Visitors were assigned to the following functional groups: large bees, medium-sized bees, small bees, flies, pollen beetles, and ants. Because there were very few visitors from some bee size classes, all bee visitors were added together in the final analysis.
The number of observations differed among plants due to weather and individual plant flowering times. To incorporate the number of observations into a measure of visitation by each functional group, we added 0·10 to the total count of visitors from a particular functional group and divided by the number of observations carried out, then log-transformed this measure of visitation to improve normality. Any functional groups for which 26 or fewer visits were observed in a given year and site were excluded from analyses because it was determined that this sample size was too small to make meaningful estimates of parameters.
Herbivory
Associations between herbivore damage, plant traits and fitness were studied. Three general types of herbivory were common at all sites: petal damage, flower bud galls and nectar robbing by ants. A fourth type of herbivore, coleopteran stem borers, was common only at WAK.
A variety of petal herbivores created visually similar damage, so there was no reliable way to determine what herbivore had damaged a flower and petal damage from all consumers was measured together. Damage levels for individual flowers were recorded. Petal limb damage was scored 1–4, and 5 indicated complete destruction of anthers and stigma. Level ‘1’ corresponded to 10 % damage or less, ‘2’ to damage between 10 % and 25 %, ‘3’ to 25–50 %, ‘4’ to 50 % or more. Then the sum of all damage scores was log-transformed from all flowers for each plant for the day of the season with the most petal damage.
Gall midge larvae (Cecidomyiidae) form galls from flower buds and typically prevent flowers from opening. Flower bud galls were counted on each observation day and the log-transformed maximum count for galls found on a single day over the observation period used in analyses because galls remained on the plant throughout observations.
Nectar removal by ants was measured as the number of ant visitors observed, as explained in the previous section.
Stem borers were unidentified beetle larvae that typically killed whole inflorescences. When this type of herbivory could be distinguished from mammalian herbivory, it was included in analyses.
Mammalian herbivores, chiefly pocket gophers and ruminants, damaged standing inflorescences in the three highest elevation locations (WAK, NED and ELK). Because both ruminants and gophers often removed tags or rendered them illegible, it was difficult to tell whether plants were still alive but had lost identifying information or if they had been killed by mammalian herbivores. Therefore, study plants were removed from analysis whether they were clearly affected by mammalian herbivores or if their tags could not be found. Overall, between 10 and 25 plants were removed from analyses for WAK, NED and ELK in each year.
Fitness
Plants were collected after senescence. The total number of flowers produced by plants was obtained by counting fruits and scars of flowers that reached anthesis but aborted. To estimate female fitness, the lengths of each silique were measured and added together. The number of seeds per silique is correlated (R2 = 0·69, P < 0·01) to the length of the siliques (C. Lay, unpubl. res.), and using this measure allowed the fitness contribution of dehisced siliques to be measured. Estimated fitness was total silique length; this value was log-transformed to improve normality.
Analysis of visitation, herbivory and fitness
For each population and year where there were sufficient visitation data, structural equation modelling (SEM) without latent variables was used to examine the relationship between visitation, herbivory and plant traits. An inclusive model was created and nested models were compared with more inclusive ones. Because GBT 2005 was missing all fitness data, it was impossible to run multigroup analysis (Grace, 2006; Rey et al., 2006) to determine whether the same model would apply to all populations. Preliminary analysis suggested that populations and years were too dissimilar in the types of visitors and herbivores present to use the same model for all populations and years, and the saturated model was unidentified in some populations and years. Because of these issues, separate most-inclusive models were created for each population and year. The most-inclusive models were intended to be as similar to one another as possible, given population differences. The most-inclusive models for each population are shown next to the best-fit models in the results. All structural equations modelling was performed in the sem package in R (Fox et al., 2010), which uses full-information maximum likelihood to estimate path strengths. Covariance matrices were calculated using pairwise complete observations, and standardized path coefficients were interpreted. Independent variables were centred (by subtracting the mean) to allow comparison of parameter estimates among populations, and some were transformed to improve normality. For each population, the simplest model that had a significantly lower X2-value than simpler models was chosen. Only significant paths are included in Figs 1–5. Models with lower X2-values are a better fit to the data than models with higher X2-values. Although transformations improved normality of the variables on which they were used, the transformed variables are not always normally distributed and this can affect the validity of P-values (Tabachnick and Fidell, 1996b). Therefore, the best-fit models are shown even when the X2-value is significant and include corrected values of Akaike's information criterion (AICc) to aid in interpretation.
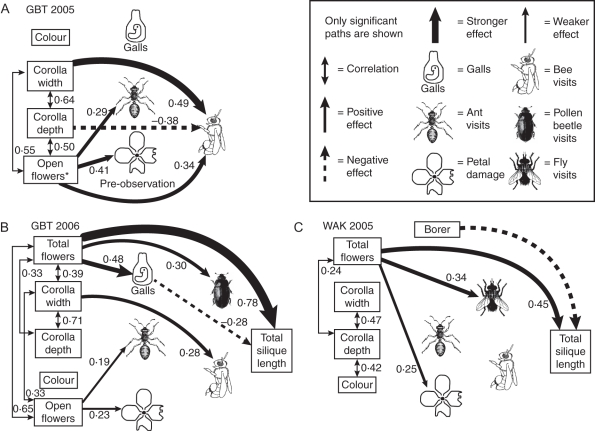
Fig. 1.
Best-fit models from structural equations analysis of GBT 2005 (A), GBT 2006 (B) and WAK 2005 (C). Numbers represent standardized path coefficients. (A) In GBT 2005, the number of open flowers (*) and petal damage were measured on the day before beginning observations. Data on galls and insect visitation were collected between 17 May and 26 May on 75 plants not included in experimental treatments. Neither total silique length nor total flowers could be measured because all plants were trampled by cattle. (B) In GBT 2006, measurements were taken for 100 plants not included in experimental treatments between 15 May and 23 May. (C) In WAK 2005 data were taken for 100 plants not included in experimental treatments between 1 June and 23 June; petal damage and open flowers were measured on 50 of these plants.
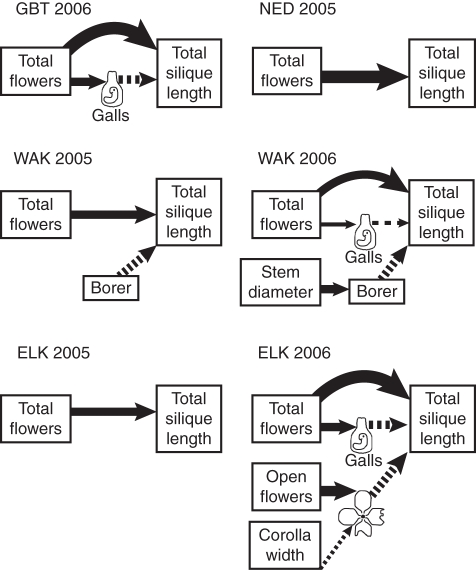
Fig. 5.
Summary diagram of individual path analyses that showed effects of plant traits and insects on fitness. There was no best-fit model that included fitness for either NED 2006 or GBT 2005. See Fig. 1 for explanation of symbols.
Experimental manipulations
To test the importance of pollinator visitation and herbivory to plant fitness and any joint effects of herbivores and pollinators, pollen and insecticide were added to 50–200 haphazardly selected plants in 2005 and 2006. Where possible, four treatments were used in a fully factorial two-way design: control, hand pollination, insecticide application, and insecticide application plus hand pollination. In GBT and ELK, insecticide was not applied due to environmental concerns, and only hand pollination and control groups were included. Plants were haphazardly assigned to treatments, and some were lost throughout the season. To add pollen, anthers were collected from 10–15 nearby plants and mixed in a glass vial. Then the pollen was applied to receptive stigmas with a paintbrush. Vials were replenished as needed from nearby plants not included in observations or experimental treatments. Insecticide was added to plants before observations and pollinations began. In 2005, pollen was added every other day, but greenhouse experiments showed that pollinating every third day was sufficient to pollinate all the flowers on a plant, so pollen was added every third day in 2006.
