Abstract
The physiological role of the TNF receptor (TNFR) family member, RANK, was investigated by generating RANK-deficient mice. RANK−/− mice were characterized by profound osteopetrosis resulting from an apparent block in osteoclast differentiation. RANK expression was not required for the commitment, differentiation, and functional maturation of macrophages and dendritic cells from their myeloid precursors but provided a necessary and specific signal for the differentiation of myeloid-derived osteoclasts. RANK−/− mice also exhibited a marked deficiency of B cells in the spleen. RANK−/− mice retained mucosal-associated lymphoid tissues including Peyer’s patches but completely lacked all other peripheral lymph nodes, highlighting an additional major role for RANK in lymph node formation. These experiments reveal that RANK provides critical signals necessary for lymph node organogenesis and osteoclast differentiation.
Keywords: RANK/RANKL, osteoclast, lymph node, osteopetrosis, knockout mice, TNF receptor
Numerous members of the tumor necrosis factor (TNF) ligand and TNF receptor (TNFR) superfamilies have been implicated in the mechanisms by which cells differentiate, proliferate, become functionally activated, or die. RANKL is a TNF ligand homolog (also called TRANCE, OPGL, and ODF) that is expressed by T cells, pro-B cells, and bone marrow stromal cells (Anderson et al. 1997; Wong et al. 1997a: Lacey et al. 1998; Yasuda et al. 1998a). RANKL has demonstrable activity in vitro on multiple cell lineages, including myeloid and T cells. Stimulation of myeloid-derived dendritic cells (DCs) with RANKL results in an enhancement of the allostimulatory activation of T cells (Anderson et al. 1997; Wong et al. 1997a; Josien et al. 1999). RANKL is also capable of acting directly on T cells leading to stimulation of c-Jun amino-terminal kinase (JNK) activity (Wong et al. 1997b) and increased survival of activated T cells (Anderson et al. 1997). RANKL, in combination with CSF-1, can substitute for bone marrow stromal factors to promote the differentiation of the bone-resorbing osteoclasts from myeloid precursors (Lacey et al. 1998; Yasuda et al. 1998a). RANK is a cognate receptor for RANKL expressed on myeloid-derived DCs, activated T cells and osteoclast progenitors (Anderson et al. 1997; Nakagawa et al. 1998; Hsu et al. 1999), and this expression pattern suggests that RANK may be responsible for the in vitro activities on these cells.
The recent discovery of a second TNF-family receptor for RANKL, osteoprotegerin (OPG), has expanded the known function of RANKL from a role in immune cell activation to include a crucial activity in the physiological process of bone resorption. OPG, which lacks a transmembrane domain, is a distinct protein from the transmembrane signaling receptor, RANK. In vivo and in vitro experiments have demonstrated that OPG functions as a secreted inhibitor of bone resorption by acting both to block osteoclast differentiation from precursor cells and to block the activation of mature osteoclasts (Simonet et al. 1997; Bucay et al. 1998; Yasuda et al. 1998b). The potent inhibition of bone resorption by OPG is a result of high-affinity binding to RANKL (Lacey et al. 1998; Yasuda et al. 1998b). Whereas OPG expression can be modulated by factors that affect osteoclastogenesis (Hofbauer et al. 1998; Takai et al. 1998), OPG expression is also affected by CD40 stimulation in B cells and DCs (Yun et al. 1998). The ability of OPG to bind at least two TNF-family ligands including RANKL and TRAIL (Emery et al. 1998) and the modulation of OPG expression in immune cells may suggest additional roles for OPG beyond its capacity to inhibit osteoclast differentiation.
The multinucleated osteoclast is the principal bone resorbing cell of the body and derives from a hematopoietic precursor of the monocytic lineage common to macrophages and myeloid DCs (Suda et al. 1992; Roodman 1996). Analysis of spontaneous mouse mutants or mice with experimental gene disruptions has highlighted key steps in the differentiation and activation of osteoclasts. The op/op mouse (Yoshida et al. 1990), which lacks functional CSF-1 protein, and animals that lack the lymphoid/myeloid transcription factor PU.1 (Tondravi et al. 1997) reveal early myeloid defects and show impaired development of both macrophages and osteoclasts. Mice lacking the nuclear protein c-Fos demonstrate a lineage-specific defect resulting in the lack of osteoclasts but enhanced numbers of bone marrow macrophages (Johnson et al. 1992; Grigoriadis et al. 1994; Okada et al. 1994). Similarly, osteoclast development is defective in the absence of p50 and p52 NF-κB subunits, whereas macrophage numbers are enhanced (Franzoso et al. 1997; Iotsova et al. 1997). Finally, activation of mature, differentiated osteoclasts is compromised in c-src and cathepsin K-deficient mice (Soriano et al. 1991; Saftig et al. 1998, respectively). The significance of each of these mutations in osteoclast development/activation is underscored by the common osteopetrotic disorder seen in each animal. In humans, common pathologies of the bone resulting from enhanced osteoclast activity include Paget’s disease, postmenopausal osteoporosis, bone loss secondary to malignancy, and rheumatoid arthritis (Roodman 1996; Guise and Mundy 1998).
The TNF/TNFR superfamilies exhibit considerable redundancy at the level of receptor/ligand binding (Smith et al. 1994), however, this redundancy does not always lead to functional compensation in vivo (for review, see Chaplin and Fu 1998). The interaction of RANKL with two TNFR superfamily members, OPG and RANK, illustrates the receptor/ligand binding promiscuity common to these protein families. The genetic ablation of RANKL has been shown to have pleiotropic effects, leading to B- and T-cell defects in addition to osteopetrosis, thereby demonstrating a broad role for this cytokine in immune cell and osteoclast function/development (Kong et al. 1999). To investigate the putative role of RANK in bone resorption directly and to analyze the relevance of RANK in multiple cell lineages, we generated RANK-deficient mice RANK−/−) by gene targeting. We find that RANK knockout mice have profound defects in bone resorption, lymph-node formation, and B-cell development. However, RANK is not required for myeloid lineage commitment to the macrophage, granulocyte, or DC pathways. RANK−/− mice retain the proper development of functional DCs and macrophages but not myeloid-derived osteoclasts, revealing an essential role for RANK in osteoclast differentiation.
Results
Generation of RANK−/−
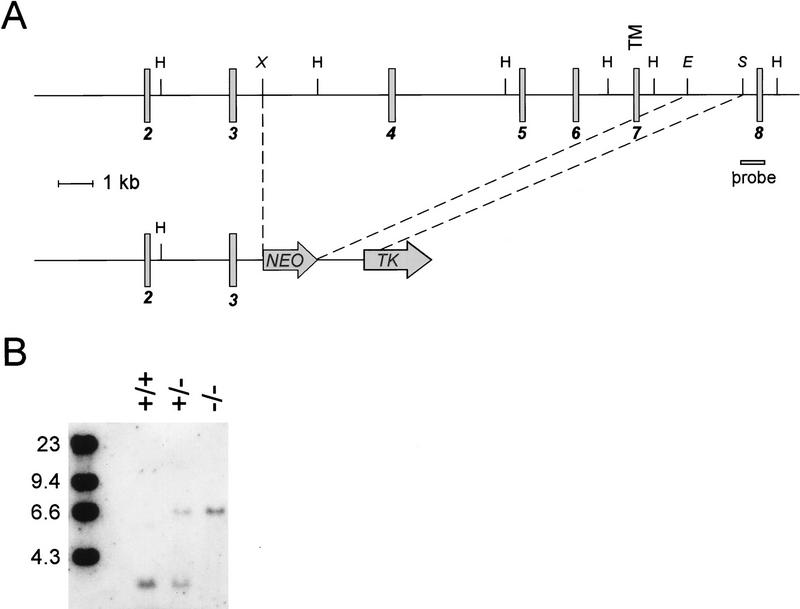
A targeted mutation in the RANK gene was introduced by homologous recombination using a vector in which exons 4–7, encoding the majority of the extracellular domain and the transmembrane region of RANK, were replaced by a PGK–NEO cassette (Fig. 1A,B). RANK−/− mice were born at the expected Mendelian frequency from RANK+/− intercrosses (data not shown), arguing against an obligate role for RANK embryonic development. Mice heterozygous for the RANK mutation were indistinguishable from wild-type mice in all parameters analyzed (data not shown). Hematopoietic precursor cells isolated from RANK−/− mice failed to respond to recombinant RANKL (see Fig. 3, below), and do not express RANK on the cell surface (data not shown), confirming that this mutation represents a null mutation in RANK.
Figure 1.
Targeted disruption of RANK. (A) The structure of the RANK gene, spanning exons 2–8 (shaded boxes), and the RANK gene-targeting vector. Relevant restriction sites used to create the targeting vector and to analyze the targeted RANK gene are shown. The position of the transmembrane domain (TM) is indicated. Restriction sites: HindIII (H); XbaI (X); EcoRV (E); StuI (S). (B) Southern blot analysis of the disrupted RANK gene. Genomic DNAs from wild-type (+/+), RANK+/−, and RANK−/− cells were digested with HindIII and subjected to Southern blot analysis using the indicated probe.
