Abstract
Vitamin D regulates calcium and immune function. While vitamin D deficiency has been associated with periodontitis, little information exists regarding its effect on wound healing and periodontal surgery outcomes. This longitudinal clinical trial assessed outcomes of periodontal surgery and teriparatide administration in vitamin-D-sufficient and -insufficient individuals. Forty individuals with severe chronic periodontitis received periodontal surgery, daily calcium and vitamin D supplements, and self-administered teriparatide or placebo for 6 wks to correspond with osseous healing time. Serum 25(OH)D was evaluated at baseline, 6 wks, and 6 mos post-surgery. Clinical and radiographic outcomes were evaluated over 1 yr. Placebo patients with baseline vitamin D deficiency [serum 25(OH)D, 16-19 ng/mL] had significantly less clinical attachment loss (CAL) gain (-0.43 mm vs. 0.92 mm, p < 0.01) and probing depth (PPD) reduction (0.43 mm vs. 1.83 mm, p < 0.01) than vitamin-D-sufficient individuals. Vitamin D levels had no significant impact on CAL and PPD improvements in teriparatide patients at 1 yr, but infrabony defect resolution was greater in teriparatide-treated vitamin-D-sufficient vs. -deficient individuals (2.05 mm vs. 0.87 mm, p = 0.03). Vitamin D deficiency at the time of periodontal surgery negatively affects treatment outcomes for up to 1 yr. Analysis of these data suggests that vitamin D status may be critical for post-surgical healing. (ClinicalTrials.gov number, CT00277706)
Keywords: vitamin D, teriparatide, parathyroid hormone, periodontitis, periodontal surgery outcomes, osseous healing
Introduction
The main function of vitamin D is to support calcium homeostasis, but it also plays an important role in immunity, the cardiovascular system, diabetes, cancer, and chronic illness (Adams and Hewison, 2010). The primary sources of vitamin D are dietary intake and sunlight exposure in the form of vitamin D2 and D3, which are metabolized to 25-hydroxyvitamin D [25(OH)D] in the liver. Further metabolism in the kidneys produces the active form of vitamin D, 1,25-dihydroxyvitamin D (Holick, 2007).
Periodontitis is characterized by alveolar bone loss induced by the host immune response to bacterial insult. Because vitamin D plays a crucial role in bone maintenance and immunity, there is biologic rationale to suspect that a vitamin D deficiency could negatively affect the periodontium. A diagnosis of vitamin D deficiency is made through serum analysis of 25(OH)D levels. The normal range of serum 25(OH) D levels is 20-74 ng/mL. No absolute threshold for deficiency status is universally accepted, although most authorities agree that levels below 20-30 ng/mL constitute at least a mild deficiency, with severe vitamin D deficiency beginning at a level of 12 ng/mL (Malabanan et al., 1998; Bischoff-Ferrari et al., 2006; Holick, 2007). Vitamin D deficiency is highly prevalent, with an estimated 1 billion people affected worldwide (Holick, 2007); however, it is difficult to estimate the prevalence due to a lack of consensus about the definition, and recent information suggests that this may be overestimated (Ross et al., 2011). In addition, mean serum 25(OH)D levels appear to be declining over the past several decades, due to changes in BMI, dietary intake, and sun exposure (Looker et al., 2008).
Calcium, phosphorus, and parathyroid hormone levels all influence the rate of conversion of 25(OH)D to its active form (DeLuca, 2004). Parathyroid hormone (PTH) is an endogenous hormone with both catabolic and anabolic properties in bone, depending on the concentration and dosing regimen (Khosla et al., 2008; Kousteni and Bilezikian, 2008). Several studies have confirmed that serum levels of PTH are inversely proportional to those of 25(OH)D, and that there is seasonal variation in these levels (Thomas et al., 1998; Cranney et al., 2007). Recently, it was determined that a minimum 25(OH)D serum concentration of 28 ng/mL was required to stabilize PTH levels and maintain normal calcium availability (Okazaki et al., 2011). Consequently, low vitamin D levels may result in high, catabolic PTH levels that could negatively affect bone health.