In 2005 and 2006, different insecticides were used. In 2005, a topical insecticide spray consisting of 0·5 % fenbutatin and 8 % acephate was used, and all other plants were sprayed with water to simulate insecticide addition. This treatment was found to be not very effective in deterring herbivory and a systemic insecticide (Marathon 1 % granular imadocloprid) was added and watered in to the bases of plants at the beginning of flowering (some plants had already bolted) in 2006. In GBT and ELK only pollen addition was performed. Estimated female fitness was measured as in the observational study. For experimental plants, the average length of siliques produced by each plant, the total number of flowers and the number of aborted flowers were also calculated. Counts of total and aborted flowers were square-root transformed to improve normality in all populations and years, and average silique length was square-root transformed where it was variable enough to create non-normal distributions of residuals.
Analysis of experimental results
Analysis of covariance (ANCOVA) with height of the tallest inflorescence at harvest a covariate was used to test the effects of treatment and, in some populations galls, on total fitness.
Analysis of compensatory response
To determine whether plants arrived at similar fitness outcomes through variation among the components of total silique length (total flowers, aborted flowers, and average silique length), these were included as dependent variables in a multivariate analysis of covariance (MANCOVA), with height as a covariate. In populations where the number of galls present on experimental plants was measured, the count was included as a covariate and square-root transformed when it was variable enough to create non-normal distributions of residuals. Inflorescence height at harvest was centred by subtracting the mean from all values. The MANCOVA allowed independent variables that affected any of the fitness components to be identified so the nature of those changes could be examined in further univariate stepdown analyses.
Because the purpose of the MANCOVA was to determine which variables to include in univariate stepdown analyses, the MANCOVA significance tests of type II sums of squares were interpreted. Significance tests of type II sums of squares are valid for all effects in models that have no significant interaction terms, and for interaction terms in models with significant interactions. They are sometimes preferable because main effects are not calculated while controlling for interactions (Fox, 2008). Potential multivariate outliers were identified using the mvoutlier package in R (Gschwandtner and Filzmoser, 2009), and univariate outliers using plot from the base package (R Development Core Team, 2010). Although there were several apparent outliers, they did not affect the outcome of significance tests so no observations were removed.
When there were significant effects of independent variables on the dependent variables, those independent variables were included in univariate ANCOVA stepdown analyses for each dependent variable. This procedure was based on Roy–Bargmann stepdown analysis (Tabachnick and Fidell, 1996a). Stepdown analysis is used to determine how independent variables affect dependent variables. Dependent variables of most interest or importance (in this case aborted flowers) are regressed on independent variables, and are then included as independent variables in analyses of dependent variables of lesser interest. Aborted flowers were analysed first, then total flowers, then average silique length. Observations were included even if it was not possible to measure one of the dependent variables, so in some cases, the sample size is different for different variables. Significance values from type III sums of squares were used in the stepdown analysis to improve interpretation of main effects. Stepdown analyses were performed with glm in R (R Development Core Team, 2010). Because Bonferonni adjustments did not change the interpretation of significance levels for total flowers and aborted flowers, and average silique length was not strongly correlated with the other two dependent variables (Supplementary Data Table S2), non-adjusted P-values were interpreted.
The details of sampling and analysis differed slightly among populations for both observational and experimental studies. These differences are explained in the results in figure and table captions for each year and population.
RESULTS
Summary of results for pollinator and herbivore preferences for each population and year
Pollinator and herbivore preferences (observation)
The best-fit models produced by observational study were used to determine pollinator and herbivore preferences and relationship between herbivory and pollinator visitation. Comparisons of most-inclusive and best-fit models for each site and year are shown in Table 1.
Table 1.
Model-fit statistics for null, most-inclusive and best-fit models in observational study
| Model | X2 | d.f. | P | AICc | |
|---|---|---|---|---|---|
| GBT 2005 | Null | 102·69 | 28 | ||
| Inclusive | 11·08 | 6 | 0·08 | 167·75 | |
| Best-fit | 25·35 | 19 | 0·15 | 76·89 | |
| GBT 2006 | Null | 317·63 | 55 | ||
| Inclusive | 29·08 | 17 | 0·03 | 217·54 | |
| Best-fit | 54·03 | 40 | 0·07 | 123·36 | |
| WAK 2005 | Null | 90 | 45 | ||
| Inclusive | 33·96 | 15 | <0·01 | 155·28 | |
| Best-fit | 47·3 | 30 | 0·03 | 109·64 | |
| WAK 2006 | Null | 412·35 | 66 | ||
| Inclusive | 102·64 | 28 | <0·001 | 252·13 | |
| Best-fit | 122·99 | 39 | <0·001 | 227·94 | |
| NED 2005 | Null | 291·13 | 45 | ||
| Inclusive | 25·08 | 14 | 0·03 | 162·67 | |
| Best-fit | 36·52 | 31 | 0·23 | 99·05 | |
| NED 2006 | – | ||||
| ELK 2005 | Null | 170·55 | 45 | ||
| Inclusive | 33·87 | 14 | <0·01 | 190·41 | |
| Best-fit | 52·35 | 34 | 0·02 | 107·48 | |
| ELK 2006 | Null | 374·33 | 55 | ||
| Inclusive | 38·44 | 17 | <0·001 | 230·57 | |
| Best-fit | 61·76 | 36 | 0·004 | 146·98 |
The null model contains only error variances and can be thought of as the least-inclusive model. Lower X2-values indicate better-fitting models.
Fitness effects of pollinators and herbivores (observation and experiment)
Best-fit models of data from the observational study show selection (paths that link traits to fitness, sometimes through pollinator visitation and herbivory). Pollinators and herbivores can affect fitness (total silique length) without selecting on measured plant traits if there are no links between traits and visitation. Paths that go from traits to pollinator visitation and herbivory to fitness are taken as evidence of selection by visitors on particular traits. Fit statistics, including AICc, for complete sets of tested models are given in Supplementary Data Tables S3 and S4 as are the most-inclusive models for each population and year (Supplementary Data Figs S1 and S2). Experimental additions of pollen and insecticide also identified fitness effects of pollinators and herbivores. Main effects of pollen, insecticide and plant size, as well as significant interaction terms, are detailed in the following sections, and full tables of the ANCOVA analyses are in Supplementary Data Table S5.
GBT 2005 (Fig. 1A)
Pollinator and herbivore preferences
Plants with more flowers at the beginning of the study received more bee visits and ant visits and had sustained more petal damage before observations began. Plants with wider, shallower flowers received more bee visits, but these characters did not affect herbivory.
Fitness effects of pollinators and herbivores
Plants were trampled by cattle and neither the fitness of observational plants nor the effect of experimental pollen manipulations could be assessed.
GBT 2006 (Fig. 1B)
Pollinator and herbivore preferences
Bees preferred plants with wider flowers. Plants with more flowers produced over the course of the season received more beetle visits but also had more galls. Ants preferred plants with more open flowers, and plants with more open flowers had more petal damage, but the number of open flowers did not affect pollinator visitation.
Fitness effects of pollinators and herbivores
Estimated fitness was directly increased by total number of flowers (Fig. 1B). Plants with more total flowers had more galls, and plants with more galls were less fit than plants with fewer galls (Fig. 1B). There were no effects of ants, petal damage or pollinator visitation on fitness (Fig. 1B). Experimental treatments (data from 47 supplemental pollen and 45 control plants were used) showed that pollination did not change fitness as measured by total silique length (β = 0·29, F1,88 = 0·10, P = 0·37) when controlling for height (β = 0·11, F1,88 = 41·99, P < 0·0001). Insecticide was not applied at this site; treatments included only control and supplemental pollen addition.
WAK 2005 (Fig. 1C)
Pollinator and herbivore preferences
Fly visits and petal damage were positively related to the total number of flowers. Galls were not common enough to measure and preferences of borers were not tested.
Fitness effects of pollinators and herbivores
Plants with more total flowers had greater fitness (Fig. 1C). Stem borers reduced fitness (Fig. 1C), but their preferences were not measured in this year, so there is no evidence for selection by borers on any measured trait. No effects of ants, petal damage or pollinator visitation on fitness were found (Fig. 1C), and galls were not common enough to include in this year.
Experimental treatments (data from 26 control, 30 supplemental pollen, 24 insecticide and 25 supplemental pollen and insecticide plants were used) showed that fitness was not limited by pollen availability (β = –0·06 F1,97 = 0·11, P = 0·97). Fitness increased with height (β = 0·06, F1,97 = 35·76, P < 0·0001) but did not change with insecticide application (β = 0·18, F1,97 = 1·51, P = 0·22).
