Abstract
Background
Colorectal cancer (CRC) screening and treatment are rapidly evolving.
Aims
To reappraise stool-based CRC screening in light of changing test performance characteristics, lower test cost, and increasing CRC care costs.
Methods
Using a Markov model, we compared fecal DNA testing every 3 years (F-DNA), annual fecal occult blood testing (FOBT) or immunochemical testing (FIT), and colonoscopy every 10 years (COLO).
Results
In the base case, FOBT and FIT gained life-years/person and cost less than no screening. F-DNA version 1.1 at $300 (the current PreGen Plus test) gained 5,323 life-years/100,000 persons at $16,900/life-year gained, and F-DNA version 2 (enhanced test) gained 5,795 life-years/100,000 persons at $15,700/life-year gained vs. no screening. In the base case and most sensitivity analyses, FOBT and FIT were preferred over F-DNA. F-DNA version 2 cost $100,000/life-year gained vs. FIT when per-cycle adherence with FIT was 22%. FIT with excellent adherence was superior to COLO.
Conclusions
As novel biological therapies increase CRC treatment costs, FOBT and FIT could become cost-saving. The cost-effectiveness of F-DNA compared with no screening has improved, but FOBT and FIT are preferred over F-DNA when patient adherence is high. FIT may be comparable to COLO in persons adhering to yearly testing.
INTRODUCTION
Colorectal cancer (CRC) affects up to 6% of the population and is the second leading cause of cancer-related death in the U.S.(1) Each year, approximately 145,000 new cases of CRC are diagnosed and approximately 55,000 deaths are attributed to CRC in the U.S.(2) Screening decreases CRC incidence and mortality and is cost-effective,(3–13) but only a minority of the population has been screened.(14, 15) Patient preferences for invasive vs. non-invasive screening tests vary,(16–18) and the availability of some tests may be limited.(19)
In 2004, we first explored the potential role of fecal DNA testing in average-risk persons.(20) We concluded that it could not be considered a substitute for traditional screening methods, but that it could have an important impact if it attracted persons who are not currently screened for CRC.(20) A prospective trial of the original PreGen Plus fecal DNA test (Exact Sciences Corporation, Marlboro, MA and LabCorp, Burlington, NC) subsequently found the test to be superior to fecal occult blood testing in detecting CRC and large adenomas,(21) but its performance was inferior to our original estimates and its projected effectiveness and cost-effectiveness declined.(22).
CRC screening is a rapidly evolving field and key variables that affect estimates of effectiveness and cost-effectiveness are changing, including test performance characteristics and cost, and costs of CRC care. Technical advances in DNA stabilization,(23) DNA extraction from stool,(24) and use of gene-specific methylation(25) have improved the fecal DNA test.(26) Test cost has decreased to approximately $300 after write-offs (personal communication, Barry Berger, Exact Sciences Corporation). At the same time, bevacizumab (an antibody targeting vascular endothelial growth factor, a known regulator of tumor cell angiogenesis) and cetuximab (an antibody targeting the epidermal growth factor receptor, a tyrosine kinase important in the regulation of growth and survival pathways in CRC cells)(27–29) have emerged as novel treatments that enhance the efficacy of chemotherapy for advanced CRC,(28, 30) but also markedly increase treatment costs.(31)
Our aims were to reappraise noninvasive stool-based screening for colorectal neoplasia in persons unwilling or unable to undergo invasive screening with sigmoidoscopy or colonoscopy in light of changing fecal DNA test performance characteristics,(21, 26) lower test cost, and increasing costs of CRC care. We compared fecal DNA testing, guaiac-based fecal occult blood testing, and fecal immunochemical testing. Because adherence with yearly guaiac-based fecal occult blood testing is poor,(15, 32–44) we examined in detail the potential impact of imperfect adherence on the effectiveness and cost-effectiveness of screening strategies. We have previously examined the cost-effectiveness of other modalities, including colonoscopy.(11, 20, 22, 45) While here we focus on stool-based testing, we report results for screening colonoscopy for purposes of comparison.
MATERIALS AND METHODS
Literature Review and Data Sources
The sources for most model inputs have been described previously.(11, 20, 22, 45) For updated clinical information on fecal DNA testing and FIT, we searched PubMed using the terms fecal DNA, colorectal cancer, fecal immunohistochemistry, detection, sensitivity, specificity, and test performance, we reviewed national meeting abstracts, and we obtained data from EXACT Sciences Corporation (Marlboro, MA) and FDA submission data from Enterix Inc. (Edison, NJ), maker of InSure FIT. For updated cost data, we searched PubMed using the terms colorectal cancer, chemotherapy, and cost, we reviewed national meeting abstracts, we obtained data from EXACT Sciences Corporation, and we used 2006 Medicare fee schedules, as detailed below.(46)
Decision Analytic Model
Our decision analytic model and its calibration and validation have been described in detail.(11, 20, 22, 45, 47) The model is constructed in TreeAge (TreeAge Software, Inc., Williamston, MA) and the Natural History model is calibrated to reproduce the natural history and age-specific incidence and prevalence of colorectal adenomas and CRC in the U.S. without screening.(11, 20, 22, 45, 47) Screening strategies are then superimposed on the Natural History model. As described in detail previously, the model’s predictions for conventional strategies are consistent with available clinical data.(11, 20, 22, 45, 47) For the current analysis, the model was modified to allow variable adherence rates every time a screening test was offered. To validate this modification, we have modeled a cohort representing the one studied by Mandel et al.(32, 33) with FOBT offered and followed up as in that study.(22) Our model predicts a 21% reduction in CRC incidence over 18 years vs. 20% observed in the study,(33) and a 36% reduction in CRC mortality over 16 years vs. 33% observed in the study.(32)
Natural History
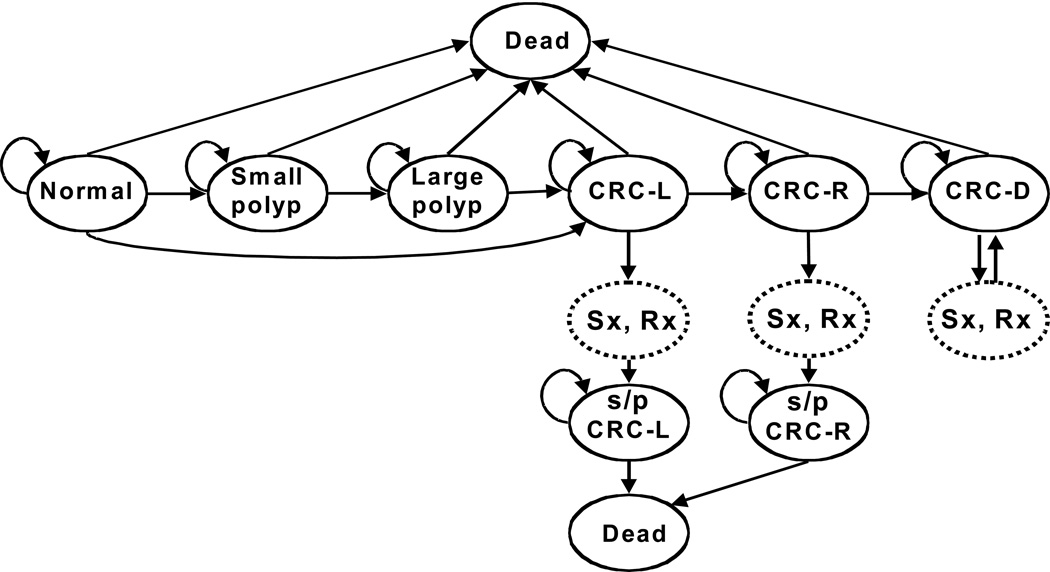
The principal health states in the model are (Figure 1): normal; small (<10 mm) adenomatous polyp; large (≥10 mm) adenomatous polyp; localized, regional, or distant CRC; and dead. Approximately 85% of CRCs develop through a polypoid adenoma. In the Natural History model, CRCs are diagnosed with colonoscopy once they lead to symptoms. Diagnosed CRCs are treated, resulting in stage-specific survival.(11, 20, 28, 30, 45, 48–51) Persons surviving CRC treatment enter surveillance (see below). Beginning at age 50 years, average-risk persons progress through the model for 50 1-year cycles, until age 100 years or death. Age-specific non-CRC mortality rates reflect U.S. life table data.(52) Model inputs are shown in Table 1.
Figure 1.
Markov states in the natural history model. Persons cycle between states every year from age 50 to 100. Screening strategies were superimposed on the natural history model.
Table 1.