Figure 3.
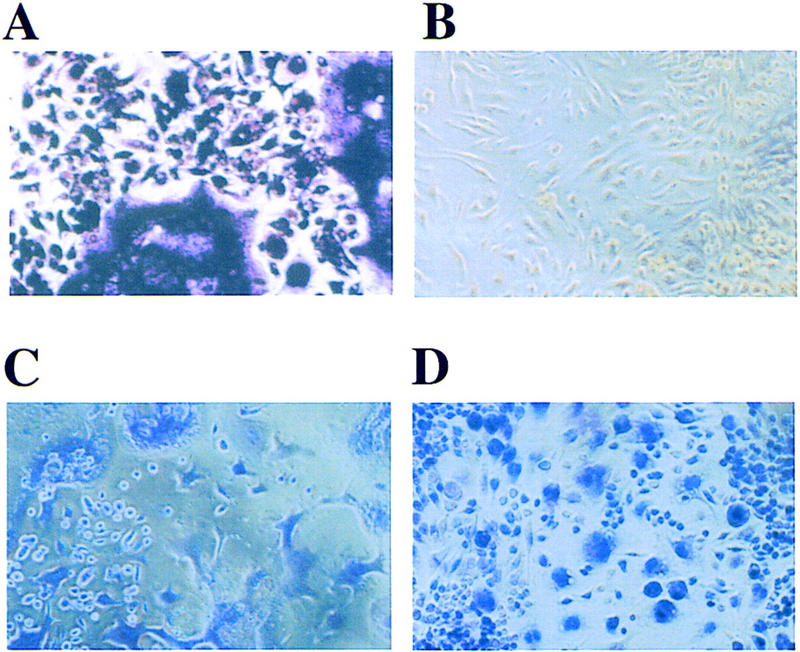
RANK−/− hematopoietic precursors fail to form osteoclasts in vitro. Splenocytes from RANK+/+ and RANK−/− animals were cultured with 40 ng/ml mCSF-1 and 200 ng/ml mRANKL. After 7 days in culture, osteoclasts were identified by staining for TRAP, which reveals morphologically distinct osteoclasts with a purple stain in situ. TRAP+ osteoclasts are only seen from RANK+/+ cultures (A) and not in cultures from RANK−/− mice (B). Staining with hematoxylin (C,D) reveals cells in both cultures, including cells with monocytic/macrophage morphology in the RANK−/− cultures (D).
RANK−/− mice are runted and have profound osteopetrosis
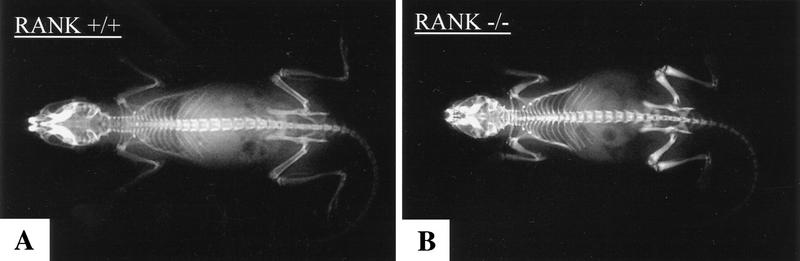
The RANK−/− mice were typified by small body size, shortened limbs, and doming of the skull. Neither incisor teeth nor molars were visible up to 80 days of age. The retarded growth became apparent after weaning at ∼3 weeks after birth. Radiographic examination of the RANK−/− mice revealed a severe osteopetrotic phenotype as evidenced by shortened long bones and metaphyseal flaring with radio-dense regions within the bone marrow cavity (Fig. 2A,B). Moreover, the base of the skull and the axial skeleton, including the vertebrae and the ribs, also showed increased radio-density relative to control age-matched animals (Fig. 2A,B). Qualitative increases in bone density observed by microradiography were verified using ex vivo pDXA measurements (data not shown).
Figure 2.
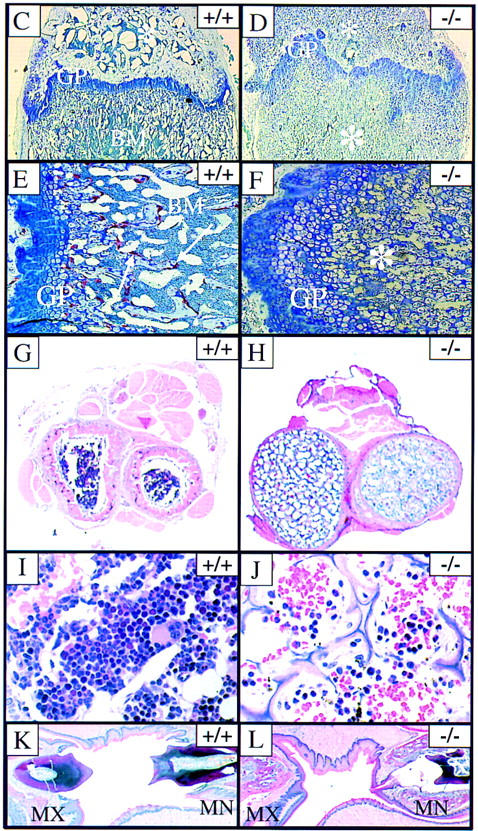
Osteopetrosis in RANK−/− mice. (A,B) Radiograph of 6-week-old RANK+/+ (left) and RANK−/− (right) mice. Note the absence of the bone marrow cavity, shortening of the long bones, and overall increase in radiodensity in the RANK−/− animals. (C–L) Histological analysis of bone. (C,D) Histological staining of the femur (4× magnification). The growth plate (GP) is distorted in the RANK−/− animals and the bone marrow (BM) is filled with cartilage (*). (E,F) Histological staining of the femur (20× magnification). TRAP positive (red) osteoclasts (arrows) are readily seen in the RANK+/+ bone. Note the absence of TRAP-positive osteoclasts in RANK−/− bone and the disordering of the chondrocytes at the growth plate. (G,H) Cross section of radius and ulna indicating the occlusion of the bone marrow space in RANK−/− mice. Note the thinning of the cortical bone in RANK−/− mice. (I,J) Histological sections of vertebral bone. Note osteopetrotic condition of RANK−/− vertebrae and residual, small hematopoietic foci in the RANK−/− animal. (K,L) Histological section of the jaw, illustrating the maxilla (MX) and mandible (MN). The teeth are erupting through the wild-type jaw; in the RANK−/− mice, teeth are present but impacted in abnormal bone.
Histological analysis of forelimbs and hindlimbs in the RANK−/− mice confirmed the osteopetrotic phenotype, including disorganization of chondrocytes at the growth plate and an almost complete occlusion of the marrow cavities with a poorly remodeled, osteocartilaginous structure (Fig. 2C,D). Although osteoclasts were readily detected in the primary and secondary spongiosa of wild-type mice by staining for the osteoclast marker tartrate-resistant acid phosphatase (TRAP) (Fig. 2E), the RANK−/− bone sections failed to reveal any positive-staining osteoclasts (Fig. 2F). The absence of mature or preosteoclast differentiation was also determined using in vitro analysis of RANK−/− hematopoietic precursors (see below). Other osteopetrotic features of the RANK−/− mice included increased cross-sectional diameter of long bones (Fig. 2G,H), thin and poorly mineralized cortical bone with some osteophyte formation, abnormal mineralization and formation of alveolar bone, and widening of all growth plates. The vertebral bodies also exhibited osteopetrotic features (Fig. 2I,J) and illustrate reduced hematopoietic activity in RANK−/− mice including small, hypocellular clusters with myeloid, erythroid, and megakaryocytic differentiation. Doming of the skull resulted from abnormal shortening and ossification of the bones at the base of the skull. RANK−/− mice had small teeth with normal layers of enamel, dentin, cementum, odontoblasts, and pulp cavity found in the mandible and maxilla but embedded in an excess of poorly ossified and abnormally thin alveolar bone and osteoid (Fig. 2K,L). The failure of tooth eruption is a hallmark of osteopetrotic animals (Johnson et al. 1992; Franzoso et al. 1997) and resulted from bone completely surrounding the incisors and cheek teeth except at the point of epithelial downgrowth through the gingival sulcus into the dental sac (Fig. 2M,N).