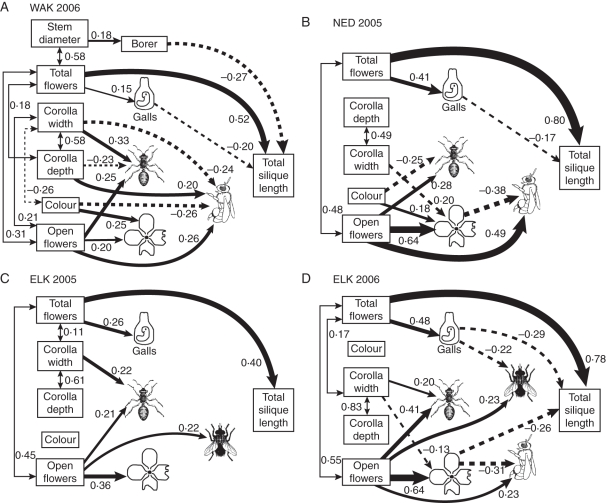
WAK 2006 (Fig. 2A)
Fig. 2.
Best-fit models from structural equations analysis of WAK 2006 (A) NED 2005 (B), ELK 2005 (C) and ELK 2006 (D). Numbers represent standardized path coefficients. (A) In WAK 2006, data were collected on 270 plants, including plants treated with supplemental pollen when it was shown not to affect fitness, were taken between 3 June and 20 June. (B) In NED 2005, measurements were taken on 99 plants not included in experimental treatments between 18 June and 30 June. (C) In ELK 2005 data were collected on 100 control plants between 1 July and 20 July. (D) In ELK 2006, data were collected on 187 plants, including plants treated with supplemental pollen between 20 June and 30 July. For 98 plants on which pollen was not added, insect visitation was observed. See Fig. 1 for explanation of symbols.
Pollinator and herbivore preferences
Plants with more open flowers had more bee visits, ant visits and damage. Bees preferred redder, narrower, deeper flowers, while ants preferred wider, shallower flowers. Plants with more total flowers had more galls. Borers tended to attack plants with thicker stems.
Fitness effects of pollinators and herbivores
Total flowers increased fitness (Fig. 2A). Stem borers preferred thicker stems and reduced fitness. Therefore, borers select for plants with narrower stems (Fig. 2A). There was no selection by pollinators, ants or petal herbivores (Fig. 2A). Analysis of effects of experimental treatments on total fitness included 51 control, 22 supplemental pollen, 53 insecticide and 23 supplemental pollen and insecticide plants for which total fruit length could be measured. Galls were included as a predictor variable in analyses of treatment effects. In general, female reproduction was not pollen limited (β = 0·43, F1,140 = 0·90, P = 0·35), although supplemental pollen improved fitness for plants with galls (β = 0·45, F1,140 = 9·07, P < 0·01). Fitness decreased with galls (β = –0·18, F1,140 = 18·93, P < 0·0001). There was no main effect of insecticide (β = 0·06, F1,140 = 0·08, P = 0·77), but fitness improvements associated with height at harvest (β = 0·12, F1,140 = 55·06, P < 0·0001) increased on plants treated with insecticide (β = 0·12, F1,140 = 12·67, P < 0·001).
NED 2005 (Fig. 2B)
Pollinator and herbivore preferences
Bees and ants preferred plants with more flowers, and damage was more common on those plants. In addition, ants visited flowers that were wider and redder more frequently. Petal damage was higher on plants with wider, yellower flowers. Galls were more common on plants that produced more flowers overall. Although both bee visits and petal damage were associated with greater numbers of open flowers, bees were less likely to visit plants with petal damage.
Fitness effects of pollinators and herbivores
Fitness increased with total flowers but was not otherwise affected by variables included in models (Fig. 2B). Experimental treatments included 27 control plants, 26 supplemental pollen plants, 27 insecticide plant and 24 plants to which both pollen and insecticide were added. In these plants, fitness increased with height at harvest (β = 0·04, F1,96 = 47·47, P < 0·0001). There were no effects of pollination (β = 0·11, F1,96 = 0·45, P = 0·50) or insecticide (β = –0·01, F1,96 = 0·08, P = 0·92).
NED 2006
Pollinator and herbivore preferences
Observations of visitors were precluded by wet and cool weather in this year and site, so preferences were not modelled.
Fitness effects of pollinators and herbivores
Preferences of pollinators and herbivores were not measured in this year, and observation plants were used as controls in experimental treatments due to poor weather conditions for insect observations. Experimental treatments included 22 control plants, 20 supplemental pollen plants, 22 insecticide plants and 21 plants to which both pollen and insecticide were added. Galls were included as a predictor variable in analyses of treatment effects. Experimental results showed that fitness increased with height at harvest (β = 0·11 F1,75 = 57·76, P < 0·0001) and decreased with galls (β = –0·21, F1,75 = 22·52, P < 0·0001), but was not affected by supplemental pollination (β = –0·09, F1,75 = 0·24, P = 0·63) or insecticide (β = –0·23, F1,75 = 1·37, P = 0·25).
ELK 2005 (Fig. 2C)
Pollinator and herbivore preferences
Too few bees visited to be included in this year. Flies and ants visited plants with more open flowers, and these plants had more petal damage. Flies visited plants with yellower flowers and ants visited plants with wider flowers more often. Galls were positively associated with total flowers.
Fitness effects of pollinators and herbivores
Fitness was affected only by the total number of flowers (Fig. 2C). Weather and phenology precluded pollination treatments in this site and year.
ELK 2006 (Fig. 2D)
Pollinator and herbivore preferences
Flies, bees and ants preferred plants that had more flowers on observation days. Ants visited plants with wider flowers more frequently. More damage occurred on plants that had narrower flowers. Flies were less likely to visit plants with galls, and bees less likely to visit plants with substantial petal damage.
Fitness effects of pollinators and herbivores
Petal damage was associated with reduced fitness (Fig. 2D). Plants with more total flowers had more galls and galls reduced fitness (Fig. 2D). Experimental treatments included 81 control plants and 76 plants to which supplemental pollen was added. Insecticide was not used in this site. Galls were included as a predictor variable and were variable enough to require square-root transformation. For experimental plants, fitness decreased with galls (β = –0·33, F1,151 = 10·53, P < 0·01). Pollination did not improve fitness (β = 0·51, F1,151 = 3·09, P > 0·05). The positive effect of height (β = 0·08, F1,151 = 53·14, P < 0·0001) was reduced by added pollen (β = –0·09, F1,151 = 6·45, P < 0·05), suggesting shorter plants may have been pollen limited although taller plants were not.
Analysis of plant compensatory responses to herbivory and pollination (experiment)
To determine how plant compensatory responses contributed to fitness in the manipulative experiments, we tested how inflorescence height at harvest, galls, supplemental pollination and insecticide changed at least one of the fitness components including flower abortion, flower production and allocation to individual fruits (aborted flowers, total flowers and average silique length). This was done using MANCOVA and stepdown analysis for three components of fitness (total flowers, aborted flowers and average silique length) from experimental plants. Sample sizes differ slightly between dependent variables in stepdown analyses, and from sample sizes for total fitness because for some plants average silique length could not be calculated, and for others, the number of aborted flowers was unclear. Fruit number was not included because the numbers of fruit and aborted flowers together determine total flower number. Full tables of MANCOVA results are in Supplementary Data Table S6.
GBT 2005
Plants were trampled by cattle and no fitness components were measured.
GBT 2006
When controlling for inflorescence height at harvest (Wilk's λ = 0·78, P < 0·0001), there was no effect of pollination (Wilk's λ = 0·98, P = 0·69) on total flowers, aborted flowers or average silique length. Even though the interaction between pollination and height did not significantly affect dependent variables (Wilk's λ = 0·91, P > 05), it was tested in the univariate stepdown analysis. Inflorescence height at harvest was associated with an increase in the number of aborted flowers, total flowers and average silique length (Table 2). Main effects of pollination on flower abortion were not significant, but supplemental pollen decreased the positive association between height at harvest and aborted flowers (Table 2). The number of total flowers increased with aborted flowers (Table 2).
Table 2.