Analysis of cross-sectional data from the third National Health and Nutrition Examination Survey (NHANES III) revealed that individuals with the highest 25(OH)D levels experienced 20% less bleeding on probing than those with the lowest levels, suggesting that vitamin D may reduce the risk of gingival inflammation by exerting anti-inflammatory effects (Dietrich et al., 2005). Analysis of data from this study also demonstrated an inverse relationship between clinical attachment loss (CAL) and 25(OH)D levels in persons aged 50 yrs or older (Dietrich et al., 2004). A 5-year prospective study found that calcium and vitamin D supplementation decreased the risk of tooth loss in elderly men and women (Krall et al., 2001). Similarly, periodontal maintenance patients taking calcium and vitamin D supplements had better periodontal health than those who did not (Miley et al., 2009; Garcia et al., 2011). A case-control study found that vitamin D insufficiency was associated with periodontal disease among pregnant women (Boggess et al., 2010). However, no study to date has evaluated long-term outcomes of surgical intervention based on a person’s vitamin D status. The aim of this study was to evaluate the effect of pre-surgical vitamin D status on periodontal surgery outcomes with or without concomitant administration of anabolic doses of a commercially available form of PTH (teriparatide, PTH 1-34).
Materials & Methods
The University of Michigan Institutional Review Board approved the study, which was conducted from January 20, 2005 – June 25, 2009. Written informed consent was obtained from all participants prior to enrollment. A detailed study protocol along with separate outcomes data was published previously (Bashutski et al., 2010). Briefly, 40 individuals with severe periodontal disease received open flap debridement surgery in one sextant of the mouth and were followed for 1 yr post-surgery. The primary outcome variable was infrabony defect resolution, comparing those taking teriparatide with those receiving placebo medication. A post hoc analysis of the effect of vitamin D status on clinical and radiographic outcomes was then completed.
Study medications were randomized by the pharmacy, and each patient received either teriparatide (20 µg) or placebo that was self-administered daily via subcutaneous injection to the thigh or abdomen. Dosing of the study medication, along with daily 1000 mg calcium and 800 IU vitamin D oral supplements, was initiated 3 days prior to surgery and continued for 6 wks to correspond with osseous healing. To assess drug adherence, we collected all unused medication and monitored serum bone alkaline phosphatase levels throughout the drug administration phase.
Periodontal surgery consisted of an open flap debridement procedure in the study quadrant by 2 blinded operators (R.M.E. and J.D.B.). To maintain blinding, the operators did not evaluate the individuals on whom they performed surgery. Supra- and sub-gingival scaling, polishing, and oral hygiene instruction were provided every 3 mos post-surgically.
Serum samples were collected to determine 25(OH)D levels at baseline, 6 wks, and 6 mos post-surgery and analyzed independently at the Mayo Clinic. Clinical parameters were evaluated at baseline, 6 wks, and 3, 6, 9, and 12 mos by three blinded examiners (J.D.B., R.M.E., and J.S.K.) and included probing depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP). Standardized periapical and bitewing radiographs of the treatment area were taken at baseline, 3, 6, 9, and 12 mos (Duckworth et al., 1983; Reddy and Jeffcoat, 1993) and analyzed with digital software (Emago™, Oral Diagnostic Systems, Amsterdam, Netherlands). Independent analyses of linear defect resolution, assessed radiographically, were completed by two calibrated and blinded examiners (J.D.B. and E.B.). Linear defect resolution was measured from the deepest point of the initial defect to the first point at which complete bone fill occurred.
The study participants were stratified by treatment group (teriparatide vs. placebo) and by whether they were vitamin-D-deficient at baseline, defined as a serum vitamin D less than 20 ng/mL. For each of the four groups (vitamin-D-deficient/ placebo, vitamin-D-sufficient/placebo, vitamin-D-deficient/teriparatide, vitamin-D-sufficient/teriparatide), changes in CAL, PD, and linear bone gain were evaluated for the surgical sites at each time-point.
A paired t test was used for within-patient comparisons, and a two-sample t test was used for between-patient group comparisons. Statistical significance was defined as a p-value less than 0.05. We also used generalized estimating equations to compare treatment groups at all time-points simultaneously, but these gave results similar to those reported when two-sample t tests were used at each time-point.
Results
Baseline demographics of the study population are presented in Table 1 with no significant differences between groups. The compliance rate of the 40 enrolled individuals was high, with only 2 missed follow-up appointments—one 9-month (vitamin-D-sufficient teriparatide patient) and one 12-month (vitamin-D-sufficient teriparatide patient) (Appendix Fig.). At baseline, 11 participants were vitamin-D-deficient (28%); of these, four received teriparatide as part of the study. At 6 wks, five vitamin-D-deficient placebo patients converted to sufficiency; of those, four returned to a deficient status at 6 mos. All of the vitamin-D-deficient teriparatide patients achieved sufficiency at 6 wks, and only one became deficient again at 6 mos. Table 2 shows the mean serum 25(OH) vitamin D levels over time for each group.