RANK is essential for osteoclast differentiation in vitro
RANKL, which has been shown previously to be an essential stromal-derived factor for osteoclastogenesis in vivo (Kong et al. 1999), binds to a receptor on hematopoietic osteoclast precursors in the bone marrow (Lacey et al. 1998) that derive from CSF-1 responsive cells in the monocyte/macrophage lineage (Suda et al. 1992; Roodman 1996). To determine whether the failure of osteoclasts to form in the RANK−/− mice is due to the loss of RANKL responsiveness by RANK−/− hematopoietic precursors, the osteoclast-forming ability of hematopoietic precursors from RANK−/− mice was tested in vitro using recombinant RANKL and CSF-1. Spleen cells (free of stromal elements) from the RANK−/− mice cultured with CSF-1 and RANKL failed to form large multinucleated TRAP-positive cells under conditions that promote the generation of mature, TRAP-positive (Fig. 3) and calcitonin receptor-positive (data not shown) osteoclasts from wild-type hematopoietic cells. RNA markers indicative of different stages of osteoclast development were also assayed to determine whether immature preosteoclasts were being formed from RANK−/− progenitors. Whereas expression of mRNAs for calcitonin receptor, MMP-9, cathepsin K, and TRAP were significantly induced in RANK+/+ cells treated with RANKL and CSF-1, these changes did not occur with RANK−/− precursors (data not shown), indicating an arrest in preosteoclast development. To further verify that the osteoclast defect in RANK−/− animals lies in the hematopoietic compartment and is not a defect in stromal cells, retroviral-mediated gene transfer of RANK into RANK−/− splenic hematopoietic cells was used to rescue the efficient formation of TRAP-positive osteoclasts in the presence of recombinant RANKL and CSF-1 (A. Armstrong, M. Tometsko, and W. Dougall, in prep.). These data confirm the existence of osteoclast progenitors in RANK−/− mice and indicate that RANK expression on hematopoietic osteoclast progenitors is essential for osteoclast differentiation in vitro and in vivo.
RANK−/− mice have impaired B-cell development
The RANK−/− mice showed a number of alterations in the hematopoietic system including a dramatic reduction of the femoral marrow cellularity (∼10% of normal; data not shown). As noted in histological sections of the RANK−/− bones, only a few hematopoietic foci were found in long bones and vertebral bodies. We have observed no recovery of bone marrow cellularity in older RANK−/− animals (data not shown) in contrast to that observed in op/op mice (Begg et al. 1993). Spleens of RANK−/− mice were visibly enlarged, and after normalizing for the smaller body weight of the RANK−/− mice, the relative spleen-to-body weight ratios were twice that of age-matched littermates. Flow cytometric analysis of spleens from RANK−/− mice using antibodies against cell-surface markers revealed a 50% reduction in B220+ B cells (Fig. 4). Reduction of mature B cells was evident by the reduced levels of IgM+/IgD+ and B220+/IgM+ cells in the RANK−/− mice (Fig. 4). The frequency of CD4+ and CD8+ T cells (Fig. 4) appeared to be normal in RANK−/− spleens. Analysis of the myeloid lineage in the RANK−/− spleens showed slightly elevated proportions of CD11b+/GR-1+ cells (Fig. 4). The CD11b+/GR-1bright staining indicates a greater number of mature granulocytes/neutrophils and not immature myeloid cells (Hestdal et al. 1991). Normal frequencies of mature macrophages in RANK−/− spleen were evident by staining for the macrophage-specific marker F4/80 (data not shown) as well as by histological analysis of the spleen (see below). The percentage of erythroid precursor cells (identified by Ter-119 staining) was significantly increased in the RANK−/− spleens (Fig. 4) presumably to compensate for reduced bone marrow erythropoiesis. Differential analysis of peripheral blood showed that total white blood cell counts varied from normal to low normal with normal differentials compared to the wild-type values. Red blood cell counts and indices were low to low normal and were compatible with a regenerative anemia.
Figure 4.
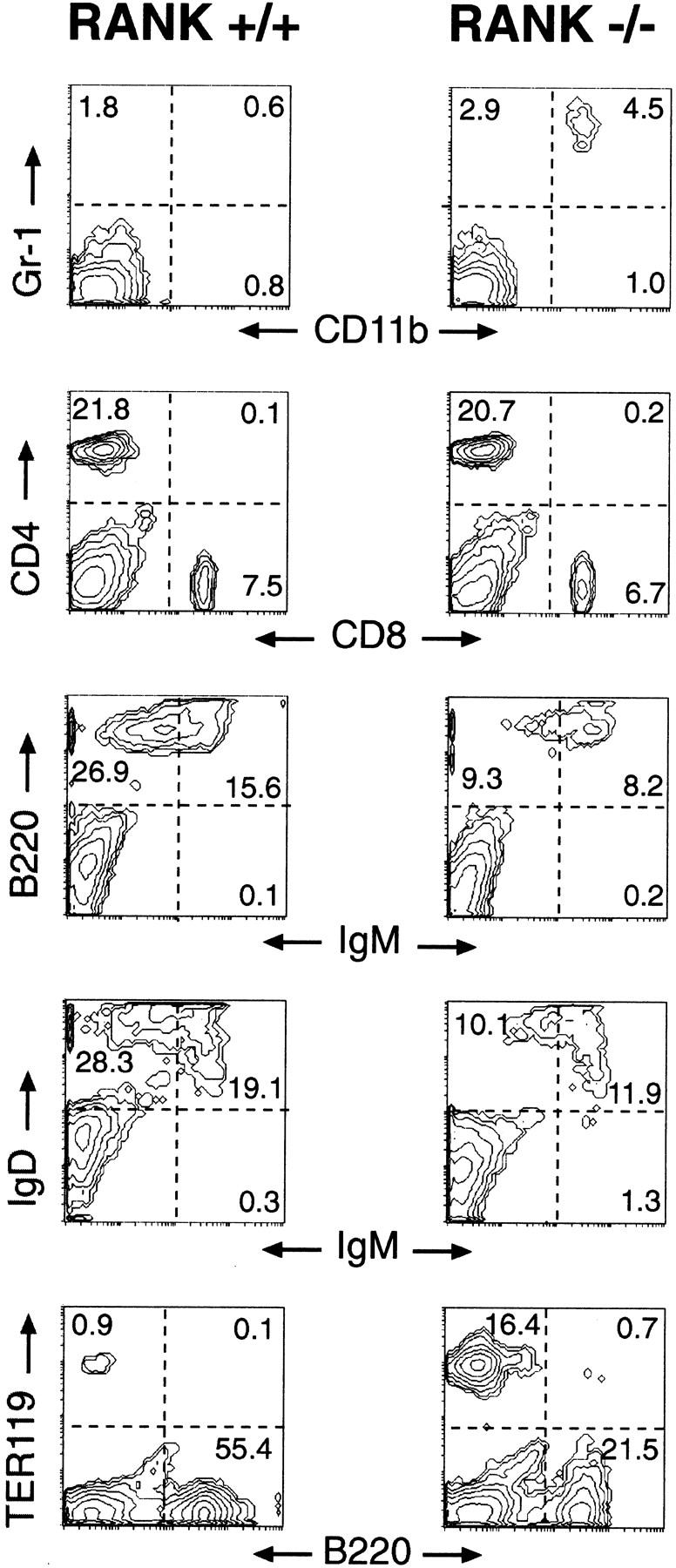
Defective B-lymphocyte development in RANK−/− mice. Flow cytometric analysis of spleen cells from 6–8-week-old RANK+/+ (left) and RANK−/− (right) mice. Details of the cytometric analyses are described in Materials and Methods. The histograms are representative of multiple RANK+/+ (n = 6) and RANK−/− (n = 7) mice. The numbers represent the percentage positive cells within each gated quadrant.
RANK−/− mice have significant extramedullary hematopoiesis
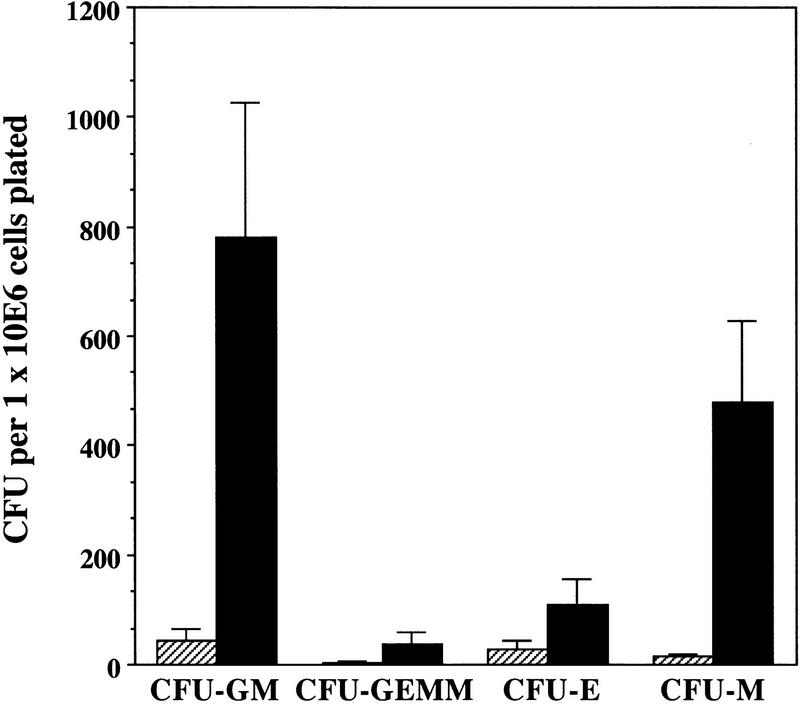
To determine if the changes observed in the myeloid (osteoclast and CD11b+/GR1+cells), erythroid, and B-cell compartments in spleens of RANK−/− mutant mice resulted from differences in the frequency of hematopoietic progenitors, the numbers of lineage-committed progenitors were assessed using in vitro clonogenic assays. Numbers of colony-forming units–granulocyte/erythrocyte/megakaryocyte/macrophage (CFU-GEMM), CFU–erythrocyte (CFU-E), and CFU–granulocyte/macrophage (CFU-GM) colonies were elevated from spleens of 6- to 8-week-old RANK−/− mice compared with control mice (Fig. 5). The ratios between mixed, macrophage, granulocyte, or erythroid colonies appeared to be the same in all cultures suggesting the lineage potentials in RANK−/− animals were not disturbed. Because osteoclasts share a precursor to the monocyte/macrophage lineage that expresses the lineage-specific CSF-1 receptor (Suda et al. 1992), colony-forming assays were also performed with CSF-1 to determine the frequency of lineage-restricted, committed precursors. The RANK−/− spleens contained 30-fold higher CFU–macrophage (CFU-M) relative to control animals (Fig. 5). These results demonstrate that RANK−/− animals exhibit high levels of erythroid and granulocyte/macrophage progenitors in the spleen presumably because of an altered bone marrow environment. Interestingly, histological examination revealed no obvious evidence of extramedullary hematopoiesis in the livers of RANK−/− mice (data not shown).
