Abstract
Programmed cell death, or apoptosis, is one of the fates of the medial edge epithelium (MEE) during palatal fusion. Transforming growth factor β (Tgf-β) signaling (such as Tgf-β3) is required for the disappearance of the MEE, but the relationship between Tgf-β3 and apoptosis remains unclear. Here we show that the Fas ligand (FasL)-Fas-Caspase extrinsic apoptosis pathway functions during palatal fusion in wild-type mice, but is not detectable in mice lacking Tgf-β3 (Tgf-β3 −/− ) or Tgfβr2 in the MEE (K14-Cre;Tgfbr2 fl/fl). Inhibition of the FasL-Fas system results in persistence of the midline epithelial seam (MES) and inhibition of caspase activity during palatal organ culture. Moreover, ectopic FasL protein induces apoptosis in MES of K14-Cre;Tgfbr2 fl/fl mice. Thus, we conclude that the FasL-Fas-caspase extrinsic apoptosis pathway is regulated by the Tgf-β3 signaling cascade and is essential for palatal fusion during craniofacial development.
Keywords: palatogenesis, medial edge epithelium (MEE), program cell death (PCD), transforming growth factor β (Tgf-β) signaling, Fas ligand, caspase
Introduction
Embryonic palatogenesis is a critical and complex event during mammalian craniofacial development (Chai and Maxson, 2006). In mice, the secondary palatal shelves, which arise from maxillary processes, grow vertically beside the tongue from embryonic days 11.5 (E11.5) to E13.5 and then reach a horizontal position touching each other at the midline after E13.5. After contact and adhesion, the medial edge epithelium (MEE) of each palatal shelf fuses together to form the midline epithelial seam (MES) (Ferguson, 1988; Shuler, 1995; Chai and Maxson, 2006). Regression of MEE cells is required for palatal fusion. MEE persistence at the midline of the palate results in cleft palate and submucous cleft (Kaartinen et al., 1995; Proetzel et al., 1995; Chai and Maxson, 2006). The fate of MEE cells has been subject to much debate (Chai and Maxson, 2006; Dudas et al., 2007). Cell death, cell migration, epithelial-mesenchymal transformation, and other mechanisms have been proposed to explain the process of MEE regression (Carette and Ferguson, 1992; Shuler, 1995; Nawshad and Hay, 2003; Cuervo and Covarrubias, 2004; Nawshad et al., 2004). Accumulating evidence demonstrates that programmed cell death (PCD)/apoptosis and epithelial cell migration are the primary reasons for loss of the MEE during palatal fusion (Carette and Ferguson, 1992; Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005; Xu et al., 2006; Dudas et al., 2007).
PCD is essential for embryonic development, homeostasis, and defense against viral infection, dysregulated immune disease, and uncontrolled cell growth. Caspases, the intracellular apoptotic proteases, are the primary drivers of PCD (Hengartner, 2000). Two main caspase-dependent pathways have been identified (Green, 1998): the intrinsic pathway, in which diverse stress pathways cause release of mitochondrial proteins to form a complex called the apoptosome to induce apoptosis; and the extrinsic pathway, which is activated by ligand-bound death receptors such as Fas. The extrinsic pathway has previously been proposed to be much simpler and better understood (Rathmell and Thompson, 2002). Fas, also named TNFRSF6/Apo-1/CD95, and Fas ligand (FasL), the prototypic members of the TNF family, play a role in T-cell-mediated cytotoxicity, apoptosis induction in activated lymphocytes (Brunner et al., 1995; Dhein et al., 1995), and maintenance of the immunoprivileged state of eyes and testis (Griffith et al., 1995). FasL-induced apoptosis initiates when membrane-bound FasL triggers multimerization of Fas and the formation of a death-inducing signaling complex that includes Fas-associated Death Domain (FADD) and the FADD-interacting initiator caspase-8 (Krammer, 2000). The death-inducing signaling complex promotes procaspase-8 auto-cleavage and activation. Active caspase-8 activates effector caspases, such as caspase-3, causing the cell to undergo initiation of the apoptotic caspase cascade (Hengartner, 2000).