Inputs in the Cost-Effectiveness Model
| Variable | Base Case Value (Range)* | References |
|---|---|---|
| Clinical | ||
| Polyp prevalence at age 50, %† | 15 | (91–93) |
| small polyp, % † | 95 | (93–95) |
| large polyp % † | 5 | (93–95) |
| Annual transition rate to small polyp from normal, %† | Age specific, 1.1–1.9 | (91–95) |
| Annual transition rate to large polyp from small polyp, %† | 1.5 | (93–96) |
| Annual transition rate to cancer without polypoid precursor, %† | Age specific, 0.006–0.086 | (9, 91–93, 97) |
| Annual transition rate to cancer from large polyp, %† | 5 | (9, 91–93, 97) |
| Symptomatic presentation of localized cancer, %† | 22/y over 2y | (97) |
| Symptomatic presentation of regional cancer, %† | 40/y over 2y | (97) |
| Mortality rate from treated localized cancer, %¶ | 1.74/y in first 5y | (97) |
| Mortality rate from treated regional caner, %¶ | 8.6/y in first 5y | (97) |
| Mean survival from distant cancer, y | 1.9 | (28, 30, 31, 48–51, 78, 79, 97) |
| Mortality rate from cancer treatment, % | 2 | (9, 10) |
| Fecal occult blood testing sensitivity for cancer, % | 40 (30–60) | (9, 10) |
| Fecal occult blood testing sensitivity for large polyp, % | 10 (5 – 15) | (9, 10) |
| Fecal occult blood testing specificity, %‡ | 92 (90–97) | (9, 10) |
| FIT testing sensitivity for cancer, % | 76 (62–88) | (56–66) |
| FIT testing sensitivity for large polyp, % | 40 (20–67) | (56–66) |
| FIT testing specificity, %‡ | 91 (86–98) | (56–66) |
| Fecal DNA version 1 testing sensitivity for cancer, % | 52 (35–68) | (21) |
| Fecal DNA version 1 testing sensitivity for large polyp, % | 18 (14–22) | (21) |
| Fecal DNA version 1 testing specificity, %‡ | 94 (93–96) | (21) |
| Fecal DNA version 1.1 testing sensitivity for cancer, % | 73 (57–84) | (26) |
| Fecal DNA version 1.1 testing sensitivity for large polyp, % | 18 (14–22) | (26) |
| Fecal DNA version 1.1 testing specificity, %‡ | 89 (83–94) | (26) |
| Fecal DNA version 2 testing sensitivity for cancer, % | 88 (74–95) | (26) |
| Fecal DNA version 2 testing sensitivity for large polyp, % | 18 (14–22) | (26) |
| Fecal DNA version 2 testing specificity, %‡ | 82 (74–88) | (26) |
| Colonoscopy sensitivity for cancer, % | 95 (90–97) | (9, 10) |
| Colonoscopy sensitivity for large polyp, % | 90 (85–95) | (9, 10) |
| Colonoscopy sensitivity for small polyp, % | 85 (80–90) | (9, 10) |
| Colonoscopy major complication rate, % | 0.1 (0.05–0.5) | (9, 10) |
| Colonoscopy mortality rate, % | 0.01 (0.005–0.03) | (9, 10) |
| Cost, $ | ||
| Fecal occult blood testing | 15 (15–56) | (46) |
| FIT testing | 22 (22–95) | (46) |
| Fecal DNA testing | 300 (300–495) | # |
| Colonoscopy | 920 (710–1350) | (7, 9, 11, 20, 47, 98) |
| Colonoscopy with lesion removal | 1350 (990–2030) | (7, 9, 11, 20, 47, 98) |
| Endoscopy complication | 29,000 (16,000 – 43,000) | (71, 72) |
| Colorectal cancer care by stage§ | ||
| Localized | 51,000 (40,000–62,000) | (5, 73–75) |
| Regional | 98,000 (85,000 – 105,000) | (5, 28, 30, 31, 48–51, 73–75, 78, 79) |
| Distant | 200,000 (175,000 – 230,000) | (5, 28, 30, 31, 48–51, 73–75, 78, 79) |
Range for test sensitivity and specificity used in Monte Carlo simulation
Derived from epidemiologic and autopsy data
The annual mortality rate applies to those surviving to the beginning of each year, reflecting exponential decay since the fraction of persons surviving decreases at a rate proportional to its value
Sensitivity for small polyp set at (1-specificity)
Derived from Centers for Medicare and Medicaid Services and published data
Derived from LabCorp list price and average reimbursement (personal communication, Barry Berger, Exact Sciences Corp.)
Screening Strategies and Surveillance
We compared Natural History, fecal DNA testing (F-DNA), annual guaiac-based fecal occult blood testing (FOBT) and annual fecal immunochemical testing (FIT). First, a screening interval for F-DNA was selected that could be considered cost-effective compared to a shorter screening interval, as described below. Because our focus was noninvasive stool-based screening strategies, flexible sigmoidoscopy and colonoscopy are not presented as alternatives.
Screening strategies were superimposed on the Natural History model. In the base case, in all strategies, screening and surveillance with perfect adherence were performed up to and including age 80. Variable adherence was a principal focus of sensitivity analyses. After age 80, colonoscopy was performed only to evaluate symptoms. With colonoscopy, polyps were removed and CRCs were biopsied if detected. If F-DNA, FOBT or FIT were positive then colonoscopy followed with polypectomy and biopsy as necessary. If colonoscopy was normal after a positive noninvasive test, the noninvasive test was assumed to be a false-positive and screening resumed in 10 years with the primary screening strategy. CRC was managed, and symptomatic CRC could be detected, as in the Natural History model.
In all strategies, after adenoma detection, patients underwent surveillance colonoscopy every 5 years.(53, 54) Persons developing CRC underwent colonoscopy at diagnosis, 3 years later and then every 5 years thereafter.(53, 54)
Fecal Occult Blood Testing and Fecal Immunochemical Testing
In the FOBT strategy, annual testing(3, 53, 55) was offered with test performance characteristics as modeled previously (Table 1).(22) FIT was evaluated with annual testing and test performance characteristics based on available literature(56–66) and FDA submission data from Enterix Inc. (Edison, NJ), maker of InSure FIT.(67) Reported FIT sensitivities range from 30–100% for CRC and 20–71% for large adenoma, with specificities of 86–99%.(56–66) In the base case for FIT, we assumed sensitivity of 76% for CRC, 40% for large adenoma, and specificity of 91%.
Fecal DNA Testing
F-DNA version 1 was defined as the strategy using the prototype test evaluated by Imperiale et al.(21) This test had sensitivities of 52% for CRC and 18% for large adenoma, and specificity of 94%.(21) F-DNA version 2 was defined as the strategy using the test recently reported by Itzkowitz et al.(26) This test represents the optimal marker combination of vimentin methylation and a DNA integrity assay, with sensitivity of 88% for CRC, and specificity of 82%.(26) The sensitivity of F-DNA version 2 for large adenoma has not been reported formally. We assumed that the sensitivity for large adenoma of F-DNA version 2 was 18%, the same as for version 1. For F-DNA versions 1 and 2, we assumed that F-DNA could not distinguish normal from small adenoma. Thus, F-DNA was positive when the most advanced lesion was a small adenoma at a rate defined as (100%-specificity).
The test currently available on the market is version 1.1 (PreGen Plus, LabCorp, Burlington, NC). Compared with version 1, version 1.1 includes a DNA stabilization buffer and an improved gel capture method for isolating DNA.(18, 23–25). When the version 1 test was enhanced in these ways in the recent study by Itzkowitz et al., sensitivity for CRC was 73% and specificity was 89%.(26) We assumed that the sensitivity for large adenoma of F-DNA version 1.1 was 18%, the same as for the other versions of the test.
Before evaluating fecal DNA testing strategies, an appropriate screening interval was selected. As described previously,(20) we examined F-DNA at progressively shorter screening intervals ranging from 1 to 5 years. Screening at a given interval (e.g. 4 years) was compared to screening at a longer interval (e.g. 5 years), yielding the incremental cost per life-year gained when shortening the interval. For the base case, we selected a screening interval consistent with the commonly accepted “willingness to pay” threshold of $50,000/life-year gained.(68–70) Thus, in the base case fecal DNA testing was offered every 3 years (see Results).
Screening Colonoscopy
The screening colonoscopy strategy included colonoscopy every 10 years if no adenomas were detected (COLO). Polyps were removed upon detection and masses underwent biopsy. Test performance characteristics and costs are presented in Table 1. After detection of adenomas, surveillance was performed as described for all strategies above.
Cost Inputs
Procedure cost estimates ranged from those derived from Medicare fee schedules (including professional fees and procedure reimbursement) to those reported from a health maintenance organization.(7–13, 20, 47) Based on Medicare schedules, we assumed a base case cost of $15 for each cycle of FOBT and $22 for each cycle of FIT.(46) The PreGen Plus test list price is $495 (LabCorp, Burlington, NC; test number 512094), but the average reimbursement for the test is approximately $300 after write-offs (personal communication, Barry Berger, Exact Sciences Corporation). In the base case, we assumed a cost of $300 for each fecal DNA test. Complication costs were derived from relevant diagnostic related groups (DRG 148, major small and large bowel procedures). (9, 11, 20, 47, 71, 72)
Stage-specific costs of care for CRC were taken from published reports and available data on the costs of newer therapies for advanced CRC.(5, 9, 11, 20, 31, 47, 73–75) Our Natural History model is calibrated to SEER data on CRC stage distribution of 39% localized, 39% regional and 22% disseminated CRC.(22) After comparisons with data on CRC TNM stage distribution, we assumed that disseminated CRC in our model represented TNM Stage IV disease and that 2/3 of patients with regional CRC in our model had TNM Stage III disease.(76, 77) To account for the increasing costs of CRC care for advanced disease, we assumed that patients with TNM Stage III disease received three 8-week cycles of FOLFOX (oxaliplatin, infusional fluorouracil and leucovorin) chemotherapy,(78) resulting in an increased cost of $34,800 over the costs assumed in our previous analyses.(31) We assumed that patients with TNM Stage IV disease received four to six cycles of treatment including the emerging biological agents bevacizumab and cetuximab,(28, 30, 48–51, 78, 79) resulting in an increased cost to $200,000.(31) Base case cost inputs incorporate these assumptions (Table 1).