Figure 5.
Elevated extramedullary hematopoiesis in RANK−/− mice. Cells were cultured in methylcellulose in the presence of rmuIL-3 plus rmuMGF (c-kit ligand) plus rmu EPO (CFU-E, CFU-GM, and CFU-GEMM) or with rmu CSF-1 (CFU-M), as described in Materials and Methods. Absolute numbers of clonogenic precursors observed with RANK+/− cells were similar to wild-type levels (data not shown). (Hatched bars) RANK+/+ mice; (solid bars) RANK−/− mice. Values represent the means ± s.e.m. for four experiments done in triplicate.
RANK is not essential for the development and function of differentiated macrophages and dendritic cells
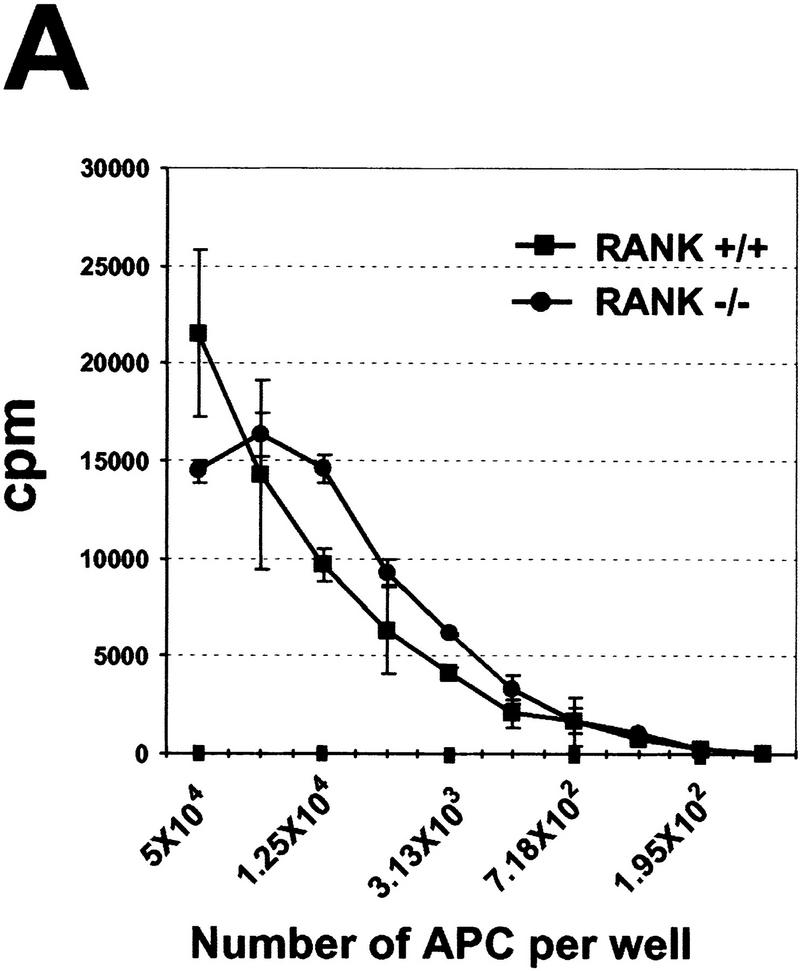
RANKL has been shown to activate the allostimulatory capacity of myeloid-derived DCs in vitro (Anderson et al. 1997) through mechanisms that include enhanced DC survival (Wong et al. 1997a) and production of cytokines (Josien et al. 1999). We analyzed various DC populations to determine the integrity of DC development and function in the RANK−/− animals. Analysis of splenic cells showed similar frequencies of CD11c+ DCs in RANK+/+ and RANK−/− animals, and equivalent expression of the lymphoid DC marker CD8α was observed within the CD11c+ fraction of either RANK+/+ or RANK−/− DCs (data not shown) indicating that DC development is intact. Moreover, DCs isolated from both RANK+/+ or RANK−/− mice showed similar expression patterns of several other DC markers (including CD40, CD80, CD86, and class II MHC), which were appropriately upregulated upon activation with GM-colony stimulating factors (GM-CSF) (data not shown). Purified CD11c+ DCs from RANK+/+ and RANK−/− mice were equally capable of stimulating allogeneic T cells in a mixed lymphocyte reaction (MLR) (Fig. 6A). Identification of class II MHC-positive epidermal Langerhans cells in the skin (not shown) and other DC subsets residing in the spleen (see below) confirms the normal distribution of multiple DC populations in tissue microenvironments of RANK−/− mice. These data indicate that the frequency of DCs from RANK−/− mice was equal to that from RANK+/+ mice, and these cells express typical DC phenotypic/costimulatory markers and function to efficiently stimulate alloreactive T cells.
Figure 6.
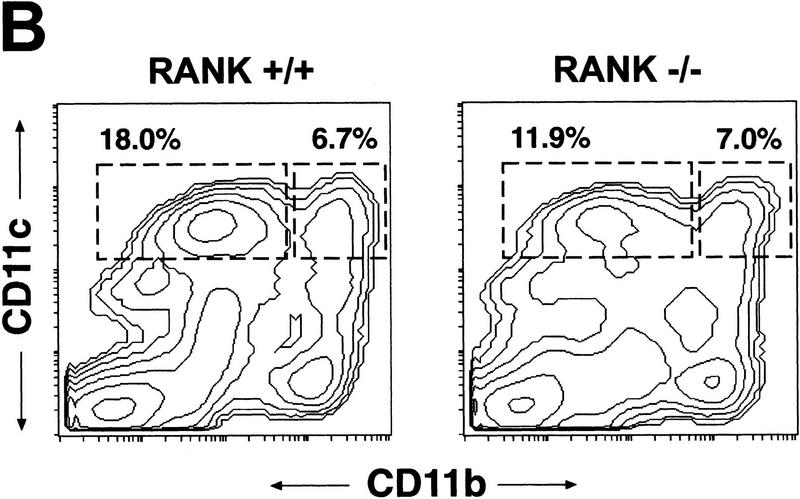
RANK−/− mice show normal DC development and function. (A) The allostimulatory capacity of CD11c+ DCs from untreated RANK+/+ (█) and RANK−/− (●) mice was measured in a mixed lymphocyte reaction (MLR) as described in Materials and Methods. Values represent the mean ± s.e.m. of triplicate cultures and are representative of experiments from multiple mice (n = 3). (B) Flow cytometric analysis of splenic DCs from FL-treated RANK+/+ (left) and RANK−/− (right) animals. Mice were treated with FL for nine days (see Materials and Methods) to mobilize DC, and single cell spleen suspensions were stained for CD11b and CD11c. The percentages of the lymphoid-related DC (CD11blow CD11c+) and myeloid-related DC (CD11b+ CD11c+) are indicated above the gated areas.
Treatment of mice with the hematopoietic growth factor flt3 ligand (FL) induces a large expansion of mature and immature DCs in the spleen and bone marrow (Maraskovsky et al. 1996), facilitating further functional and developmental analysis of DCs. FL induces the expansion of CD11b+ CD11c+ myeloid- and CD11blow CD11c+ lymphoid-related DCs in murine spleen (Pulendran et al. 1997). The relative distribution of splenic DCs from FL-treated RANK−/− mice was similar to that seen in wild-type mice with the exception of slightly reduced frequencies of CD11blow CD11c+ lymphoid-related DCs (Fig. 6B). There was also no functional difference in the ability of RANK−/− and RANK+/+ lymphoid- and myeloid-DC subsets to phagocytose or endocytose particles in vitro (not shown).