Transforming growth factor β (Tgf-β) signaling plays important roles during palatogenesis. Loss of Tgf-β3 (Kaartinen et al., 1995; Proetzel et al., 1995) or loss of Tgf-β receptor II (Tgfbr2) in the palatal epithelium (Xu et al., 2006) results in persistence of the MEE and cleft palate. Cell death is almost undetectable in MEE cells of Tgf-β3 −/− and K14-Cre;Tgfbr2 fl/fl mutant mice, whereas it is easily detectable in MEE cells of wild-type mice (Xu et al., 2006). Although Tgf-β signaling is required for MEE degeneration, the mechanism linking Tgf-β3 signaling and PCD in MEE cells during palatogenesis remains unclear.
In the present study, we investigated the FasL-Fas-caspase apoptosis pathway in MEE cells during palatal fusion. We found that the FasL-Fas-caspase pathway, which is detectable in the MEE during palatal fusion in wild-type mice, is compromised in Tgf-β3 −/− and K14-Cre;Tgfbr2 fl/fl mice. Further, we inhibited the FasL-Fas pathway using a blocking FasL antibody in wild-type palatal shelves in vitro and found that caspase activity and PCD in MEE cells was dramatically reduced, the MES persisted, and the palatal shelves failed to fuse. Moreover, ectopic FasL induced MEE cells to undergo apoptosis in K14-Cre;Tgfbr2 fl/fl mice. Analysis of these data suggests that PCD controlled by the FasL-Fas-caspase extrinsic apoptosis pathway is essential in the disappearance of the MEE during palatogenesis.
Materials & Methods
Generation of Tgf-β3 −/− and K14-Cre;Tgfbr2 fl/fl Mutant Mice
Male and female Tgf-β3 +/− mice (Kaartinen et al., 1995; Proetzel et al., 1995) were mated to generate Tgf-β3 −/− mice, which were genotyped with PCR primers as previously described (Taya et al., 1999). Male mice carrying the K14-Cre allele were crossed with Tgfbr2 fl/fl females to generate K14-Cre;Tgfbr2 fl/+ mice (Xu et al., 2006). Male K14-Cre;Tgfbr2 fl/+ mice were mated with Tgfbr2 fl/fl female mice to generate K14-Cre;Tgfbr2 fl/fl mice that were genotyped with PCR primers as previously described (Xu et al., 2006).
Sample Preparation and H&E Staining
All samples were fixed in 4% paraformaldehyde at 4 °C and processed into serial paraffin-embedded sections by routine procedures. Coronal sections (6 µm) were mounted in serial order on poly-L-lysine-coated slides. For general morphology, deparaffinized sections were stained with hematoxylin and eosin by standard procedures.
Cell Death Detection
Following treatment with 20 mg/mL of proteinase K for 15 min at room temperature, apoptotic cells were assayed by the TUNEL procedure with use of the In Situ Cell Death Detection (fluorescein) kit (Roche, South San Francisco, CA, USA) according to the manufacturer’s protocol.
Palatal Shelf Organ Cultures
Palatal shelves were microdissected from E13.5 wild-type and K14-Cre;Tgfbr2 fl/fl mice, at which point the palatal shelves have not yet come into contact with each other. The dissected palatal shelves were placed on 0.8 µm Millipore filters in Trowell-type organ cultures, keeping the paired shelves with their medial edge epithelia (MEE) in close apposition without apparent distortion of the tissue shape. We cultured the palatal shelves of wild-type mice in serum-free, BGJb culture medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% ascorbic acid. BSA (800 ng/mL), or blocking FasL antibody (800 ng/mL, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), or z-VAD (100 µM, Biomol, Plymouth, PA, USA) was added directly to the culture medium. We also cultured the palatal shelves of wild-type and K14-Cre;Tgfbr2 fl/fl mice from E13.5 in BGJb culture medium with BSA (100 ng/mL) or FasL protein (100 ng/mL, Santa Cruz Biotechnology Inc.).Tissues were harvested after 2 days (48 hrs) and 4 days (96 hrs) of culture, fixed in 4% paraformaldehyde, and processed for histological analysis (Xu et al., 2006; Huang et al., 2010).
Immunohistochemistry
Tissues were fixed with 4% paraformaldehyde for immunohistochemistry. The slides were heated in a 95°C oven for 30 min and subsequently hydrated through a series of decreasing concentrations of ethanol. The immunohistochemical staining was performed with the Zymed HistoStain SP kit (Invitrogen) according to the manufacturer’s instructions, with anti-Fas and anti FasL antibodies (Santa Cruz Biotechnology Inc.) and anti-caspases 3, 8, and 9 antibodies (Cell Signaling Technology, Inc., Boston, MA, USA) (Huang et al., 2009).