Table 1.
Demographics of the Study Population
| Placebo | Teriparatide | |||
|---|---|---|---|---|
| Characteristic | Deficient | Sufficient | Deficient | Sufficient |
| Vitamin D Status | (N = 7) | (N = 13) | (N = 4) | (N = 16) |
| Median age in yrs (range) | 43 (31-64) | 57 (38-65) | 48 (43-61) | 47 (30-61) |
| Gender | ||||
| Male | 2 | 4 | 3 | 6 |
| Female | 5 | 9 | 1 | 10 |
| Race/ethnicity | ||||
| Caucasian | 4 | 11 | 2 | 13 |
| African-American | 2 | 2 | 0 | 2 |
| Asian | 1 | 0 | 0 | 1 |
| Hispanic | 0 | 0 | 1 | 0 |
| Arabic | 0 | 0 | 1 | 0 |
| Smoking status | ||||
| Current | 4 | 2 | 3 | 6 |
| Former | 2 | 5 | 0 | 5 |
| Never | 1 | 6 | 1 | 5 |
Table 2.
Serum 25(OH) Vitamin D Changes over Time Based on Status (Deficient or Sufficient) at Baseline
| Placebo | Teriparatide | |||||
|---|---|---|---|---|---|---|
| Mean Serum Vitamin D Level (ng/mL) ± Standard Error | Deficient | Sufficient | Deficient | Sufficient | ||
| Time-point | (N = 7) | (N = 13) | p-value | (N = 4) | (N = 16) | p-value |
| 0 | 17.29 ± 0.42 | 33.92 ± 2.76 | < 0.001 | 17.75 ± 0.63 | 31.69 ± 1.53 | < 0.001 |
| 6 wks | 26.43 ± 4.44 | 40.54 ± 5.65 | 0.050 | 38.25 ± 8.23 | 31.19 ± 2.41 | 0.410 |
| 6 mos | 20 ± 2.54 | 31.08 ± 2.37 | 0.001 | 26.25 ± 5.94 | 29.69 ± 1.81 | 0.580 |
For individuals who received placebo, periodontal surgery resulted in improved CAL gain and PD reduction at the surgical site if they had sufficient vitamin D levels prior to surgery (Figs. A, C). Those who were initially vitamin-D-deficient lost clinical attachment post-surgically. Significant differences in clinical outcomes between vitamin-D-deficient and -sufficient participants were noted at all follow-up time-points in the study, beginning 6 wks post-operatively. At 12 mos, vitamin-D-sufficient individuals had greater CAL gain (0.92 mm vs. −0.43 mm, p < 0.01) and PD reduction (1.83 mm vs. 0.43 mm, p < 0.01) compared with -deficient individuals. Radiographic linear infrabony defect resolution was minimal, and there were no significant differences between groups at any time-point (Fig. E). Bleeding upon probing (BOP) was reduced by 36% in deficient participants and 42% in sufficient participants at 12 mos, with no significant differences between groups.
Figure.