The clonogenic progenitor assays demonstrated that RANK−/− mice contain both primitive and mononuclear phagocyte-committed, CSF-1-responsive cells in the spleen (Fig. 5). To verify the macrophage/monocyte identity of RANK−/− cells that responded to CSF-1, expression of multiple macrophage markers was analyzed by flow cytometry. Nearly all of the RANK−/− spleen cells treated with CSF-1 for 7 days expressed the macrophage markers F4/80, BM-8, and MOMA-2 (Table 1) establishing that macrophage differentiation is apparently normal. To test whether differentiated macrophages from RANK−/− animals are functionally intact, these cells were tested for their responsiveness to LPS and IFN-γ. Activation of both RANK−/− and wild-type macrophages enhanced B7-2 surface expression as well as production of IL-1β and TNFα (data not shown). F4/80- and BM-8-positive macrophages were also localized in the spleen (see Fig. 8, below), indicating that macrophages are normally distributed in the tissues of RANK−/− animals.
Table 1.
Antibody staining of RANK+/+ and RANK−/− spleen cells cultured with CSF-1
| Cell population
|
Percentage of cells positive for antibody
|
||||
|---|---|---|---|---|---|
| CD11b
|
F4/80
|
BM-8
|
MOMA-2
|
class II
|
|
| RANK+/+ | 100 | 94 | 100 | 95 | N.D. |
| RANK−/− | 97 | 87 | 97 | 97 | 68 |
Results represent the percentages of cells positive for the antibody as determined by flow cytometry. No background staining was observed with isotype-matched antibody controls. Values are from one experiment and are typical of multiple experiments using spleen cells from 6- to 8-week old mice. RANK+/+ (n = 4); RANK−/− (n = 6). Cells were cultured with CSF-1 for 7 days prior to analysis, as described in Materials and Methods and cells from both RANK+/+ and RANK−/− cultures had the morphology of mature macrophages. (N.D.) Not determined.
Figure 8.
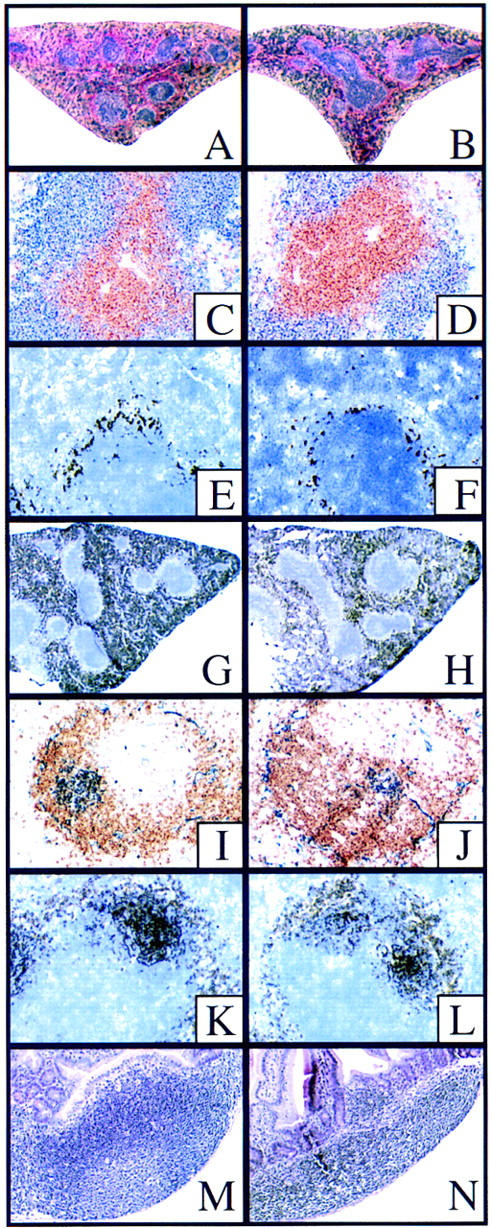
Histological staining of spleen and Peyer’s patches. Wild-type (A) and RANK−/− (B) spleens stained with hematoxylin and Eosin; both spleens show normal lymphocytic follicles. (C,D) Immunostaining for B- and T-cell organization. Anti-CD3 (brown) and anti-B220 (blue) reveals normal B/T-cell segregation in spleens of both wild-type (C) and RANK−/− (D) mice. (E,F) MOMA-1 staining (black) to indicate splenic white pulp marginal zone macrophages. The RANK−/− spleen shows slightly reduced staining for MOMA-1 positive cells (F). (G,H) Immunohistology of macrophage populations in the spleen using anti-BM8 (black) indicating slightly reduced staining for BM-8-positive macrophages in RANK−/− spleen (H) relative to control (G). Immunohistology of germinal centers (I–L). Anti-B220 (brown) and PNA (blue) indicate PNA-positive regions centered within B-cell areas in both wild-type (I) and RANK−/− (J) spleens. The size and density of the germinal center-like PNA+ regions was slightly reduced in RANK−/− spleens. (K,L) Anti-CD35 staining (black) indicates intact follicular dendritic cell networks around PNA+ germinal centers in both wild-type (K) and RANK−/− (L) spleens. Histology of Peyer’s patches (M,N). H&E stain of Peyer’s patches of wild-type (M) and RANK−/− (N) mice. Note reduced size and cellularity of RANK−/− Peyer’s patches.
Thymic development is normal in RANK−/− mice
Thymic developmental defects have been observed in some osteopetrotic mice as a result of both intrinsic lineage defects (Franzoso et al. 1997) and indirect, stress-related effects (Milhaud et al. 1983). Importantly, the osteopetrotic RANKL−/− mice show significantly reduced thymic size and cellularity and a block in thymocyte development (Kong et al. 1999). In this study, the cellularity of thymi from RANK−/− mice was reduced relative to wild-type thymi (Fig. 7A); however, when normalized for the smaller body weight of RANK−/− mice, there was no significant difference in thymic size (Fig. 7B). Phenotypic analysis of thymic cell suspensions revealed the appropriate relative percentages of double positive CD4+/CD8+ immature thymocytes and single CD4+ or CD8+ mature thymocytes (Fig. 7C). T-cell precursors were examined by gating out Lin+ cells (CD3ε+, CD4+, CD8+, B220+, CD11b+, GR-1+, and TCRαβ+) and were analyzed for CD25 and CD44 expression to assess thymocyte maturation. The development of CD25−/CD44+ precursors to CD25−/CD44− thymocytes appears phenotypically normal in RANK−/− mice (Fig. 7C).
Figure 7.
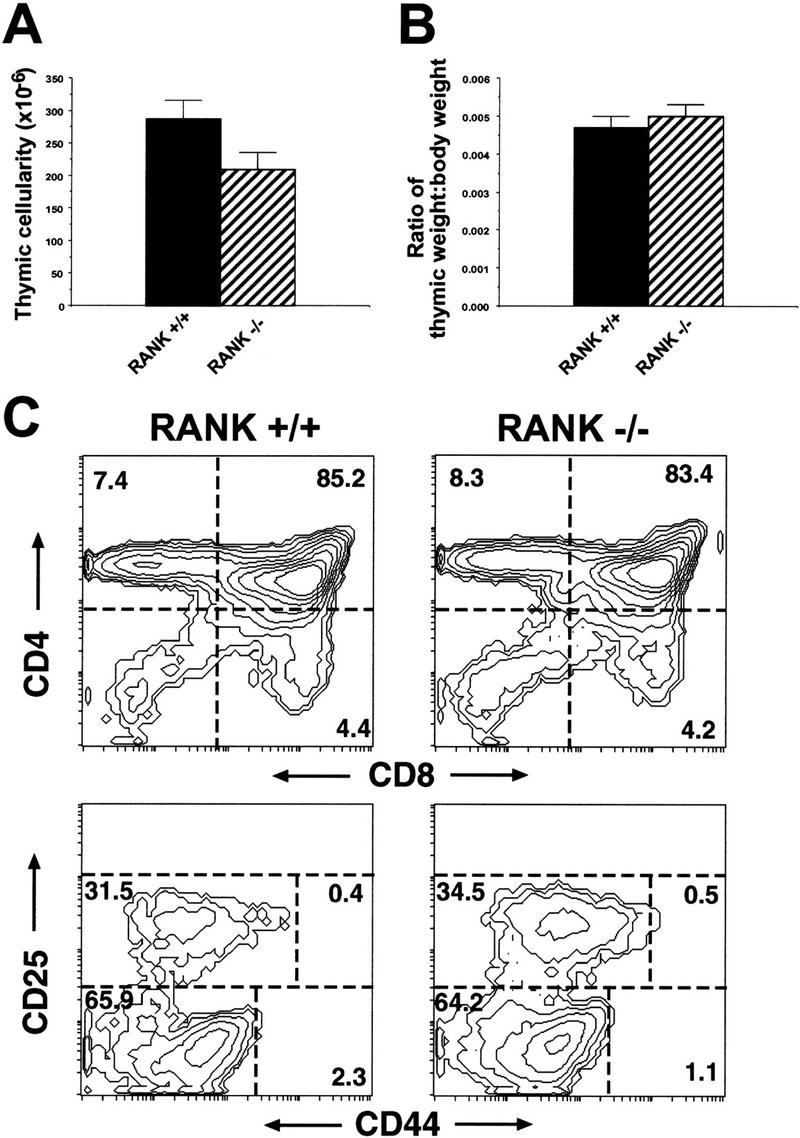
Intact thymocyte development in RANK−/− mice. (A) Cell counts of thymi from 6- to 8-week-old RANK+/+ (n = 14) and RANK−/− (n = 14) mice. (B) The thymic weights were expressed as a proportion of total body weight. Note the equivalent thymic weight/body weight ratios observed in RANK+/+ and RANK−/− mice. (C) Flow cytometric analysis of thymi from 6-week-old RANK+/+ or RANK−/− mice. Thymocyte suspensions were stained for CD4 and CD8 (top) or gated, Lin− (CD3ε−, CD4−, CD8−, B220−, GR-1−, CD11b−, TCRγδ−) thymocyte precursors were stained for CD25 and CD44 (bottom).