Stepdown results for fitness components in GBT 2006
| Height | Pollination | Pollination* height | Aborted flowers | Total flowers | |
|---|---|---|---|---|---|
| Aborted flowers | β = 0·10 | β = 0·02 | β = –0·14 | – | – |
| F1,88 = 20·29 | F1,88 < 0·01 | F1,88 = 5·01 | |||
| P < 0·0001 | P = 0·97 | P = 0·03 | |||
| Total flowers | β = 0·11 | β = 0·32 | β = –0·06 | β = 0·39 | – |
| F1,87 = 50·10 | F1,87 = 1·46 | F1,87 = 1·72 | F1,87 = 31·81 | ||
| P < 0·0001 | P = 0·23 | P = 0·19 | P < 0·0001 | ||
| Average silique length | β = 1·04 | β = –0·62 | β = –0·71 | β = –2·43 | β = 1·81 |
| F1,86 = 10·76 | F1,86 = 0·02 | F1,86 = 1·12 | F1,86 = 3·76 | F1,86 = 1·17 | |
| P < 0·01 | P = 0·88 | P = 0·29 | P > 0·05 | P = 0·28 |
All fitness components increase with inflorescence height at harvest. Total flowers increase with aborted flowers, and pollination reduces the number of aborted flowers on tall plants. β is the standardized regression coefficient, or effect size, for each relationship.
WAK 2005
Height at harvest (Wilk's λ = 0·47, P < 0·0001) and insecticide (Wilk's λ = 0·88, P < 0·01) significantly affected one or more of the three fitness components, but neither pollination (Wilk's λ = 0·99, P = 0·98) nor the interaction between pollination and insecticide (Wilk's λ = 0·98, P = 0·72) had an effect. Height at harvest was associated with an increase in the number of aborted flowers, total flowers and average silique length (Table 3). Flower production was higher on plants with more aborted flowers and on plants treated with insecticide (Table 3).
Table 3.
Stepdown results for fitness components in WAK 2005
| Height | Insecticide | Aborted flowers | Total flowers | |
|---|---|---|---|---|
| Aborted flowers | β = 0·03 | β = 0·27 | – | – |
| F1,102 = 8·41 | F1,102 = 2·69 | |||
| P < 0·01 | P = 0·10 | |||
| Total flowers | β = 0·08 | β = 0·48 | β = 0·40 | – |
| F1,101 = 70·22 | F1,101 = 10·60 | F1,101 = 20·30 | ||
| P < 0·0001 | P < 0·01 | P < 0·0001 | ||
| Average silique length | β = 0·69 | β = –1·57 | β = –1·07 | β = –1·02 |
| F1,100 = 15·05 | F1,100 = 0·53 | F1,100 = 0·64 | F1,100 = 0·55 | |
| P < 0·001 | P = 0·47 | P = 0·43 | P = 0·46 |
All fitness components increased with inflorescence height at harvest. Total flowers increased with insecticide and aborted flowers.
WAK 2006
Average silique length of these data was square-root transformed to improve normality. Galls (Wilk's λ = 0·81, P < 0·0001) and the interaction between insecticide and height at harvest (Wilk's λ = 0·90, P < 0·01) affected the dependent variables. The interaction between pollination and galls was tested in the post-hoc stepdown analysis, even though it did not significantly influence variables (Wilk's λ = 0·94, P = 0·08), because it affected total silique length. Total sample sizes are greater for the analyses of total flower number and aborted flowers than for the analyses of total fitness because broken siliques precluded measurement of total fitness. Height at harvest was associated with an increase in total flowers and average silique length (Table 4). Galls increased the number of aborted flowers, and decreased average silique length, but pollination attenuated the negative effects of galls on silique length (Table 4). Total flowers increased with the number of aborted flowers (Table 4). Insecticide increased the positive effects of height on silique length.
Table 4.
Stepdown results for WAK 2006
| Height | Insecticide | Pollination | Galls | Insecticide × height | Pollination × galls | Aborted flowers | Total flowers | |
|---|---|---|---|---|---|---|---|---|
| Aborted flowers | β = –1·88 × 10−3 | β = 0·22 | β = –0·13 | β = 0·10 | β = –5·8 × 10−3 | β = 0·12 | – | – |
| F1,158 = 0·02 | F1,158 = 1·97 | F1,158 = 0·09 | F1,158 = 7·12 | F1,158 = 0·046 | F1,158 = 0·70 | |||
| P = 0·89 | P = 0·16 | P = 0·76 | P < 0·01 | P = 0·83 | P = 0·40 | |||
| Total flowers | β = 0·10 | β = 0·03 | β = 0·09 | β = –0·01 | β = 0·03 | β = –0·02 | β = 0·61 | – |
| F1,157 = 7·12 | F1,157 = 0·10 | F1,157 = 0·11 | F1,157 = 0·36 | F1,157 = 2·43 | F1,157 = 0·04 | F1,157 = 144·89 | ||
| P < 0·01 | P = 0·74 | P = 0·75 | P = 0·55 | P = 0·12 | P = 0·84 | P < 0·0001 | ||
| Average silique length | β = 0·09 | β = 0·07 | β = –1·39 | β = –0·31 | β = 0·12 | β = 0·72 | β = –5·8 × 10−3 | β = –0·09 |
| F1,122 = 6·59 | F1,122 = 0·05 | F1,122 = 2·64 | F1,122 = 14·75 | F1,122 = 4·33 | F1,122 = 7·82 | F1,122 = 7 × 10−4 | F1,122 = 0·11 | |
| P = 0·01 | P = 0·82 | P = 0·11 | P < 0·001 | P = 0·03 | P < 0·01 | P = 0·98 | P = 0·74 |
Height at harvest increased total flowers and silique length but not aborted flowers. Some siliques were broken at harvest and it was impossible to count floral scars to determine the number of aborted and total flowers on some plants so sample sizes were smaller for measures of silique length. Supplemental pollen attenuated the negative relationship between galls and silique length.
NED 2005
Insecticide (Wilk's λ = 0·87, P < 0·01) and height at harvest (Wilk's λ = 0·51, P < 0·001) significantly affected the three dependent variables, but pollination did not (Wilk's λ = 0·71, P = 0·55). Insecticide decreased aborted flowers (Table 5). Height at harvest increased total flowers (Table 5). Total flowers increased with aborted flowers (Table 5). Silique length increased with aborted flowers and height at harvest (Table 5).
Table 5.
Stepdown results for NED 2005
| Height | Insecticide | Aborted flowers | Total flowers | |
|---|---|---|---|---|
| Aborted flowers | β = 0·01 | β = –0·44 | – | – |
| F1,101 = 3·18 | F1,101 = 9·44 | |||
| P = 0·08 | P < 0·01 | |||
| Total flowers | β = 0·06 | β = –0·29 | β = 0·35 | – |
| F1,100 = 83·34 | F1,100 = 2·75 | F1,100 = 143·73 | ||
| P < 0·0001 | P = 0·10 | P < 0·0001 | ||
| Average silique length | β = 0·68 | β = 2·74 | β = 4·46 | β = –2·37 |
| F1,99 = 1·10 | F1,99 = 6·26 | F1,99 = 6·27 | F1,99 = 2·52 | |
| P < 0·0001 | P = 0·30 | P = 0·0139 | P = 0·12 |
Total flowers and silique length both increased with height at harvest. Plants to which insecticide was added aborted fewer flowers. Total flowers increased with the number of aborted flowers. Flower abortions were associated with longer siliques.
NED 2006
Height at harvest (Wilk's λ = 0·47, P < 0·001) significantly affected the three dependent variables, as did the interaction between pollination and galls (Wilk's λ = 0·85, P = 0·02). In stepdown analysis, galls increased aborted flowers, and pollination also increased aborted flowers on plants with no galls (Table 6). Pollination reduced flower abortions on plants with galls, and the effect increased with the number of galls (Table 6). Total flowers increased with aborted flowers and height at harvest, but decreased with galls (Table 6). Average silique length increased with height at harvest, but decreased with galls (Table 6).
Table 6.