Costs were updated to 2006 dollars as necessary, using the medical services component of the consumer price index.(80) For each base case cost input, we used the average of the published values. Indirect costs were not included. We used a third-party payer perspective.
Clinical and Economic Outcomes
For each strategy, we determined CRC cases by stage in a cohort of 100,000 persons, deaths by cause, and average life-years and costs per person (both discounted at 3% annually).(81)
Cost-Effectiveness of Screening Strategies
If one strategy afforded more life-years than another at higher expense, an incremental cost-effectiveness ratio was calculated. One-way sensitivity analyses were performed on all model inputs, including test performance characteristics and costs. Two-way sensitivity analyses were performed on variables determined to be influential on one-way sensitivity analyses. Threshold analyses were performed to identify critical values for variables at which specific conditions of interest were met (e.g. clinical equivalence, or cost-effectiveness at a willingness to pay of $50,000–$100,000/life-year gained). A Monte Carlo simulation with 1,000 trials was performed with sampling for the test performance characteristics for FOBT, FIT, and F-DNA versions 1, 1.1 and 2 from uniform distributions representing the 95% confidence interval ranges reported in the literature (Table 1).
In controlled trials of FOBT, adherence has been less than perfect.(32, 33, 36, 38) Initial screening rates have ranged from 53% to 78%(32, 33, 36, 38) and repeat screening has ranged from 77%(82) to 94%.(38) Adherence is lower outside of controlled trials. Data from the Behavioral Risk Factor Surveillance System (BRFSS) in 2001 reported that 45% of adults aged 50 or greater had ever had FOBT and 24% had FOBT within the past 12 months.(15) Others have reported initial rates of screening with FOBT from 35% to 47%(34, 41–43, 61) and rates of FOBT within one year (considered up to date) from 10% to 26%.(37, 39, 40, 43, 44) Data on annual follow up, or serial screening, are very limited. Myers et al. reported initial response to a screening program of 41% (647 of 1,565 subjects) and then subsequent serial screening by 56% of initial responders (362 of 647).(42) Using data from Liang et al., adherence to annual screening can be estimated at 61%.(39) Thus, imperfect adherence was explored in detail in sensitivity analyses.
In the base case, we assumed perfect adherence for all strategies. This reflects the optimal possible “efficacy” of the strategies. The results are useful because they reflect a strategy’s impact in persons who adhere to it. Because imperfect adherence limits true “efficacy” in larger cohorts, we performed extensive sensitivity analyses on adherence in order to estimate real-world “effectiveness” with imperfect adherence.
RESULTS
Base Case
Selection of Screening Interval for F-DNA
F-DNA version 1 every 3 years compared with every 4 years cost $39,200/life-year gained, and every 2 years compared with every 3 years it cost $52,600/life-year gained (Table 2). Similarly, F-DNA version 2 every 3 years compared with every 4 years cost $47,700/life-year gained, and every 2 years compared with every 3 years it cost $57,100/life-year gained. Therefore, we selected a screening interval of 3 years for F-DNA.
Table 2.
Effectiveness, Cost and Incremental Cost-Effectiveness of F-DNA version 1 at progressively shorter intervals.
| F-DNA Interval |
Discounted Life- Years/Person |
Discounted Cost/Person |
Incremental Cost/Life-Year Gained compared to next shorter interval |
|---|---|---|---|
| 5 years | 18.7197 | $3,531 | -- |
| 4 years | 18.7244 | $3,627 | $20,400 |
| 3 years | 18.7305 | $3,867 | $39,200 |
| 2 years | 18.7394 | $4,339 | $52,600 |
| 1 year | 18.7478 | $5,658 | $158,000 |
Clinical Outcomes with Perfect Adherence
Compared with no screening, all strategies reduced CRC incidence and mortality (Table 3). FIT yielded the greatest number of discounted life-years/person, followed by COLO, F-DNA version 2, FOBT, F-DNA version 1.1 and F-DNA version 1. Without screening, a cohort of 100,000 persons experienced 5,927 CRC cases, and CRC accounted for 2.4% of deaths. Compared with no screening, F-DNA version 1 decreased CRC incidence by 33% and CRC-related mortality by 49%, F-DNA version 1.1 decreased CRC incidence by 37% and CRC-related mortality by 57%, FOBT decreased CRC incidence by 49% and CRC-related mortality by 66%, F-DNA version 2 decreased CRC incidence by 43% and CRC-related mortality by 63%, COLO decreased CRC incidence by 73% and CRC-related mortality by 80%, and FIT decreased CRC incidence by 66% and CRC-related mortality by 78%.
Table 3.
Base case clinical and economic results and incremental cost-effectiveness ratios
| Natural History |
F-DNA version 1 |
F-DNA version 1.1 |
FOBT | F-DNA version 2 |
Colonoscopy | FIT | |
|---|---|---|---|---|---|---|---|
| CRC cases per 100,000 persons from age 50 to 100 years | 5,927 | 3,989 | 3,711 | 3,009 | 3,403 | 1,584 | 2,015 |
| CRC stage | |||||||
| Local | 2,373 | 2,191 | 2,231 | 1,876 | 2,148 | 882 | 1,291 |
| Regional | 2,210 | 1,266 | 1,086 | 813 | 943 | 509 | 504 |
| Distant | 1,345 | 532 | 393 | 320 | 312 | 193 | 220 |
| Deaths attributable to CRC | 2.4% | 1.2% | 1.0% | 0.8% | 0.9% | 0.5% | 0.5% |
| Life yrs/person† | 18.686 | 18.730 | 18.739 | 18.742 | 18.744 | 18.748 | 18.751 |
| Cost/person† | $2,921 | $3,867 | $3,821 | $2,683 | $3,833 | $3,489 | $2,428 |
| Incremental life-yrs gained per 100,000 persons compared to: | |||||||
| Natural History | - | 4,466 | 5,323 | 5,623 | 5,795 | 6,185 | 6,542 |
| F-DNA version 1 | - | - | 857 | 1,157 | 1,329 | 1,719 | 2,076 |
| F-DNA version 1.1 | - | - | - | 300 | 472 | 862 | 1,219 |
| FOBT | - | - | - | - | 172 | 562 | 919 |
| F-DNA version 2 | - | - | - | - | - | 390 | 747 |
| Colonoscopy | - | - | - | - | - | - | 357 |
| Increment cost per life-yr gained compared to: | |||||||
| Natural History | - | $21,200 | $16,900 | Dominates‡ | $15,700 | $9,200 | Dominates‡ |
| F-DNA version 1 | - | - | Dominates‡ | Dominates‡ | Dominates‡ | Dominates‡ | Dominates‡ |
| F-DNA version 1.1 | - | - | - | Dominates‡ | $2,700 | Dominates‡ | Dominates‡ |
| FOBT | - | - | - | - | $669,000 | $144,000 | Dominates‡ |
| F-DNA version 2 | - | - | - | - | - | Dominates‡ | Dominates‡ |
| Colonoscopy | - | - | - | - | - | - | Dominates‡ |
CRC=Colorectal cancer; F-DNA=Fecal DNA testing every 3 years; FOBT=Annual guaiac-based fecal occult blood testing; FIT=Annual fecal immunochemical testing
Discounted at 3% per year
Strategy in top row is more effective and less costly than strategy in left column to which it is being compared
Cost-Effectiveness with Perfect Adherence
Compared with no screening, all screening strategies increased life expectancy at reasonable costs (Table 3). FOBT and FIT yielded more average life-years per person than no screening, and achieved this at a lower cost—that is, they were dominant compared with no screening. Compared with no screening, F-DNA version 1 gained 4,466 life-years/100,000 persons at an incremental cost of $21,200/life-year gained, F-DNA version 1.1 gained 5,323 life-years/100,000 persons at an incremental cost of $16,900/life-year gained, and F-DNA version 2 gained 5,795 life-years/100,000 persons at an incremental cost of $15,700/life-year gained. COLO gained 6,185 life-years/100,000 persons at an incremental cost of $9,200/life-year gained.
FOBT and FIT were preferred over all F-DNA versions. F-DNA versions 1 and 1.1 were dominated by FOBT and FIT. F-DNA version 2 was slightly more effective than FOBT, but at a very high incremental cost of $669,000/life-year gained. FIT was dominant over all other strategies, including F-DNA version 2 (Table 3). COLO was dominated by FIT and it cost $144,000/life-year gained compared to FOBT.