RNA Preparation and RT-PCR
RNA was isolated from E14.0 palatal shelves. First-strand cDNA was synthesized from 1 µg of total RNA with an oligo (dT) 20 primer and SuperScript III reverse transcriptase (Invitrogen), and PCR was performed by standard procedures. PCR primers were as following: FasL-a, 5′-GACAGCAGTGCCACTTCATC-3′; FasL-b, 5′-TTAAGGCTTTGGTTGGTGAA-3′; GAPDH-a, AGGCCGGTGCTGAGTATGTC; and GAPDH-b, TGCCTGCTTCACCACCTTCT.
Results
Mouse palatogenesis starts at E11.5. Before E13.5, palatal shelves are located on both sides of the tongue (Chai and Maxson, 2006). At E14.5, the palatal shelves turn horizontally above the tongue, they come into contact with each other along the midline, and fusion begins to take place (Ferguson, 1988; Shuler, 1995; Chai and Maxson, 2006) (Figs. 1A, 1B; arrow indicates the MEE). As expected, we detected apoptotic cells in the MES of wild-type mice at E14.5 (Figs. 1C, 1F, 1I). Caspase 3, FasL, and Fas were also detected in MEE cells (Figs. 1D, 1G, 1J). After merging the images, we found that all TUNEL-positive cells expressed caspase 3, FasL, and Fas (Figs. 1F, 1G), consistent with a role for the FasL-Fas-caspase-induced apoptotic pathway in the disappearance of MEE cells during palatal fusion.
Figure 1.
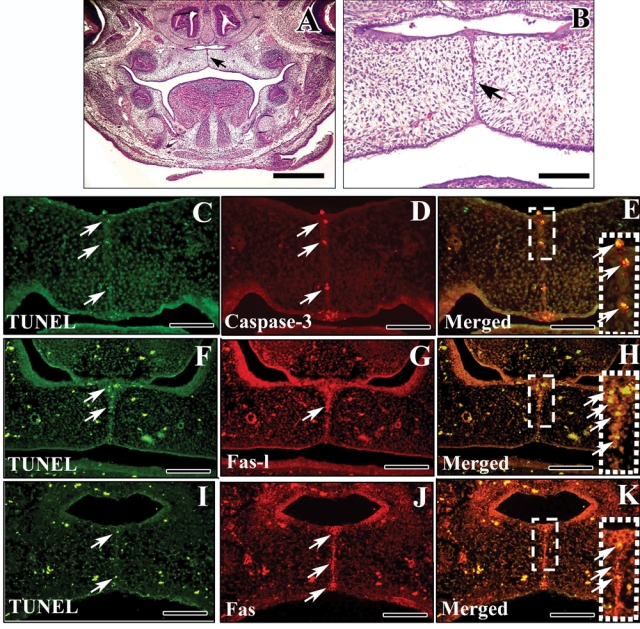
Apoptosis in the medial edge epithelium (MEE) at E14.5. (A,B) H&E staining of palatal shelves of E14.5 wild-type mice. Note that the palatal shelves have rotated horizontally above the tongue to come into contact with each other along the midline. Arrow indicates the MEE. (C-K) TUNEL staining (C, F, I), caspase-3, FasL, and Fas immunofluorescence (D, G, J) and merged images (E, H, K) of E14.5 wild-type palatal shelves. Note that TUNEL-positive, caspase 3-, FasL-, and Fas-positive cells are detectable in MEE cells. Merged images show overlap of TUNEL-positive cells and cells that express caspase 3 (E), FasL (H), and Fas (K). Scale bars: A, 250 µm; B-K, 100 µm.