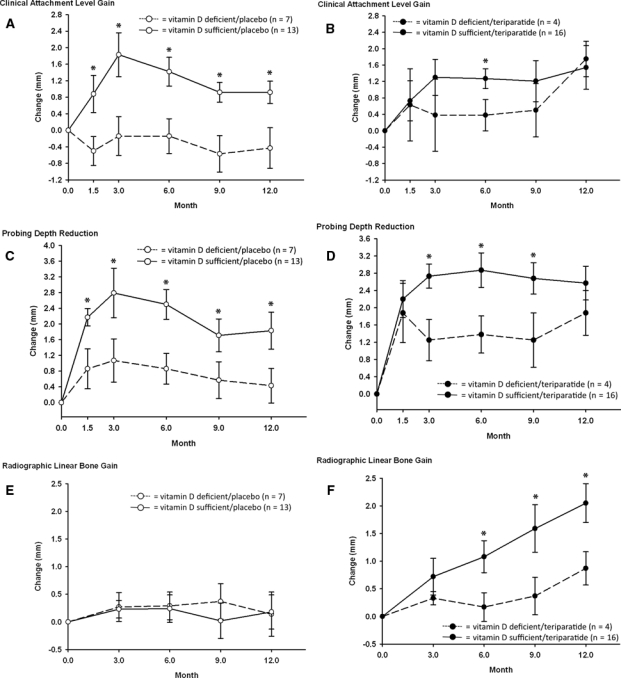
Clinical and radiographic outcomes of vitamin-D-sufficient and -deficient participants supplemented with placebo or teriparatide for 6 wks. Mean (± SE) changes from baseline for clinical attachment level gain (A, B), probing depth reduction (C, D), and linear defect resolution as measured radiographically with bitewing radiographs (E, F). In participants taking placebo, clinical attachment level gain was significantly greater in vitamin-D-sufficient individuals at all time-points, with vitamin-D-deficient persons experiencing a loss of attachment compared with baseline (A, p < 0.01). Probing depth reductions were significantly greater in vitamin-D-sufficient participants compared with vitamin-D-deficient participants at all time-points (C, p < 0.01). Neither group experienced significant radiographic defect resolution compared with baseline, or between groups (E, p = NS). For those supplemented with teriparatide, both vitamin-D-sufficient and -deficient groups experienced significant improvement in all parameters at 12 mos compared with baseline (B,D,F, p < 0.01). Clinical attachment level gain and probing depth reduction were not significantly different between vitamin D groups at the 12-month time-point, although vitamin-D-deficient participants had significantly less improvement in clinical attachment gain at 6 mos (B, p < 0.01) and significantly less improvement in probing depth reduction at 3, 6, and 9 mos (D, p < 0.01). Teriparatide recipients who were vitamin-D-sufficient experienced significantly greater radiographic linear defect resolution than -deficient participants beginning at 6 mos (F, p = 0.03).
For individuals who received teriparatide, open flap debridement surgery resulted in improved clinical and radiographic outcomes at 12 mos (Figs. B, D, F; Bashutski et al., 2010). Vitamin-D-sufficient participants had significantly greater CAL gain at 6 mos (p < 0.01; Fig. B), and significantly greater PD reduction from the 3-month time-point to the 9-month time-point (p < 0.01; Fig. D). However, at 1 yr, the outcomes for vitamin-D-deficient and -sufficient participants were similar for CAL gain (1.54 mm vs. 1.75 mm, NS) and PD reduction (2.57 mm vs. 1.88 mm, NS). In contrast, those who were vitamin-D-sufficient at baseline experienced more radiographic infrabony defect resolution compared with those who were deficient at baseline (Fig. F, 2.05 mm vs. 0.87 mm, p = 0.03). Teriparatide patients who were vitamin-D-deficient at baseline had a 12% increase in BOP at 12 mos, compared with a 39% decrease in vitamin-D-sufficient teriparatide patients (p < 0.01).
Discussion
Vitamin D is necessary for bone formation and proper immune function, which are also important to the success of periodontal therapy. The prevalence of vitamin D deficiency in this study was high and comparable with that of the general population (Chapuy et al., 1997; Guardia et al., 2008), with 28% of enrolled participants presenting with mild deficiency [16-19 ng/mL serum 25(OH)D level]. 25OHD values lower than 16 ng/mL have been shown to have a drastic effect on PTH levels (Carnevale et al., 2010). Participants with moderate to severe deficiency were excluded from the study, and constituted 9.7% of all individuals screened.
Previously, only a few studies had assessed the role of vitamin D on periodontal disease status. A recent survey of periodontal maintenance patients found that only 7% had vitamin D intake levels that met published guidelines (Dixon et al., 2009). In cross-sectional studies, low vitamin D levels have been associated with increased gingival inflammation, tooth loss, clinical attachment loss, and maternal periodontal disease during pregnancy (Krall et al., 2001; Dietrich et al., 2004, 2005; Miley et al., 2009; Boggess et al., 2010). However, ours was the first study to report on the effect of vitamin D status on periodontal surgery. Vitamin D sufficiency in placebo patients at the time of surgery resulted in an average of 1.35 mm greater CAL gain and 1.4 mm greater PD reduction compared with deficient patients at 12 mos. Cross-sectional studies evaluating non-treatment situations found mean differences of 0.21-0.39 mm in CAL between vitamin-D-sufficient and -deficient patients (Dietrich et al., 2004; Miley et al., 2009).
