Abstract
The use of FeCl3 resulted in a fast homocoupling of aryllithiums, and this enabled its integration with the halogen–lithium exchange reaction of aryl halides in a flow microreactor. This system allows the homocoupling of two aryl halides bearing electrophilic functional groups, such as CN and NO2, in under a minute.
Keywords: homocoupling, iron salts, microreactor, organolithiums
Introduction
Biaryl structures often occur in various organic compounds including natural products, bioactive compounds, functional polymers, ligands in catalysts and theoretically interesting molecules, and the oxidative homocoupling of arylmetals is one of the most useful methods for the construction of biaryl frameworks [1]. Stoichiometric amounts of transition metal salts such as TiCl4 [2], TlCl [3], VO(OEt)Cl2 [4], CoCl2 [5], CuCl2 [6] and Pd(OAc)2 [7] have been used for homocoupling of arylmetals. In some cases catalytic processes in the presence of a reoxidant, such as oxygen or other organic oxidants, are effective. Recently, iron salts have been also used because of their low costs and lack of toxicity [8–18]. For example, Hayashi et al. reported the iron-catalyzed oxidative homocoupling of Grignard reagents, using 1,2-dihalogenoethanes as an oxidant [19]. Cahiez et al. have also reported the FeCl3-catalyzed homocoupling reaction of Grignard reagents bearing functional groups, using atmospheric oxygen [20]. The use of aryllithium compounds instead of Grignard reagents is very interesting, because they are easily generated by halogen–lithium exchange under homogeneous conditions, thus enabling the generation in a flow. However, to the best of our knowledge, oxidative homocoupling of aryllithiums using iron salts has not been reported so far. One of the major reasons for this seems to be the instability of aryllithiums, especially of those bearing electrophilic functional groups such as cyano and nitro groups [21], making the subsequent homocoupling difficult or impossible.
Recently, we have reported that flow microreactor systems [22–85] are quite effective for the generation and reaction of highly reactive organolithiums such as functionalized aryllithiums, oxiranyllithums, aziridinyllithiums, and allenyllithiums [86–98]. Herein we report that integration [99–100] of the generation of aryllithiums, especially those bearing electrophilic functional groups, by halogen–lithium exchange and FeCl3 promoted homocoupling has been effectively accomplished in an integrated flow microreactor system.
Results and Discussion
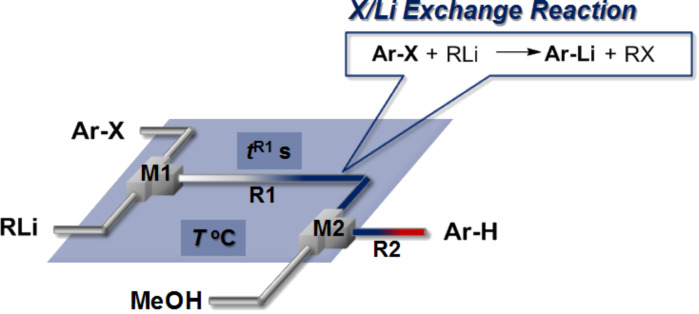
First, we focused on the generation of p-methoxyphenyllithium from p-bromoanisole (Scheme 1). A flow microreactor system, consisting of two T-shaped micromixers (M1 and M2) and two microtube reactors (R1 and R2) shown in Figure 1, was used. A solution of p-bromoanisole (Ar–X) (0.10 M in THF, flow rate: 6.0 mL/min) and a solution of n-butyllithium (0.40 M in hexane, flow rate: 1.5 mL/min) were introduced to M1 (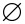 = 250 μm) by syringe pumps. The resulting mixture was passed through R1 to conduct the bromine–lithium exchange reaction. Methanol (neat, flow rate: 3.0 mL/min) was added in M2 (
= 250 μm) by syringe pumps. The resulting mixture was passed through R1 to conduct the bromine–lithium exchange reaction. Methanol (neat, flow rate: 3.0 mL/min) was added in M2 (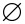 = 500 μm) and the mixture was passed through R2 (
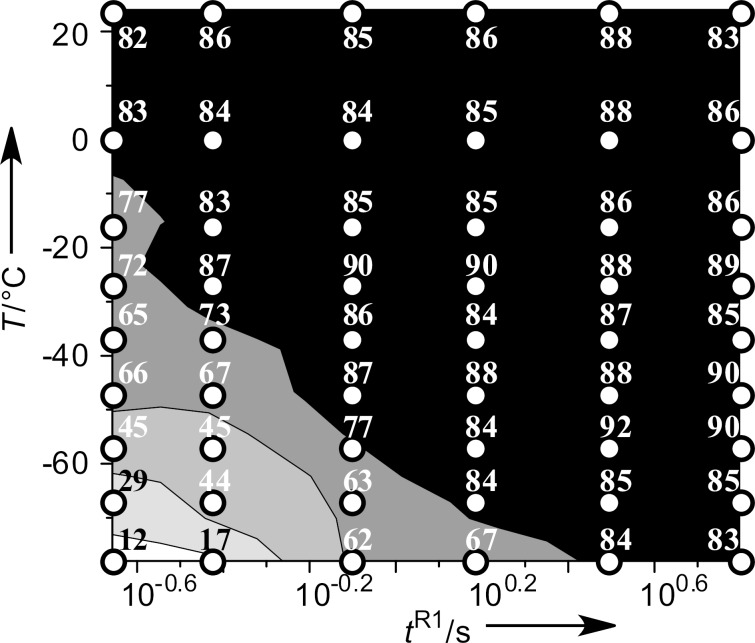
= 500 μm) and the mixture was passed through R2 (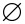 = 1000 μm, L = 50 cm) to protonate p-methoxyphenyllithium. The reactions were carried out with varying residence time in R1 (tR1: 0.2–6.3 s) and varying temperature (T: −78 to 24 °C). The temperature (T) was controlled by adjusting the bath temperature. The residence time (tR1) was adjusted by changing the inner diameter and the length in the microtube reactor R1 with a fixed flow rate. After a steady state was reached, the product solution was collected for 30 s. As shown in Figure 2, the yield of the protonated product, anisole, depends on both T and tR1. The reaction at low temperatures (T < −48 °C) with short residence times (tR1 < 0.79 s) resulted in very low yields, because the Br–Li exchange reaction was not complete. The increase in T and tR1 caused an increase in the yield, and high yields (>85%) were obtained through the appropriate choice of T and tR1.