Lymphoid organs of RANK−/− mice
The general architecture of the spleen of RANK−/− mice was intact except for a reduction in the apparent cellularity, number, and size of lymphoid aggregates; small foci suggestive of germinal centers; and a slight-to-moderate increase in extramedullary hematopoiesis compared to littermates (Fig. 8A,B). Immunohistochemical analyses for B and T cells in spleen sections (using anti-CD3 and anti-B220 antibodies) showed an overall normal architecture with well-organized B/T-cell segregation in the white pulp in RANK−/− mice (Fig. 8C,D). The red pulp was somewhat expanded relative to control animals, reflecting the enhanced extramedullary erythropoiesis seen in RANK−/− mice.
In all cases, the immunohistochemistry indicated that the spleens of RANK−/− mice had an intact T- and B-cell architecture, but, compared to littermates, showed slightly reduced staining with all markers for T and B cells (lymphoid follicle and red pulp) and macrophages including marginal zone subpopulations (MOMA-1 positive staining, Fig. 8E,F) and BM-8-positive macrophages (Fig. 8G,H). Analysis of germinal center morphology in the spleens of unimmunized animals indicated that peanut agglutin-specific germinal centers were present, but were smaller than those in littermate controls (Fig. 8I,J). Follicular DCs and B cells positive for CD35 were also found in RANK−/− mice (Fig. 8K,L). Similarly, IgM- and IgD-positive cells were present and normally distributed, but reduced in number in the lymphoid follicles and red pulp of RANK−/− mice (data not shown).
Lymph nodes were identified in control mice, but could not be found grossly or by serial sectioning of the appropriate anatomic sites of multiple areas in the RANK−/− mice. All components of normal lymph node architecture were completely absent in these areas including mammary glands and mesenteries. By both gross and histological examination, blood vessels and lymphatics were found to be small but present in all tissues. The lack of lymph node organogenesis was not due to a defect in cellular homing of RANK−/− cells, as RANK−/− lymphoid cells were evident in the lymph nodes of chimeras generated using RANK−/− embryonic stem (ES) cells (data not shown). Unlike lymph nodes, mucosa-associated lymphoid tissue was present in RANK−/− mice, although reduced in comparison to littermates. Whereas multiple obvious Peyer’s patches extended beyond the serosa of the duodenum, jejunum, and ileum of wild-type mice, RANK−/− mice had consistently fewer Peyer’s patches that were slightly raised above the serosa and visible as small hypocellular clusters of milary white foci in the duodenum and ileum (Fig. 8M,N). By immunophenotyping, Peyer’s patches from RANK−/− mice were similar to the spleen in a general reduction of B220+ cells. The nasal-associated lymphoid tissue was present but small and hypocellular.
Discussion
The results of this study show that RANK is an essential receptor for the development of osteoclasts and lymph nodes and also influences the development of the B-cell lineage in vivo. The arrest in osteoclast generation is not due to a lack of myeloid stem or progenitor cells but is specific for osteoclast differentiation. Although RANK was initially identified as a gene expressed in myeloid-derived DCs, expression of this receptor is not critical for the differentiation and function of cells of the myeloid compartment including macrophages and DCs. Our data reveal that RANK−/− mice retain mucosal-associated lymphoid tissues, but completely lack all other peripheral lymph nodes, highlighting an additional major function for RANK in lymph node organogenesis. The phenotypic features of RANK−/− mice are similar to those observed with the RANKL−/− (OPGL) mice (Kong et al. 1999), with the notable exception that thymic differentiation is intact in RANK−/− mice but is defective in RANKL−/− mice (see below). The phenotypic similarities and differences between RANK−/− and RANKL−/− mice are illustrated in Table 2.
Table 2.
Comparative phenotypes of RANK−/− and RANKL (OPGL)−/− mice
|
|
RANK−/− micea
|
RANKL (OPGL)−/− miceb
|
|---|---|---|
| Bone | osteopetrotic; no osteoclasts present | osteopetrotic; no osteoclasts present |
| B lymphocytes | reduced number; B-cell development N.D. | reduced number; B-cell development impaired |
| T lymphocytes | CD4+/CD8+ ratio normal; T-cell activation N.D. | CD4+/CD8+ ratio normal; T-cell activation impaired |
| Thymus | normal thymus size; thymocyte development normal | reduced thymus size; thymocyte development impaired |
| Dendritic cells | normal development and function/activation | normal development and function/activation |
| Lymph nodes (LN) | peripheral LN absent; Peyer’s patches smaller | peripheral LN absent; Peyer’s patches smaller |
| Spleen | normal architecture; extramedullary hematopoiesis | normal architecture; extramedullary hematopoiesis |
(N.D.) Not determined; (LN) lymph nodes.
Results from this study.
Results from Kong et al. (1999).
Bones form from either a cartilagenous intermediate (endochondral ossification) or direct calcification of mesenchyme (intramembranous ossification) (Karsenty 1998). Remodeling of bone occurs throughout adult life and is regulated by the interaction of bone marrow stromal cells/osteoblasts and osteoclasts. It is now clear that CSF-1 and RANKL can substitute for stromal cell-derived osteoclastogenic signals and are sufficient to cause the differentiation of osteoclasts from purified hematopoietic precursors, and RANKL expression can be upregulated on stromal cells by bone-resorbing factors (Tsukii et al. 1998). In this paper, we show that in the absence of RANK, the generation of osteoclasts from their myeloid progenitors, both in vitro and in vivo, is blocked, resulting in a complete absence of bone resorption and severe osteopetrosis in RANK−/− mice. RANK has been shown to be expressed on hematopoietic cells and osteoclasts, but not osteoblast or stromal cells (Jimi et al. 1999), supporting the evidence that the absence of osteoclast differentiation observed in the RANK−/− mice is an intrinsic defect in the hematopoietic lineage. The doming of the skull, foreshortening of limbs, abnormal growth plates, and abnormal vertebrae observed in these mice are related to abnormal ossification of a cartilagenous intermediate in all of these sites. Failure of osteoblasts to invade these sites and osteoclasts to modify the osseous precursors may account for the observed abnormalities in the skull and appendicular skeleton (Erlebacher et al. 1995). Cortical bone of long bones is not dependent on endochondral ossification, yet cortical bones of RANK−/− mice are abnormally thin and may exhibit osteophyte formation. Paradoxically, the excess bone in osteopetrosis results in enhanced spontaneous and traumatic fractures. Evidence of fractures was not found in the RANK−/− mice, but the bones were abnormally fragile under digital pressure. The abnormal mineralization, thin cortical bone, and weak spongiosa-like bone accounts for the lack of structural integrity despite the apparent increase in bone mineral density.
Several studies have defined a role for two cognate pairs of the TNF and TNFR families, CD40L/CD40 and RANKL/RANK, in the activation of antigen-presenting cells (APC) (including DCs), which mediate potent signals resulting in the activation of T cells (for review, see Green and Flavell 1999). RANKL and CD40L both upregulate the antiapoptotic protein BCL-xl and block spontaneous cell death of DCs (Wong et al. 1997a). Activation of DCs with either RANKL or CD40L leads to the production of IL-1, IL-6, and IL-12 (van Kooten and Banchereau 1997; Josien et al. 1999), which can influence TH1 differentiation. In this study, we showed by phenotypic and functional analyses that the development and function of both DCs and macrophages in RANK−/− mice are intact. The reduction of CD8α+ lymphoid DC subset after FL treatment of RANK−/− mice presumably correlates to the reduced overall lymphocyte content of RANK−/− spleen. Whereas RANKL may have potent activity on DCs in vitro, CD40L/CD40 may be able to functionally compensate in the absence of RANK during DC development and activation in vivo. By using CD40-deficient mice, Bachmann et al. (1999) have shown that blockade of RANKL using soluble RANK significantly inhibits viral-specific CD4+ T-cell responses. Further experiments are necessary to reveal other in vivo RANKL-dependent APC responses.