Stepdown results for NED 2006
| Height | Pollination | Galls | Pollination × galls | Aborted flowers | Total flowers | |
|---|---|---|---|---|---|---|
| Aborted flowers | β = 0·03 | β = 0·66 | β = 0·22 | β = –0·18 | – | – |
| F1,81 = 4·07 | F1,81 = 11·04 | F1,81 = 32·59 | F1,81 = 7·06 | |||
| P < 0·05 | P < 0·01 | P < 0·0001 | P < 0·01 | |||
| Total flowers | β = 0·05 | β = –0·32 | β = –0·09 | β = 0·09 | β = 0·64 | – |
| F1,80 = 83·34 | F1,80 = 3·86 | F1,80 = 6·87 | F1,80 = 2·55 | F1,80 = 56·66 | ||
| P < 0·0001 | P = 0·053 | P = 0·0105 | P = 0·11 | P < 0·0001 | ||
| Average silique length | β = 1·76 | β = –2·99 | β = –2·29 | β = –0·03 | β = 0·01 | β = –0·55 |
| F1,76 = 40·58 | F1,76 = 0·63 | F1,76 = 7·09 | F1,76 = 4·0 × 10−4 | F1,76 = 4·0 × 10−5 | F1,76 = 0·04 | |
| P < 0·0001 | P = 0·43 | P < 0·01 | P = 0·98 | P = 0·99 | P = 0·83 |
All fitness components increased on taller plants. The number of aborted flowers increased with galls, and galls were related to a significant reduction in total flowers when controlling for the number of aborted flowers. Pollination had complex effects. Some siliques were broken at harvest, so sample sizes were smaller for measures of silique length.
ELK 2005
Extremely dry weather precluded pollination experiments in ELK 2005, so components of fitness were not examined.
ELK 2006
Galls (Wilk's λ = 0·65, P < 0·001) affected the three dependent variables, as did the interaction between pollination and height at harvest (Wilk's λ = 0·91, P < 0·001). Stepdown analysis showed that galls increased aborted flowers (Table 7). Height at harvest increased the number of total flowers as did the number of aborted flowers (Table 7). Silique length increased with height at harvest, but this effect decreased with pollination (Table 7).
Table 7.
Stepdown results for ELK 2006
| Height | Pollination | Galls | Pollination × height | Aborted flowers | Total flowers | |
|---|---|---|---|---|---|---|
| Aborted flowers | β = 4·3 × 10−3 | β = 0·05 | β = 1·02 | β = 0·03 | – | – |
| F1,149 < 0·01 | F1,149 < 0·01 | F1,149 = 76·44 | F1,149 = 3·24 | |||
| P = 0·95 | P = 0·98 | P < 0·0001 | P = 0·07 | |||
| Total flowers | β = 0·004 | β = 0·05 | β = 0·05 | β = 1·99 × 10−4 | β = 0·50 | – |
| F1,148 = 40·69 | F1,148 = 0·20 | F1,148 = 2·12 | F1,148 = 0·03 | F1,148 = 82·33 | ||
| P < 0·0001 | P = 0·62 | P = 0·15 | P = 0·88 | P < 0·0001 | ||
| Average silique length | β = 0·10 | β = 8·34 | β = –6·48 | β = –0·14 | β = –3·40 | β = –3·44 |
| F1,147 = 0·25 | F1,147 = 5·80 | F1,147 = 4·56 | F1,147 = 11·52 | F1,147 = 2·48 | F1,147 = 1·79 | |
| P < 0·0001 | P = 0·02 | P = 0·03 | P < 0·001 | P = 0·12 | P = 0·18 |
Height at harvest was associated with more total flowers and longer siliques, but not with aborted flowers. The number of aborted flowers increased with galls, silique length decreased with galls. Supplemental pollen increased fitness for some plants. Total flowers could not be counted on some plants, so sample sizes are slightly smaller than for analyses of total fitness.
Summary figures
To clarify the discussion, results from multiple populations and years are consolidated in Figs 3–7.
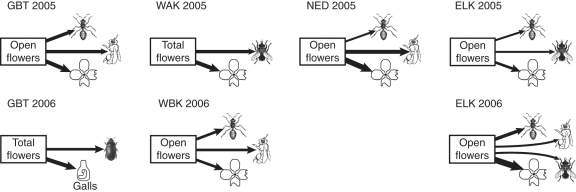
Fig. 3.
Summary diagram of best-fit models showing traits that attracted both pollinators and herbivores. Arrow thickness indicates the relative strength of an effect. See Fig. 1 for explanation of symbols.
Fig. 7.
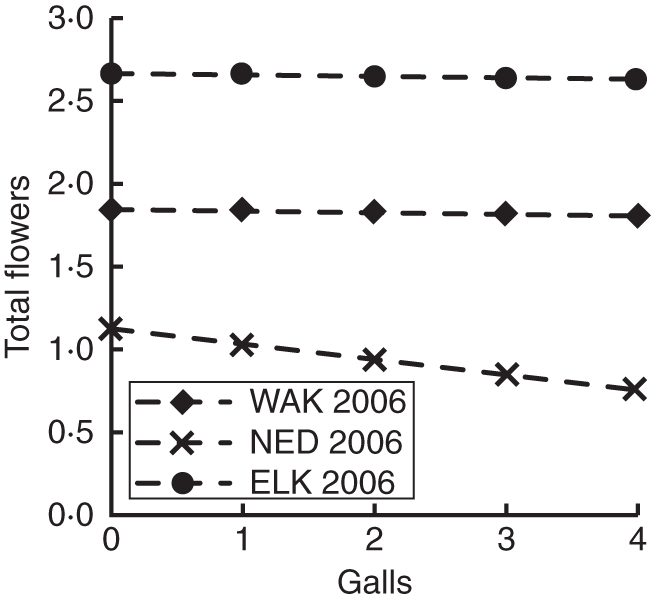
Relationship between galls and total number of flowers produced over the season. When controlling for the effect of aborted flowers on total flowers, galls and total flowers are not positively related on experimental plants in any of the three populations. This suggests that the positive associations between galls and total flowers in best-fit models of observational data are due to plant compensatory responses. Lines show predicted values of total flowers for gall counts in each population. 95 % confidence intervals of effect sizes were too small to be seen on the graphs. The negative relationship is significant in NED 2006, but not in the other two populations.
DISCUSSION
Pollinator and herbivore preferences
Pollinators and herbivores may be attracted by similar traits (Strauss and Armbruster, 1997; Irwin et al., 2004; McCall and Irwin, 2006), particularly large floral displays (Brody and Mitchell, 1997; Collin et al., 2002; Ehrlen et al., 2002). In the current study, E. capitatum plants that produced more flowers over the course of the season and that had more open flowers per day experienced more visitation by both pollinators (flies, bees, beetles) and most herbivores (ants, gall midges and petal consumers; Fig. 3). Other than flower production, no measured traits simultaneously attracted both pollinators and herbivores (Figs 1 and 2). For instance, in GBT, wider flowers attracted more pollinators but not more herbivores, whereas in other populations, wider flowers attracted more ants but had no effect on pollinators. Divergent pollinator and herbivore preferences should allow E. capitatum to escape potential trade-offs between attracting pollinators and herbivores (Adler, 2000; Herrera et al., 2002; Irwin et al., 2004).
Herbivory reduced pollinator visits to E. capitatum in some populations. Bees in NED and ELK preferred plants with less petal damage, and flies in ELK preferred plants with fewer galls (Fig. 4). In WAK, plants with galls were sometimes pollen limited while plants without galls were not, suggesting that galls may have reduced pollinator service to individual flowers even though whole-plant visitation was not affected. Reduced attractiveness to pollinators is a commonly observed outcome of floral herbivory (e.g. Kudoh and Whigham, 1998; Krupnick et al., 1999; Adler, 2000; Sanchez-Lafuente, 2007; Danderson and Molano-Flores, 2010), and is one of the primary reasons to measure both pollination and herbivory when examining selection on floral traits.
Fig. 4.
Summary diagram of best-fit models that showed effects of herbivory on pollinator visitation. See Fig. 1 for explanation of symbols.
Pollinator and herbivore effects on fitness, selection and plant compensatory responses
Pollinators
While selection by pollinators can occur when plants are not pollen limited (Parachnowitsch and Kessler, 2010), pollen limitation makes pollinator-mediated selection more likely to occur. The study populations of E. capitatum were not pollen limited during 2005 and 2006. Erysimum capitatum requires out-crossed pollen to set fruit (Price, 1984) and greenhouse-grown plants from the four study populations did not set fruit unless hand-pollinated (C. Lay, unpubl. res.); thus, pollinator visitation is required for reproduction. Differences in pollinator visitation, however, were not related to variation in estimated female fitness in any site or year (Fig. 5), and experimental pollen addition did not result in increased female fitness. The lack of pollen limitation suggests that plant reproductive success, as measured by total silique length, was primarily limited by resource availability, though this was not explicitly tested by adding nutrients or water. Observed levels of visitation were quite low in all populations, yet appear to have been adequate given the level of resources available for fruit set. In contrast, populations of Erysimum mediohispanicum in Spain have similar levels of visitation but are pollen limited (Gomez et al., 2010). Pollinators in the Rocky Mountain sites may be more effective, or E. capitatum may be more capable of avoiding pollen limitation than E. mediohispanicum.