= 1000 μm, L = 50 cm) to protonate p-methoxyphenyllithium. The reactions were carried out with varying residence time in R1 (tR1: 0.2–6.3 s) and varying temperature (T: −78 to 24 °C). The temperature (T) was controlled by adjusting the bath temperature. The residence time (tR1) was adjusted by changing the inner diameter and the length in the microtube reactor R1 with a fixed flow rate. After a steady state was reached, the product solution was collected for 30 s. As shown in Figure 2, the yield of the protonated product, anisole, depends on both T and tR1. The reaction at low temperatures (T < −48 °C) with short residence times (tR1 < 0.79 s) resulted in very low yields, because the Br–Li exchange reaction was not complete. The increase in T and tR1 caused an increase in the yield, and high yields (>85%) were obtained through the appropriate choice of T and tR1.
Scheme 1.
Halogen–lithium exchange of p-bromoanisole followed by reaction with methanol.
Figure 1.
Flow microreactor system for halogen–lithium exchange of aryl halide followed by reaction with methanol. T-shaped micromixer: M1 (inner diameter: 250 μm), and M2 (inner diameter: 500 μm), microtube reactor: R1 and R2 (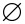 = 1000 μm, length = 50 cm), a solution of aryl halides: 0.10 M in THF (6.0 mL/min), a solution of lithium reagent: 0.40 M or 0.42 M in hexane (n-BuLi) or Et2O (PhLi) (1.5 mL/min), a solution of methanol: Neat (3.0 mL/min).
= 1000 μm, length = 50 cm), a solution of aryl halides: 0.10 M in THF (6.0 mL/min), a solution of lithium reagent: 0.40 M or 0.42 M in hexane (n-BuLi) or Et2O (PhLi) (1.5 mL/min), a solution of methanol: Neat (3.0 mL/min).
Figure 2.
Effects of the temperature (T) and the residence time in R1 (tR1) on the yield of anisole in the Br–Li exchange reaction of p-bromoanisole followed by reaction with methanol in the flow microreactor system. The contour plot with scatter overlay shows the yields of anisole (%), which are indicated by small circles.
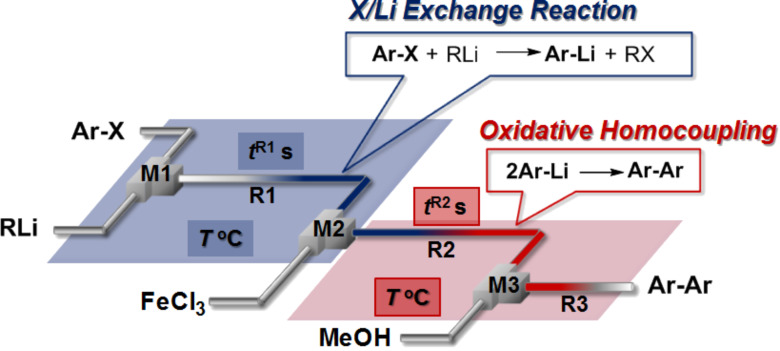
Next, we examined the integration of the halogen–lithium exchange reaction with FeCl3 promoted homocoupling (Scheme 2). Integrated flow microreactor systems consisting of three micromixers (M1, M2, and M3) and three microtube reactors (R1, R2, and R3) were used, as shown in Figure 3. A solution of p-bromoanisole (Ar–X) (0.10 M in THF, flow rate: 6.0 mL/min) and a solution of n-butyllithium (0.42 M in hexane, flow rate: 1.5 mL/min) were introduced to M1 (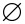 = 250 μm) by syringe pumps. The resulting mixture was passed through R1 (tR1 = 13 s (−78 °C), tR1 = 13 s (−48 °C), tR1 = 3.1 s (−28 °C), tR1 = 3.1 s (0 °C), tR1 = 3.1 s (24 °C)) at the corresponding temperatures and was mixed with a solution of FeCl3 (0.10 M in THF, flow rate: 6.0 mL/min) in M2 (