T-lymphocyte differentiation is initiated in the thymus from fetal liver- or bone marrow-derived hematopoietic cells, and the thymocyte developmental pathway can be assessed according to the expression of the cell-surface markers CD25 and CD44 (Godfrey and Zlotnik 1993). In contrast to the RANKL knockout animals, RANK−/− mice do not have apparent defects in thymocyte differentiation. The early differentiation of thymocyte precursors, as defined by these markers, progress normally into CD25−/CD44− thymocytes. Thus, although the RANKL knockout mice have markedly reduced thymus size (Kong et al. 1999), the size and cellularity of RANK−/− thymi (expressed as a function of organ weight/body weight) are normal. The thymocyte developmental block attributable to the loss of RANKL expression was an intrinsic defect in bone marrow-derived cells as verified by fetal liver transfer into irradiated mice (Kong et al. 1999). RANKL and RANK both are expressed in the thymus. Specifically, RANKL has been shown to be expressed in single positive (CD4+ or CD8+) thymocytes (Josien et al. 1999), and RANK mRNA has been shown in total thymus preparations (D. Anderson, K. Charrier, and W.C. Dougall, unpubl.) or by in situ hybridization of thymus sections (Kong et al. 1999). However, it is not clear whether RANK protein is expressed in a T-cell, DC, or stromal component of the thymus. That early, pre-TCR, thymocyte development progressed independently of the receptor RANK but was arrested in the absence of RANKL suggests that RANKL may function through another receptor. Alternatively, as multiple TNF-family ligands are capable of ‘reverse-signaling’ to the ligand-expressing cell (Stuber et al. 1995; van Essen et al. 1995; Wiley et al. 1996), RANKL, activated by a receptor other than RANK, may be essential for thymocyte development in this capacity.
The osteopetrotic condition severely affected hematopoiesis in the RANK−/− mice leading to extramedullary hematopoiesis in the spleen with an increase in clonogenic progenitors for granulocyte, erythroid, and macrophage lineages. Despite the lack of visible bone marrow in all sites, the small islands of hematopoietic activity in bone and the extramedullary hematopoiesis in the spleen were sufficient to prevent leucopenia or a dramatic anemia, and the existing anemia was regenerative. The observed lack of extramedullary hematopoiesis in the liver further supports that the anemia was marginal in RANK−/− mice. The loss of RANK expression impairs B-cell development, resulting in a drastic reduction in mature B cells. However, in contrast to the myeloid precursors, the overall frequencies of B cells were not fully compensated in the RANK−/− spleen relative to levels seen in wild-type bone marrow. The defect in B-cell development in the RANKL knockout mice tracked as a cell-autonomous defect within a radiation-sensitive compartment of the bone marrow (Kong et al. 1999). RANKL−/− mice have normal frequencies of B220+/CD25− pro-B cells, but a selective block in the progression to B220+/CD25+ pre-B cells. Detailed analysis of the pro- and pre-B cells in the RANK−/− animals is necessary to reveal the stage in which B-cell development is affected. B-cell deficiencies resulting from inadequate bone marrow hematopoiesis due to osteopetrosis have also been observed with the c-fos−/− mice (Okada et al. 1994) and hck−/−/src−/− mice (Lowell et al. 1996). However, transfer of fetal liver RANK−/− cells into irradiated wild-type recipients will be required to clarify whether the B-cell deficiency seen in RANK−/− mice is intrinsic to the hematopoietic cell lineage or reflects alterations in the stromal environment.
Induced mutations that impinge on the activities of several members of the TNF and TNFR gene families result in aberrant development and function of primary and secondary lymphoid organs (for review, see Chaplin and Fu 1998). Interestingly, mutations that affect both the RANK and the lymphotoxin β receptor (LT β R) systems all lead to defects in lymph node organogenesis (Chaplin and Fu 1998; Futterer et al. 1998), revealing essential requirements for these genes in the development of lymph nodes. It is formally possible that RANK/RANKL interactions are dependent on signals received by the LT β R or that interactions between the LT β R and LT are dependent on signals received by RANK. However, several lines of evidence argue against an obligate interaction between these two pathways. First, mice lacking LT β R, LTα, or LT β are not osteopetrotic. Second, mice lacking RANK have Peyer’s patches, whereas these structures are absent in mice lacking LT β R, LTα, or LT β. Third, germinal centers, marginal zones and follicular dendritic cell networks are affected in spleens from mice lacking LT β R, LTα, or LT β and yet appear grossly unaffected in mice lacking RANK (this paper) or RANKL (Kong et al. 1999). These data suggest that unique signaling pathways essential for the development of lymphoid structures are activated by RANK and the LT β R.
The signal transduction pathways activated by RANK incorporate the tumor necrosis receptor-associated factor (TRAF) family of cytoplasmic adaptor proteins. Studies of RANK signaling in model cell lines have demonstrated the binding of multiple TRAF proteins to distinct regions in the RANK cytoplasmic domain (Darnay et al. 1998, 1999; Galibert et al. 1998; Wong et al. 1998) but have highlighted a crucial role for TRAF6 binding in subsequent NF-κB and JNK activation (Galibert et al. 1998; Hsu et al. 1999). The targeted disruption of TRAF6 leads to osteopetrosis (Lomaga et al. 1999) confirming an essential role for this TRAF protein in bone resorption. It remains to be defined whether the bone resorption defects observed in the TRAF6 knockout mice are intrinsic to the same cells and signaling pathways used by RANK. However, because TRAF6 knockout mice can still form osteoclasts, other TRAF6-independent RANK signaling pathways are crucial for osteoclast differentiation. Similar to RANK−/− animals, double mutant p50−/p52− NF-κB and c-fos mutant mice also have osteoclast defects and highlight the potential importance of JNK- and NF-κB-dependent pathways in the physiology of bone. However, as none of the osteopetrotic mouse mutants or TRAF2, TRAF3, and TRAF6 knockouts have the same deficiency in lymph node formation seen in the RANK−/− animals (Xu et al. 1996; Yeh et al. 1997), TRAF-independent RANK signaling pathways may be crucial for lymph node development.
Materials and methods
Gene targeting and generation of RANK−/− mice
Genomic clones encoding RANK−/− were isolated from a 129-derived λ library (Statagene, La Jolla, CA) and mapped by a combination of restriction digest, PCR, and sequence analyses. A targeting vector was generated by replacing 12 kb of the RANK gene, spanning exons 4–7 and encoding amino acids 95–244, with a PGK–neo cassette. A thymidine kinase cassette was inserted into the 5′ end of the vector. 129-derived ES cells were electroporated with the targeting vector and selected in G418 and ganciclovir. ES clones carrying a RANK allele disrupted by homologous recombination were identified by a combination of PCR and genomic Southern blot analyses. Targeted clones were injected into day-3.5 C57BL/6 blastocysts and transferred to day-2.5 pseudopregnant Swiss–Webster recipients. The resulting male chimeras were bred to C57BL/6 females and analyzed for germ-line transmission. Mice heterozygous for the targeted RANK mutation (RANK+/−) were intercrossed to generate RANK deficient mice (RANK−/−). The mice used throughout these experiments represent random C57BL/6 × 129 hybrids. Age-matched RANK+/+ and RANK+/− littermates were used as controls.
Mouse husbandry
RANK−/− mice lack incisor tooth eruption and are unable to eat standard mouse chow. RANK−/− mice, and all littermates used as controls, were weaned onto a diet composed of rodent chow pellets (no. 8656, Harlan-Teklad, Madison, WI) wetted in 18% (wt/vol) Good Start infant formula (Nestle-Carnation) prepared in sterile water. Swiss-Webster and C57BL/6 mice were obtained from Taconic (Germantown, NY). Genotyping was performed by PCR analysis of ear biopsy genomic DNA.
Osteoclast formation assay
Mouse spleen cells were cultured at 5 × 105 cells/ml in Dulbecco’s modified Eagle medium containing 10% FBS in eight-chamber cover slides (Becton Dickinson/Falcon, Franklin Lakes, NJ). Murine CSF-1 (R&D Systems, Minneapolis, MN) was added at 40 ng/ml and mRANKL LZ (Anderson et al. 1997) was added at 200 ng/ml where indicated. Cells were cultured for 7 days and stained for TRAP (Sigma, St. Louis, MO).
DC isolation and functional assays
DCs were isolated from nontreated animals using collagenase/EDTA treatment of spleens followed by enrichment for CD11c+ cells (De Smedt et al. 1998). Allogeneic T cells (1 × 105) were incubated with varying numbers of CD11c+ DCs for 5 days and cultures were pulsed with 0.5 μCi of [3H]thymidine as described (Maraskovsky et al. 1996). Isolation and analysis of DCs from FL-treated mice was performed as described previously (Maraskovsky et al. 1996).