After the palatal shelves come into contact, MEE cells undergo PCD. All MEE cells are gradually eliminated, and the palatal mesenchymes fuse completely after E16.5 (Chai and Maxson, 2006). In contrast to wild-type palates, a midline epithelial seam persists in the secondary palate in K14-Cre;Tgfbr2 fl/fl and Tgf-β3 −/− mutant mice (Kaartinen et al., 1995; Proetzel et al., 1995; Xu et al., 2006). To investigate the relationship between Tgf-β signaling and PCD, we analyzed the components of the FasL-Fas-caspase pathway in wild-type, K14-Cre;Tgfbr2 fl/fl, and Tgf-β3 −/− mice. We found that FasL was primarily detectable in the MEE of wild-type but not in that of K14-Cre;Tgfbr2 fl/fl and Tgf-β3 −/− mice (Figs. 2A-2C). Using RT-PCR, we found that the gene expression of FasL in the palatal shelves of K14-Cre;Tgfbr2 fl/fl and Tgf-β3 −/− mutant mice was decreased compared with that in wild-type mice (Fig. 2D). Similarly, Fas and caspases 3, 8, and 9 were clearly expressed in MEE cells in wild-type mice (Figs. 2E, 2H, 2K, 2N), but were not detectable in K14-Cre;Tgfbr2 fl/fl and Tgf-β3 −/− mice (Figs. 2F, 2G, 2I, 2J, 2L, 2M, 2O, 2P). Therefore, we conclude that the FasL-Fas-caspase extrinsic pathway is regulated by Tgf-β signaling to control MEE disintegration.
Figure 2.
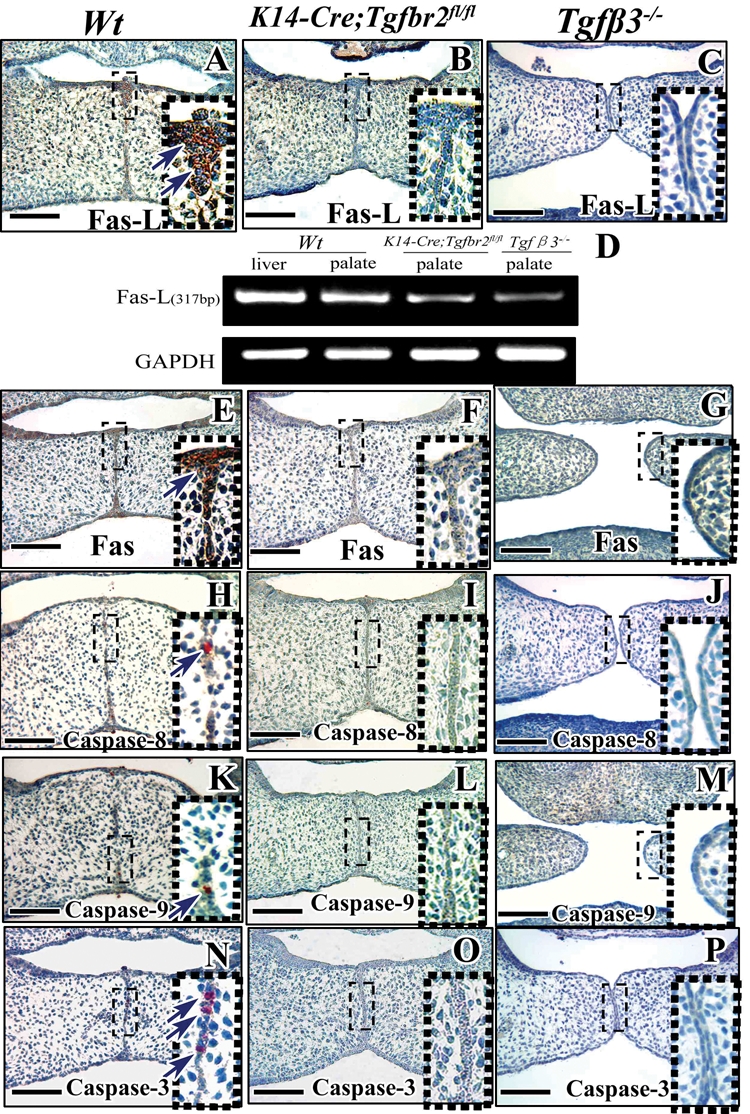
FasL-Fas-caspase pathway expression during palatogenesis is Tgf-β-dependent. Immunohistochemistry of Fas ligand (Fas-L; A-C), Fas (E-G), caspase-8 (H-J), caspase-9 (K-M), and caspase-3 (N-P) in E14.5 wild-type (Wt), K14-Cre;Tgfbr2 fl/fl, and Tgf-β3 −/− mice. MEE cells outlined with red dotted boxes are shown magnified to the right. (D) RT-PCR of Fas-L in E14.5 wild-type (Wt), K14-Cre;Tgfbr2 fl/fl, and Tgf-β3 −/− mice. Scale bars: A-C, E-P, 100 µm.