One-way and Two-way Sensitivity Analyses
Changes in most variables did not significantly affect the comparisons between the F-DNA strategies and FOBT or FIT (Table 4). If we assumed significantly worse test performance characteristics for FOBT than in the base case, the F-DNA strategies compared more favorably but still cost >$50,000/life-year gained compared with FOBT. When we examined the low end of reported values for FIT test performance, it was still dominant over the F-DNA strategies. If FIT test cost increased to $95, the strategy was no longer cost-saving compared with no screening (it cost $8,300/life-year gained) and it cost $135,000/life-year gained compared with FOBT, but it was still dominant over the F-DNA strategies. Changes in colonoscopy test performance, complication rate, and costs did not affect the results significantly.
Table 4.
One-way sensitivity analyses
|
Subject of sensitivity analysis |
Base case value(s) |
Value(s) in sensitivity analysis |
FOBT vs. F- DNA version 1 |
FOBT vs. F-DNA version 1.1 |
F-DNA version 2 vs. FOBT |
FIT vs. F- DNA version 2 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Life-yrs gained per 100,000 persons |
Cost/life-yr gained |
Life-yrs gained per 100,000 persons |
Cost/life-yr gained |
Life-yrs gained per 100,000 persons |
Cost/life-yr gained |
Life-yrs gained per 100,000 persons |
Cost/life-yr gained |
|||
| Fecal DNA test | ||||||||||
| Cost | $300 | $200 | 1,157 | Dominates | 300 | Dominates | 172 | $410,000 | 747 | Dominates |
| $495 | 1,157 | Dominates | 300 | Dominates | 172 | $1,170,000 | 747 | Dominates | ||
| Colonoscopy | ||||||||||
| Sensitivity for CRC | 95% | 90% | 1,136 | Dominates | 273 | Dominates | 192 | $596,000 | 720 | Dominates |
| 97% | 1,156 | Dominates | 242 | Dominates | 240 | $474,000 | 612 | Dominates | ||
| Sensitivity for large polyp | 90% | 85% | 1,157 | Dominates | 289 | Dominates | 194 | $592,000 | 658 | Dominates |
| 95% | 1,166 | Dominates | 313 | Dominates | 154 | $745,000 | 818 | Dominates | ||
| Sensitvity for small polyp | 85% | 80% | 1,135 | Dominates | 286 | Dominates | 178 | $647,000 | 745 | Dominates |
| 90% | 1,168 | Dominates | 308 | Dominates | 167 | $688,000 | 748 | Dominates | ||
| Probability of complication | 0.1% | 0.05% | 1,157 | Dominates | 300 | Dominates | 172 | $670,000 | 747 | Dominates |
| 0.5% | 1,157 | Dominates | 300 | Dominates | 172 | $663,000 | 747 | Dominates | ||
| Probability of death related to colonoscopy | 0.01% | 0.005% | 1,204 | Dominates | 328 | Dominates | 169 | $690,000 | 761 | Dominates |
| 0.05% | 751 | Dominates | 61 | Dominates | 217 | $531,000 | 632 | Dominates | ||
| Cost (diagnostic/with lesion removal) | $920/$1350 | $710/$990 | 1,157 | Dominates | 300 | Dominates | 172 | $688,000 | 747 | Dominates |
| $1350/$2030 | 1,157 | Dominates | 300 | Dominates | 172 | $632,000 | 747 | Dominates | ||
| Cost of complication | $29,000 | $16,000 | 1,157 | Dominates | 300 | Dominates | 172 | $669,000 | 747 | Dominates |
| $43,000 | 1,157 | Dominates | 300 | Dominates | 172 | $668,000 | 747 | Dominates | ||
| FOBT | ||||||||||
| Sensitivity for CRC/large polyp/small polyp/Specificity | 40%/10%/8%/92% | 30%/5%/3%/97% | 527 | Dominates | −330(F-DNA version 1.1 is more effective) | $385,000(F-DNA version 1.1 vs. FOBT) | 802 | $160,000 | 747 | Dominates |
| 60%/15%/10%/90% | 1,747 | Dominates | 890 | Dominates | −418 (FOBT is more effective) | $286,000 (FOBT vs. F-DNA version 2) | 747 | Dominates | ||
| 13%/11%/5%/95%(as in Imperiale et al. (21)) | −407 (F-DNA version 1 is more effective) | $303,000 (F-DNA version 1 vs. FOBT) | −1,264 (F-DNA version 1.1 is more effective) | $94,000 (F-DNA version 1.1 vs. FOBT) | 1,736 | $69,000 | 747 | Dominates | ||
| Cost | $15 | $56 | 1,157 | Dominates | 300 | Dominates | 172 | $413,000 | 747 | Dominates |
| FIT | ||||||||||
| Sensitivity for CRC/large polyp/small polyp/ specificity | 76%/40%/91% | 62%/20%/14%/86% | 1,157 | Dominates | 300 | Dominates | 172 | $669,000 | 385 | Dominates |
| Cost | $22 | $95 | 1,157 | Dominates | 300 | Dominates | 172 | $669,000 | 747 | Dominates |
| CRC care costs | ||||||||||
| Local/regional/distant | $51,000/98,000/200,000 | $40,000/85,000/175,000 | 1,157 | Dominates | 300 | Dominates | 172 | $657,000 | 747 | Dominates |