Two separate mechanisms can explain the absence of pollen limitation despite self-incompatibility and low visitation in all populations. First, E. capitatum has a long period of stigmatic receptivity, up to 7 d when measured by peroxidase assay (C. Lay, unpubl. res.). Prolonged stigmatic receptivity allows plants to tolerate low frequencies of pollinator visitation without loss of fitness (Ashman and Schoen, 1994; Bingham and Orthner, 1998; Navarro et al., 2007). Secondly, variation in pollination may be compensated by changes in components of fitness (aborted flowers, flower production and silique length). In the experimental study, the number of aborted flowers (flowers that reach anthesis but fail to produce fruit) was associated with a greater number of total flowers (after controlling for plant height; Fig. 6). This relationship suggests that flowers that do not produce fruit are partially replaced by the subsequent production of additional flowers on these indeterminate inflorescences. Pollination also may cause changes in the components of fitness. For instance, in ELK 2006, supplemental pollination led to increased allocation to individual fruits as measured by average silique length (Table 7). Also, in GBT 2006, taller plants aborted more flowers, but supplemental pollination reduced the number of aborted flowers in taller plants (Table 2). These data suggest that E. capitatum may shift resources between flower production and developing fruits in response to changes in pollen quality or quantity.
Fig. 6.
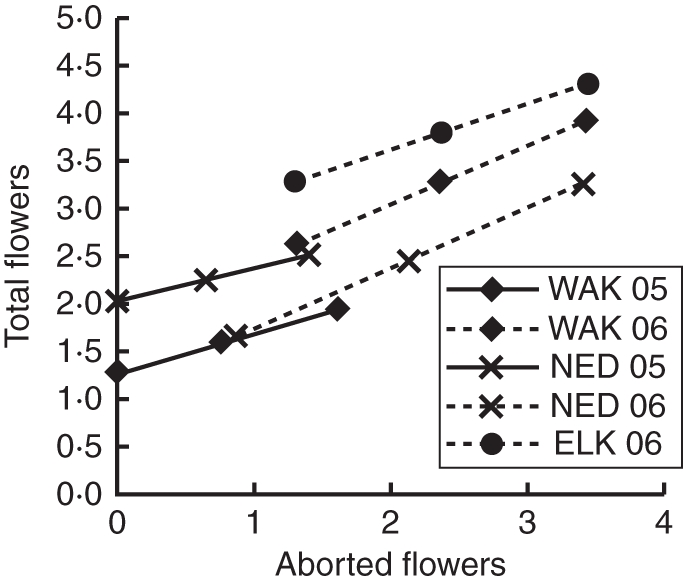
Relationship between aborted and total flowers. Total flowers increased with the number of aborted flowers in all populations and years. Lines show predicted values of total flowers for values of aborted flowers (both are square-root transformed) when accounting for other independent variables. 95 % confidence intervals of effect sizes are too small to be visible on the graph.
Burd (Burd, 2008; Burd et al., 2009) suggested that the general prevalence of pollen limitation could be the result of stochastic pollination environments that have selected many plants to overproduce ovules. However, the iterative and modular nature of plant reproduction was only briefly addressed in Burd's analysis, and the inclusion of plant response strategy in studying pollen limitation is important (Wesselingh, 2007). Whereas large numbers of ovules per flower allow plants to take advantage of high levels of pollen delivered by single pollinator visits, the number of flowers (and the ovules they contain) produced by indeterminate inflorescences is free to change over time with varying resource conditions as the flowering period unfolds (Lloyd, 1980; Stephenson, 1992; Diggle and Miller, 2004). Sequential, indeterminate flowering could be favoured over initial overproduction of ovules in some cases (Wesselingh, 2007). Mechanisms such as prolonged stigmatic receptivity, indeterminate flowering and reallocation of reproductive resources may be particularly important for E. capitatum, which is visited by a diverse group of pollinators and multiple herbivores that may differ in time and space. The reproductive success of individuals with a generalized pollination system typically depends more on the frequency and effectiveness of individual visitors than the diversity of visitors (Perfectti et al., 2009). Observed visitation rates and variation among sites in the species composition of common visitors (C. Lay, unpubl. res.) suggest that not all E. capitatum populations have dependable and constant pollinators. The reallocation of resources to production of new flowers and to surviving fruits may reduce the impact of spatiotemporal variation in pollinator assemblages as selective agents.
Herbivores
Unlike pollinator visitation, herbivory influenced total fitness in several populations and years (Fig. 5). Moreover, well-supported paths between herbivore preferences, traits and fitness provide evidence of selection. For instance, stem borers were most common in WAK and preferred plants with thicker stems when their preferences were measured in 2006. Plants at that site had the narrowest stems of the four populations (C. Lay, unpubl. res.), suggesting that selection by borers in WAK may have resulted in narrower stems compared with plants in other populations.
Petal damage was greater on plants with narrower flowers and more open flowers, and because petal damage reduced fitness, petal herbivores selected for fewer open flowers and wider flowers in this population and year. However, the reason for reduced fitness of plants with damaged petals was ambiguous. Petal damage commonly affects plant fitness through reduced pollinator visitation (McCall and Irwin, 2006). Bees were less likely to visit E. capitatum plants with more petal damage, but the best-fit model for ELK 2006 did not show effects of pollinators on fitness.
Ants never affected plant fitness despite their prevalence as flower visitors, removal of nectar and occasional agonistic interactions with potential pollinators (Fig. 5), and ant exclusion had no effect on fitness (C. Lay, unpubl. res.). Therefore, while ants preferred larger display sizes and wider flowers (Figs 1–3), they were not effective agents of selection on these characters in the populations studied. Species such as E. capitatum that are not pollen limited are less likely to experience a reduction in fitness when nectar is removed without pollination service (Burkle et al., 2007).
Galls reduced fitness in all populations in 2006, though not in 2005 (Fig. 5), and plants with more total flowers had more galls. Total flowers were related to galls only through flower abortion (Figs 6 and 7), however, so it is unlikely that gall midges prefer plants with more flowers (and hence, select for fewer flowers). Instead, this association can be explained by plant compensatory response. Galled flowers were almost always aborted, and plants offset this loss by increasing flower production (Figs 6 and 7). Plants did not fully compensate for galling; galled flowers were only partially replaced by new flowers, and plant investment in individual fruits was reduced on plants with galls, so overall fitness effects of galls were negative. In addition, galls appeared to reduce pollination success, even without affecting visitation. Added pollen increased the length of surviving siliques on plants with galls in WAK 2006, suggesting that plants with galls experienced pollen limitation (that is, flowers on galled plants received insufficient or inferior pollen) while plants without galls did not.
Plant compensatory responses moderate fitness consequences of both pollinators and herbivores
Multiple herbivores and damage to multiple tissues are a perennial problem for plants (Linhart, 1991; Strauss et al., 2005), as is pollinator unpredictability (Ashman et al., 2004; Knight et al., 2005; Burd et al., 2009) and E. capitatum is no exception. Several herbivores strongly reduced female fitness, although their selective influences on plant traits were varied and relatively independent of one another. Surprisingly, although pollinator visitation was rare and pollinator communities were variable, pollinators did not affect fitness or select on plant traits in the study populations (Fig. 5). The effects of herbivores (e.g. gall midges) and infrequent pollinators appeared to be moderated by compensatory responses in E. capitatum. Plants produce flowers sequentially and indeterminately, allowing individuals to replace damaged and unpollinated flowers over the course of the season. The development of new flowers in response to herbivory is hardly unique to E. capitatum. Removal of flower buds increases the production of new flowers in Brassica napus (Williams and Free, 1979), a close relative of E. capitatum, and many plants are able to respond to herbivory and uneven pollen receipt among flowers by changing patterns of fruit and seed set (Marshall et al., 1985; Stephenson, 1992; Knight et al., 2005; Wise and Cummins, 2006; Nunez-Farfan et al., 2007; Wesselingh, 2007; Wise and Abrahamson, 2007). The heritability of plant resource allocation strategies is difficult to measure (but see Juenger and Bergelson, 2000; Wise et al., 2008). However, plant compensatory strategies are likely to be key traits that allow plants to succeed in unpredictable environments with selection pressures from multiple interacting partners.