= 250 μm) by syringe pumps. The resulting mixture was passed through R1 (tR1 = 13 s (−78 °C), tR1 = 13 s (−48 °C), tR1 = 3.1 s (−28 °C), tR1 = 3.1 s (0 °C), tR1 = 3.1 s (24 °C)) at the corresponding temperatures and was mixed with a solution of FeCl3 (0.10 M in THF, flow rate: 6.0 mL/min) in M2 (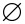 = 500 μm). The resulting mixture was passed through R2 and was then mixed with methanol (neat, flow rate: 1.5 mL/min) in M3 (
= 500 μm). The resulting mixture was passed through R2 and was then mixed with methanol (neat, flow rate: 1.5 mL/min) in M3 (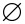 = 500 μm) to protonate the unchanged p-methoxyphenyllithium. The resulting solution was passed through R3 (
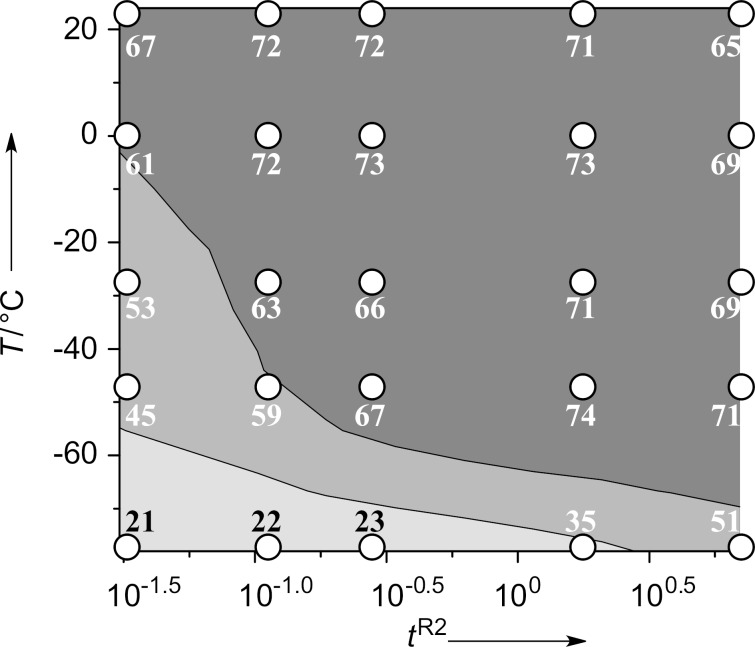
= 500 μm) to protonate the unchanged p-methoxyphenyllithium. The resulting solution was passed through R3 (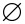 = 1000 μm, L = 50 cm). The temperature (T) was controlled by adjusting the bath temperature, and the residence time in R2 (tR2) by changing the inner diameter and the length in R2 with the fixed flow rate. After a steady state was reached, the product solution was collected for 30 s. The results obtained from varying tR2 and T are summarized in Figure 4, in which the yield of 4,4'-dimethoxybiphenyl is plotted against T and tR2 as a contour map with scattered overlay (see Supporting Information File 1 for details). The yield depends on both T and tR2. At −78 °C, the yield increased with tR2 because of the progress of the homocoupling. At 0 °C, the homocoupling product was obtained in reasonable yields for a wide range of tR2. The productivity of the present system is acceptable for large scale laboratory synthesis (6.2 g/h). It is noteworthy that the integrated reactions were complete within the overall residence time of 14.7 s, even at low temperatures such as −48 °C. Thus, we envisaged that the reaction could also be applied to less stable aryllithium compounds that decompose very quickly.
= 1000 μm, L = 50 cm). The temperature (T) was controlled by adjusting the bath temperature, and the residence time in R2 (tR2) by changing the inner diameter and the length in R2 with the fixed flow rate. After a steady state was reached, the product solution was collected for 30 s. The results obtained from varying tR2 and T are summarized in Figure 4, in which the yield of 4,4'-dimethoxybiphenyl is plotted against T and tR2 as a contour map with scattered overlay (see Supporting Information File 1 for details). The yield depends on both T and tR2. At −78 °C, the yield increased with tR2 because of the progress of the homocoupling. At 0 °C, the homocoupling product was obtained in reasonable yields for a wide range of tR2. The productivity of the present system is acceptable for large scale laboratory synthesis (6.2 g/h). It is noteworthy that the integrated reactions were complete within the overall residence time of 14.7 s, even at low temperatures such as −48 °C. Thus, we envisaged that the reaction could also be applied to less stable aryllithium compounds that decompose very quickly.
Scheme 2.
Halogen-lithium exchange of p-bromoanisole followed by oxidative homocoupling with FeCl3.
Figure 3.