| $62,000/105,000/230,000 | 1,157 | Dominates | 300 | Dominates | 172 | $678,000 | 747 | Dominates | ||
| $51,000/75,000/78,000 (without use of novel chemotherapy) | 1,157 | Dominates | 300 | Dominates | 172 | $674,000 | 747 | Dominates | ||
CRC=Colorectal cancer; F-DNA=Fecal DNA testing every 3 years; FOBT=Annual guaiac-based fecal occult blood testing; FIT=Annual fecal immunochemical testing
“Dominates” denotes situation where first strategy is more effective and less costly than second strategy
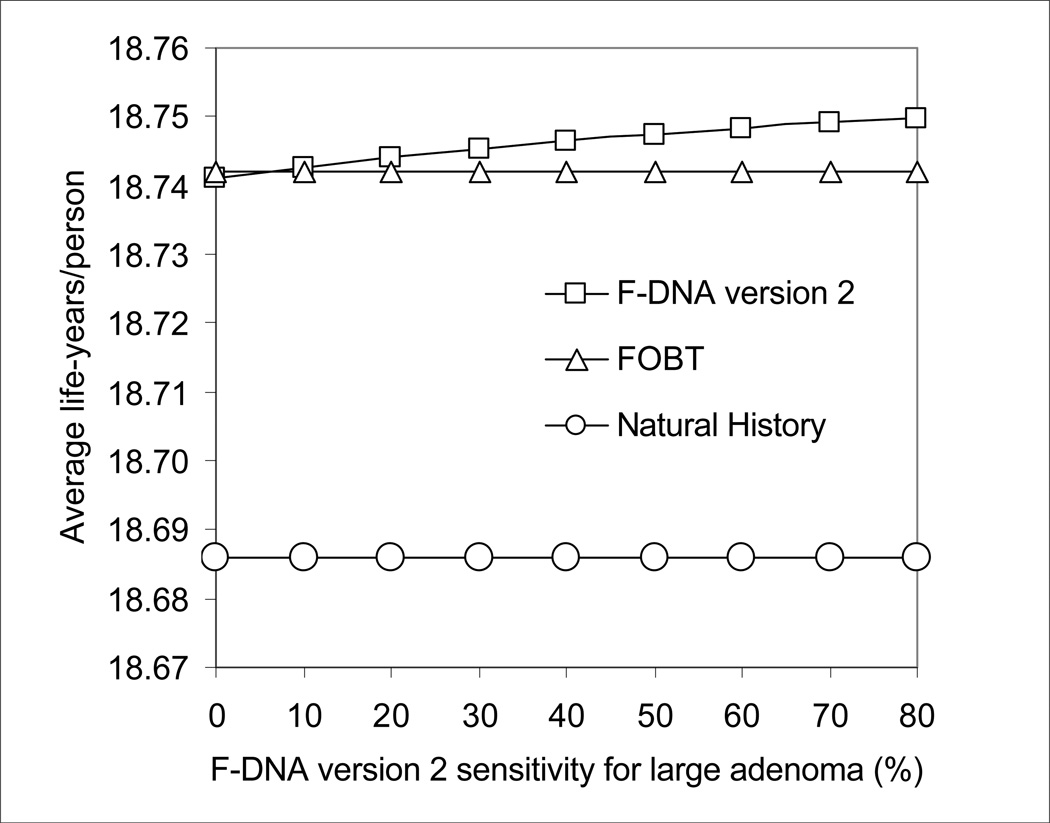
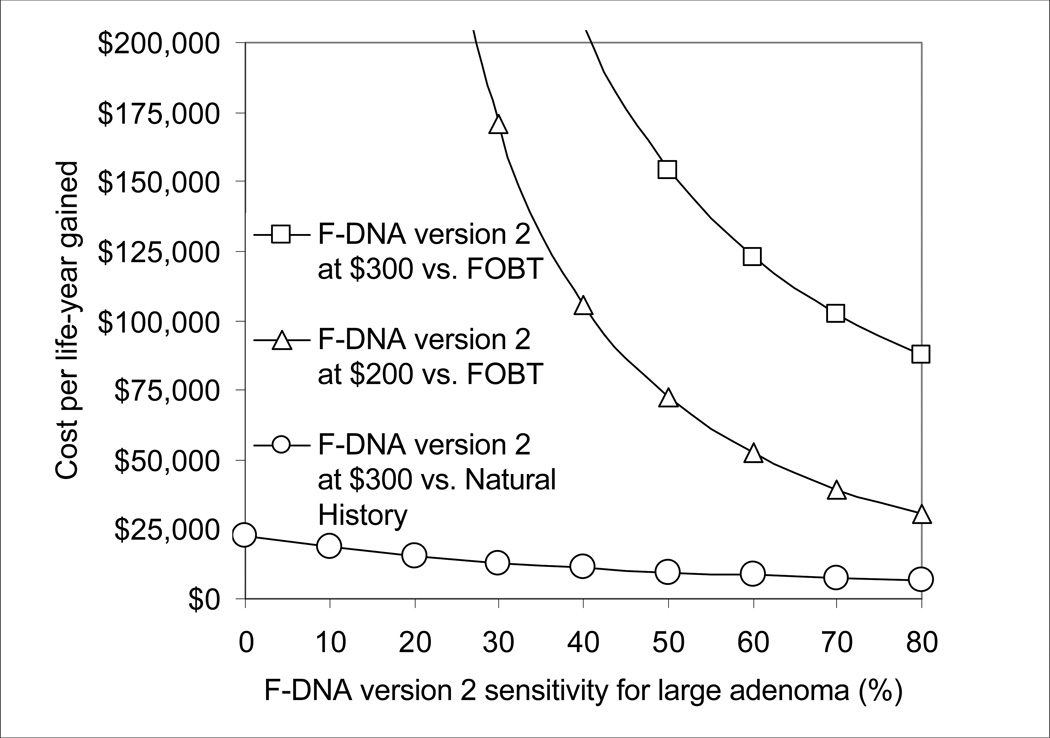
As the sensitivity for large adenoma of the F-DNA version 2 test improved, this strategy became progressively more effective than FOBT (Figure 2A). With a sensitivity for large adenoma of 80%, F-DNA version 2 cost $87,500/life-year gained compared with FOBT, but this incremental cost/life-year gained rose sharply as sensitivity for large adenoma decreased (Figure 2B). At a test cost of $200, F-DNA version 2 cost <$50,000/life-year gained compared with FOBT when F-DNA test sensitivity for large adenoma was >60% (Figure 2B).
Figure 2.
A. Impact of sensitivity for large adenoma on the effectiveness of F-DNA. The effectiveness of F-DNA increases as sensitivity for large adenoma improves.
B. Impact of sensitivity for large adenoma and test cost on the cost-effectiveness of F-DNA. At a test cost of $200 and test sensitivity for large adenoma of >60%, F-DNA version 2 cost <$50,000/life-year gained compared with FOBT.
If we assumed lower CRC care costs because the novel, costly therapies were not used, no screening strategy was cost-saving anymore. Compared with no screening, FOBT cost $8,000/life-year gained, FIT cost $4,3000/life-year gained, F-DNA version 1 cost $33,100/life-year gained, F-DNA version 1.1 cost $28,800/life-year gained, and F-DNA version 2 cost $27,700/life-year gained. However, the incremental cost-effectiveness ratios comparing the F-DNA strategies to FOBT and FIT were not affected significantly (Table 4).
Threshold Analyses on F-DNA Test Cost
F-DNA test cost would need to be significantly lower than the $300 assumed in the base case in order to make any of the F-DNA strategies competitive with FOBT. F-DNA test cost would need to fall to $40 for FOBT to cost >$50,000/life-year gained compared to F-DNA version 1.1. F-DNA test cost would need to fall to $60 for F-DNA version 2 to cost <$50,000/life-year gained compared to FOBT.
Even when the F-DNA test was assumed to be free, FIT cost only $9,200/life-year gained compared to F-DNA version 1 and $8,100/life-year gained compared to F-DNA version 1.1, and it still dominated F-DNA version 2.
Monte Carlo Simulation Focusing on Test Performance Characteristics
When test performance characteristics for all stool-based tests were varied within the ranges reported in the literature (Table 1), FOBT was dominant over no screening in >95% of iterations, and FIT was dominant over no screening in 100% of iterations. Compared with no screening, the mean (and 95% confidence interval) for the cost/life-year gained was $21,500 ($16,000–$29,200) for F-DNA version 1, $17,600 ($13,900–$21,700) for F-DNA version 1.1, and $16,500 ($13,700–$19,200) for F-DNA version 2.
Compared with F-DNA version 1.1, FOBT was dominant in 88% of iterations, it cost between $100,000 and $1,000,000/life-year gained in 18% of iterations, and it was more costly in the remainder. Compared with FOBT, F-DNA version 2 was dominant in 64% of iterations, it cost <$100,000/life-year gained in 1% of iterations, it cost between $100,000 and $1,000,000/life-year gained in 28% of iterations, and it was more costly in the remainder. Compared with F-DNA version 2, FIT was dominant in 100% of iterations.
Sensitivity Analyses on Adherence with Testing
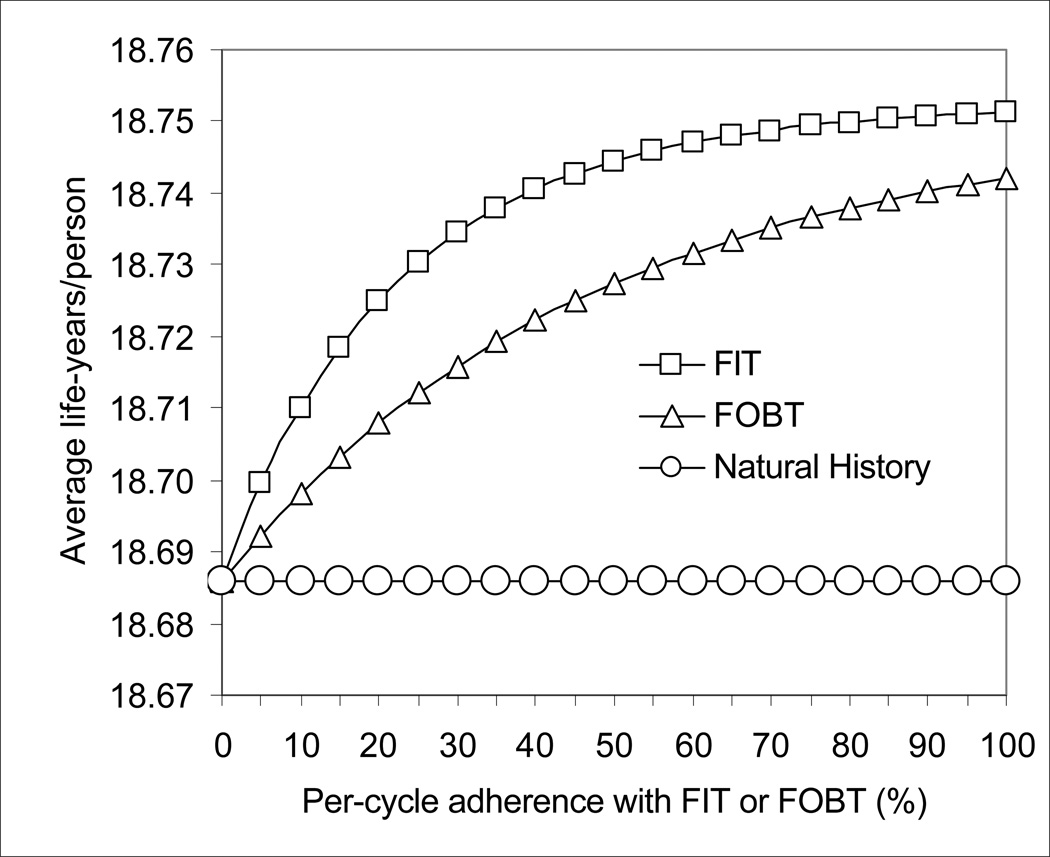
As the per-cycle (per-year) adherence with testing decreased with FOBT and FIT, the effectiveness of FOBT decreased steadily, and the effectiveness of FIT began to decrease significantly when the per-cycle adherence fell below approximately 60% (Figure 3).
Figure 3.
Impact of adherence on the effectiveness of FOBT and FIT. As adherence with yearly testing decreased, the effectiveness of FOBT decreased steadily, and the effectiveness of FIT decreased significantly with per-cycle adherence below 60%.