Spatiotemporal variation in the selective landscape
A recent meta-analysis by Harder and Johnson (2009) found that directional selection by pollinators is often weak or non-existent and tends to be inconsistent over time and space. In addition, they found that herbivores often produce stronger selection on plant traits than pollinators, and positive directional selection for flower number occurs three times more often than other commonly measured traits (Harder and Johnson, 2009). The present results are consistent with these general patterns (see also Strauss and Whittall, 2006).
Selective effects of pollinators on measured traits of E. capitatum were not identified in any population or year, and elevational gradients were not associated with any gradual biotic gradients (Figs 1–5). Herbivores selected on specific plant traits, but their prevalence and preferences varied over time and space. For instance, stem-boring beetle larvae selected for narrower stems but were only common in WAK (Fig. 5). Insects that caused petal damage selected for fewer, wider flowers in ELK 2006, but in no other population or year. Although the prevalence of galls differed somewhat among populations, their effects were similar among populations; gall midges reduced fitness in 2006 in all populations and did not affect fitness in any population in 2005 (Fig. 5). Some of this temporal variation may have been driven by water availability or other abiotic factors; 2005 was very dry and many plants at the three highest-elevation populations in 2005 wilted during observation periods. Under such conditions, water availability was likely to be the primary determinant of fitness, with pollinators and herbivores playing a more minor role.
As in the meta-analysis (Harder and Johnson, 2009), total flower number was the only trait that was under selection in all populations and years. Flower number was always directly and positively associated with plant female fitness (Fig. 5) so overall, selection favoured plants with more flowers. The relationship between flower number, insects, and fitness was not always straightforward, however. Structural equation models of observational data suggested that the selective benefit of increased flower production was attenuated by gall midges in 2006, as galls reduced fitness and were found in greater numbers on plants with more flowers (Fig. 5). Furthermore, experimental evidence suggests that increased flower production may actually have been a compensatory response to gall midge attack (Figs 6 and 7). If increased flower production in response to herbivory is common, negative effects of herbivores may obscure the positive effects of flower number on fitness. This in turn suggests that selection for increased flower number may be even more frequent than was suggested by Harder and Johnson's meta-analysis. Compensatory responses such as flower production may vary among individuals and result in increased phenotypic variation. Such variation can mediate selection by pollinators and herbivores in both time and space (Fordyce, 2006).
Conclusions
Individuals of Erysimum capitatum face an adaptive landscape that is shaped by diverse generalist interacting partners that shift in abundance and importance in time and space. Evidence from both experimentation and observation was used to determine how herbivores and pollinators alter this landscape. Neither pollen limitation nor selection by pollinators was evident in E. capitatum, though selection by herbivores was occasionally important. Compensatory reproductive mechanisms may allow E. capitatum to succeed in this complex selective environment. In addition to flower longevity, plants respond to both galls and low pollinator visitation by altering flower abortion, new flower production and allocation to individual fruits. Developmental mechanisms that allow plants to make iterative decisions about reproductive resource allocation over the course of the flowering season may be under selection in E. capitatum.
SUPPLEMENTARY DATA
LITERATURE CITED
- Adler LS. Alkaloid uptake increases fitness in a hemiparasitic plant via reduced herbivory and increased pollination. American Naturalist. 2000;156:92–99. doi: 10.1086/303374. [DOI] [PubMed] [Google Scholar]
- Adler LS, Bronstein JL. Attracting antagonists: does floral nectar increase leaf herbivory? Ecology. 2004;85:1519–1526. [Google Scholar]
- Agrawal AA. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science. 2000;5:309–313. doi: 10.1016/s1360-1385(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Aigner PA. Variation in pollination performance gradients in a Dudleya species complex: can generalization promote floral divergence? Functional Ecology. 2005;19:681–689. [Google Scholar]
- Armbruster WS. Exaptations link evolution of plant–herbivore and plant–pollinator interactions: a phylogenetic inquiry. Ecology. 1997;78:1661–1672. [Google Scholar]
- Armbruster WS, Lee J, Baldwin BG. Macroevolutionary patterns of defense and pollination in Dalechampia vines: adaptation, exaptation, and evolutionary novelty. Proceedings of the National Academy of Sciences of the USA. 2009;106:18085–18090. doi: 10.1073/pnas.0907051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman TL, Schoen DJ. How long should flowers live? Nature. 1994;371:788–791. [Google Scholar]
- Ashman TL, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Bingham RA, Orthner AR. Efficient pollination of alpine plants. Nature. 1998;391:238–239. [Google Scholar]
- Bradley KL, Damschen EI, Young LM, et al. Spatial heterogeneity, not visitation bias, dominates variation in herbivory. Ecology. 2003;84:2214–2221. [Google Scholar]
- Brody AK, Mitchell RJ. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed predation in the hummingbird-pollinated plant Ipomopsis aggregata. Oecologia. 1997;110:86–93. doi: 10.1007/s004420050136. [DOI] [PubMed] [Google Scholar]
- Burd M. The Haig–Westoby model revisited. American Naturalist. 2008;171:400–404. doi: 10.1086/527499. [DOI] [PubMed] [Google Scholar]
- Burd M, Ashman TL, Campbell DR, et al. Ovule number per flower in a world of unpredictable pollination. American Journal of Botany. 2009;96:1159–1167. doi: 10.3732/ajb.0800183. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Irwin RE, Newman DA. Predicting the effects of nectar robbing on plant reproduction: implications of pollen limitation and plant mating system. American Journal of Botany. 2007;94:1935–1943. doi: 10.3732/ajb.94.12.1935. [DOI] [PubMed] [Google Scholar]
- Collin CL, Pennings PS, Rueffler C, Widmer A, Shykoff JA. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia. 2002;131:94–102. doi: 10.1007/s00442-001-0854-8. [DOI] [PubMed] [Google Scholar]
- Danderson CA, Molano-Flores B. Effects of herbivory and inflorescence size on insect visitation to Eryngium yuccifolium (Apiaceae) a prairie plant. American Midland Naturalist. 2010;163:234–246. [Google Scholar]
- Diggle PK, Miller JS. Architectural effects mimic floral sexual dimorphism in Solanum (Solanaceae) American Journal of Botany. 2004;91:2030–2040. doi: 10.3732/ajb.91.12.2030. [DOI] [PubMed] [Google Scholar]
- Dilley JD, Wilson P, Mesler MR. The radiation of Calochortus: generalist flowers moving through a mosaic of potential pollinators. Oikos. 2000;89:209–222. [Google Scholar]
- Ehrlen J. Assessing the lifetime consequences of plant–animal interactions for the perennial herb Lathyrus vernus (Fabaceae) Perspectives in Plant Ecology Evolution and Systematics. 2002;5:145–163. [Google Scholar]
- Ehrlen J, Kack S, Agren J. Pollen limitation, seed predation and scape length in Primula farinosa. Oikos. 2002;97:45–51. [Google Scholar]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. Journal of Experimental Biology. 2006;209:2377–2383. doi: 10.1242/jeb.02271. [DOI] [PubMed] [Google Scholar]
- Fox J. Analysis of variance. In: Fox J, editor. Applied regression analysis and generalized linear models. 2nd edn. Sage Publications; 2008. [Google Scholar]
- Fox J, Kramer A, Friendly M. 2010 sem: structural equations models. R package version 0·9-20. http://CRAN.R-project.org/package=sem . [Google Scholar]
- Galen C, Butchart B. Ants in your plants: effects of nectar-thieves on pollen fertility and seed-siring capacity in the alpine wildflower. Polemonium viscosum. Oikos. 2003;101:521–528. [Google Scholar]
- Galen C, Cuba J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot. Polemonium viscosum. Evolution. 2001;55:1963–1971. doi: 10.1111/j.0014-3820.2001.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Gomez JM, Zamora R. Spatial variation in the selective scenarios of Hormathophylla spinosa (Cruciferae) American Naturalist. 2000;155:657–668. doi: 10.1086/303353. [DOI] [PubMed] [Google Scholar]
- Gomez JM, Perfectti F, Bosch J, Camacho JPM. A geographic selection mosaic in a generalized plant–pollinator–herbivore system. Ecological Monographs. 2009;79:245–263. [Google Scholar]