Integrated flow microreactor system for oxidative homocoupling reaction of aryllithium with FeCl3. T-shaped micromixer: M1 (inner diameter: 250 μm), M2 (inner diameter: 500 μm), and M3 (inner diameter: 500 μm), microtube reactor: R1, R2 and R3 ( = 1000 μm, length = 50 cm), a solution of aryl halides: 0.10 M in THF (6.0 mL/min), a solution of lithium reagent: 0.42 M in hexane (n-BuLi) or Et2O (PhLi) (1.5 mL/min), a solution of FeCl3: 0.10 M in THF (6 mL/min), a solution of methanol: Neat (1.5 mL/min).
= 1000 μm, length = 50 cm), a solution of aryl halides: 0.10 M in THF (6.0 mL/min), a solution of lithium reagent: 0.42 M in hexane (n-BuLi) or Et2O (PhLi) (1.5 mL/min), a solution of FeCl3: 0.10 M in THF (6 mL/min), a solution of methanol: Neat (1.5 mL/min).
Figure 4.
Effects of the temperature (T) and the residence time in R2 (tR2) on the yield of 4,4'-dimethoxybiphenyl in the oxidative homocoupling of p-methoxyphenyllithium with FeCl3 in the integrated flow microreactor system. Contour plot with scatter overlay of the yields of 4,4'-dimethoxybiphenyl (%), which are indicated by small circles.
One of the major benefits of flow microreactor synthesis is the ability to use highly unstable reactive intermediates. Such intermediates can be rapidly generated and transferred to another location to be used in a subsequent reaction before they decompose. We have already reported the generation and reactions of unstable aryllithium species such as o-bromophenyllithiums, and aryllithiums bearing alkoxycarbonyl, cyano, nitro, and ketone carbonyl groups [86–87,93,95–96,98], which are difficult to use in conventional macro batch reactors. As shown in Table 1, reactions of aryllithiums bearing cyano and nitro groups proceeded successfully to give the corresponding homocoupling products, where in contrast it is very difficult to achieve such reactions using conventional batch reactors. A mechanism involving transmetalation of the aryl group from lithium to iron followed by reductive elimination of the homo-coupling product seems to be plausible, while a similar mechanism is proposed for homo-coupling of organomagnesium compounds with FeCl3 [19–20]. The regiospecificity of the coupling is consistent with this mechanism. Radical coupling seems to be less likely.
Table 1.
Homocoupling of aryl halides using the integrated flow microreactor system.
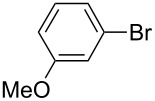
| Ar–X | T (°C) | tR1 (s) | Ar–Ar | Yield (%) |
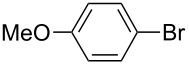 |
24 | 3.100 | 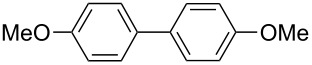 |
72 |
 |
24 | 3.100 | 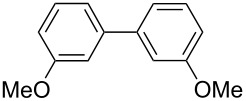 |
69 |
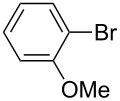 |
0 | 3.100 | 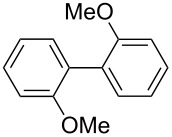 |
76 |
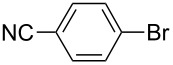 |
−28 | 0.055 | 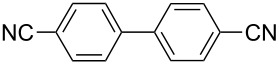 |
75 |
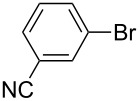 |
0 | 0.055 | 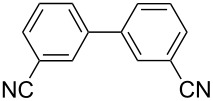 |
66 |
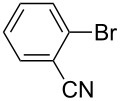 |
24 | 0.055 | 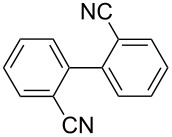 |
76 |
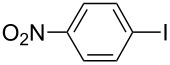 |
−48 | 0.014 | 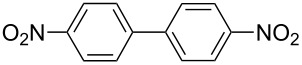 |
53a |
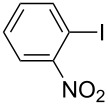 |
−48 | 0.014 | 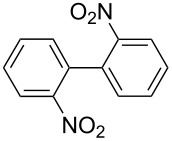 |
63a |
aPhLi instead of n-BuLi was used as lithiating reagent.
Conclusion
In conclusion, we found that the use of FeCl3 results in fast oxidative homocoupling of aryllithiums, which enables its integration with the halogen–lithium exchange of aryl halides. Various aryl halides, including those bearing electrophilic functional groups, can be used for this transformation in the integrated flow microreactor system. Hence, the method greatly enhances the synthetic utility of aryllithium compounds and adds a new dimension to the chemistry of coupling reactions.
Supporting Information
Supporting Information features experimental procedures and full spectroscopic data for all new compounds.
Experimental details.
Acknowledgments
This work was financially supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and NEDO projects.
This article is part of the Thematic Series "Chemistry in flow systems II".
Contributor Information
Aiichiro Nagaki, Email: anagaki@sbchem.kyoto-u.ac.jp.
Jun-ichi Yoshida, Email: yoshida@sbchem.kyoto-u.ac.jp.