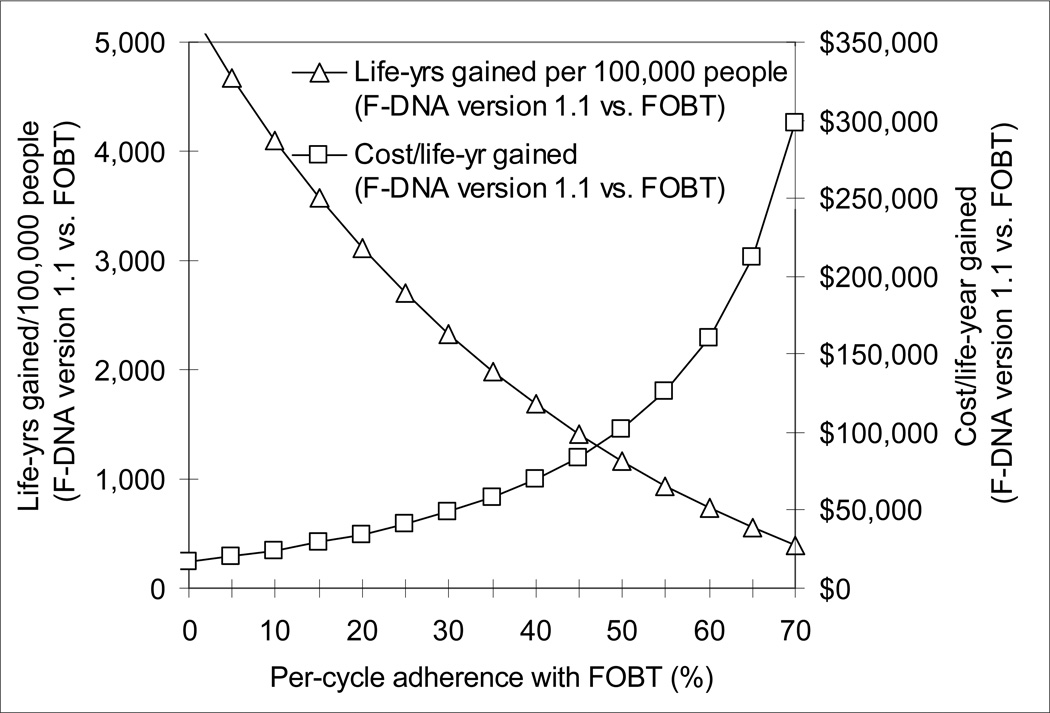
F-DNA version 1.1 (with 100% adherence) became more effective than FOBT when the per-cycle adherence with FOBT fell below 85%. F-DNA version 1.1 cost $100,000/life-year gained compared with FOBT when per-cycle adherence with FOBT was 49%, and $50,000/life-year gained when the per-cycle adherence with FOBT was 31% (Figure 4).
Figure 4.
Impact of adherence on the effectiveness and cost-effectiveness of F-DNA version 1.1 compared with FOBT. F-DNA version 1.1 became more effective than FOBT when the per-cycle adherence with FOBT fell below 85%, and it cost an incremental $50,000/life-year gained when the per-cycle adherence with FOBT was 31%.
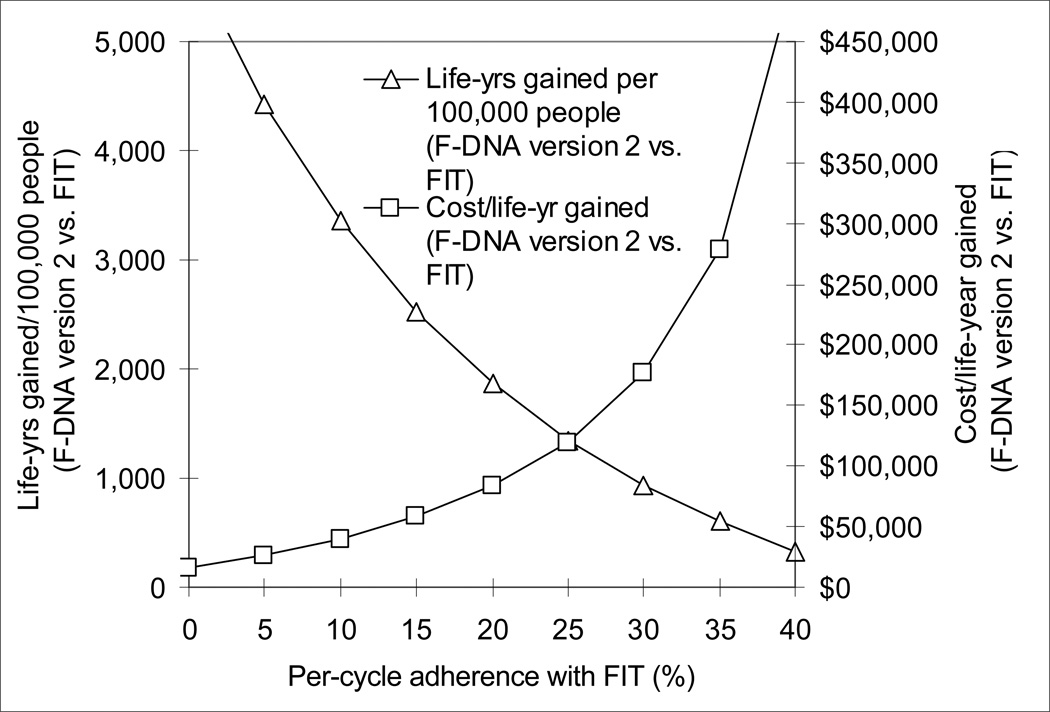
F-DNA version 2 (with 100% adherence) became more effective than FIT when the per-cycle adherence with FIT fell below 50%. F-DNA version 2 cost $100,000/life-year gained compared with FIT when per-cycle adherence with FIT was 22%, and $50,000/life-year gained when the per-cycle adherence with FIT was 13% (Figure 5).
Figure 5.
Impact of adherence on the effectiveness and cost-effectiveness of F-DNA version 2 compared with FIT. F-DNA version 2 became more effective than FIT when the per-cycle adherence with FIT fell below 50%, and it cost an incremental $100,000/life-year gained when per-cycle adherence with FIT was 22%
Imperfect adherence with F-DNA affected the comparisons with FOBT and FIT. To illustrate, when the per-cycle adherence with F-DNA version 1.1 was 50%, F-DNA version 1.1 became more effective than FOBT when the per-cycle adherence with FOBT fell below 35% and it cost $100,000/life-year gained compared with FOBT when per-cycle adherence with FOBT was 26. Similarly, when the per-cycle adherence with F-DNA version 2 was 50%, F-DNA version 2 became more effective than FIT when the per-cycle adherence with FIT fell below 19% and it cost $100,000/life-year gained compared with FIT when per-cycle adherence with FIT was 12%.
DISCUSSION
CRC screening and treatment are rapidly evolving fields, necessitating reappraisal of the effectiveness and cost-effectiveness of screening strategies as key variables change. Our current analyses focused on the latest test performance characteristics and costs of noninvasive, stool-based tests, and the increasing costs of care for advanced CRC. Our results lead to four major conclusions. First, if CRC treatment costs increase significantly due to the use of novel biological therapies, FOBT and FIT could improve clinical outcomes while also achieving cost savings. Second, recent improvements in test performance and lower test cost have translated into enhanced cost-effectiveness for F-DNA compared with no screening, but FOBT and FIT are likely to be preferred over F-DNA when patient adherence with yearly testing is high. Third, adherence over time is a key determinant of the effectiveness of strategies that rely on frequent testing, and F-DNA with screening every 3 years could be cost-effective compared with FOBT and FIT in populations with poor adherence to yearly testing. Fourth, in persons who can adhere to yearly testing, highly sensitive and relatively inexpensive stool-based testing such as FIT may be comparable to screening colonoscopy every 10 years.
Before the current era of novel but costly treatments for advanced CRC, multiple analyses concluded that CRC screening is cost-effective.(3–13, 20, 22) Screening had been estimated to be cost-saving only when very low screening costs were assumed.(83) Our current analyses demonstrate how FOBT and FIT could not only decrease CRC incidence and mortality, but could actually decrease total overall CRC-related costs (screening, testing, complications and CRC care) if advanced CRC is treated with novel, costly therapies.(28, 30, 31, 48–51, 78, 79) It is rare for medical interventions to improve outcomes as well as decrease costs. Therefore, the question is often whether an intervention is “cost-effective.” We have previously estimated that screening 75% of the U.S. population with conventional methods could increase overall CRC-related costs by $1–3 billion/year, accounting for savings in CRC care.(22) However, if costly therapies for advanced CRC become widely used, the economic benefit of prevention and early detection may become large enough that overall savings could be realized by screening.
With current test cost of $300, F-DNA version 1.1 (the currently available test PreGen Plus, LabCorp, Burlington, NC) and F-DNA version 2 (the refined test as in Itzkowitz et al.(26)) were both cost-effective compared with no screening. Assuming the high advanced CRC care costs associated with novel biological therapies, these strategies cost approximately $17,000/life-year gained (upper 95% confidence interval of approximately $22,000/life-year gained). Without the use of novel therapies for advanced CRC, these strategies were still cost-effective compared with no screening (<$30,000/life-year gained). However, FOBT and FIT were preferred over all F-DNA strategies when they were not compromised by poor adherence.