Interestingly, vitamin D supplementation at the time of surgery failed to prevent the negative clinical outcomes associated with baseline deficiency. Patients were supplemented with vitamin D for only a six-week period, and it takes up to 3 mos for serum 25(OH)D levels to stabilize after vitamin D intake is increased (Vieth et al., 2001). Six-week vitamin D supplementation alone did not exert long-term effects, since serum 25(OH)D levels returned to baseline levels in placebo patients by 6 mos. However, vitamin D levels remained elevated in teriparatide patients, which may account for the lack of significant differences in clinical outcomes in the teriparatide groups. The transient increase in serum levels of 25(OH)D that coincided with the six-week healing phase may also have had important anti-inflammatory effects. This phenomenon may explain the early improvements in CAL gain and PD decreases in the vitamin-D-deficient teriparatide patients. However, this increase did not reach the threshold level of 28 ng/mL required to stabilize PTH levels and maintain normal calcium availability for all patients and may explain the greater improvement in bone defects in the vitamin-D-sufficient teriparatide patients.
In participants taking teriparatide, vitamin D status was not a modifier of the clinical findings at the one-year time-point. However, there was a trend toward better outcomes in participants who were vitamin-D-sufficient. Among teriparatide patients, vitamin-D-deficient individuals had significantly less improvement in CAL gain at 6 mos (p < 0.01) and significantly less improvement in PD reduction at 3, 6, and 9 mos (p < 0.01). Thus, the lack of significance at 1 yr may be due to the small sample size in this subset. Alternatively, analysis of these data could suggest that teriparatide overrides the negative effects of vitamin D deficiency. In a previous study, vitamin D and calcium supplementation was shown to have a positive effect on outcomes of fracture healing in osteoporotic women during the first 6 wks of healing, although the effects were no longer significant at the 12-week time-point (Doetsch et al., 2004).
Open flap debridement surgery does not result in appreciable bone gain (Dybvik et al., 2007; Shirakata et al., 2008), explaining the lack of significance of vitamin D status on radiographic outcomes in the placebo group. In contrast, for participants taking teriparatide and for whom we previously reported a significant benefit in radiographic linear bone gain (Bashutski et al., 2010), baseline vitamin D deficiency resulted in less infrabony defect resolution compared with that in participants with sufficient levels. Results from animal studies suggest that vitamin D may play a predominant role in mandibular anabolic bone formation (Liu et al., 2009), and may exert positive effects on fracture healing (Fu et al., 2009). Vitamin D deficiency has also been associated with compromised osseous healing in the oral cavity in bisphosphonate-associated osteonecrosis of the jaw pre-clinical studies, supporting a role for vitamin D in bone healing in the oral cavity (Hokugo et al., 2010; Yamashita et al., 2010).
Analysis of these data suggests that if an individual is vitamin-D-deficient, minimal benefits can be obtained from periodontal surgery. Furthermore, vitamin D supplementation at the time of surgery is unable to prevent this effect. Since vitamin D deficiency is highly prevalent, it may be advisable to ensure adequate vitamin D levels well in advance of periodontal surgery, to attain the best possible results. Radiographic outcomes were better in vitamin-D-sufficient patients taking teriparatide, suggesting that an anabolic agent, such as teriparatide, benefits from vitamin D sufficiency to promote oral bone formation. However, due to the small sample size of this pilot study and, particularly, the vitamin-D-deficient groups, larger-scale trials are needed to confirm these initial results.