In vitro colony assays
The in vitro colony assays for splenic CFUs were performed as described previously (Brasel et al. 1996). For enumeration of CFU-GM, CFU-GEMM, and CFU-E, spleen cells were seeded in methylcellulose-based medium (HCC-3230; Stem Cell Technologies, Vancouver, Canada), supplemented with 100 ng/ml of mMGF (Immunex), 50 ng/ml of mIL-3 (Immunex), 2 U/ml of hEPO (R&D Systems; Minneapolis, MN), and 0.2 nmol/liter of hemin (Eastman Kodak; Rochester, NY). Colonies consisting of >50 cells were scored after 9 days of culture. CFU-M were set up using spleen cells cultured in media supplemented with 100 ng/ml of hCSF-1 (Immunex), and colonies were scored after 7 days of culture.
Gross histopathology
Blood samples were collected in EDTA and in serum tubes and analyzed by Phoenix Central Laboratory for Veterinarians (Everett, WA) for a complete blood count and serum biochemistry using a CellDyne 3500 and Hitachi analyzer, respectively. Tissue samples were collected in 10% neutral buffered formalin, processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Histopathological analysis was performed on 38–44 tissues representing all major tissues within all organ systems, including multiple sections of the appendicular and axillary skeleton and serial sections of areas with expected lymph nodes. Bones were decalcified overnight in Immunocal. Additionally, in a subset of mice, inguinal and axillary mammary glands and intestinal mesenteries were removed in toto, fixed in Carnoy’s fixative for 2 hr, progressively hydrated through graded alcohols, stained overnight in carmine stain (Sigma, St. Louis, MO), progressively dehydrated to absolute alcohol, and temporarily cover slipped to identify possible residual lymph nodes (see http://mammary.nih.gov/tools/). Peyer’s patches and the lack of lymph nodes were also visualized and confirmed by intraluminal injection and immersion of tissues in Carnoy’s fixative.
Whole-body microradiographs were performed with a Hewlett Packard Faxitron machine while animals were under anesthesia. For TRAP staining of bone sections, intact femur and L2–L4 vetebral bodies were dissected out and fixed in 10% neutral formalin. Excised bones were decalcified by 5% formic acid, dehydrated, sectioned, and stained for TRAP according to Liu et al. (1987).
Immunohistochemistry
Spleen and Peyer’s patches were immediately quick frozen in optimal cutting temperature compound (OCT; Miles Laboratories Inc., Elkhart, IN) using liquid nitrogen or 2-methyl butane/liquid nitrogen. Sections were cut in a cryostat at 5 μm onto charged slides, air dried overnight, fixed in cold acetone, and dried prior to storage −70°C or placed in PBS for immediate use. Immunophenotypic markers for macrophages included BM8 and ER-TR9 (both Bachem Bioscience, King of Prussia, PA), F4/80, and MOMA-1 (both Serotec). Follicular dendritic cells were identified with CD35 (CR1), B cells with CD45R (B220) (all PharMingen, San Diego, CA); biotinylated peanut agglutin was used for germinal centers (Vector, Burlingame, CA). All primary antibodies were diluted in a 1% blocker in PBS (Boehringer Mannheim, Indianapolis, IN) and revealed as reported previously (De Smedt et al. 1998). Other blockers included 0.3% hydrogen peroxide for endogenous peroxidase and endogenous avidin–biotin blocking kit (Vector), and all washes were performed with a commercial wash buffer (Biogenex, San Ramon, CA). Digital images were captured with an AX-70 microscope (Olympus, Lake Success, NY) attached to a Sony video camera and a personal computer using Flash Point software (Integral Technologies). Controls included parallel staining with isotype-specific irrelevant antibodies and staining of spleen sections from a C57BL/6 mouse.
Flow cytometry and immunohistochemistry
Single-cell suspensions of spleen cells were depleted of RBC with NH4Cl. Two- and three-color flow cytometric analysis of single cell suspensions of spleen and thymi were performed with the following antibodies: anti-B220 phycoerythrin (PE), anti-CD11b, anti-CD3ε, anti-CD4, anti-CD8, TCRαβ, TCRγδ, CD25, CD44, anti-TER-119, GR-1, anti-IgM, anti-IgD-biotin, F4/80-PE, CD4, CD8, anti-CD43; the biotin-conjugated antibodies were followed by the addition of streptavidin-PE, -FITC, or -APC. For analysis of T-cell precursors, thymocytes were stained with the following biotinylated or APC-conjugated lineage markers: CD3ε, CD4, CD8, B220, CD11b, GR-1, and TCRγδ (plus streptavidin-APC) and with FITC-conjugated anti-CD25 and PE-conjugated anti-CD44. Lineage negative cells were then analyzed for their expression of CD25 and CD44. All antibodies were purchased from PharMingen (San Diego, CA), except for the F4/80 and MOMA-2 antibodies, which were from Biosource International (Camarillo, CA) and the BM-8 antibodies from Bachem (Philadelphia, PA).
Acknowledgments
We thank Daniel Hirchstein, Steve Braddy, Bill Billingsley, Kim Stocking, Dan Horovitz, John Marken, Martha Strachan, Matt Heggem, and The Immunex Animal Facility for their technical assistance; Doug Williams, Hillary McKenna, Lisa Sedger, and Laurent Galibert for helpful discussions and critical reading of the manuscript; and Anne Aumell and Gary Carlton for editorial and graphics assistance.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL wdougall@immunex.com; FAX (206) 233-9733.
References
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg SK, Radley JM, Pollard JW, Chisholm OT, Stanley ER, Bertoncello I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993;177:237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel K, McKenna HJ, Morrissey PJ, Charrier K, Morris AE, Lee CC, Williams DE, Lyman SD. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Moroney S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes & Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD, Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of Receptor Activator of NF-κB (RANK) J Biol Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-κB by RANK requires Tumor Necrosis Factor-Associated Factor (TRAF) 6 and NF-κB-inducing kinase. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- De Smedt T, Pajak B, Klaus GG, Noelle RJ, Urbain J, Leo O, Moser M. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J Immunol. 1998;161:4476–4479. [PubMed] [Google Scholar]
- Emery JG, McDonnell P, Brigham Burke M, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzo R, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes & Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC. The involvement of multiple Tumor Necrosis Factor Receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-κB, a member of the TNFR superfamily. J Biol Chem. 1998;273:34120–34127. doi: 10.1074/jbc.273.51.34120. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Green EA, Flavell RA. TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation. J Exp Med. 1999;189:1017–1020. doi: 10.1084/jem.189.7.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang Z-Q, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SEW, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression of murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast Differentiation Factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999;163:434–442. [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Josien R, Wong BR, Li H, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562–2568. [PubMed] [Google Scholar]
- Karsenty G. Genetics of skeletogenesis. Dev Genet. 1998;22:301–313. doi: 10.1002/(SICI)1520-6408(1998)22:4<301::AID-DVG1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos A, Van G, Itie A, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Sanghvi R, Burnell JM, Howard GA. Simultaneous demonstration of bone alkaline and acid phosphatase activities in plastic-embedded sections and differential inhibition of the activities. Histochemistry. 1987;86:559–565. doi: 10.1007/BF00489547. [DOI] [PubMed] [Google Scholar]
- Lomaga MA, Yeh W-C, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40 and LPS signaling. Genes & Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell CA, Niwa M, Soriano P, Varmus HE. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87:1780–1792. [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhaud G, Labat ML, Moricard Y. Dichloromethylene-diphosphate induced impairment of T-lymphocyte function. Proc Natl Acad Sci. 1983;80:4469–4473. doi: 10.1073/pnas.80.14.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differntiation factor in osteoclastogenesis. Biochem Biophy Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- Okada S, Wang Z-Q, Grigoriadis AE, Wagner EF, von Ruden T. Mice lacking c-fos have normal hematopoietic stem cells but exhibit altered B-cell differentiation due to an impaired bone marrow environment. Mol Cell Biol. 1994;14:382–390. doi: 10.1128/mcb.14.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- Roodman GD. Advances in bone biology: The osteoclast. Endoc Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Detlev Moritz J, Schu P, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation, and death. Cell. 1994;76:959–960. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Crosslinking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation of murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takai H, Kanematsu M, Yano K, Tsuda E, Higashio K, Ikeda K, Watanabe K, Yamada Y. Transforming growth factor-β stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem. 1998;273:27091–27096. doi: 10.1074/jbc.273.42.27091. [DOI] [PubMed] [Google Scholar]
- Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386:81–84. doi: 10.1038/386081a0. [DOI] [PubMed] [Google Scholar]
- Tsukii K, Shima N, Mochizuki S, Yamaguchi K, Kinosaki M, Yano K, Shibata O, Udagawa N, Yasuda H, Suda T, Higashio K. Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1 alpha, 25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun. 1998;246:337–341. doi: 10.1006/bbrc.1998.8610. [DOI] [PubMed] [Google Scholar]
- van Essen D, Kikutani H, Gray D. CD40 ligand transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997a;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997b;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci. 1998a;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki S, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998b;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, et al. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage-colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, Pascual V, Hood LE, Clark EA. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161:6113–6121. [PubMed] [Google Scholar]