With current test performance characteristics and good adherence, substantial decreases in test cost would be required for any F-DNA test to become cost-effective compared with FOBT. F-DNA test cost would need to be $40–60 for F-DNA versions 1.1 and 2 to compare favorably with FOBT at a threshold of $50,000/life-year gained. More dramatically, FIT dominated F-DNA strategies in most sensitivity analyses, and it was preferred even when the F-DNA test was assumed to be free.
Early detection of CRC as well as CRC prevention through removal of adenomas underlie the benefit of screening. In the base case, we assumed low F-DNA sensitivity for large adenoma. Better sensitivity for large adenoma would improve F-DNA’s effectiveness (Figure 2A), but the effect appears less dramatic than we expected initially. This result depends on the assumption that most CRCs remain localized or regional for several years, and can therefore be detected at a high rate with a relatively sensitive test that is performed every 3 years. Similarly, for adenomas that “dwell” for many years, repeated testing with only a fair test has a reasonably high cumulative sensitivity. Our model’s predictions for FOBT’s effectiveness are very close to the results of clinical trials,(22, 32, 33) giving us confidence regarding our predictions for F-DNA. However, if the fraction of rapidly advancing adenomas or tumors is higher than reflected in our current model, the benefit of improved sensitivity for large adenoma may be underestimated.
Not surprisingly, we found that adherence over time is a key determinant of the effectiveness of strategies that rely on frequent testing (Figure 3). Even in the idealized setting of a controlled trial, adherence with annual or biannual FOBT is less than ideal.(32, 33, 36, 38) In clinical practice, it has been difficult to achieve ongoing high rates of adherence with FOBT,(39, 42) and the follow-up of abnormal tests is difficult to ensure.(32, 33, 36, 38, 41, 61) Furthermore, patient preferences for screening options vary.(16, 84–90) Because changing the adherence rates of multiple strategies simultaneously is cumbersome, we compared F-DNA with perfect adherence against FOBT and FIT with imperfect adherence (Figures 4 and 5). It is conceivable that F-DNA could be considered cost-effective compared with FOBT or FIT in populations that demonstrate good to excellent adherence with testing every 3 years, but who would otherwise have very poor adherence with yearly testing. Further study is required in this area.
In persons adhering perfectly with screening, which reflects optimal efficacy, screening colonoscopy every 10 years decreased CRC incidence more than annual FIT, but the average life-expectancy with FIT was higher than with screening colonoscopy. This is explained by the fact that most CRCs were diagnosed at treatable stages. The generalizable conclusion is that among persons who can comply with frequent testing, highly sensitive and inexpensive non-invasive testing may be comparable to much less frequent screening with colonoscopy.
The current reappraisal raises important points when compared with our first analysis of F-DNA.(20) As F-DNA’s test performance has improved and its cost has decreased, it has become more cost-effective when compared with no screening, an effect that is accentuated as the cost of CRC care increases. However, colonoscopy remains preferred over F-DNA with current parameters. In our first analysis, we did not focus on the comparison between stool-based tests, which is the principal subject of our current reappraisal. Our current results highlight that, in the setting of good adherence, FOBT and FIT are likely to be preferred over F-DNA.
Our analysis has some limitations. Indirect costs were not included. Patterns of adherence over time are likely to be complex, and such considerations are beyond the scope of the current analyses. Finally, as in all decision analytic exercises, there is uncertainty surrounding important inputs. However, we have addressed the key variables in extensive sensitivity analyses in order to be able to draw conclusions that may focus future clinical research and inform policy decisions.
In conclusion, our analyses suggest that as the costs of care for advanced CRC increase due to use of novel but costly biological therapies, screening with reasonably effective and inexpensive methods such as FOBT and FIT could be not only cost-effective, but potentially cost-saving. The evolution of test performance characteristics and decrease in test cost for F-DNA have translated into improved cost-effectiveness for F-DNA compared with no screening, but presently FOBT and FIT remain preferred over F-DNA in populations with high adherence to yearly testing. F-DNA with excellent adherence could be considered cost-effective compared with FOBT or FIT in populations with very poor adherence to yearly testing. With excellent annual adherence, sensitive and inexpensive stool-based testing such as FIT may be comparable to screening colonoscopy.
Acknowledgments
Funding Sources: Unrestricted research grant from Exact Sciences Corp. NIH R01 CA101849-01A1 (PI: K. A. Phillips)
Footnotes
Presented in part at Digestive Disease Week, Los Angeles, California, May 2006
Potential conflicts of interest: Dr. Fendrick serves on the Scientific Advisory Board of Exact Sciences Corporation and Dr. Ladabaum serves as consultant for GeneNews and Epigenomics.
REFERENCES
- 1.Levin B, Brooks D, Smith RA, Stone A. Emerging technologies in screening for colorectal cancer: CT colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA Cancer J Clin. 2003;53(1):44–55. doi: 10.3322/canjclin.53.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 5.Eddy DM. Screening for colorectal cancer. Ann Intern Med. 1990;113(5):373–384. doi: 10.7326/0003-4819-113-5-373. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman DA. Cost-effectiveness model for colon cancer screening. Gastroenterology. 1995;109(6):1781–1790. doi: 10.1016/0016-5085(95)90744-0. [DOI] [PubMed] [Google Scholar]
- 7.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. Jama. 2000;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg A, Delco F, Inadomi J. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133(8):573–584. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JL, Tunis S, Brown M, Ching A, Almeida R. Cost-effectiveness of colorectal cancer screening in average risk adults. In: Young G, Rozen P, Levin B, editors. Prevention and early detection of colorectal cancer. Philadelphia: WB Saunders; 1996. pp. 321–356. [Google Scholar]
- 10.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 11.Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, Fendrick AM. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer. A cost-effectiveness analysis. Ann Intern Med. 2001;135(9):769–781. doi: 10.7326/0003-4819-135-9-200111060-00007. [DOI] [PubMed] [Google Scholar]
- 12.Vijan S, Hwang EW, Hofer TP, Hayward RA. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001;111(8):593–601. doi: 10.1016/s0002-9343(01)00977-9. [DOI] [PubMed] [Google Scholar]
- 13.Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000;16(3):799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 14.Chao A, Connell CJ, Cokkinides V, Jacobs EJ, Calle EE, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of US adults. Am J Public Health. 2004;94(10):1775–1781. doi: 10.2105/ajph.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colorectal cancer test use among persons aged > or = 50 years--United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(10):193–196. [PubMed] [Google Scholar]
- 16.Schroy PC, Heeren TC. A comparative study of patient perceptions and screening preferences for stool-based DNA testing (SBDNA), fecal occult blood testing (FOBT) or colonoscopy (CS) (Abstract) Gastroenterology. 2003;124(4):A-77. [Google Scholar]
- 17.Schroy PC, Heeren TC. Patient perceptions of stool-based DNA testing for colorectal cancer screening. Am J Prev Med. 2005;28(2):208. doi: 10.1016/j.amepre.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Berger BM, Schroy PC, 3rd, Rosenberg JL, Lai-Goldman M, Eisenberg M, Brown T, et al. Colorectal cancer screening using stool DNA analysis in clinical practice: early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal tests. Clin Colorectal Cancer. 2006;5(5):338–343. doi: 10.3816/CCC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 19.Seeff LC, Manninen DL, Dong FB, Chattopadhyay SK, Nadel MR, Tangka FK, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127(6):1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 20.Song K, Fendrick AM, Ladabaum U. Fecal DNA testing compared to conventional colorectal cancer ccreening methods: A decision analysis. Gastroenterology. 2004;126(5):1270–1279. doi: 10.1053/j.gastro.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351(26):2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 22.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129(4):1151–1162. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 23.Olson J, Whitney DH, Durkee K, Shuber AP. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagn Mol Pathol. 2005;14(3):183. doi: 10.1097/01.pas.0000176768.18423.7e. [DOI] [PubMed] [Google Scholar]
- 24.Whitney D, Skoletsky J, Moore K, Boynton K, Kann L, Brand R, et al. Enhanced retrieval of DNA from human fecal samples results in improved performance of colorectal cancer screening test. J Mol Diagn. 2004;6(4):386. doi: 10.1016/S1525-1578(10)60536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127(5):1578. doi: 10.1053/j.gastro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy Iii PC, Sontag S, et al. Improved Fecal DNA Test for Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–680. [PubMed] [Google Scholar]
- 28.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21(14):2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 31.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 32.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 33.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 34.Bat L, Pines A, Ron E, Niv Y, Arditi E, Shemesh E. A community-based program of colorectal screening in an asymptomatic population: evaluation of screening tests and compliance. Am J Gastroenterol. 1986;81(8):647–651. [PubMed] [Google Scholar]