To test this model, we utilized a blocking FasL antibody to block FAS-L action. After 4 days’ organ culture, E13.5 palatal shelves treated with BSA fused together (Fig. 3A). In contrast, none of the samples treated with 800 ng/mL blocking FasL antibody showed complete fusion. No fusion of the palatal shelves was detectable in 75% (6/8) of the samples (Fig. 3C), and 25% (2/8) of the samples exhibited only partial fusion (Fig. 3B). Treatment with the pan-caspase-inhibitor z-VAD also resulted in no fusion of the palatal shelves (Fig. 3D).
Figure 3.
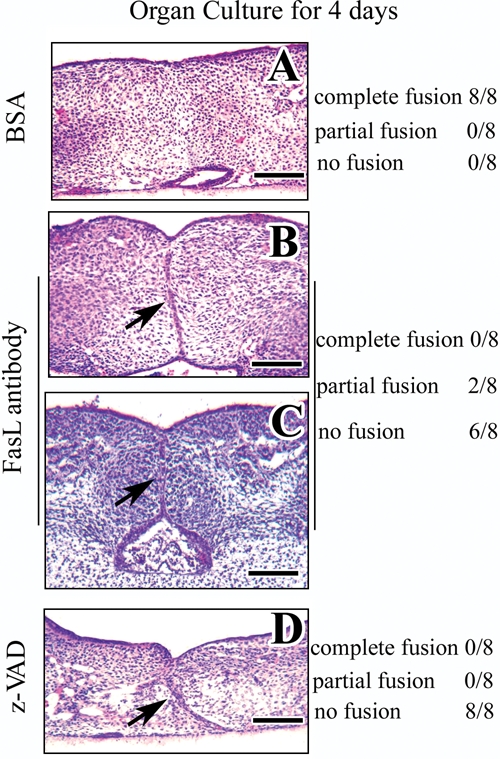
Blocking the Fas ligand-Fas-caspase pathway interferes with palatal fusion in organ culture. H&E staining after organ culture of E13.5 palatal shelves from wild-type mice treated with BSA (A), blocking Fas-L antibody (B, C), or pan-caspase-inhibitor z-VAD (D) for 4 days. All of the samples treated with BSA fused together completely (A), whereas samples treated with Fas ligand antibody fused partially (B) or not at all (C), and all samples treated with z-VAD failed to fuse (D). Scale bars: A-D, 50 µm.
Next, we investigated caspase expression after inhibiting the FasL-Fas system with blocking antibody or z-VAD in palatal shelf organ culture. After 2 days’ culture, palatal shelves had begun to fuse in samples treated with BSA, but not z-VAD or blocking Fas-L antibody (Figs. 4A-4C). Expression of caspases 3 and 8 was detectable in the MEE cells of samples treated with BSA medium (Figs. 4D, 4G), whereas it was not detectable in samples treated with z-VAD or blocking FasL antibody (Figs. 4E, 4F, 4H, 4I). Apoptotic cells were also detectable in the MES treated with BSA medium; however, few apoptotic signals were found in samples with z-VAD or blocking FasL antibody (Figs. 4J-4L). Quantitative analysis demonstrated that the number of TUNEL-positive cells was dramatically decreased in samples with z-VAD or blocking FasL antibody (Fig. 4P). Thus, analysis of our data demonstrated that a failure of the FasL-Fas-caspase-dependent apoptotic pathway in the MEE leads to persistence of the MES and lack of palatal fusion.
Figure 4.