- Gomez JM, Abdelaziz M, Lorite J, Munoz-Pajares AJ, Perfectti F. Changes in pollinator fauna cause spatial variation in pollen limitation. Journal of Ecology. 2010;98:1243–1252. [Google Scholar]
- Grace JB. Structural equation modeling and natural systems. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Gschwandtner M, Filzmoser P. 2009 mvoutlier: multivariate outlier detection based on robust methods. R package version 1·4. ed http://www.statistik.tuwien.ac.at/public/filz/ [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist. 2009;183:530–545. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Variation in mutualisms: the spatio-temporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society. 1988;35:95–125. [Google Scholar]
- Herrera CM. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology. 2000;81:2170–2176. [Google Scholar]
- Herrera CM, Medrano M, Rey PJ, et al. Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proceedings of the National Academy of Sciences of the USA. 2002;99:16823–16828. doi: 10.1073/pnas.252362799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RE, Strauss SY, Storz S, Emerson A, Guibert G. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology. 2003;84:1733–1743. [Google Scholar]
- Irwin RE, Adler LS, Brody AK. The dual role of floral traits: pollinator attraction and plant defense. Ecology. 2004;85:1503–1511. [Google Scholar]
- Juenger T, Bergelson J. Pollen and resource limitation of compensation to herbivory in scarlet gilia. Ipomopsis aggregata. Ecology. 1997;78:1684–1695. [Google Scholar]
- Juenger T, Bergelson J. The evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution. 2000;54:764–777. doi: 10.1111/j.0014-3820.2000.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Juenger T, Lennartsson T, Tuomi J. The evolution of tolerance to damage in Gentianella campestris: natural selection and the quantitative genetics of tolerance. Evolutionary Ecology. 2000;14:393–419. [Google Scholar]
- Kearns CA. Anthophilous fly distribution across an elevation gradient. American Midland Naturalist. 1992;127:172–182. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Krupnick GA, Weis AE, Campbell DR. The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology. 1999;80:125–134. [Google Scholar]
- Kudoh H, Whigham DF. The effect of petal size manipulation on pollinator/seed-predator mediated female reproductive success of Hibiscus moscheutos. Oecologia. 1998;117:70–79. doi: 10.1007/s004420050633. [DOI] [PubMed] [Google Scholar]
- Linhart YB. Disease, parasitism and herbivory – multidimensional challenges in plant evolution. Trends in Ecology & Evolution. 1991;6:392–396. doi: 10.1016/0169-5347(91)90160-Y. [DOI] [PubMed] [Google Scholar]
- Linhart YB, Grant MC. Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics. 1996;27:237–277. [Google Scholar]
- Lloyd DG. Sexual strategies in plants: an hypothesis of serial adjustment of maternal investment during one reproductive season. New Phytologist. 1980;86:69–79. [Google Scholar]
- Lomolino MV. Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography. 2001;10:3–13. [Google Scholar]
- Louda SM, Potvin MA. Effect of inflorescence-feeding insects on the demography and lifetime fitness of a native plant. Ecology. 1995;76:229–245. [Google Scholar]
- McCall AC. Florivory affects pollinator visitation and female fitness in Nemophila menziesii. Oecologia. 2008;155:729–737. doi: 10.1007/s00442-007-0934-5. [DOI] [PubMed] [Google Scholar]
- McCall AC. Does dose-dependent petal damage affect pollen limitation in an annual plant? Botany-Botanique. 2010;88:601–606. [Google Scholar]
- McCall AC, Irwin RE. Florivory: the intersection of pollination and herbivory. Ecology Letters. 2006;9:1351–1365. doi: 10.1111/j.1461-0248.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- Marr JW. Ecosystems of the east slope of the front range in Colorado. Boulder, CO: University Press of Colorado; 1967. [Google Scholar]
- Marshall DL, Levin DA, Fowler NL. Plasticity in yield components in response to fruit predation and date of fruit initation in three species of Sesbania (Leguminosae) Journal of Ecology. 1985;73:71–81. [Google Scholar]
- Navarro L, Ayensa G, Guitian P. Adaptation of floral traits and mating system to pollinator unpredictibility: the case of Disterigma stereophyllum (Ericaceae) in southwestern Colombia. Plant Systematics and Evolution. 2007;266:165–174. [Google Scholar]
- Nunez-Farfan J, Fornoni J, Valverde PL. The evolution of resistance and tolerance to herbivores. Annual Review of Ecology, Evolution and Systematics. 2007;38:541–566. [Google Scholar]
- Paige KN, Whitham TG. Overcompensation in response to mammalian herbivory: the advantage of being eaten. American Naturalist. 1987;129:407–416. [Google Scholar]
- Parachnowitsch AL, Kessler A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytologist. 2010;188:393–402. doi: 10.1111/j.1469-8137.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- Perfectti F, Gomez JM, Bosch J. The functional consequences of diversity in plant–pollinator interactions. Oikos. 2009;118:1430–1440. [Google Scholar]
- Price RA. 1984 The systematics of the Erysimum capitatum alliance (Brassicaceae) in North America. PhD Thesis, University of California, Davis. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. http://www.r-project.org/ [Google Scholar]
- Rand TA. Variation in insect herbivory across a salt marsh tidal gradient influences plant survival and distribution. Oecologia. 2002;132:549–558. doi: 10.1007/s00442-002-0989-2. [DOI] [PubMed] [Google Scholar]
- Rey PJ, Herrera CM, Guitian J, et al. The geographic mosaic in predispersal interactions and selection on Helleborus foetidus (Ranunculaceae) Journal of Evolutionary Biology. 2006;19:21–34. doi: 10.1111/j.1420-9101.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lafuente AM. Corolla herbivory, pollination success and fruit predation in complex flowers: an experimental study with Linaria lilacina (Scrophulariaceae) Annals of Botany. 2007;99:355–364. doi: 10.1093/aob/mcl267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf KE, Price MV. Does pollination limit tolerance to browsing in Ipomopsis aggregata? Oecologia. 2004;138:396–404. doi: 10.1007/s00442-003-1436-8. [DOI] [PubMed] [Google Scholar]
- Stephenson AG. The regulation of maternal investment in plants. In: Marshall C, Grace J, editors. Fruit and seed production: aspects of development, environmental physiology and ecology. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Strauss SY, Armbruster WS. Linking herbivory and pollination – new perspectives on plant and animal ecology and evolution. Ecology. 1997;78:1617–1618. [Google Scholar]
- Strauss SY, Whittall J. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Strauss SY, Sahli H, Conner JK. Toward a more trait-centred approach to diffuse (co)evolution. New Phytologist. 2005;165:81–89. doi: 10.1111/j.1469-8137.2004.01228.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. New York, NY: HarperCollins Publishers; 1996a. Roy–Bargmann stepdown analysis. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. New York, NY: HarperCollins Publishers; 1996b. Assessing the fit of the model. [Google Scholar]
- Thompson JN. Variation in interspecific interactions. Annual Review of Ecology and Systematics. 1988;19:65–87. [Google Scholar]
- Turner BL. Taxonomy and nomenclature of the Erysimum asperum–E.capitatum complex (Brassicaceae) Phytologia. 2006;88:279–287. [Google Scholar]
- USDA. 2004 The PLANTS Database, Version 3·5. National Plant Data Center, Baton Rouge, LA 70874-4490, USA. http://plants.usda.gov . [Google Scholar]
- Weber WA. Colorado flora: eastern slope. Boulder, CO: University Press of Colorado; 1990. [Google Scholar]
- Wesselingh RA. Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytologist. 2007;174:26–37. doi: 10.1111/j.1469-8137.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Whittall J, Carlson M. Plant defense: a pre-adaptation for pollinator shifts. New Phytologist. 2009;182:5–8. doi: 10.1111/j.1469-8137.2009.02796.x. [DOI] [PubMed] [Google Scholar]
- Williams IH, Free JB. Compensation of oil-seed rape (Brassica napus L.) plants after damage to their buds and pods. Journal of Agricultural Science. 1979;92:53–59. [Google Scholar]
- Wise MJ, Abrahamson WG. Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. American Naturalist. 2007;169:443–454. doi: 10.1086/512044. [DOI] [PubMed] [Google Scholar]
- Wise MJ, Cummins JJ. Strategies of Solanum carolinense for regulating maternal investment in response to foliar and floral herbivory. Journal of Ecology. 2006;94:629–636. [Google Scholar]
- Wise MJ, Cummins JJ, De Young C. Compensation for floral herbivory in Solanum carolinense: identifying mechanisms of tolerance. Evolutionary Ecology. 2008;22:19–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.