Supplementary Material
Footnotes
This study was supported by Eli Lilly through an investigator-initiated study, the American Academy of Periodontology Foundation Tarrson Regeneration Scholarship, and the University of Michigan Clinical Research Unit (NIH/NCRR UL1RR024986). Lea Franco and Jim Sugai provided invaluable assistance. Jonathan Ee, Henry Mallett, and JooHyung Choi provided significant data entry support. Lindsay Rayburn offered expert advice regarding radiographic analysis.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Adams JS, Hewison M. (2010). Update in vitamin D. J Clin Endocrinol Metab 95:471-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. (2010). Teriparatide and osseous regeneration in the oral cavity. N Engl J Med 363:2396-2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. (2006). Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18-28 [DOI] [PubMed] [Google Scholar]
- Boggess KA, Espinola JA, Moss K, Beck J, Offenbacher S, Camargo CA. (2010). Vitamin D status and periodontal disease among pregnant women. J Periodontol 82:195-200 [DOI] [PubMed] [Google Scholar]
- Carnevale V, Nieddu L, Romagnoli E, Battista C, Mascia ML, Chiodini I, et al. (2010). Regulation of PTH secretion by 25-hydroxyvitamin D and ionized calcium depends on vitamin D status: a study in a large cohort of healthy subjects. Bone 47:626-630 [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. (1997). Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439-443 [DOI] [PubMed] [Google Scholar]
- Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. (2007). Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 158:1-235 [PMC free article] [PubMed] [Google Scholar]
- DeLuca HF. (2004). Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80(6 Suppl):1689S-1696S [DOI] [PubMed] [Google Scholar]
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. (2004). Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 80:108-113 [DOI] [PubMed] [Google Scholar]
- Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. (2005). Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr 82:575-580 [DOI] [PubMed] [Google Scholar]
- Dixon D, Hildebolt CF, Miley DD, Garcia MN, Pilgram TK, Couture R, et al. (2009). Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br Dent J 206:627-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch AM, Faber J, Lynnerup N, Watjen I, Bliddal H, Danneskiold-Samsoe B. (2004). The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo-controlled study. Calcif Tissue Int 75:183-188 [DOI] [PubMed] [Google Scholar]
- Duckworth JE, Judy PF, Goodson JM, Socransky SS. (1983). A method for the geometric and densitometric standardization of intraoral radiographs. J Periodontol 54:435-440 [DOI] [PubMed] [Google Scholar]
- Dybvik T, Leknes KN, Boe OE, Skavland RJ, Albandar JM. (2007). Bioactive ceramic filler in the treatment of severe osseous defects: 12-month results. J Periodontol 78:403-410 [DOI] [PubMed] [Google Scholar]
- Fu L, Tang T, Miao Y, Hao Y, Dai K. (2009). Effect of 1,25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone 44:893-898 [DOI] [PubMed] [Google Scholar]
- Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, et al. (2011). One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82:25-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia G, Parikh N, Eskridge T, Phillips E, Divine G, Rao DS. (2008). Prevalence of vitamin D depletion among subjects seeking advice on osteoporosis: a five-year cross-sectional study with public health implications. Osteoporos Int 19:13-19 [DOI] [PubMed] [Google Scholar]
- Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, et al. (2010). Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 25:1337-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. (2007). Vitamin D deficiency. N Engl J Med 357:266-281 [DOI] [PubMed] [Google Scholar]
- Khosla S, Westendorf JJ, Oursler MJ. (2008). Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118:421-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bilezikian JP. (2008). Cellular actions of parathyroid hormone. In: Principles of bone biology. San Diego, CA: Academic Press/Elsevier, pp. 639-663 [Google Scholar]
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. (2001). Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med 111:452-456 [DOI] [PubMed] [Google Scholar]
- Liu H, Guo J, Wang L, Chen N, Karaplis A, Goltzman D, et al. (2009). Distinctive anabolic roles of 1,25-dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. J Endocrinol 203:203-213 [DOI] [PubMed] [Google Scholar]
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. (2008). Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr 88:1519-1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabanan A, Veronikis IE, Holick MF. (1998). Redefining vitamin D insufficiency. Lancet 351:805-806 [DOI] [PubMed] [Google Scholar]
- Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, et al. (2009). Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol 80:1433-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R, Sugimoto T, Kaji H, Fujii Y, Shiraki M, Inoue D, et al. (2011). Vitamin D insufficiency defined by serum 25-hydroxyvitamin D and parathyroid hormone before and after oral vitamin D(3) load in Japanese subjects. J Bone Miner Metab 29:103-110 [DOI] [PubMed] [Google Scholar]
- Reddy MS, Jeffcoat MK. (1993). Digital subtraction radiography. Dent Clin North Am 37:553-565 [PubMed] [Google Scholar]
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. (2011). The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J Clin Endocrinol Metab 96:53-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata Y, Setoguchi T, Machigashira M, Matsuyama T, Furuichi Y, Hasegawa K, et al. (2008). Comparison of injectable calcium phosphate bone cement grafting and open flap debridement in periodontal intrabony defects: a randomized clinical trial. J Periodontol 79:25-32 [DOI] [PubMed] [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. (1998). Hypovitaminosis D in medical inpatients. N Engl J Med 338:777-783 [DOI] [PubMed] [Google Scholar]
- Vieth R, Chan PC, MacFarlane GD. (2001). Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73:288-294 [DOI] [PubMed] [Google Scholar]
- Yamashita J, McCauley LK, Van Poznak C. (2010). Updates on osteonecrosis of the jaw. Curr Opin Support Palliat Care 4:200-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