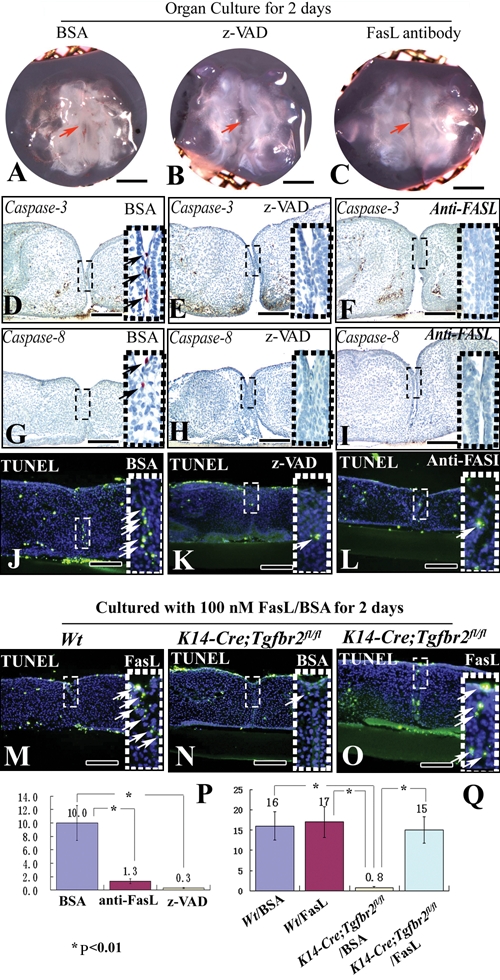
Blocking the Fas ligand-Fas-caspase pathway inhibits caspase expression. (A-C) Organ culture of E13.5 palatal shelves from wild-type mice treated with BSA (A), pan-caspase-inhibitor z-VAD (B), or blocking Fas-L antibody (C) for 2 days. Red arrows indicate fusion of the palatal shelves (A) or no fusion (B, C). (D-L, P) Immunohistochemistry of caspases 3 (D-F) and 8 (G-I) and TUNEL staining (J-L) after organ culture of E13.5 wild-type palatal shelves treated with BSA (D, G, J), pan-caspase-inhibitor z-VAD (E, H, K), or blocking Fas-L antibody (F, I, L) for 2 days. Arrows indicate positive signal. Quantitation of the number of TUNEL-positive cells in the MEE from J-L (P). (M-O, Q) TUNEL staining after organ culture of E13.5 palatal shelves from wild-type and K14-Cre;Tgfbr2 fl/fl mice treated with BSA (J, N) and FasL protein (M, O) for 2 days. Arrows indicate TUNEL-positive cells. Quantitation of the number of TUNEL-positive cells in the MEE from J, M-O (Q). Scale bars: A-C, 500 µm; D-I, 80 µm; J-L, N-P, 100 µm.
Finally, we utilized ectopic FasL to induce cell death in the MES of wild-type and K14-Cre;Tgfbr2 fl/fl mice. After 2 days’ culture, TUNEL-positive cells were detectable in the MEE cells of wild-type samples treated with BSA and FasL medium (Figs. 4J, 4M), whereas few apoptotic signals were detectable in samples of K14-Cre;Tgfbr2 fl/fl mice treated with BSA (Fig. 4N). Although the MES persisted between the 2 palatal shelves, many apoptotic cells were detectable in K14-Cre;Tgfbr2 fl/fl mutant mice treated with FasL medium (Fig. 4O). After quantitation of the TUNEL-positive cells, we found that the number of apoptotic cells in the MEE was statistically indistinguishable in K14-Cre;Tgfbr2 fl/fl mice treated with FasL and wild-type mice with BSA or FasL (Fig. 4Q). Therefore, analysis of our data suggests that FasL can partially rescue an apoptotic defect in the MES, due to lack of Tgf-β signaling during palatal fusion.
Discussion
Programmed cell death is essential for the development and health of multicellular organisms. Cells die in response to a variety of stimuli and in a highly regulated fashion. The FasL-Fas-caspase pathway, known as the extrinsic or death receptor pathway, is utilized throughout physiological activities and diseases. Moreover, the extrinsic pathway plays important roles in the proper development of a variety of tissues and organs, including the neural crest, the interdigital fields of the limb, and the mammary gland ductal system (Nguyen and Pollard, 2000).