References
- 1.Cepanec I. Synthesis of Biaryls. Amsterdam, Boston: Elsevier; 2004. [Google Scholar]
- 2.Inoue A, Kitagawa K, Shinokubo H, Oshima K. Tetrahedron. 2000;56:9601–9605. doi: 10.1016/S0040-4020(00)00929-7. [DOI] [Google Scholar]
- 3.McKillop A, Elsom L F, Taylor E C. J Am Chem Soc. 1968;90:2423–2424. doi: 10.1021/ja01011a041. [DOI] [Google Scholar]
- 4.Ishikawa T, Ogawa A, Hirao T. Organometallics. 1998;17:5713–5716. doi: 10.1021/om980607c. [DOI] [Google Scholar]
- 5.Kharasch M S, Fields E K. J Am Chem Soc. 1941;63:2316–2320. doi: 10.1021/ja01854a006. [DOI] [Google Scholar]
- 6.Sakellarios E, Kyrimis T. Ber Dtsch Chem Ges. 1924;57:322–326. doi: 10.1002/cber.19240570233. [DOI] [Google Scholar]
- 7.Lei A, Srivastava M, Zhang X. J Org Chem. 2002;67:1969–1971. doi: 10.1021/jo011098i. [DOI] [PubMed] [Google Scholar]
- 8.Cahiez G, Marquais S. Pure Appl Chem. 1996;68:53–60. doi: 10.1351/pac199668010053. [DOI] [Google Scholar]
- 9.Cahiez G, Marquais S. Tetrahedron Lett. 1996;37:1773–1776. doi: 10.1016/0040-4039(96)00116-5. [DOI] [Google Scholar]
- 10.Cahiez G, Avedissian H. Synthesis. 1998:1199–1205. doi: 10.1055/s-1998-2135. [DOI] [Google Scholar]
- 11.Dohle W, Kopp F, Cahiez G, Knochel P. Synlett. 2001:1901–1904. doi: 10.1055/s-2001-18748. [DOI] [Google Scholar]
- 12.Fürstner A, Leitner A, Méndez M, Krause H. J Am Chem Soc. 2002;124:13856–13863. doi: 10.1021/ja027190t. [DOI] [PubMed] [Google Scholar]
- 13.Fürstner A, Leitner A. Angew Chem, Int Ed. 2002;41:609–612. doi: 10.1002/1521-3773(20020215)41:4<609::AID-ANIE609>3.0.CO;2-M. [DOI] [Google Scholar]
- 14.Quintin J, Franck X, Hocquemiller R, Figadère B. Tetrahedron Lett. 2002;43:3547–3549. doi: 10.1016/S0040-4039(02)00568-3. [DOI] [Google Scholar]
- 15.Martin R, Fürstner A. Angew Chem, Int Ed. 2004;43:3955–3957. doi: 10.1002/anie.200460504. [DOI] [PubMed] [Google Scholar]
- 16.Scheiper B, Bonnekessel M, Krause H, Fürstner A. J Org Chem. 2004;69:3943–3949. doi: 10.1021/jo0498866. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Matsuo K, Ito S, Nakamura E. J Am Chem Soc. 2004;126:3686–3687. doi: 10.1021/ja049744t. [DOI] [PubMed] [Google Scholar]
- 18.Nagano T, Hayashi T. Org Lett. 2004;6:1297–1299. doi: 10.1021/ol049779y. [DOI] [PubMed] [Google Scholar]
- 19.Nagano T, Hayashi T. Org Lett. 2005;7:491–493. doi: 10.1021/ol047509+. [DOI] [PubMed] [Google Scholar]
- 20.Cahiez G, Chaboche C, Mahuteau-Betzer F, Ahr M. Org Lett. 2005;7:1943–1946. doi: 10.1021/ol050340v. [DOI] [PubMed] [Google Scholar]
- 21.Knochel P, editor. Handbook of Functionalized Organometallics. Weinheim, Germany: Wiley-VCH; 2005. [DOI] [Google Scholar]
- 22.Yoshida J. Flash Chemistry: Fast Organic Synthesis in Microsystems. Hoboken, N.J.: Wiley; 2008. [Google Scholar]
- 23.Fletcher P D I, Haswell S J, Pombo-Villar E, Warrington B H, Watts P, Wong S Y F, Zhang X. Tetrahedron. 2002;58:4735–4757. doi: 10.1016/S0040-4020(02)00432-5. [DOI] [Google Scholar]
- 24.Jähnisch K, Hessel V, Löwe H, Baerns M. Angew Chem, Int Ed. 2004;43:406–446. doi: 10.1002/anie.200300577. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida J. Chem Commun. 2005:4509–4516. doi: 10.1039/b508341a. [DOI] [PubMed] [Google Scholar]
- 26.Doku G N, Verboom W, Reinhoudt D N, van den Berg A. Tetrahedron. 2005;61:2733–2742. doi: 10.1016/j.tet.2005.01.028. [DOI] [Google Scholar]
- 27.Yoshida J, Nagaki A, Iwasaki T, Suga S. Chem Eng Technol. 2005;28:259–266. doi: 10.1002/ceat.200407127. [DOI] [Google Scholar]