- 35.Bond JH. The place of fecal occult blood test in colorectal cancer screening in 2006: the U.S. perspective. Am J Gastroenterol. 2006;101(2):219–221. doi: 10.1111/j.1572-0241.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 36.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 37.Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. Am J Gastroenterol. 2003;98(9):2082–2091. doi: 10.1111/j.1572-0241.2003.07574.x. [DOI] [PubMed] [Google Scholar]
- 38.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 39.Liang SY, Phillips KA, Nagamine M, Ladabaum U, Haas JS. Rates and predictors of colorectal cancer screening. Prev Chronic Dis. 2006;3(4):A117. [PMC free article] [PubMed] [Google Scholar]
- 40.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 41.Morris JB, Stellato TA, Guy BB, Gordon NH, Berger NA. A critical analysis of the largest reported mass fecal occult blood screening program in the United States. Am J Surg. 1991;161(1):101–105. doi: 10.1016/0002-9610(91)90368-n. discussion 105-6. [DOI] [PubMed] [Google Scholar]
- 42.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Adherence to continuous screening for colorectal neoplasia. Med Care. 1993;31(6):508–519. doi: 10.1097/00005650-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21(2):132–137. doi: 10.1016/s0749-3797(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 45.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Clin Gastroenterol Hepatol. 2004;2(7):554–563. doi: 10.1016/s1542-3565(04)00247-2. [DOI] [PubMed] [Google Scholar]
- 46.CMS Clinical laboratory fee schedule. [Accessed April 19, 2007]; In http://www.cms.hhs.gov/ClinicalLabFeeSched/
- 47.Ladabaum U, Scheiman JM, Fendrick AM. Potential effect of cyclooxygenase-2-specific inhibitors on the prevention of colorectal cancer: a cost-effectiveness analysis. Am J Med. 2003;114(7):546–554. doi: 10.1016/s0002-9343(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 48.Bernold DM, Sinicrope FA. Advances in chemotherapy for colorectal cancer. Clin Gastroenterol Hepatol. 2006;4(7):808–821. doi: 10.1016/j.cgh.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 50.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 51.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 52.Vital Statistics of the United States. Hyattsville, MD: National Center for Health Statistics; 1998. Life Tables. Preprint of Vol II, Mortality, part A, section 6. [Google Scholar]
- 53.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 54.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130(6):1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin. 2003;53(1):27–43. doi: 10.3322/canjclin.53.1.27. [DOI] [PubMed] [Google Scholar]
- 56.Allison JE. Colon Cancer Screening Guidelines 2005: the fecal occult blood test option has become a better FIT. Gastroenterology. 2005;129(2):745–748. doi: 10.1016/j.gastro.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 57.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334(3):155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 58.Rozen P, Knaani J, Samuel Z. Performance characteristics and comparison of two immunochemical and two guaiac fecal occult blood screening tests for colorectal neoplasia. Dig Dis Sci. 1997;42(10):2064–2071. doi: 10.1023/a:1018866400973. [DOI] [PubMed] [Google Scholar]
- 59.Greenberg PD, Bertario L, Gnauck R, Kronborg O, Hardcastle JD, Epstein MS, et al. A prospective multicenter evaluation of new fecal occult blood tests in patients undergoing colonoscopy. Am J Gastroenterol. 2000;95(5):1331–1338. doi: 10.1111/j.1572-0241.2000.02032.x. [DOI] [PubMed] [Google Scholar]
- 60.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129(2):422–428. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 61.Ko CW, Dominitz JA, Nguyen TD. Fecal occult blood testing in a general medical clinic: comparison between guaiac-based and immunochemical-based tests. Am J Med. 2003;115(2):111–114. doi: 10.1016/s0002-9343(03)00294-8. [DOI] [PubMed] [Google Scholar]
- 62.Nakama H, Yamamoto M, Kamijo N, Li T, Wei N, Fattah AS, et al. Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterology. 1999;46(25):228–231. [PubMed] [Google Scholar]
- 63.Rozen P, Knaani J, Samuel Z. Comparative screening with a sensitive guaiac and specific immunochemical occult blood test in an endoscopic study. Cancer. 2000;89(1):46–52. [PubMed] [Google Scholar]
- 64.Vilkin A, Rozen P, Levi Z, Waked A, Maoz E, Birkenfeld S, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005;100(11):2519–2525. doi: 10.1111/j.1572-0241.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 65.Wong BC, Wong WM, Cheung KL, Tong TS, Rozen P, Young GP, et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment Pharmacol Ther. 2003;18(9):941–946. doi: 10.1046/j.1365-2036.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 66.Young GP, St John DJ, Cole SR, Bielecki BE, Pizzey C, Sinatra MA, et al. Prescreening evaluation of a brush-based faecal immunochemical test for haemoglobin. J Med Screen. 2003;10(3):123–128. doi: 10.1177/096914130301000305. [DOI] [PubMed] [Google Scholar]
- 67.Enterix Corporation. In http://www.insuretest.com/professionals/invitro.html. [Google Scholar]
- 68.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Cmaj. 1992;146(4):473–481. [PMC free article] [PubMed] [Google Scholar]
- 69.Laupacis A, Feeny D, Detsky AS, Tugwell PX. Tentative guidelines for using clinical and economic evaluations revisited. Cmaj. 1993;148(6):927–929. [PMC free article] [PubMed] [Google Scholar]
- 70.Tengs TO, Adams ME, Pliskin JS, Safran DG, Siegel JE, Weinstein MC, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal. 1995;15(3):369–390. doi: 10.1111/j.1539-6924.1995.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 71.Eddy DM, Nugent FW, Eddy JF, Coller J, Gilbertsen V, Gottlieb LS, et al. Screening for colorectal cancer in a high-risk population. Results of a mathematical model. Gastroenterology. 1987;92(3):682–692. doi: 10.1016/0016-5085(87)90018-7. [DOI] [PubMed] [Google Scholar]
- 72.The DRG Handbook: Comparative Clinical and Financial Standards. Baltimore, MD: HCIA, Inc. and Ernst & Young LLP; 1997. [Google Scholar]
- 73.Brown ML, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;37(12):1249–1259. doi: 10.1097/00005650-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Fireman BH, Quesenberry CP, Somkin CP, Jacobson AS, Baer D, West D, et al. Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 1997;18(4):51–76. [PMC free article] [PubMed] [Google Scholar]
- 75.Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87(6):417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 76.Greene FL. AJCC cancer staging manual. 6th ed. New York: Springer; 2002. American Joint Committee on Cancer., American Cancer Society. [Google Scholar]
- 77.NCDB Site by stage distribution of cancer - 2003. [Accessed April 20, 2007]; In http://www.facs.org/cancer/ncdb/ver7_site_stage_2003.htm. [Google Scholar]
- 78.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 79.Giantonio BJ. Bevacizumab in the treatment of metastatic colorectal cancer (mCRC) in second- and third-line settings. Semin Oncol. 2006;33(5) Suppl 10:S15–S18. doi: 10.1053/j.seminoncol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 80.Bureau of Labor Statistics 2006. U.S. Department of Labor. [Accessed April 20, 2007]; Available at: In http://www.bls.gov/cpi/home.htm.
- 81.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. pp. 214–235. [Google Scholar]
- 82.Hardcastle JD, Thomas WM, Chamberlain J, Pye G, Sheffield J, James PD, et al. Randomised, controlled trial of faecal occult blood screening for colorectal cancer. Results for first 107,349 subjects. Lancet. 1989;1(8648):1160–1164. doi: 10.1016/s0140-6736(89)92750-5. [DOI] [PubMed] [Google Scholar]
- 83.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Endoscopic Colorectal Cancer Screening: a Cost-SavingAnalysis. J Natl Cancer Inst. 2000;92(7):557–563. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 84.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. J Gen Intern Med. 2001;16(12):822–830. doi: 10.1111/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dominitz JA, Provenzale D. Patient preferences and quality of life associated with colorectal cancer screening. Am J Gastroenterol. 1997;92(12):2171–2178. [PubMed] [Google Scholar]
- 86.Leard LE, Savides TJ, Ganiats TG. Patient preferences for colorectal cancer screening. J Fam Pract. 1997;45(3):211–218. [PubMed] [Google Scholar]
- 87.Nelson RL, Schwartz A. A survey of individual preference for colorectal cancer screening technique. BMC Cancer. 2004;4:76. doi: 10.1186/1471-2407-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14(7):432–437. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 90.Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: a patient demographic study. South Med J. 2004;97(3):224–230. doi: 10.1097/01.SMJ.0000078619.39604.3D. [DOI] [PubMed] [Google Scholar]
- 91.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 92.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49(4):819–825. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 93.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23(10):835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arminski TC, McLean DW. Incidence And Distribution Of Adenomatous Polyps Of The Colon And Rectum Based On 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;19:249–261. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 95.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43(5):1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 96.Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology. 2001;219(3):685–692. doi: 10.1148/radiology.219.3.r01jn40685. [DOI] [PubMed] [Google Scholar]
- 97.Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK, editors. SEER Cancer Statistics Review, 1973–1994. Bethesda, MD: National Cancer Institute; 1997. NIH Pub. No. 97-2789. [Google Scholar]
- 98.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]