In our study, we found that all apoptotic cells (TUNEL-positive) in the MEE during palatogenesis expressed caspase-3, which is an activated effector caspase that directly cleaves the DNA into fragments. In an organ culture system, blocking caspase activity via the pan-caspase-inhibitor z-VAD resulted in persistence of the MES (Cuervo et al., 2002; Takahara et al., 2004). Therefore, we conclude that MEE cells undergo PCD through a caspase-dependent apoptotic pathway during palatal fusion. To determine whether the extrinsic or intrinsic pathway mediates PCD in MEE cells, we used immunohistochemistry to examine FasL, Fas, and caspases 8 and 3 expression and found that they are detectable in MEE cells at E14.5. Treatment with a blocking FasL antibody in organ culture to inhibit the activities of FasL prevented palatal fusion and inhibited the expression of caspases 8 and 3. Then, we tried to utilize ectopic FasL with different levels to rescue the fusion defect in K14-Cre;Tgfbr2 fl/fl mutant mice. Unfortunately, the MES of palatal shelves cannot be degenerated in the mutant. There might be some unknown technical or biological problems, such as, insufficient levels of FasL being absorbed by the cells to rescue palatal fusion, or that the deletion of Tgfbr2 in epithelial cells might affect additional processes parallel to the FasL-Fas pathway. However, FasL can dramatically induce MEE cells to undergo apoptosis in K14-Cre;Tgfbr2 fl/fl mice, in which epithelial cells of the MES persist with reduced apoptosis. Therefore, we suggest that MEE cells, like neural crest cells and mammary cells, undergo cell death via the FasL-Fas-caspase extrinsic apoptotic pathway.
In studies of carcinoma development, Tgf-β is a potent inducer of growth inhibition in several cell types, including epithelial cells (Moses et al., 1990). The key events that lead to Tgf-β-induced growth arrest and cell death are the induction of expression of the CDK inhibitors p15 and/or p21, the induction of the expression of c-Myc, CDK4, and CDK6, and the induction of apoptosis by activation of caspase (Hannon and Beach, 1994; Chipuk et al., 2001). During development, TGF-β, as a pro-apoptotic factor and a key signal, mediates PCD in the developing retina (Dünker et al., 2002), neuron (Krieglstein et al., 2000), interdigital webs of the limb (mouse), and gastrointestinal tract (Dünker et al., 2002). Therefore, the endogenous Tgf-β plays a key pro-apoptotic role in a diverse spectrum of biological processes, including palatogenesis.
Tgf-β3 signaling is important for the disintegration of MEE cells during palatal fusion. Loss of Tgf-β3 and its type II receptor in the palatal epithelium leads to cleft palate (Kaartinen et al., 1995; Proetzel et al., 1995; Xu et al., 2006). Recent reports highlight the multiple roles of TGFβ3 in palatogenesis: (1) TGFβ3 functions in MEE adhesion (Choi et al., 2009); (2) TGFβ3 plays important roles in degradation of the underlying basement membrane (Xu et al., 2006); (3) TGFβ3 signaling is capable of promoting epithelial-mesenchymal transformation (EMT) and cell migration in the MES epithelia (Nawshad and Hay, 2003; Nawshad et al., 2004); (4) TGFβ3 induces cell-cycle arrest prior to cell migration (Choi et al., 2009); and (5) at later stages of palatogenesis, TGFβ3 triggers MES cells to undergo apoptosis to achieve confluence in palatal mesenchymal cells (Cuervo and Covarrubias, 2004; Xu et al., 2006).
In the present study, we found that FasL, Fas, and caspases 8 and 3, which play important roles in MEE cells during palatal development, are not expressed in K14-Cre;Tgfbr2 fl/fl and Tgf-β3 −/− mutant mice. Moreover, ectopic FasL induces MEE cells to undergo apoptosis in K14-Cre;Tgfbr2 fl/fl mice, in which MES persists with reduced apoptosis. Thus, we suggest that the FasL-Fas-caspase extrinsic apoptotic pathway is the mechanism by which Tgf-β3 signaling triggers PCD, resulting in palatal fusion. This study represents the first report that the extrinsic apoptotic pathway is triggered by Tgf-β in the MES during palatogenesis. We hope that our findings will improve the understanding of the molecular and cellular regulatory mechanisms of palatal formation and ultimately will provide strategies for the prevention of, and alternative treatment for, congenital birth defects.
Acknowledgments
We thank Drs. Sarah Millar and Harold Moses for K14-Cre and Tgfbr2fl/fl mice, respectively, and thank Julie Mayo for critical reading of the manuscript.