- 28.Geyer K, Codee J D C, Seeberger P H. Chem–Eur J. 2006;12:8434–8442. doi: 10.1002/chem.200600596. [DOI] [PubMed] [Google Scholar]
- 29.Whitesides G. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 30.deMello A J. Nature. 2006;442:394–402. doi: 10.1038/nature05062. [DOI] [PubMed] [Google Scholar]
- 31.Song H, Chen D L, Ismagilov R F. Angew Chem, Int Ed. 2006;45:7336–7356. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi J, Mori Y, Kobayashi S. Chem–Asian J. 2006;1:22–35. doi: 10.1002/asia.200600058. [DOI] [PubMed] [Google Scholar]
- 33.Mason B P, Price K E, Steinbacher J L, Bogdan A R, McQuade D T. Chem Rev. 2007;107:2300–2318. doi: 10.1021/cr050944c. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed-Omer B, Brandtand J C, Wirth T. Org Biomol Chem. 2007;5:733–740. doi: 10.1039/b615072a. [DOI] [PubMed] [Google Scholar]
- 35.Watts P, Wiles C. Chem Commun. 2007:443–467. doi: 10.1039/b609428g. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida J, Nagaki A, Yamada T. Chem–Eur J. 2008;14:7450–7459. doi: 10.1002/chem.200800582. [DOI] [PubMed] [Google Scholar]
- 37.Fukuyama T, Rahman M T, Sato M, Ryu I. Synlett. 2008:151–163. doi: 10.1055/s-2007-1000884. [DOI] [Google Scholar]
- 38.McMullen J P, Jensen K F. Annu Rev Anal Chem. 2010;3:19–42. doi: 10.1146/annurev.anchem.111808.073718. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida J. Chem Rec. 2010;10:332–341. doi: 10.1002/tcr.201000020. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida J, Kim H, Nagaki A. ChemSusChem. 2011;4:331–340. doi: 10.1002/cssc.201000271. [DOI] [PubMed] [Google Scholar]
- 41.Watts P, Wiles C, Haswell S J, Pombo-Villar E, Styring P. Chem Commun. 2001:990–991. doi: 10.1039/b102125g. [DOI] [Google Scholar]
- 42.Suga S, Okajima M, Fujiwara K, Yoshida J. J Am Chem Soc. 2001;123:7941–7942. doi: 10.1021/ja015823i. [DOI] [PubMed] [Google Scholar]
- 43.Fukuyama T, Shinmen M, Nishitani S, Sato M, Ryu I. Org Lett. 2002;4:1691–1694. doi: 10.1021/ol0257732. [DOI] [PubMed] [Google Scholar]
- 44.Ueno M, Hisamoto H, Kitamori T, Kobayashi S. Chem Commun. 2003:936–937. doi: 10.1039/b301638b. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Egido E, Spikmans V, Wong S Y F, Warrington B H. Lab Chip. 2003;3:73–76. doi: 10.1039/b302381h. [DOI] [PubMed] [Google Scholar]
- 46.Lai S M, Martin-Aranda R, Yeung K L. Chem Commun. 2003:218–219. doi: 10.1039/b209297b. [DOI] [PubMed] [Google Scholar]
- 47.Mikami K, Yamanaka M, Islam M N, Kudo K, Seino N, Shinoda M. Tetrahedron Lett. 2003;44:7545–7548. doi: 10.1016/S0040-4039(03)01835-5. [DOI] [Google Scholar]
- 48.Kobayashi J, Mori Y, Okamoto K, Akiyama R, Ueno M, Kitamori T, Kobayashi S. Science. 2004;304:1305–1308. doi: 10.1126/science.1096956. [DOI] [PubMed] [Google Scholar]
- 49.Nagaki A, Kawamura K, Suga S, Ando T, Sawamoto M, Yoshida J. J Am Chem Soc. 2004;126:14702–14703. doi: 10.1021/ja044879k. [DOI] [PubMed] [Google Scholar]
- 50.Lee C-C, Sui G, Elizarov A, Shu C J, Shin Y-S, Dooley A N, Huang J, Daridon A, Wyatt P, Stout D, et al. Science. 2005;310:1793–1796. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 51.Horcajada R, Okajima M, Suga S, Yoshida J. Chem Commun. 2005:1303–1305. doi: 10.1039/b417388k. [DOI] [PubMed] [Google Scholar]
- 52.Kawaguchi T, Miyata H, Ataka K, Mae K, Yoshida J. Angew Chem, Int Ed. 2005;44:2413–2416. doi: 10.1002/anie.200462466. [DOI] [PubMed] [Google Scholar]