Footnotes
This study was supported by grants from the NIDCR, NIH (R37 DE012711 and R01 DE014078) to Yang Chai and by the Beijing New Star Program (2007B54) to Xiaofeng Huang.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, et al. (1995). Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441-444 [DOI] [PubMed] [Google Scholar]
- Carette MJ, Ferguson MW. (1992). The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development 114:379-388 [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE. (2006). Recent advances in craniofacial morphogenesis. Dev Dyn 235:2353-2375 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. (2001). Bcl-xL blocks TGF-β1-induced apoptosis by inhibiting cytochrome C release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. J Biol Chem 276:26614-26621 [DOI] [PubMed] [Google Scholar]
- Choi KY, Kim HJ, Cho BC, Kim IS, Kim HJ, Ryoo HM. (2009). A TGF-β-induced gene, big-h3, is crucial for the apoptotic disappearance of the medial edge epithelium in palate fusion. J Cell Biochem 107:818-825 [DOI] [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. (2004). Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development 131:15-24 [DOI] [PubMed] [Google Scholar]
- Cuervo R, Valencia C, Chandraratna RA, Covarrubias L. (2002). Programmed cell death is required for palate shelf fusion and is regulated by retinoic acid. Dev Biol 245:145-156 [DOI] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. (1995). Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373:438-441 [DOI] [PubMed] [Google Scholar]
- Dudas M, Li WY, Kim J, Yang A, Kaartinen V. (2007). Palatal fusion – Where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem 109:1-14 [DOI] [PubMed] [Google Scholar]
- Dünker N, Schmitt K, Schuster N, Krieglstein K. (2002). The role of transforming growth factor beta 2 and 3 in mediating apoptosis in the murine intestinal mucosa. Gastroenterology 122:1364-1375 [DOI] [PubMed] [Google Scholar]
- Ferguson MW. (1988). Palate development. Development 103(Suppl):41-60 [DOI] [PubMed] [Google Scholar]
- Green DR. (1998). Apoptotic pathways: the roads to ruin. Cell 94:695-698 [DOI] [PubMed] [Google Scholar]
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. (1995). Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Beach D. (1994). p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature 371:257-261 [DOI] [PubMed] [Google Scholar]
- Hengartner MO. (2000). The biochemistry of apoptosis. Nature 407:770-776 [DOI] [PubMed] [Google Scholar]
- Huang X, Bringas P, Jr, Slavkin HC, Chai Y. (2009). Fate of HERS during tooth root development. Dev Biol 334:22-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Xu X, Bringas P, Jr, Hung YP, Chai Y. (2010). Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res 25:1167-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, et al. (1995). Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11:415-421 [DOI] [PubMed] [Google Scholar]
- Krammer PH. (2000). CD95’s deadly mission in the immune system. Nature 407:789-795 [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Richter S, Farkas L, Schuster N, Dünker N, Oppenheim RW, et al. (2000). Reduction of endogenous transforming growth factors beta prevents ontogenetic neuron death. Nat Neurosci 3:1085-1090 [DOI] [PubMed] [Google Scholar]
- Moses HL, Yang EY, Pietenpol JA. (1990). TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63:245-247 [DOI] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. (2003). TGF-beta 3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. Cell Biol 163:1291-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, LaGamba D, Olsen BR, Hay ED. (2004). Laser capture microdissection (LCM) for analysis of gene expression in specific tissues during embryonic epithelial–mesenchymal transformation. Dev Dyn 230:529-534 [DOI] [PubMed] [Google Scholar]
- Nguyen AV, Pollard JW. (2000). Transforming growth factor-3 induces cell death during the first stage of mammary gland involution. Development 127:3107-3118 [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, et al. (1995). Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet 11:409-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Thompson CB. (2002). Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109(Suppl):97-107 [DOI] [PubMed] [Google Scholar]
- Shuler CF. (1995). Programmed cell death and cell transformation in craniofacial development. Crit Rev Oral Biol Med 6:202-217 [DOI] [PubMed] [Google Scholar]
- Takahara S, Takigawa T, Shiota K. (2004). Programmed cell death is not a necessary prerequisite for fusion of the fetal mouse palate. Int J Dev Biol 48:39-46 [DOI] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MW. (1999). Pathogenesis of cleft palate in TGFbeta-3 knock out mice. Development 126:3869-3879 [DOI] [PubMed] [Google Scholar]
- Vaziri Sani F, Hallberg K, Harfe BD, McMahon AP, Linde A, Gritli-Linde A. (2005). Fate-mapping of the epithelial seam during palatal fusion rules out epithelial-mesenchymal transformation. Dev Biol 285:490-495 [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Urata MM, Chai Y. (2006). Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol 297:238-248 [DOI] [PubMed] [Google Scholar]