- 53.Ducry L, Roberge D M. Angew Chem, Int Ed. 2005;44:7972–7975. doi: 10.1002/anie.200502387. [DOI] [PubMed] [Google Scholar]
- 54.Nagaki A, Togai M, Suga S, Aoki N, Mae K, Yoshida J. J Am Chem Soc. 2005;127:11666–11675. doi: 10.1021/ja0527424. [DOI] [PubMed] [Google Scholar]
- 55.He P, Watts P, Marken F, Haswell S J. Angew Chem, Int Ed. 2006;45:4146–4149. doi: 10.1002/anie.200600951. [DOI] [PubMed] [Google Scholar]
- 56.Uozumi Y, Yamada Y, Beppu T, Fukuyama N, Ueno M, Kitamori T. J Am Chem Soc. 2006;128:15994–15995. doi: 10.1021/ja066697r. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka K, Motomatsu S, Koyama K, Tanaka S, Fukase K. Org Lett. 2007;9:299–302. doi: 10.1021/ol062777o. [DOI] [PubMed] [Google Scholar]
- 58.Sahoo H R, Kralj J G, Jensen K F. Angew Chem, Int Ed. 2007;46:5704–5708. doi: 10.1002/anie.200701434. [DOI] [PubMed] [Google Scholar]
- 59.Hornung C H, Mackley M R, Baxendale I R, Ley S V. Org Process Res Dev. 2007;11:399–405. doi: 10.1021/op700015f. [DOI] [Google Scholar]
- 60.Iwasaki T, Nagaki A, Yoshida J. Chem Commun. 2007:1263–1265. doi: 10.1039/b615159k. [DOI] [PubMed] [Google Scholar]
- 61.Nagaki A, Iwasaki T, Kawamura K, Yamada D, Suga S, Ando T, Sawamoto M, Yoshida J. Chem–Asian J. 2008;3:1558–1567. doi: 10.1002/asia.200800081. [DOI] [PubMed] [Google Scholar]
- 62.Nagaki A, Tomida Y, Yoshida J. Macromolecules. 2008;41:6322–6330. doi: 10.1021/ma800769n. [DOI] [Google Scholar]
- 63.Fukuyama T, Kobayashi M, Rahman M T, Kamata N, Ryu I. Org Lett. 2008;10:533–536. doi: 10.1021/ol702718z. [DOI] [PubMed] [Google Scholar]
- 64.Nagaki A, Tomida Y, Miyazaki A, Yoshida J. Macromolecules. 2009;42:4384–4387. doi: 10.1021/ma900551a. [DOI] [Google Scholar]
- 65.Tricotet T, O’Shea D F. Chem–Eur J. 2010;16:6678–6686. doi: 10.1002/chem.200903284. [DOI] [PubMed] [Google Scholar]
- 66.Greenway G M S, Haswell J, Morgan D O, Skelton V, Styring P. Sens Actuators, B. 2000;63:153–158. doi: 10.1016/S0925-4005(00)00352-X. [DOI] [Google Scholar]
- 67.Haswell S J, O'Sullivan B, Styring P. Lab Chip. 2001;1:164–166. doi: 10.1039/b104035a. [DOI] [PubMed] [Google Scholar]
- 68.Niwa S, Eswaramoorthy M, Nair J, Raj A, Itoh N, Shoji H, Namba T, Mizukami F. Science. 2002;295:105–107. doi: 10.1126/science.1066527. [DOI] [PubMed] [Google Scholar]
- 69.Jas G, Kirschning A. Chem–Eur J. 2003;9:5708–5723. doi: 10.1002/chem.200305212. [DOI] [PubMed] [Google Scholar]
- 70.Basheer C, Hussain F S J, Lee H K, Valiyaveettil S. Tetrahedron Lett. 2004;45:7297–7300. doi: 10.1016/j.tetlet.2004.08.017. [DOI] [Google Scholar]
- 71.Liu S, Fukuyama T, Sato M, Ryu I. Org Process Res Dev. 2004;8:477–481. doi: 10.1021/op034200h. [DOI] [Google Scholar]
- 72.Kunz U, Schönfeld H, Solodenko W, Jas G, Kirschning A. Ind Eng Chem Res. 2005;44:8458–8467. doi: 10.1021/ie048891x. [DOI] [Google Scholar]
- 73.Comer E, Organ M G. J Am Chem Soc. 2005;127:8160–8167. doi: 10.1021/ja0512069. [DOI] [PubMed] [Google Scholar]
- 74.Lee C K Y, Holmes A B, Ley S V, McConvey I F, Al-Duri B, Leeke G A, Santos R C D, Sevilled J P K. Chem Commun. 2005:2175–2177. doi: 10.1039/b418669a. [DOI] [PubMed] [Google Scholar]
- 75.Mauger C, Buisine O, Caravieilhes S, Mignani G. J Organomet Chem. 2005;690:3627–3629. doi: 10.1016/j.jorganchem.2005.03.071. [DOI] [Google Scholar]
- 76.Kirschning A, Solodenko W, Mennecke K. Chem–Eur J. 2006;12:5972–5990. doi: 10.1002/chem.200600236. [DOI] [PubMed] [Google Scholar]
- 77.Shore G, Morin S, Organ M G. Angew Chem, Int Ed. 2006;45:2761–2766. doi: 10.1002/anie.200503600. [DOI] [PubMed] [Google Scholar]
- 78.Shi G, Hong F, Liang Q, Fang H, Nelson S, Weber S G. Anal Chem. 2006;78:1972–1979. doi: 10.1021/ac051844+. [DOI] [PubMed] [Google Scholar]
- 79.Rahman M T, Fukuyama T, Kamata N, Sato M, Ryu I. Chem Commun. 2006:2236–2238. doi: 10.1039/b600970k. [DOI] [PubMed] [Google Scholar]
- 80.Murphy E R, Martinelli J R, Zaborenko N, Buchwald S L, Jensen K F. Angew Chem, Int Ed. 2007;46:1734–1737. doi: 10.1002/anie.200604175. [DOI] [PubMed] [Google Scholar]
- 81.Kawanami H, Matsushima K, Sato M, Ikushima Y. Angew Chem, Int Ed. 2007;46:5129–5132. doi: 10.1002/anie.200700611. [DOI] [PubMed] [Google Scholar]
- 82.Yamada Y M A, Watanabe T, Torii K, Uozumi Y. Chem Commun. 2009:5594–5596. doi: 10.1039/b912696a. [DOI] [PubMed] [Google Scholar]
- 83.Jin J, Cai M-M, Li J. Synlett. 2009:2534–2538. doi: 10.1055/s-0029-1217730. [DOI] [Google Scholar]
- 84.Ahmed-Omer B, Barrow D A, Wirth T. Tetrahedron Lett. 2009;50:3352–3355. doi: 10.1016/j.tetlet.2009.02.133. [DOI] [Google Scholar]
- 85.McMullen J P, Stone M T, Buchwald S L, Jensen K F. Angew Chem, Int Ed. 2010;49:7076–7080. doi: 10.1002/anie.201002590. [DOI] [PubMed] [Google Scholar]
- 86.Usutani H, Tomida Y, Nagaki A, Okamoto H, Nokami T, Yoshida J. J Am Chem Soc. 2007;129:3046–3047. doi: 10.1021/ja068330s. [DOI] [PubMed] [Google Scholar]
- 87.Nagaki A, Tomida Y, Usutani H, Kim H, Takabayashi N, Nokami T, Okamoto H, Yoshida J. Chem–Asian J. 2007;2:1513–1523. doi: 10.1002/asia.200700231. [DOI] [PubMed] [Google Scholar]
- 88.Nagaki A, Takabayashi N, Tomida Y, Yoshida J. Org Lett. 2008;10:3937–3940. doi: 10.1021/ol8015572. [DOI] [PubMed] [Google Scholar]
- 89.Nagaki A, Kim H, Yoshida J. Angew Chem, Int Ed. 2008;47:7833–7836. doi: 10.1002/anie.200803205. [DOI] [PubMed] [Google Scholar]
- 90.Nagaki A, Takizawa E, Yoshida J. J Am Chem Soc. 2009;131:1654–1655. doi: 10.1021/ja809325a. [DOI] [PubMed] [Google Scholar]
- 91.Nagaki A, Takabayashi N, Tomida Y, Yoshida J. Beilstein J Org Chem. 2009;5:No. 16. doi: 10.3762/bjoc.5.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomida Y, Nagaki A, Yoshida J. Org Lett. 2009;11:3614–3617. doi: 10.1021/ol901352t. [DOI] [PubMed] [Google Scholar]
- 93.Nagaki A, Kim H, Yoshida J. Angew Chem, Int Ed. 2009;48:8063–8065. doi: 10.1002/anie.200904316. [DOI] [PubMed] [Google Scholar]
- 94.Nagaki A, Takizawa E, Yoshida J. Chem–Eur J. 2010;16:14149–14158. doi: 10.1002/chem.201000815. [DOI] [PubMed] [Google Scholar]
- 95.Nagaki A, Kim H, Moriwaki Y, Matsuo C, Yoshida J. Chem–Eur J. 2010;16:11167–11177. doi: 10.1002/chem.201000876. [DOI] [PubMed] [Google Scholar]
- 96.Nagaki A, Kim H, Matsuo C, Yoshida J. Org Biomol Chem. 2010;8:1212–1217. doi: 10.1039/b919325c. [DOI] [PubMed] [Google Scholar]
- 97.Tomida Y, Nagaki A, Yoshida J. J Am Chem Soc. 2011;133:3744–3747. doi: 10.1021/ja110898s. [DOI] [PubMed] [Google Scholar]
- 98.Kim H, Nagaki A, Yoshida J. Nat Commun. 2011;2:No. 264. doi: 10.1038/ncomms1264. [DOI] [PubMed] [Google Scholar]
- 99.Suga S, Yamada D, Yoshida J. Chem Lett. 2010;39:404–406. doi: 10.1246/cl.2010.404. [DOI] [Google Scholar]
- 100.Nagaki A, Kenmoku A, Moriwaki Y, Hayashi A, Yoshida J. Angew Chem, Int Ed. 2010;49:7543–7547. doi: 10.1002/anie.201002763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details.